Maternal Serum and Placental Metabolomes in Association with Prenatal Phthalate Exposure and Neurodevelopmental Outcomes in the MARBLES Cohort
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Child Neurodevelopmental Assessment
2.3. Urinary Phthalate Metabolite Analysis
2.4. Serum Sample Preparation and 1H NMR Metabolomics Analysis
2.5. Placenta Sample Preparation and 1H NMR Metabolomics Analysis
2.6. Statistical Analysis
3. Results
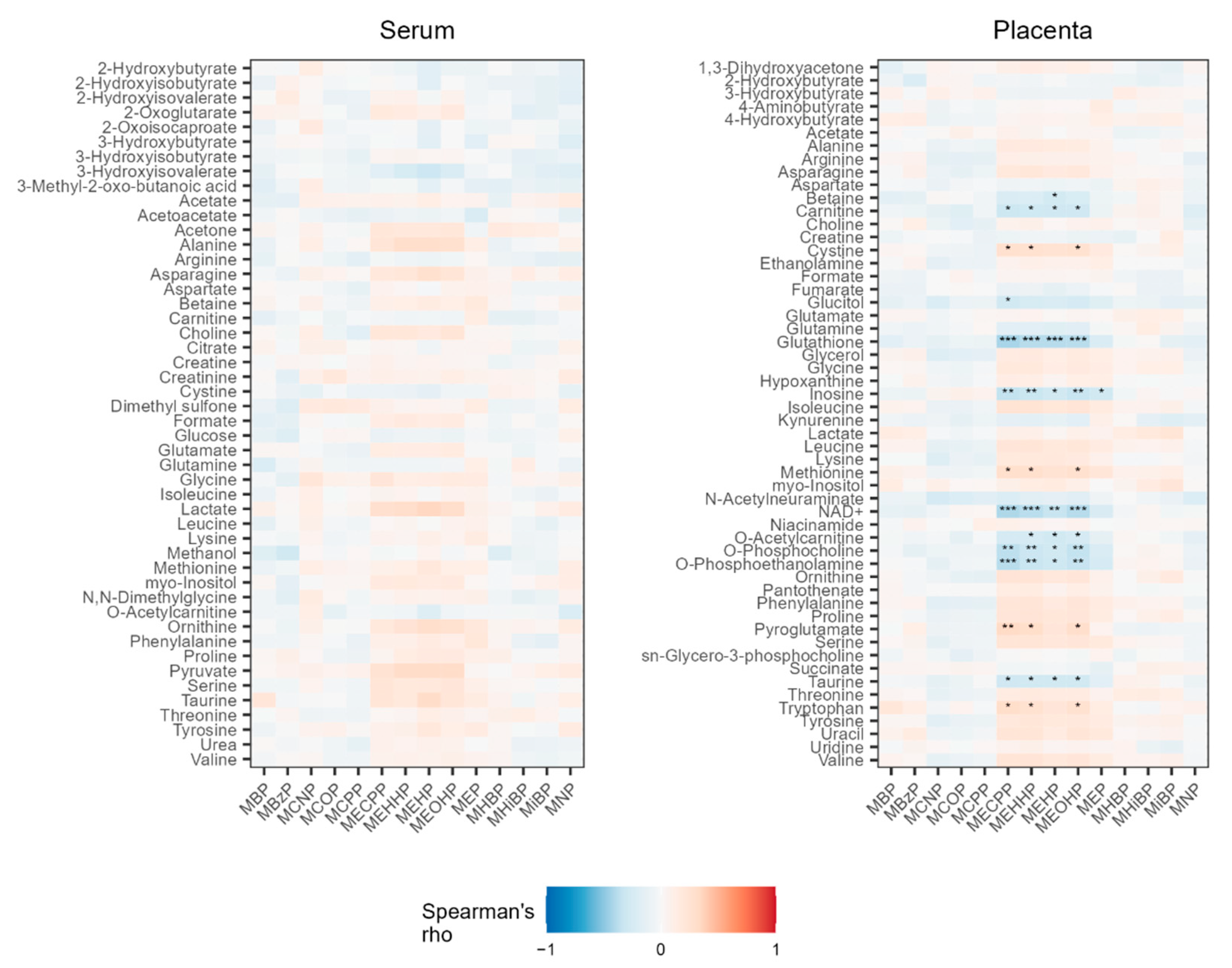
3.1. Associations between Phthalate Metabolites and Serum Metabolites
3.2. Associations between Serum Metabolites and Neurodevelopmental Outcomes
3.3. Associations between Phthalate Metabolites and Placenta Metabolites
3.4. Associations between Placenta Metabolites and Birth Year
3.5. Associations between Placenta Metabolites and Neurodevelopmental Outcomes
4. Discussion
4.1. Associations with Phthalate Exposure
4.2. Associations with Neurodevelopmental Outcome
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rowdhwal, S.S.S.; Chen, J. Toxic Effects of Di-2-Ethylhexyl Phthalate: An Overview. Bio. Med. Res. Int. 2018, 2018, 1750368. [Google Scholar] [CrossRef] [PubMed]
- Martino-Andrade, A.J.; Liu, F.; Sathyanarayana, S.; Barrett, E.S.; Redmon, J.B.; Nguyen, R.H.N.; Levine, H.; Swan, S.H.; the TIDES Study Team. Timing of Prenatal Phthalate Exposure in Relation to Genital Endpoints in Male Newborns. Andrology 2016, 4, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Dales, R.E.; Kauri, L.M.; Cakmak, S. The Associations between Phthalate Exposure and Insulin Resistance, β-Cell Function and Blood Glucose Control in a Population-Based Sample. Sci. Total Environ. 2018, 612, 1287–1292. [Google Scholar] [CrossRef] [PubMed]
- Høyer, B.B.; Lenters, V.; Giwercman, A.; Jönsson, B.A.G.; Toft, G.; Hougaard, K.S.; Bonde, J.P.E.; Specht, I.O. Impact of Di-2-Ethylhexyl Phthalate Metabolites on Male Reproductive Function: A Systematic Review of Human Evidence. Curr. Environ. Health Rep. 2018, 5, 20–33. [Google Scholar] [CrossRef]
- Kim, M.J.; Moon, S.; Oh, B.-C.; Jung, D.; Choi, K.; Park, Y.J. Association Between Diethylhexyl Phthalate Exposure and Thyroid Function: A Meta-Analysis. Thyroid 2019, 29, 183–192. [Google Scholar] [CrossRef]
- Shoshtari-Yeganeh, B.; Zarean, M.; Mansourian, M.; Riahi, R.; Poursafa, P.; Teiri, H.; Rafiei, N.; Dehdashti, B.; Kelishadi, R. Systematic Review and Meta-Analysis on the Association between Phthalates Exposure and Insulin Resistance. Environ. Sci. Pollut. Res. 2019, 26, 9435–9442. [Google Scholar] [CrossRef]
- Lee, D.-W.; Kim, M.-S.; Lim, Y.-H.; Lee, N.; Hong, Y.-C. Prenatal and Postnatal Exposure to Di-(2-Ethylhexyl) Phthalate and Neurodevelopmental Outcomes: A Systematic Review and Meta-Analysis. Environ. Res. 2018, 167, 558–566. [Google Scholar] [CrossRef]
- Koch, H.M.; Preuss, R.; Angerer, J. Di(2-Ethylhexyl)Phthalate (DEHP): Human Metabolism and Internal Exposure—An Update and Latest Results1. Int. J. Androl. 2006, 29, 155–165. [Google Scholar] [CrossRef]
- Zota, A.R.; Calafat, A.M.; Woodruff, T.J. Temporal Trends in Phthalate Exposures: Findings from the National Health and Nutrition Examination Survey, 2001–2010. Environ. Health Perspect. 2014, 122, 235–241. [Google Scholar] [CrossRef]
- Woodruff, T.J.; Zota, A.R.; Schwartz, J.M. Environmental Chemicals in Pregnant Women in the United States: NHANES 2003–2004. Environ. Health Perspect. 2011, 119, 878–885. [Google Scholar] [CrossRef] [Green Version]
- Shin, H.-M.; Dhar, U.; Calafat, A.M.; Nguyen, V.; Schmidt, R.J.; Hertz-Picciotto, I. Temporal Trends of Exposure to Phthalates and Phthalate Alternatives in California Pregnant Women during 2007–2013: Comparison with Other Populations. Environ. Sci. Technol. 2020, 54, 13157–13166. [Google Scholar] [CrossRef] [PubMed]
- Buckley, J.P.; Kuiper, J.R.; Bennett, D.H.; Barrett, E.S.; Bastain, T.; Breton, C.V.; Chinthakindi, S.; Dunlop, A.L.; Farzan, S.F.; Herbstman, J.B.; et al. Exposure to Contemporary and Emerging Chemicals in Commerce among Pregnant Women in the United States: The Environmental Influences on Child Health Outcome (ECHO) Program. Environ. Sci. Technol. 2022, 56, 6560–6573. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, A.G.; Brock, J.W.; Cruze, L.; Newman, R.B.; Unal, E.R.; Wolf, B.J.; Somerville, S.E.; Kucklick, J.R. Prevalence and Predictors of Phthalate Exposure in Pregnant Women in Charleston, SC. Chemosphere 2018, 193, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.; Mita, C.; Bellavia, A.; Parker, M.; James-Todd, T. Racial/Ethnic Disparities in Pregnancy and Prenatal Exposure to Endocrine-Disrupting Chemicals Commonly Used in Personal Care Products. Curr. Environ. Health Rep. 2021, 8, 98–112. [Google Scholar] [CrossRef] [PubMed]
- Latini, G.; De Felice, C.; Presta, G.; Del Vecchio, A.; Paris, I.; Ruggieri, F.; Mazzeo, P. In Utero Exposure to Di-(2-Ethylhexyl)Phthalate and Duration of Human Pregnancy. Environ. Health Perspect. 2003, 111, 1783–1785. [Google Scholar] [CrossRef]
- Fromme, H.; Gruber, L.; Seckin, E.; Raab, U.; Zimmermann, S.; Kiranoglu, M.; Schlummer, M.; Schwegler, U.; Smolic, S.; Völkel, W. Phthalates and Their Metabolites in Breast Milk—Results from the Bavarian Monitoring of Breast Milk (BAMBI). Environ. Int. 2011, 37, 715–722. [Google Scholar] [CrossRef]
