Metabolite Changes in Orange Dead Leaf Butterfly Kallima inachus during Ontogeny and Diapause
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Collection
2.2. Metabolite Extraction for the Untargeted Metabolomic Analysis
2.3. UPLC-MS/MS Analysis for the Untargeted Metabolomics
2.4. Bioinformatic Analysis of the Untargeted Metabolomic Dataset
2.5. Statistical Analysis
3. Results and Discussion
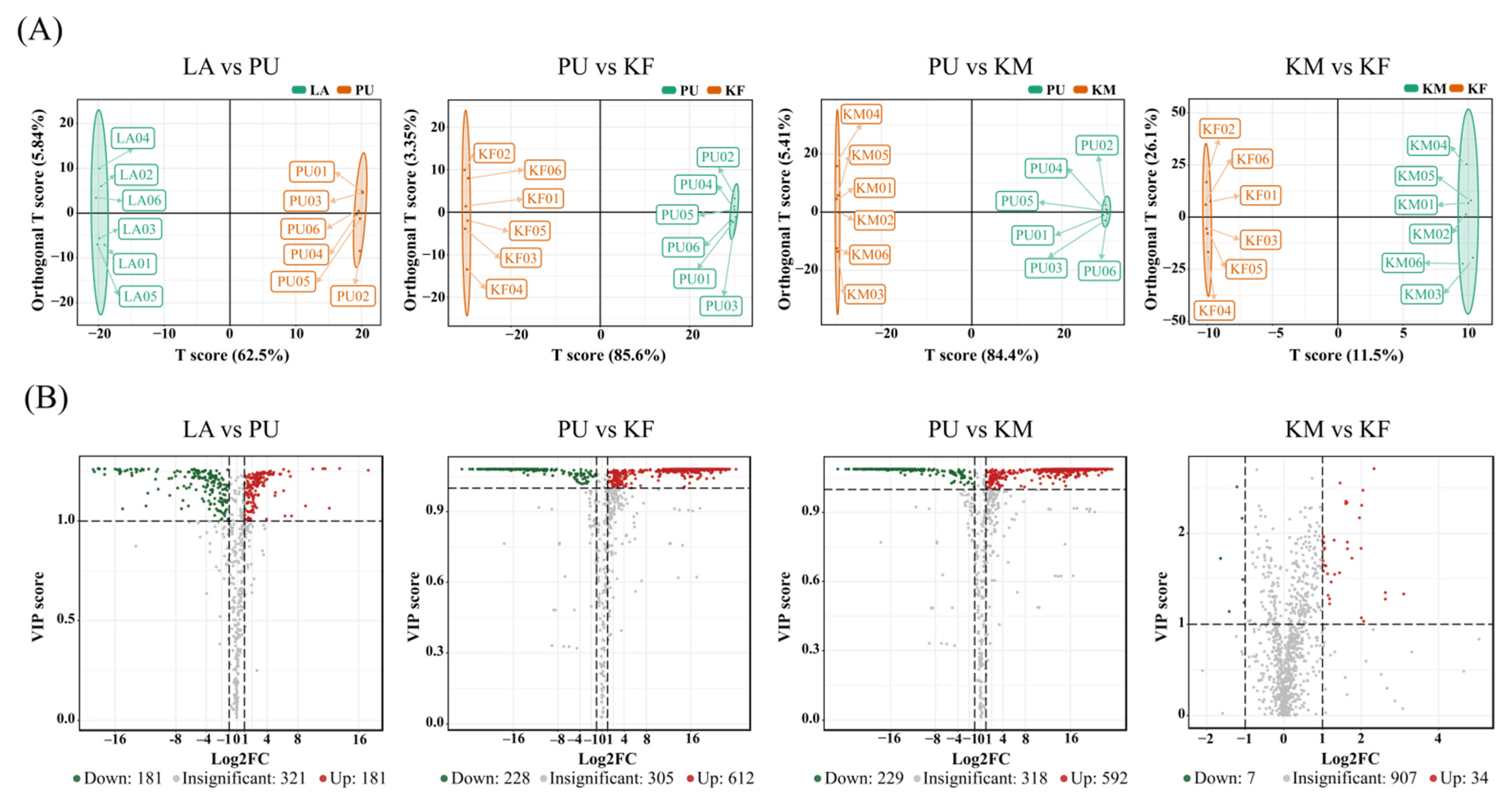
3.1. Untargeted Metabolite Analysis of Samples
3.2. Vital Metabolites for Stage Development
3.3. Vital Metabolites for Transition of Successive Stages
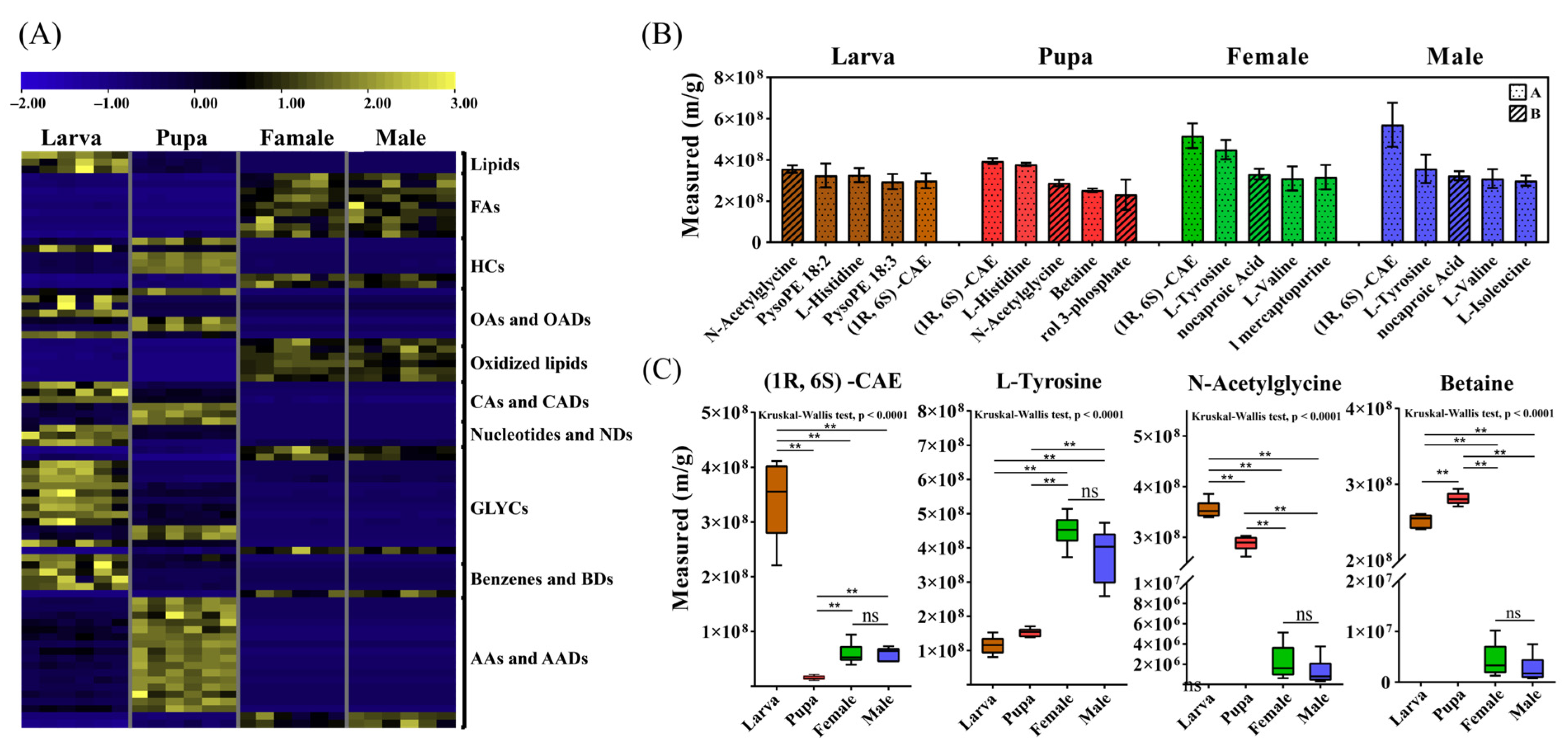
3.4. The Endogenous Metabolome of Different Developmental Stages
3.5. Diapause-Associated Changes in Metabolite Profiles
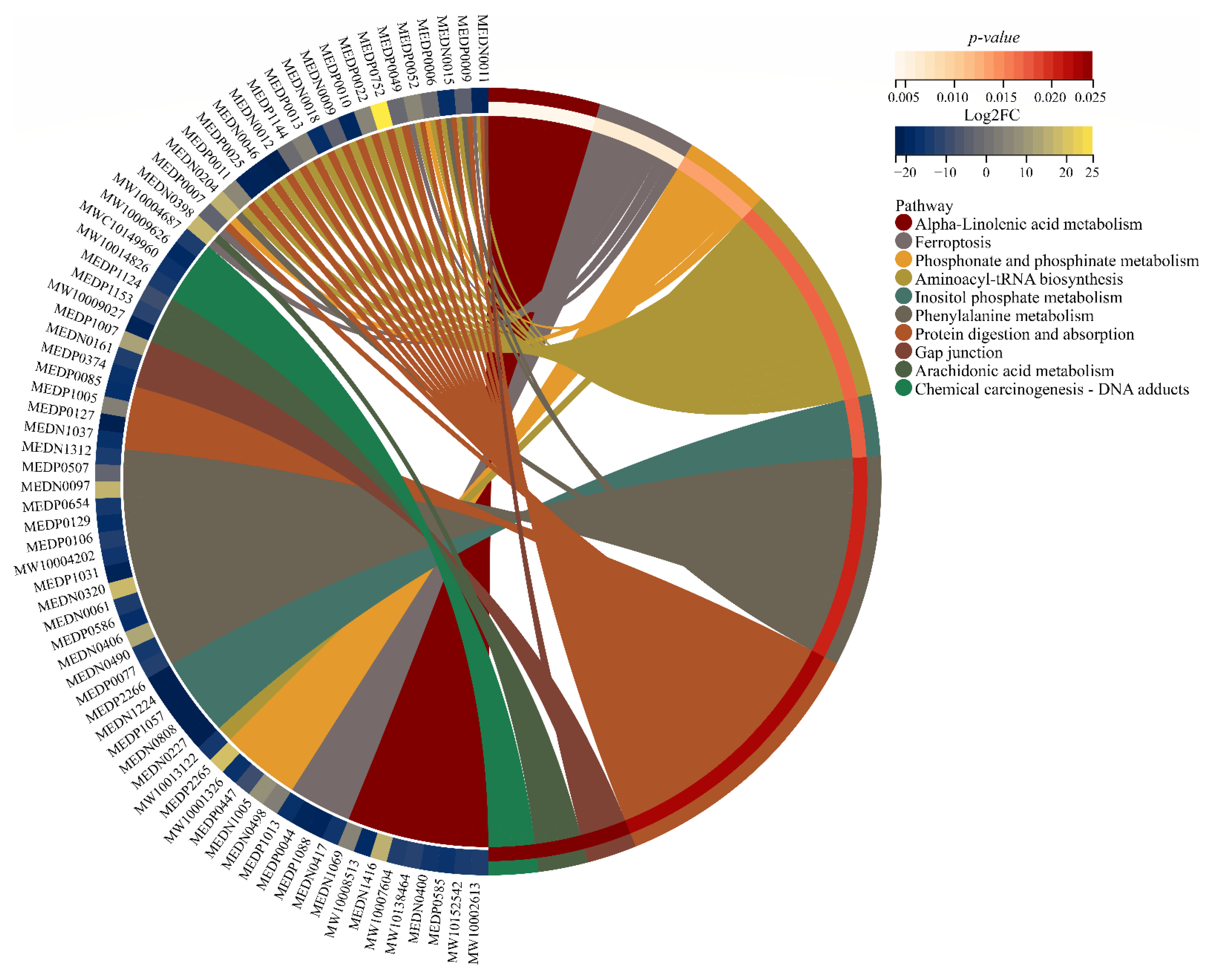
3.6. Key Pathways Affected by Entering into Adult Diapause
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TCA | Tricarboxylic acid |
| VIP | Variable importance in projection |
| AA | Amino acid |
| AAD | Amino acid derivative |
| BD | Benzene derivative |
| FA | Fatty acyl |
| OA | Organic acid |
| OAD | Organic acid derivative |
| CA | Carboxylic acid |
| CAD | Carboxylic acids derivative |
| GLYC | Glycerophospholipid |
| Gly/Glu | Glycolysis/Gluconeogenesis |
| ND | Nucleotide derivative |
| HC | Heterocyclic compound |
| Cys and Met | Cysteine and methionine |
| AKEs | Aldehyde, Ketones, Esters |
| CVs | CoEnzyme and vitamins |
| ALC and AMs | Alcohol and amines |
References
- Labandeira, C.C.; Phillips, T.L. A carboniferous insect gall: Insight into early ecologic history of the Holometabola. Proc. Natl. Acad. Sci. USA 1996, 93, 8470–8474. [Google Scholar] [CrossRef] [PubMed]
- Hammer, T.J.; Moran, N.A. Links between metamorphosis and symbiosis in holometabolous insects. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2019, 374, 20190068. [Google Scholar] [CrossRef] [PubMed]