- Huang, P.-C.; Tsai, C.-H.; Liang, W.-Y.; Li, S.-S.; Huang, H.-B.; Kuo, P.-L. Early Phthalates Exposure in Pregnant Women Is Associated with Alteration of Thyroid Hormones. PLoS ONE 2016, 11, e0159398. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, H.Y.; Bae, S.; Lim, Y.-H.; Hong, Y.-C. Diethylhexyl Phthalates Is Associated with Insulin Resistance via Oxidative Stress in the Elderly: A Panel Study. PLoS ONE 2013, 8, e71392. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, K.K.; Cantonwine, D.E.; Rivera-González, L.O.; Loch-Caruso, R.; Mukherjee, B.; AnzalotaDel Toro, L.V.; Jiménez-Vélez, B.; Calafat, A.M.; Ye, X.; Alshawabkeh, A.N.; et al. Urinary Phthalate Metabolite Associations with Biomarkers of Inflammation and Oxidative Stress Across Pregnancy in Puerto Rico. Environ. Sci. Technol. 2014, 48, 7018–7025. [Google Scholar] [CrossRef]
- Duan, Y.; Wang, L.; Han, L.; Wang, B.; Sun, H.; Chen, L.; Zhu, L.; Luo, Y. Exposure to Phthalates in Patients with Diabetes and Its Association with Oxidative Stress, Adiponectin, and Inflammatory Cytokines. Environ. Int. 2017, 109, 53–63. [Google Scholar] [CrossRef]
- Ferguson, K.K.; Chen, Y.-H.; VanderWeele, T.J.; McElrath, T.F.; Meeker, J.D.; Mukherjee, B. Mediation of the Relationship between Maternal Phthalate Exposure and Preterm Birth by Oxidative Stress with Repeated Measurements across Pregnancy. Environ. Health Perspect. 2017, 125, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.M.; Jones, D.P.; Harris, C. The Redox Theory of Development. Antioxid. Redox Signal. 2019, 32, 715–740. [Google Scholar] [CrossRef] [PubMed]
- Manivasagam, T.; Arunadevi, S.; Essa, M.M.; SaravanaBabu, C.; Borah, A.; Thenmozhi, A.J.; Qoronfleh, M.W. Role of Oxidative Stress and Antioxidants in Autism. In Personalized Food Intervention and Therapy for Autism Spectrum Disorder Management, Advances in Neurobiology; Essa, M.M., Qoronfleh, M.W., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 193–206. ISBN 978-3-030-30402-7. [Google Scholar]
- Oulhote, Y.; Lanphear, B.; Braun, J.M.; Webster, G.M.; Arbuckle, T.E.; Etzel, T.; Forget-Dubois, N.; Seguin, J.R.; Bouchard, M.F.; MacFarlane, A.; et al. Gestational Exposures to Phthalates and Folic Acid, and Autistic Traits in Canadian Children. Environ. Health Perspect. 2020, 128, 027004. [Google Scholar] [CrossRef] [PubMed]
- Day, D.B.; Collett, B.R.; Barrett, E.S.; Bush, N.R.; Swan, S.H.; Nguyen, R.H.N.; Szpiro, A.A.; Sathyanarayana, S. Phthalate Mixtures in Pregnancy, Autistic Traits, and Adverse Childhood Behavioral Outcomes. Environ. Int. 2021, 147, 106330. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.I.; Lee, J.; Lee, K.-S.; Lee, Y.A.; Shin, C.H.; Hong, Y.-C.; Kim, B.-N.; Lim, Y.-H. Association of Phthalate Exposure with Autistic Traits in Children. Environ. Int. 2021, 157, 106775. [Google Scholar] [CrossRef]
- Shin, H.-M.; Schmidt, R.J.; Tancredi, D.; Barkoski, J.; Ozonoff, S.; Bennett, D.H.; Hertz-Picciotto, I. Prenatal Exposure to Phthalates and Autism Spectrum Disorder in the MARBLES Study. Environ. Health 2018, 17, 85. [Google Scholar] [CrossRef]
- Haggerty, D.K.; Strakovsky, R.S.; Talge, N.M.; Carignan, C.C.; Glazier-Essalmi, A.N.; Ingersoll, B.R.; Karthikraj, R.; Kannan, K.; Paneth, N.S.; Ruden, D.M. Prenatal Phthalate Exposures and Autism Spectrum Disorder Symptoms in Low-Risk Children. Neurotoxicol. Teratol. 2021, 83, 106947. [Google Scholar] [CrossRef]
- Lord, C.; Brugha, T.S.; Charman, T.; Cusack, J.; Dumas, G.; Frazier, T.; Jones, E.J.H.; Jones, R.M.; Pickles, A.; State, M.W.; et al. Autism Spectrum Disorder. Nat. Rev. Dis. Primer 2020, 6, 5. [Google Scholar] [CrossRef]
- Rosen, N.E.; Lord, C.; Volkmar, F.R. The Diagnosis of Autism: From Kanner to DSM-III to DSM-5 and Beyond. J. Autism Dev. Disord. 2021, 51, 4253–4270. [Google Scholar] [CrossRef]
- Ozonoff, S.; Heung, K.; Byrd, R.; Hansen, R.; Hertz-Picciotto, I. The Onset of Autism: Patterns of Symptom Emergence in the First Years of Life. Autism Res. Off. J. Int. Soc. Autism Res. 2008, 1, 320–328. [Google Scholar] [CrossRef] [Green Version]
- Ozonoff, S.; Young, G.S.; Brian, J.; Charman, T.; Shephard, E.; Solish, A.; Zwaigenbaum, L. Diagnosis of Autism Spectrum Disorder After Age 5 in Children Evaluated Longitudinally Since Infancy. J. Am. Acad. Child Adolesc. Psychiatry 2018, 57, 849–857.e2. [Google Scholar] [CrossRef] [PubMed]
- Bennett, D.H.; Busgang, S.A.; Kannan, K.; Parsons, P.J.; Takazawa, M.; Palmer, C.D.; Schmidt, R.J.; Doucette, J.T.; Schweitzer, J.B.; Gennings, C.; et al. Environmental Exposures to Pesticides, Phthalates, Phenols and Trace Elements Are Associated with Neurodevelopment in the CHARGE Study. Environ. Int. 2022, 161, 107075. [Google Scholar] [CrossRef] [PubMed]
- Pacyga, D.C.; Sathyanarayana, S.; Strakovsky, R.S. Dietary Predictors of Phthalate and Bisphenol Exposures in Pregnant Women. Adv. Nutr. 2019, 10, 803–815. [Google Scholar] [CrossRef] [PubMed]
- Hertz-Picciotto, I.; Schmidt, R.J.; Krakowiak, P. Understanding Environmental Contributions to Autism: Causal Concepts and the State of Science. Autism Res. 2018, 11, 554–586. [Google Scholar] [CrossRef]
- Tanner, S.; Thomson, S.; Drummond, K.; O’Hely, M.; Symeonides, C.; Mansell, T.; Saffery, R.; Sly, P.D.; Collier, F.; Burgner, D.; et al. A Pathway-Based Genetic Score for Oxidative Stress: An Indicator of Host Vulnerability to Phthalate-Associated Adverse Neurodevelopment. Antioxidants 2022, 11, 659. [Google Scholar] [CrossRef]
- Zhang, X.; Barr, D.B.; Dunlop, A.L.; Panuwet, P.; Sarnat, J.A.; Lee, G.E.; Tan, Y.; Corwin, E.J.; Jones, D.P.; Ryan, P.B.; et al. Assessment of Metabolic Perturbations Associated with Exposure to Phthalates among Pregnant African American Women. Sci. Total Environ. 2021, 818, 151689. [Google Scholar] [CrossRef]
- Grindler, N.M.; Vanderlinden, L.; Karthikraj, R.; Kannan, K.; Teal, S.; Polotsky, A.J.; Powell, T.L.; Yang, I.V.; Jansson, T. Exposure to Phthalate, an Endocrine Disrupting Chemical, Alters the First Trimester Placental Methylome and Transcriptome in Women. Sci. Rep. 2018, 8, 6086. [Google Scholar] [CrossRef]
- Paquette, A.G.; MacDonald, J.; Lapehn, S.; Bammler, T.; Kruger, L.; Day, D.B.; Price, N.D.; Loftus, C.; Kannan, K.; Marsit, C.; et al. A Comprehensive Assessment of Associations between Prenatal Phthalate Exposure and the Placental Transcriptomic Landscape. Environ. Health Perspect. 2021, 129, 97003. [Google Scholar] [CrossRef]
- Orozco, J.S.; Hertz-Picciotto, I.; Abbeduto, L.; Slupsky, C.M. Metabolomics Analysis of Children with Autism, Idiopathic-Developmental Delays, and Down Syndrome. Transl. Psychiatry 2019, 9, 1–15. [Google Scholar] [CrossRef]
- Smith, A.M.; Natowicz, M.R.; Braas, D.; Ludwig, M.A.; Ney, D.M.; Donley, E.L.R.; Burrier, R.E.; Amaral, D.G. A Metabolomics Approach to Screening for Autism Risk in the Children’s Autism Metabolome Project. Autism Res. Off. J. Int. Soc. Autism Res. 2020, 13, 1270–1285. [Google Scholar] [CrossRef]
- Hollowood, K.; Melnyk, S.; Pavliv, O.; Evans, T.; Sides, A.; Schmidt, R.J.; Hertz-Picciotto, I.; Elms, W.; Guerrero, E.; Kruger, U.; et al. Maternal Metabolic Profile Predicts High or Low Risk of an Autism Pregnancy Outcome. Res. Autism Spectr. Disord. 2018, 56, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Ritz, B.; Yan, Q.; Uppal, K.; Liew, Z.; Cui, X.; Ling, C.; Inoue, K.; von Ehrenstein, O.; Walker, D.I.; Jones, D.P. Untargeted Metabolomics Screen of Mid-Pregnancy Maternal Serum and Autism in Offspring. Autism Res. 2020, 13, 1258–1269. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.J.; Liang, D.; Busgang, S.A.; Curtin, P.; Giulivi, C. Maternal Plasma Metabolic Profile Demarcates a Role for Neuroinflammation in Non-Typical Development of Children. Metabolites 2021, 11, 545. [Google Scholar] [CrossRef]
- Zhu, Y.; Gao, H.; Huang, K.; Zhang, Y.; Cai, X.; Yao, H.; Mao, L.; Ge, X.; Zhou, S.; Xu, Y.; et al. Prenatal Phthalate Exposure and Placental Size and Shape at Birth: A Birth Cohort Study. Environ. Res. 2018, 160, 239–246. [Google Scholar] [CrossRef]
- Warner, G.R.; Dettogni, R.S.; Bagchi, I.C.; Flaws, J.A.; Graceli, J.B. Placental Outcomes of Phthalate Exposure. Reprod. Toxicol. 2021, 103, 1–17. [Google Scholar] [CrossRef]
- Hertz-Picciotto, I.; Schmidt, R.; Walker, C.K.; Bennett, D.H.; Oliver, M.; Shedd-Wise, K.M.; LaSalle, J.M.; Giulivi, C.; Puschner, B.; Thomas, J.; et al. A Prospective Study of Environmental Exposures and Early Biomarkers in Autism Spectrum Disorder: Design, Protocols, and Preliminary Data from the MARBLES Study. Environ. Health Perspect. 2018, 126, 117004. [Google Scholar] [CrossRef]
- Lord, C.; Risi, S.; Lambrecht, L.; Cook, E.H.; Leventhal, B.L.; DiLavore, P.C.; Pickles, A.; Rutter, M. The Autism Diagnostic Observation Schedule-Generic: A Standard Measure of Social and Communication Deficits Associated with the Spectrum of Autism. J. Autism Dev. Disord. 2000, 30, 205–223. [Google Scholar] [CrossRef] [PubMed]
- Mullen, E.M.; American Guidance Service. Mullen Scales of Early Learning; AGS: Circle Pines, MN, USA, 1995. [Google Scholar]
- Ozonoff, S.; Young, G.S.; Belding, A.; Hill, M.; Hill, A.; Hutman, T.; Johnson, S.; Miller, M.; Rogers, S.J.; Schwichtenberg, A.J.; et al. The Broader Autism Phenotype in Infancy: When Does It Emerge? J. Am. Acad. Child Adolesc. Psychiatry 2014, 53, 398–407.e2. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Otoki, Y.; McClorry, S.; Coates, L.C.; Lombardi, R.L.; Taha, A.Y.; Slupsky, C.M. Optimization of a Method for the Simultaneous Extraction of Polar and Non-Polar Oxylipin Metabolites, DNA, RNA, Small RNA, and Protein from a Single Small Tissue Sample. Methods Protoc. 2020, 3, 61. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Keil, A.P.; Buckley, J.P.; O’Brien, K.M.; Ferguson, K.K.; Zhao, S.; White, A.J. A Quantile-Based g-Computation Approach to Addressing the Effects of Exposure Mixtures. Environ. Health Perspect. 2020, 128, 47004. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.J. Permutational Multivariate Analysis of Variance (PERMANOVA). In Wiley StatsRef: Statistics Reference Online; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 1–15. ISBN 978-1-118-44511-2. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package 2020. Available online: https://CRAN.R-project.org/package=vegan (accessed on 21 August 2021).