- Rolff, J.; Johnston, P.R.; Reynolds, S. Complete metamorphosis of insects. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2019, 374, 20190063. [Google Scholar] [CrossRef] [PubMed]
- Duneau, D.F.; Lazzaro, B.P. Persistence of an extracellular systemic infection across metamorphosis in a holometabolous insect. Biol. Lett. 2018, 14, 20170771. [Google Scholar] [CrossRef] [PubMed]
- Snart, C.J.; Hardy, I.C.; Barrett, D.A. Entometabolomics: Applications of modern analytical techniques to insect studies. Entomol. Exp. Appl. 2015, 155, 1–17. [Google Scholar] [CrossRef]
- Zhang, A.; Sun, H.; Wang, P.; Han, Y.; Wang, X. Modern analytical techniques in metabolomics analysis. Analyst 2012, 137, 293–300. [Google Scholar] [CrossRef]
- Phalaraksh, C.; Reynolds, S.E.; Wilson, I.D.; Lenz, E.M.; Nicholson, J.K.; Lindon, J.C. A metabonomic analysis of insect development: 1H-NMR spectroscopic characterization of changes in the composition of the haemolymph of larvae and pupae of the tobacco hornworm. Manduca Sexta. Sci. 2008, 34, 279–286. [Google Scholar]
- Zhou, L.; Li, H.; Hao, F.; Li, N.; Liu, X.; Wang, G.; Wang, Y.; Tang, H. Developmental changes for the hemolymph metabolome of silkworm (Bombyx mori L.). J. Proteome. Res. 2015, 14, 2331–2347. [Google Scholar] [CrossRef]
- Cao, Y.Y.; Peng, L.L.; Jiang, L.; Thakur, K.; Hu, F.; Tang, S.M.; Wei, Z.J. Evaluation of the metabolic effects of hydrogen sulfide on the development of Bombyx mori (Lepidoptera: Bombycidae), using liquid chromatography-mass spectrometry-based metabolomics. J. Insect. Sci. 2020, 20, 4. [Google Scholar] [CrossRef]
- Chowdhury, S.; Fuller, R.A.; Dingle, H.; Chapman, J.W.; Zalucki, M.P. Migration in butterflies: A global overview. Biol. Rev. Camb. Philos. Soc. 2021, 96, 1462–1483. [Google Scholar] [CrossRef]