- Anderson, M.J. Permutation Tests for Univariate or Multivariate Analysis of Variance and Regression. Can. J. Fish. Aquat. Sci. 2001, 58, 626–639. [Google Scholar] [CrossRef]
- Textor, J.; van der Zander, B.; Gilthorpe, M.S.; Liśkiewicz, M.; Ellison, G.T. Robust Causal Inference Using Directed Acyclic Graphs: The R Package ‘Dagitty’. Int. J. Epidemiol. 2016, 45, 1887–1894. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.J.; Iosif, A.-M.; Angel, E.G.; Ozonoff, S. Association of Maternal Prenatal Vitamin Use With Risk for Autism Spectrum Disorder Recurrence in Young Siblings. JAMA Psychiatry 2019, 76, 391–398. [Google Scholar] [CrossRef]
- Krakowiak, P.; Walker, C.K.; Bremer, A.A.; Baker, A.S.; Ozonoff, S.; Hansen, R.L.; Hertz-Picciotto, I. Maternal Metabolic Conditions and Risk for Autism and Other Neurodevelopmental Disorders. Pediatrics 2012, 129, e1121–e1128. [Google Scholar] [CrossRef]
- Carpenter, D.O.; Arcaro, K.; Spink, D.C. Understanding the Human Health Effects of Chemical Mixtures. Environ. Health Perspect. 2002, 110, 25–42. [Google Scholar] [CrossRef]
- Yaghjyan, L.; Sites, S.; Ruan, Y.; Chang, S.-H. Associations of Urinary Phthalates with Body Mass Index, Waist Circumference, and Serum Lipids Among Females: National Health and Nutrition Examination Survey 1999–2004. Int. J. Obes. 2015, 39, 994–1000. [Google Scholar] [CrossRef]
- Adeva-Andany, M.M.; Calvo-Castro, I.; Fernández-Fernández, C.; Donapetry-García, C.; Pedre-Piñeiro, A.M. Significance of L-Carnitine for Human Health. IUBMB Life 2017, 69, 578–594. [Google Scholar] [CrossRef]
- Xu, Y.; Knipp, G.T.; Cook, T.J. Effects of Di-(2-Ethylhexyl)-Phthalate and Its Metabolites on the Lipid Profiling in Rat HRP-1 Trophoblast Cells. Arch. Toxicol. 2006, 80, 293–298. [Google Scholar] [CrossRef]
- Xu, Y.; Agrawal, S.; Cook, T.J.; Knipp, G.T. Maternal Di-(2-Ethylhexyl)-Phthalate Exposure Influences Essential Fatty Acid Homeostasis in Rat Placenta. Placenta 2008, 29, 962–969. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Wu, Z.; Heng, J.; Tian, M.; Chen, J.; Chen, F.; Guan, W. L-Carnitine Increases Cell Proliferation and Amino Acid Transporter Expression via the Activation of Insulin-like Growth Factor I Signaling Pathway in Rat Trophoblast Cells. Food Sci. Nutr. 2020, 8, 3298–3307. [Google Scholar] [CrossRef] [PubMed]
- Arenas, J.; Rubio, J.C.; Martín, M.A.; Campos, Y. Biological Roles of L-Carnitine in Perinatal Metabolism. Early Hum. Dev. 1998, 53, S43–S50. [Google Scholar] [CrossRef]
- Bai, M.; Zeng, Q.; Chen, Y.; Chen, M.; Li, P.; Ma, Z.; Sun, D.; Zhou, H.; Zheng, C.; Zeng, S.; et al. Maternal Plasma L-Carnitine Reduction During Pregnancy Is Mainly Attributed to OCTN2-Mediated Placental Uptake and Does Not Result in Maternal Hepatic Fatty Acid β-Oxidation Decline. Drug Metab. Dispos. Biol. Fate Chem. 2019, 47, 582–591. [Google Scholar] [CrossRef] [PubMed]
- Fagerberg, L.; Hallström, B.M.; Oksvold, P.; Kampf, C.; Djureinovic, D.; Odeberg, J.; Habuka, M.; Tahmasebpoor, S.; Danielsson, A.; Edlund, K.; et al. Analysis of the Human Tissue-Specific Expression by Genome-Wide Integration of Transcriptomics and Antibody-Based Proteomics. Mol. Cell. Proteom. MCP 2014, 13, 397–406. [Google Scholar] [CrossRef]
- Chang, T.-T.; Shyu, M.-K.; Huang, M.-C.; Hsu, C.-C.; Yeh, S.-Y.; Chen, M.-R.; Lin, C.-J. Hypoxia-Mediated Down-Regulation of OCTN2 and PPARα Expression in Human Placentas and in BeWo Cells. Mol. Pharm. 2011, 8, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Cantonwine, D.E.; Meeker, J.D.; Ferguson, K.K.; Mukherjee, B.; Hauser, R.; McElrath, T.F. Urinary Concentrations of Bisphenol A and Phthalate Metabolites Measured during Pregnancy and Risk of Preeclampsia. Environ. Health Perspect. 2016, 124, 1651–1655. [Google Scholar] [CrossRef]
- Bedell, S.M.; Lyden, G.R.; Sathyanarayana, S.; Barrett, E.S.; Ferguson, K.K.; Santilli, A.; Bush, N.R.; Swan, S.H.; McElrath, T.F.; Nguyen, R.H.N. First- and Third-Trimester Urinary Phthalate Metabolites in the Development of Hypertensive Diseases of Pregnancy. Int. J. Environ. Res. Public. Health 2021, 18, 10627. [Google Scholar] [CrossRef]
- Peluso, G.; Barbarisi, A.; Savica, V.; Reda, E.; Nicolai, R.; Benatti, P.; Calvani, M. Carnitine: An Osmolyte That Plays a Metabolic Role. J. Cell. Biochem. 2000, 80, 1–10. [Google Scholar] [CrossRef]
- Brusati, V.; Józwik, M.; Józwik, M.; Teng, C.; Paolini, C.; Marconi, A.M.; Battaglia, F.C. Fetal and Maternal Non-Glucose Carbohydrates and Polyols Concentrations in Normal Human Pregnancies at Term. Pediatr. Res. 2005, 58, 700–704. [Google Scholar] [CrossRef]
- Jauniaux, E.; Hempstock, J.; Teng, C.; Battaglia, F.C.; Burton, G.J. Polyol Concentrations in the Fluid Compartments of the Human Conceptus during the First Trimester of Pregnancy: Maintenance of Redox Potential in a Low Oxygen Environment. J. Clin. Endocrinol. Metab. 2005, 90, 1171–1175. [Google Scholar] [CrossRef] [Green Version]
- Arroyo, J.A.; Garcia-Jones, P.; Graham, A.; Teng, C.C.; Battaglia, F.C.; Galan, H.L. Placental TonEBP/NFAT5 Osmolyte Regulation in an Ovine Model of Intrauterine Growth Restriction. Biol. Reprod. 2012, 86, 94. [Google Scholar] [CrossRef] [PubMed]
- Wang, B. Molecular Mechanism Underlying Sialic Acid as an Essential Nutrient for Brain Development and Cognition. Adv. Nutr. 2012, 3, 465S–472S. [Google Scholar] [CrossRef] [PubMed]
- Bian, D.; Wang, X.; Huang, J.; Chen, X.; Li, H. Maternal Neu5Ac Supplementation During Pregnancy Improves Offspring Learning and Memory Ability in Rats. Front. Nutr. 2021, 8. [Google Scholar] [CrossRef] [PubMed]
- Abeln, M.; Albers, I.; Peters-Bernard, U.; Flächsig-Schulz, K.; Kats, E.; Kispert, A.; Tomlinson, S.; Gerardy-Schahn, R.; Münster-Kühnel, A.; Weinhold, B. Sialic Acid Is a Critical Fetal Defense against Maternal Complement Attack. J. Clin. Investig. 2019, 129, 422–436. [Google Scholar] [CrossRef]
- Ma, Z.; Yang, H.; Peng, L.; Kuhn, C.; Chelariu-Raicu, A.; Mahner, S.; Jeschke, U.; von Schönfeldt, V. Expression of the Carbohydrate Lewis Antigen, Sialyl Lewis A, Sialyl Lewis X, Lewis X, and Lewis Y in the Placental Villi of Patients with Unexplained Miscarriages. Front. Immunol. 2021, 12, 679424. [Google Scholar] [CrossRef] [PubMed]
- Marini, M.; Bonaccini, L.; Thyrion, G.D.Z.; Vichi, D.; Parretti, E.; Sgambati, E. Distribution of Sugar Residues in Human Placentas from Pregnancies Complicated by Hypertensive Disorders. Acta Histochem. 2011, 113, 815–825. [Google Scholar] [CrossRef]
- Chiodelli, P.; Rezzola, S.; Urbinati, C.; Federici Signori, F.; Monti, E.; Ronca, R.; Presta, M.; Rusnati, M. Contribution of Vascular Endothelial Growth Factor Receptor-2 Sialylation to the Process of Angiogenesis. Oncogene 2017, 36, 6531–6541. [Google Scholar] [CrossRef]
- Chen, D.; Zheng, J. Regulation of Placental Angiogenesis. Microcirculation 2014, 21, 15–25. [Google Scholar] [CrossRef]
- Seymore, T.N.; Rivera-Núñez, Z.; Stapleton, P.A.; Adibi, J.J.; Barrett, E.S. Phthalate Exposures and Placental Health in Animal Models and Humans: A Systematic Review. Toxicol. Sci. 2022, 188, kfac060. [Google Scholar] [CrossRef]
- Landaas, S. The Formation of 2-Hydroxybutyric Acid in Experimental Animals. Clin. Chim. Acta Int. J. Clin. Chem. 1975, 58, 23–32. [Google Scholar] [CrossRef]
- Lord, R.S.; Bralley, J.A. Clinical Applications of Urinary Organic Acids. Part I: Detoxification Markers. Altern. Med. Rev. J. Clin. Ther. 2008, 13, 205–215. [Google Scholar]
- Amara, I.; Salah, A.; Timoumi, R.; Annabi, E.; Scuto, M.; Trovato, A.; Neffati, F.; Calabrese, V.; Abid-Essefi, S. Effect of Di(2-Ethylhexyl) Phthalate on Nrf2-Regulated Glutathione Homeostasis in Mouse Kidney. Cell Stress Chaperones 2020, 25, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, H.; Ashari, S. Mechanistic Insight into Toxicity of Phthalates, the Involved Receptors, and the Role of Nrf2, NF-ΚB, and PI3K/AKT Signaling Pathways. Environ. Sci. Pollut. Res. 2021, 28, 35488–35527. [Google Scholar] [CrossRef]
- Wagner-Golbs, A.; Neuber, S.; Kamlage, B.; Christiansen, N.; Bethan, B.; Rennefahrt, U.; Schatz, P.; Lind, L. Effects of Long-Term Storage at −80 °C on the Human Plasma Metabolome. Metabolites 2019, 9, 99. [Google Scholar] [CrossRef] [PubMed]
- Serkova, N.; Bendrick-Peart, J.; Alexander, B.; Tissot van Patot, M.C. Metabolite Concentrations in Human Term Placentae and Their Changes Due to Delayed Collection After Delivery. Placenta 2003, 24, 227–235. [Google Scholar] [CrossRef]
- Walejko, J.M.; Chelliah, A.; Keller-Wood, M.; Gregg, A.; Edison, A.S. Global Metabolomics of the Placenta Reveals Distinct Metabolic Profiles between Maternal and Fetal Placental Tissues Following Delivery in Non-Labored Women. Metabolites 2018, 8, 10. [Google Scholar] [CrossRef]
- Mitro, S.D.; Wu, J.; Rahman, M.L.; Cao, Y.; Zhu, Y.; Chen, Z.; Chen, L.; Li, M.; Hinkle, S.N.; Bremer, A.A.; et al. Longitudinal Plasma Metabolomics Profile in Pregnancy—A Study in an Ethnically Diverse U.S. Pregnancy Cohort. Nutrients 2021, 13, 3080. [Google Scholar] [CrossRef]
- Hertz-Picciotto, I.; Delwiche, L. The Rise in Autism and the Role of Age at Diagnosis. Epidemiol. Camb. Mass 2009, 20, 84–90. [Google Scholar] [CrossRef]
- Bai, D.; Yip, B.H.K.; Windham, G.C.; Sourander, A.; Francis, R.; Yoffe, R.; Glasson, E.; Mahjani, B.; Suominen, A.; Leonard, H.; et al. Association of Genetic and Environmental Factors with Autism in a 5-Country Cohort. JAMA Psychiatry 2019, 76, 1035–1043. [Google Scholar] [CrossRef]
- Newschaffer, C.J.; Croen, L.A.; Fallin, M.D.; Hertz-Picciotto, I.; Nguyen, D.V.; Lee, N.L.; Berry, C.A.; Farzadegan, H.; Hess, H.N.; Landa, R.J.; et al. Infant Siblings and the Investigation of Autism Risk Factors. J. Neurodev. Disord. 2012, 4, 7. [Google Scholar] [CrossRef]
- Hollowood-Jones, K.; Adams, J.B.; Coleman, D.M.; Ramamoorthy, S.; Melnyk, S.; James, S.J.; Woodruff, B.K.; Pollard, E.L.; Snozek, C.L.; Kruger, U.; et al. Altered Metabolism of Mothers of Young Children with Autism Spectrum Disorder: A Case Control Study. BMC Pediatr. 2020, 20, 557. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, C.S. The Placenta-Brain-Axis. J. Neurosci. Res. 2021, 99, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Loomes, R.; Hull, L.; Mandy, W.P.L. What Is the Male-to-Female Ratio in Autism Spectrum Disorder? A Systematic Review and Meta-Analysis. J. Am. Acad. Child Adolesc. Psychiatry 2017, 56, 466–474. [Google Scholar] [CrossRef]