- Radchuk, V.; Turlure, C.; Schtickzelle, N. Each life stage matters: The importance of assessing the response to climate change over the complete life cycle in butterflies. J. Anim. Ecol. 2013, 82, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Posledovich, D.; Toftegaard, T.; Wiklund, C.; Ehrlén, J.; Gotthard, K. Latitudinal variation in diapause duration and post-winter development in two pierid butterflies in relation to phenological specialization. Oecologia 2015, 177, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, T.; Aoki, S.; Shimizu, I. Neuroendocrine control of diapause hormone secretion in the silkworm. Bombyx Mori. J. Insect Physiol. 1997, 43, 1101–1109. [Google Scholar] [PubMed]
- Emerson, K.J.; Bradshaw, W.E.; Holzapfel, C.M. Complications of complexity: Integrating environmental, genetic and hormonal control of insect diapause. Trends Genet. 2009, 25, 217–225. [Google Scholar] [CrossRef]
- Lehmann, P.; Pruisscher, P.; Koštál, V.; Moos, M.; Šimek, P.; Nylin, S.; Agren, R.; Väremo, L.; Wiklund, C.; Wheat, C.W.; et al. Metabolome dynamics of diapause in the butterfly Pieris napi: Distinguishing maintenance, termination and post-diapause phases. J. Exp. Biol. 2018, 221, jeb169508. [Google Scholar] [CrossRef]
- Mikucki, E.E.; Lockwood, B.L. Local thermal environment and warming influence supercooling and drive widespread shifts in the metabolome of diapausing Pieris rapae butterflies. J. Exp. Biol. 2021, 224, jeb243118. [Google Scholar] [CrossRef]
- Huang, Q.; Ma, Q.; Li, F.; Zhu-Salzman, K.; Cheng, W. Metabolomics reveals changes in metabolite profiles among pre-diapause, diapause and post-diapause larvae of Sitodiplosis mosellana (Diptera: Cecidomyiidae). Insects 2022, 13, 339. [Google Scholar] [CrossRef]
- Batz, Z.A.; Armbruster, P.A. Diapause-associated changes in the lipid and metabolite profiles of the asian tiger mosquito, Aedes albopictus. J. Exp. Biol. 2018, 221, jeb189480. [Google Scholar] [CrossRef]
- Papanastasiou, S.A.; Nestel, D.; Diamantidis, A.D.; Nakas, C.T.; Papadopoulos, N.T. Physiological and biological patterns of a highland and a coastal population of the European cherry fruit fly during diapause. J. Insect Physiol. 2011, 57, 83–93. [Google Scholar] [CrossRef]
- Michaud, M.R.; Denlinger, D.L. Oleic acid is elevated in cell membranes during rapid cold-hardening and pupal diapause in the flesh fly, Sarcophaga crassipalpis. J. Insect Physiol. 2006, 52, 1073–1082. [Google Scholar] [CrossRef]
- Yocum, G.D.; Kemp, W.P.; Bosch, J.; Knoblett, J.N. Temporal variation in overwintering gene expression and respiration in the solitary bee Megachile rotundata. J. Insect Physiol. 2005, 51, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Hiroyoshi, S.; Reddy, G.V.P. Field and laboratory studies on the ecology, reproduction, and adult diapause of the asian comma butterfly, Polygonia c-aureum L. (Lepidoptera: Nymphalidae). Insects 2018, 9, 169. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhou, C.; Chen, X.; Zheng, H. Foraging behavior of the dead leaf butterfly, Kallima inachus. J. Insect Sci. 2013, 13, 58. [Google Scholar] [CrossRef]
- Wan, W.T.; Dong, Z.W.; Ren, Y.D.; Yang, J.; Pan, X.Y.; He, J.W.; Chang, Z.; Liu, W.; Liu, G.C.; Zhao, R.P.; et al. Chromatin accessibility profiling provides insights into larval cuticle color and adult longevity in butterflies. Zool. Res. 2021, 42, 614–619. [Google Scholar] [CrossRef]