- Ferri, S.L.; Abel, T.; Brodkin, E.S. Sex Differences in Autism Spectrum Disorder: A Review. Curr. Psychiatry Rep. 2018, 20, 9. [Google Scholar] [CrossRef] [PubMed]
- Meakin, A.S.; Cuffe, J.S.M.; Darby, J.R.T.; Morrison, J.L.; Clifton, V.L. Let’s Talk about Placental Sex, Baby: Understanding Mechanisms That Drive Female- and Male-Specific Fetal Growth and Developmental Outcomes. Int. J. Mol. Sci. 2021, 22, 6386. [Google Scholar] [CrossRef]
- Inkster, A.M.; Fernández-Boyano, I.; Robinson, W.P. Sex Differences Are Here to Stay: Relevance to Prenatal Care. J. Clin. Med. 2021, 10, 3000. [Google Scholar] [CrossRef]
- DiPietro, J.A.; Voegtline, K.M. The Gestational Foundation of Sex Differences in Development and Vulnerability. Neuroscience 2017, 342, 4–20. [Google Scholar] [CrossRef]
- Shin, H.-M.; Bennett, D.H.; Barkoski, J.; Ye, X.; Calafat, A.M.; Tancredi, D.; Hertz-Picciotto, I. Variability of Urinary Concentrations of Phthalate Metabolites during Pregnancy in First Morning Voids and Pooled Samples. Environ. Int. 2019, 122, 222–230. [Google Scholar] [CrossRef]
- James-Todd, T.; Meeker, J.; Huang, T.; Hauser, R.; Seely, E.; Ferguson, K.; Rich-Edwards, J.; McElrath, T. Racial and Ethnic Variations in Phthalate Metabolite Concentrations across Pregnancy. J. Expo. Sci. Environ. Epidemiol. 2017, 27, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Kupsco, A.J.; Deierlein, A.L.; Just, A.C.; Calafat, A.M.; Oken, E.; Braun, J.M.; Mercado-Garcia, A.; Cantoral, A.; Téllez-Rojo, M.M.; et al. Trends and Patterns of Phthalates and Phthalate Alternatives Exposure in Pregnant Women from Mexico City during 2007–2010. Environ. Sci. Technol. 2020, 54, 1740–1749. [Google Scholar] [CrossRef]
| Serum | Placenta | |||||||
|---|---|---|---|---|---|---|---|---|
| TD (n = 66) | ASD (n = 26) | Non-TD (n = 14) | p | TD (n = 83) | ASD (n = 32) | Non-TD (n = 17) | p | |
| Maternal age at birth, years | 35 ± 5 | 36 ± 5 | 34 ± 4 | 0.38 | 35 ± 5 | 35 ± 5 | 33 ± 4 | 0.40 |
| Birth weight, g | 3412 ± 448 | 3465 ± 428 | 3287 ± 268 | 0.20 | 3435 ± 486 | 3445 ± 443 | 3344 ± 378 | 0.81 |
| Birth year | 2011 ± 2 | 2011 ± 2 | 2012 ± 2 | 0.53 | 2010 ± 2 | 2010 ± 2 | 2010 ± 2 | 0.15 |
| Pre-pregnancy BMI | 25.7 ± 5.0 | 27.1 ± 7.4 | 27.6 ± 10.5 | 0.85 | 26.3 ± 6.0 | 28.1 ± 8.2 | 29.2 ± 9.8 | 0.45 |
| Time since last meal or snack, minutes | 102 ± 75 | 101 ± 66 | 94 ± 68 | 0.95 | - | - | - | - |
| Gestational age at birth, weeks | 38.8 ± 1.5 | 39.4 ± 0.8 | 39.1 ± 0.9 | 0.35 | 38.9 ± 1.3 | 39.6 ± 1.0 | 38.9 ± 1.3 | 0.04 |
| Gestational age at serum collection, days | 233 ± 19 | 230 ± 18 | 238 ± 19 | 0.50 | - | - | - | - |
| Prenatal vitamin use in the first month of pregnancy | 36 (54.5%) | 11 (42.3%) | 8 (57.1%) | 0.52 | 50 (60.2%) | 10 (31.2%) | 8 (47.1%) | 0.02 |
| Male child | 34 (51.5%) | 18 (69.2%) | 8 (57.1%) | 0.30 | 43 (51.8%) | 23 (71.9%) | 10 (58.8%) | 0.15 |
| Cesarean delivery | 27 (40.9%) | 9 (34.6%) | 4 (28.6%) | 0.64 | 48 (57.8%) | 24 (75%) | 11 (64.7%) | 0.23 |
| Delivery payer: Government | 16 (24.2%) | 6 (23.1%) | 4 (28.6%) | 0.87 | 17 (20.5%) | 7 (21.9%) | 6 (35.3%) | 0.34 |
| Homeowner | 42 (63.6%) | 15 (57.7%) | 8 (57.1%) | 0.82 | 47 (56.6%) | 17 (53.1%) | 11 (64.7%) | 0.74 |
| At least a bachelor’s degree | 37 (56.1%) | 13 (50%) | 5 (35.7%) | 0.37 | 49 (59%) | 14 (43.8%) | 5 (29.4%) | 0.05 |
| Maternal race/ethnicity | 0.43 | 0.44 | ||||||
| Non-Hispanic, White | 36 (54.5%) | 14 (53.8%) | 5 (35.7%) | 50 (60.2%) | 16 (50%) | 8 (47.1%) | ||
| Historically marginalized groups | ||||||||
| Black/African-American | 1 (1.5%) | 1 (3.8%) | 2 (14.3%) | 3 (3.6%) | 3 (9.4%) | 2 (11.8%) | ||
| Asian | 12 (18.2%) | 5 (19.2%) | 3 (21.4%) | 11 (13.3%) | 4 (12.5%) | 3 (17.6%) | ||
| Hispanic, white | 14 (21.2%) | 4 (15.4%) | 3 (21.4%) | 17 (20.5%) | 7 (21.9%) | 3 (17.6%) | ||
| Hispanic, non-white | 1 (1.5%) | 1 (3.8%) | 1 (7.1%) | 0 (0%) | 1 (3.1%) | 1 (5.9%) | ||
| Multi-racial | 2 (3.0%) | 1 (3.8%) | 0 (0%) | 2 (2.4%) | 1 (3.1%) | 0 (0%) | ||
| Type 2 diabetes * | 2 (3%) | 0 (0%) | 0 (0%) | >0.9 | 2 (2.4%) | 0 (0%) | 0 (0%) | >0.9 |
| Gestational diabetes * | 15 (22.7%) | 5 (19.2%) | 1 (7.1%) | 0.43 | 15 (18.1%) | 8 (25%) | 1 (5.9%) | 0.27 |
| Hypertension * | 6 (9.1%) | 3 (11.5%) | 2 (14.3%) | 0.72 | 5 (6%) | 3 (9.4%) | 2 (11.8%) | 0.57 |
| Preeclampsia * | 3 (4.5%) | 1 (3.8%) | 1 (7.1%) | 0.81 | 4 (4.8%) | 2 (6.2%) | 2 (11.8%) | 0.35 |
| Maternal metabolic condition * | 0.37 | 0.23 | ||||||
| BMI ≤ 25 and no metabolic conditions | 25 (37.9%) | 8 (30.8%) | 7 (50.0%) | 34 (41.0%) | 8 (25.0%) | 7 (41.2%) | ||
| 25 < BMI < 30 and no metabolic conditions | 14 (21.2%) | 6 (23.1%) | 5 (35.7%) | 16 (19.3%) | 7 (21.9%) | 5 (29.4%) | ||
| BMI ≥ 30 and no other metabolic conditions | 8 (12.1%) | 4 (15.4%) | 0 (0%) | 14 (16.9%) | 5 (15.6%) | 2 (11.8%) | ||
| Any hypertensive disorder (without any diabetes) at any BMI | 2 (3.0%) | 3 (11.5%) | 1 (7.1%) | 2 (2.4%) | 4 (12.5%) | 2 (11.8%) | ||
| Diabetes at any BMI | 17 (25.8%) | 5 (19.2%) | 1 (7.1%) | 17 (20.5%) | 8 (25.0%) | 1 (5.9%) | ||