- Fei, Y.H.; Yang, J.T. Importance of body rotation during the flight of a butterfly. Phys. Rev. E 2016, 93, 033124. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.K.; Tomita, S.; Sezutsu, H. Multicomponent structures in camouflage and mimicry in butterfly wing patterns. J. Morphol. 2019, 280, 149–166. [Google Scholar] [CrossRef]
- Qin, X.M.; Guan, Q.X.; Zeng, D.L.; Qin, F.; Li, H.M. Complete mitochondrial genome of Kallima inachus (Lepidoptera: Nymphalidae: Nymphalinae): Comparison of K. inachus and Argynnis hyperbius. Mitochondr. DNA 2012, 23, 318–320. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wan, W.; Xie, M.; Mao, J.; Dong, Z.; Lu, S.; He, J.; Xie, F.; Liu, G.; Dai, X.; et al. Chromosome-level reference genome assembly and gene editing of the dead-leaf butterfly Kallima inachus. Mol. Ecol. Resour. 2020, 20, 1080–1092. [Google Scholar] [CrossRef]
- Watson, D.; Timofeeff-Ressovsky, N.W.; Salisbury, E.J.; Turrill, W.B.; Jenkin, T.J.; Ruggles Gates, R.; Fisher, R.A.; Diver, C.; Hale Carpenter, G.D.; Haldane, J.B.S. A Discussion on the present state of the theory of natural selection. Pro. R. Soc. Lond. B Biol. Sci. 1936, 121, 43–73. [Google Scholar]
- Li, J. A Study on Characteristics of Reproductive Dormancy in Overwintering Kallima inachus; Chinese Academy of Forestry: Beijing, China, 2019. (In Chinese) [Google Scholar]
- Zhou, C.L. Studies on the Biological Deatures and Molecular Intraspecific Fenetic Differentiation of Kallima inachus (Lepidoptera: Nymphalidae); Chinese Academy of Forestry: Beijing, China, 2008. (In Chinese) [Google Scholar]
- Yi, C.H.; Chen, X.M.; Shi, J.Y.; Zhou, C.L. Change of nucleic acid contents in the non-diapause and over-winter adult of Kallima inachus. Shandong For. Sci. Technol. 2009, 182, 165–167. (In Chinese) [Google Scholar]