| Total Effect | Direct Effect | |||||
|---|---|---|---|---|---|---|
| Metabolite | Estimate (95% CI) | p | FDR p | Estimate (95% CI) | p | FDR p |
| 1,3-Dihydroxyacetone | 0.0034 (−0.1237, 0.1306) | 0.9579 | 0.9579 | −0.0019 (−0.1452, 0.1415) | 0.9795 | 0.9983 |
| 2-Hydroxybutyrate | −0.0804 (−0.1378, −0.0230) | 0.0071 | 0.0896 | −0.0925 (−0.1562, −0.0287) | 0.0053 | 0.0907 |
| 3-Hydroxybutyrate | −0.0600 (−0.1955, 0.0754) | 0.3868 | 0.8106 | −0.0655 (−0.2129, 0.0820) | 0.3860 | 0.9046 |
| 4-Aminobutyrate | 0.0081 (−0.0747, 0.0910) | 0.8476 | 0.9579 | 0.0223 (−0.0632, 0.1079) | 0.6103 | 0.9679 |
| 4-Hydroxybutyrate | −0.0103 (−0.1715, 0.1508) | 0.9002 | 0.9579 | −0.0166 (−0.1975, 0.1643) | 0.8574 | 0.9781 |
| Acetate | −0.0231 (−0.1363, 0.0901) | 0.6899 | 0.9292 | −0.0240 (−0.1405, 0.0925) | 0.6871 | 0.9764 |
| Alanine | −0.0146 (−0.0659, 0.0367) | 0.5780 | 0.9292 | −0.0122 (−0.0669, 0.0425) | 0.6632 | 0.9679 |
| Arginine | −0.0250 (−0.0814, 0.0313) | 0.3853 | 0.8106 | −0.0285 (−0.0954, 0.0384) | 0.4058 | 0.9046 |
| Asparagine | −0.0175 (−0.0706, 0.0356) | 0.5192 | 0.8869 | −0.0156 (−0.0729, 0.0416) | 0.5935 | 0.9679 |
| Aspartate | −0.0323 (−0.0800, 0.0153) | 0.1863 | 0.6288 | −0.0372 (−0.0891, 0.0147) | 0.1634 | 0.5882 |
| Betaine | −0.0473 (−0.0983, 0.0036) | 0.0714 | 0.3613 | −0.0445 (−0.1051, 0.0162) | 0.1537 | 0.5882 |
| Carnitine | −0.0738 (−0.1207, −0.0270) | 0.0025 | 0.0783 | −0.0785 (−0.1306, −0.0265) | 0.0038 | 0.0907 |
| Choline | −0.0231 (−0.0756, 0.0294) | 0.3903 | 0.8106 | −0.0237 (−0.0791, 0.0317) | 0.4040 | 0.9046 |
| Creatine | −0.0132 (−0.0538, 0.0274) | 0.5256 | 0.8869 | −0.0047 (−0.0546, 0.0452) | 0.8537 | 0.9781 |
| Cystine | 0.0588 (−0.0470, 0.1646) | 0.2786 | 0.8106 | 0.0647 (−0.0471, 0.1765) | 0.2593 | 0.7779 |
| Ethanolamine | −0.0173 (−0.0679, 0.0333) | 0.5039 | 0.8869 | −0.0214 (−0.0773, 0.0344) | 0.4542 | 0.9084 |
| Formate | −0.0382 (−0.1018, 0.0255) | 0.2425 | 0.7703 | −0.0336 (−0.0955, 0.0283) | 0.2894 | 0.8225 |
| Fumarate | −0.0628 (−0.1519, 0.0263) | 0.1697 | 0.6185 | −0.0622 (−0.1668, 0.0424) | 0.2463 | 0.7779 |
| Glucitol | −0.0912 (−0.1498, −0.0326) | 0.0029 | 0.0783 | −0.0956 (−0.1596, −0.0315) | 0.0042 | 0.0907 |
| Glutamate | −0.0160 (−0.0608, 0.0288) | 0.4855 | 0.8869 | −0.0191 (−0.0684, 0.0302) | 0.4486 | 0.9084 |
| Glutamine | −0.0185 (−0.0561, 0.0191) | 0.3364 | 0.8106 | −0.0257 (−0.0637, 0.0124) | 0.1893 | 0.6389 |
| Glutathione | −0.1151 (−0.2481, 0.0180) | 0.0928 | 0.4176 | −0.1437 (−0.2729, −0.0144) | 0.0315 | 0.2430 |
| Glycerol | −0.0149 (−0.0791, 0.0493) | 0.6503 | 0.9292 | −0.0160 (−0.0837, 0.0516) | 0.6432 | 0.9679 |
| Glycine | −0.0105 (−0.0579, 0.0369) | 0.6655 | 0.9292 | −0.0045 (−0.0585, 0.0494) | 0.8694 | 0.9781 |
| Hypoxanthine | −0.0167 (−0.0657, 0.0324) | 0.5070 | 0.8869 | −0.0173 (−0.0712, 0.0366) | 0.5310 | 0.9679 |
| Inosine | −0.0733 (−0.1778, 0.0312) | 0.1718 | 0.6185 | −0.0995 (−0.2103, 0.0112) | 0.0810 | 0.3365 |
| Isoleucine | −0.0099 (−0.0652, 0.0454) | 0.7261 | 0.9294 | −0.0032 (−0.0637, 0.0572) | 0.9167 | 0.9900 |
| Kynurenine | −0.0718 (−0.1461, 0.0025) | 0.0608 | 0.3613 | −0.0776 (−0.1594, 0.0042) | 0.0656 | 0.2952 |
| Lactate | 0.0089 (−0.0317, 0.0495) | 0.6687 | 0.9292 | 0.0082 (−0.0360, 0.0525) | 0.7164 | 0.9781 |
| Leucine | −0.0118 (−0.0693, 0.0458) | 0.6894 | 0.9292 | −0.0037 (−0.0666, 0.0591) | 0.9076 | 0.9900 |
| Lysine | −0.0307 (−0.0935, 0.0320) | 0.3395 | 0.8106 | −0.0192 (−0.0866, 0.0482) | 0.5779 | 0.9679 |
| Methionine | 0.0147 (−0.0464, 0.0758) | 0.6379 | 0.9292 | 0.0202 (−0.0459, 0.0864) | 0.5497 | 0.9679 |
| myo-Inositol | 0.0223 (−0.0222, 0.0668) | 0.3286 | 0.8106 | 0.0176 (−0.0357, 0.0709) | 0.5187 | 0.9679 |
| N-Acetylneuraminate | −0.0844 (−0.1460, −0.0228) | 0.0083 | 0.0896 | −0.0901 (−0.1559, −0.0244) | 0.0084 | 0.0907 |
| NAD+ | −0.1044 (−0.2397, 0.0309) | 0.1333 | 0.5537 | −0.1315 (−0.2700, 0.0070) | 0.0654 | 0.2952 |
| Niacinamide | 0.0255 (−0.2252, 0.2762) | 0.8422 | 0.9579 | −0.0034 (−0.2684, 0.2616) | 0.9798 | 0.9983 |
| O-Acetylcarnitine | −0.0665 (−0.1150, −0.0181) | 0.0082 | 0.0896 | −0.0733 (−0.1256, −0.0209) | 0.0071 | 0.0907 |
| O-Phosphocholine | −0.1516 (−0.2954, −0.0077) | 0.0412 | 0.3178 | −0.1647 (−0.3153, −0.0140) | 0.0344 | 0.2430 |
| O-Phosphoethanolamine | −0.1191 (−0.2315, −0.0067) | 0.0401 | 0.3178 | −0.1212 (−0.2405, −0.0019) | 0.0490 | 0.2940 |
| Ornithine | −0.0055 (−0.1014, 0.0903) | 0.9098 | 0.9579 | 0.0129 (−0.0879, 0.1137) | 0.8024 | 0.9781 |
| Pantothenate | −0.0384 (−0.1141, 0.0373) | 0.3226 | 0.8106 | −0.0406 (−0.1219, 0.0407) | 0.3297 | 0.8902 |
| Phenylalanine | −0.0141 (−0.0703, 0.0421) | 0.6232 | 0.9292 | −0.0085 (−0.0702, 0.0532) | 0.7878 | 0.9781 |
| Proline | 0.0019 (−0.0495, 0.0533) | 0.9429 | 0.9579 | 0.0078 (−0.0470, 0.0627) | 0.7803 | 0.9781 |
| Pyroglutamate | −0.0272 (−0.0888, 0.0344) | 0.3890 | 0.8106 | −0.0281 (−0.0961, 0.0398) | 0.4188 | 0.9046 |
| Serine | −0.0044 (−0.0643, 0.0555) | 0.8862 | 0.9579 | 0.0001 (−0.0665, 0.0667) | 0.9983 | 0.9983 |
| sn-Glycero−3-phosphocholine | −0.0390 (−0.1353, 0.0574) | 0.4295 | 0.8590 | −0.0388 (−0.1299, 0.0523) | 0.4054 | 0.9046 |
| Succinate | −0.0096 (−0.1030, 0.0838) | 0.8403 | 0.9579 | −0.0243 (−0.1149, 0.0662) | 0.5995 | 0.9679 |
| Taurine | −0.0366 (−0.0761, 0.0028) | 0.0714 | 0.3613 | −0.0406 (−0.0819, 0.0008) | 0.0570 | 0.2952 |
| Threonine | 0.0048 (−0.0500, 0.0596) | 0.8635 | 0.9579 | 0.0103 (−0.0495, 0.0701) | 0.7363 | 0.9781 |
| Tryptophan | 0.0877 (−0.0075, 0.1829) | 0.0736 | 0.3613 | 0.1052 (0.0081, 0.2022) | 0.0360 | 0.2430 |
| Tyrosine | 0.0027 (−0.0511, 0.0565) | 0.9225 | 0.9579 | 0.0080 (−0.0508, 0.0668) | 0.7901 | 0.9781 |
| Uracil | 0.0090 (−0.0656, 0.0835) | 0.8141 | 0.9579 | 0.0070 (−0.0744, 0.0884) | 0.8671 | 0.9781 |
| Uridine | −0.0154 (−0.0953, 0.0644) | 0.7055 | 0.9292 | −0.0199 (−0.1088, 0.0690) | 0.6619 | 0.9679 |
| Valine | −0.0087 (−0.0597, 0.0424) | 0.7401 | 0.9294 | −0.0013 (−0.0575, 0.0549) | 0.9650 | 0.9983 |
| Metabolite | Estimate (95% CI) | r2 | p | FDR p |
|---|---|---|---|---|
| Arginine | −0.0502 (−0.0965, −0.0038) | 0.0681 | 0.0344 | 0.0978 |
| Creatine | 0.0405 (0.0040, 0.0771) | 0.0712 | 0.0338 | 0.0978 |
| Cystine | −0.1050 (−0.1792, −0.0308) | 0.1110 | 0.0064 | 0.0432 |
| Glutathione | 0.1238 (0.0390, 0.2087) | 0.1172 | 0.0052 | 0.0401 |
| Inosine | 0.1328 (0.0678, 0.1978) | 0.2063 | 0.0000 | 0.0000 |
| Isoleucine | −0.0407 (−0.0765, −0.0049) | 0.0745 | 0.0278 | 0.0945 |
| Leucine | −0.0478 (−0.0842, −0.0114) | 0.0972 | 0.0122 | 0.0549 |
| Lysine | −0.0483 (−0.0912, −0.0054) | 0.0734 | 0.0280 | 0.0945 |
| Methionine | −0.0539 (−0.0941, −0.0137) | 0.1007 | 0.0074 | 0.0444 |
| NAD+ | 0.2018 (0.1186, 0.2849) | 0.2686 | 0.0000 | 0.0000 |
| O-Phosphocholine | 0.1244 (0.0402, 0.2086) | 0.1198 | 0.0040 | 0.0360 |
| O-Phosphoethanolamine | 0.0935 (0.0324, 0.1545) | 0.1275 | 0.0038 | 0.0360 |
| Ornithine | −0.0536 (−0.1026, −0.0045) | 0.0693 | 0.0320 | 0.0978 |
| Phenylalanine | −0.0461 (−0.0815, −0.0108) | 0.0960 | 0.0110 | 0.0549 |
| Pyroglutamate | −0.0974 (−0.1457, −0.0490) | 0.2018 | 0.0000 | 0.0000 |
| Serine | −0.0527 (−0.0933, −0.0121) | 0.0951 | 0.0116 | 0.0549 |
| Tryptophan | −0.0934 (−0.1659, −0.0208) | 0.0937 | 0.0138 | 0.0573 |
| Tyrosine | −0.0420 (−0.0773, −0.0067) | 0.0812 | 0.0218 | 0.0841 |
| Uracil | −0.0802 (−0.1318, −0.0286) | 0.1309 | 0.0028 | 0.0360 |
| All Children | Females | Males | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Term | df | F | R2 | p | df | F | R2 | p | df | F | R2 | p |
| Birth year | 1 | 11.22 | 0.0777 | 0.0001 | 1 | 4.19 | 0.0713 | 0.0013 | 1 | 7.12 | 0.0852 | 0.0001 |
| Delivery mode | 1 | 1.33 | 0.0092 | 0.2064 | 1 | 0.68 | 0.0116 | 0.7105 | 1 | 1.23 | 0.0147 | 0.2595 |
| Gestational age at delivery, weeks | 1 | 1.15 | 0.0079 | 0.2895 | 1 | 0.95 | 0.0162 | 0.4333 | 1 | 1.11 | 0.0132 | 0.3224 |
| Homeownership | 1 | 0.56 | 0.0039 | 0.8152 | 1 | 1.13 | 0.0192 | 0.3087 | 1 | 1.59 | 0.0190 | 0.1361 |
| Maternal age, years | 1 | 0.74 | 0.0051 | 0.6227 | 1 | 0.91 | 0.0156 | 0.4727 | 1 | 0.47 | 0.0057 | 0.8711 |
| Maternal race/ethnicity | 1 | 0.86 | 0.0059 | 0.5091 | 1 | 0.57 | 0.0097 | 0.8241 | 1 | 0.9 | 0.0108 | 0.4689 |
| Maternal metabolic condition | 4 | 0.6 | 0.0167 | 0.9596 | 4 | 0.85 | 0.0578 | 0.6787 | 4 | 0.53 | 0.0251 | 0.9843 |
| Birth weight, grams | 1 | 0.38 | 0.0026 | 0.9579 | 1 | 0.8 | 0.0136 | 0.5581 | 1 | 0.63 | 0.0076 | 0.7155 |
| Prenatal vitamin use in the first month of pregnancy | 1 | 1.81 | 0.0126 | 0.0793 | 1 | 0.64 | 0.0110 | 0.7473 | 1 | 2.34 | 0.0280 | 0.0337 |
| Neurodevelopmental outcome | 2 | 1.45 | 0.0201 | 0.127 | 2 | 1 | 0.0342 | 0.4164 | 2 | 1.84 | 0.0440 | 0.0451 |
| Residual | 117 | 0.8099 | 41 | 0.6980 | 61 | 0.7296 | ||||||
| Total | 131 | 1.0000 | 55 | 1.0000 | 75 | 1.0000 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parenti, M.; Schmidt, R.J.; Ozonoff, S.; Shin, H.-M.; Tancredi, D.J.; Krakowiak, P.; Hertz-Picciotto, I.; Walker, C.K.; Slupsky, C.M. Maternal Serum and Placental Metabolomes in Association with Prenatal Phthalate Exposure and Neurodevelopmental Outcomes in the MARBLES Cohort. Metabolites 2022, 12, 829. https://doi.org/10.3390/metabo12090829
Parenti M, Schmidt RJ, Ozonoff S, Shin H-M, Tancredi DJ, Krakowiak P, Hertz-Picciotto I, Walker CK, Slupsky CM. Maternal Serum and Placental Metabolomes in Association with Prenatal Phthalate Exposure and Neurodevelopmental Outcomes in the MARBLES Cohort. Metabolites. 2022; 12(9):829. https://doi.org/10.3390/metabo12090829
Chicago/Turabian StyleParenti, Mariana, Rebecca J. Schmidt, Sally Ozonoff, Hyeong-Moo Shin, Daniel J. Tancredi, Paula Krakowiak, Irva Hertz-Picciotto, Cheryl K. Walker, and Carolyn M. Slupsky. 2022. "Maternal Serum and Placental Metabolomes in Association with Prenatal Phthalate Exposure and Neurodevelopmental Outcomes in the MARBLES Cohort" Metabolites 12, no. 9: 829. https://doi.org/10.3390/metabo12090829
APA StyleParenti, M., Schmidt, R. J., Ozonoff, S., Shin, H.-M., Tancredi, D. J., Krakowiak, P., Hertz-Picciotto, I., Walker, C. K., & Slupsky, C. M. (2022). Maternal Serum and Placental Metabolomes in Association with Prenatal Phthalate Exposure and Neurodevelopmental Outcomes in the MARBLES Cohort. Metabolites, 12(9), 829. https://doi.org/10.3390/metabo12090829