- Yi, C.H.; Chen, X.M.; Shi, J.Y.; Zhou, C.L. Change of carbohydrate content in the non-diapause and over-winter adult of Kallima inachus. Shandong For. Sci. Technol. 2009, 182, 162–164. (In Chinese) [Google Scholar]
- Wu, C.; Wang, H.; Liu, Z.; Xu, B.; Li, Z.; Song, P.; Chao, Z. Untargeted metabolomics coupled with chemometrics for leaves and stem barks of dioecious Morus alba L. Metabolites 2022, 12, 106. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Y.; Zheng, R.Q.; Wang, Y.; Liu, Y.H.; Jiang, S.; Wang, X.Z.; He, K.; Pan, X.; Zhou, T.; Li, T. The endogenous metabolite glycerophosphocholine promotes longevity and fitness in Caenorhabditis elegans. Metabolites 2022, 12, 177. [Google Scholar] [CrossRef] [PubMed]
- Lozano, J.; Belles, X. Conserved repressive function of Krüppel homolog 1 on insect metamorphosis in hemimetabolous and holometabolous species. Sci. Rep. 2011, 1, 163. [Google Scholar] [CrossRef]
- Zhang, Q.; Dou, W.; Song, Z.H.; Jin, T.J.; Yuan, G.R.; De Schutter, K.; Smagghe, G.; Wang, J.J. Identification and profiling of Bactrocera dorsalis microRNAs and their potential roles in regulating the developmental transitions of egg hatching, molting, pupation and adult eclosion. Insect Biochem. Mol. Biol. 2020, 127, 103475. [Google Scholar] [CrossRef]
- Li, M.; Wang, G.; Shang, R.; Xu, Q.; Zhang, J.; Sun, R.; Li, L. Comparative lipid profile analysis of Hermetia illucens larvae fed food waste at different days of age using an LC-MS-based lipidomics approach. J. Insect Sci. 2021, 21, 17. [Google Scholar] [CrossRef]
- Adu-Gyamfi, E.; Johnson, K.A.; Fraser, M.E.; Scott, J.L.; Soni, S.P.; Jones, K.R.; Digman, M.A.; Gratton, E.; Tessier, C.R.; Stahelin, R.V. Host cell plasma membrane phosphatidylserine regulates the assembly and budding of ebola virus. J. Virol. 2015, 89, 9440–9453. [Google Scholar] [CrossRef]
- Suzuki, T.; Suzuki, Y. Virus infection and lipid rafts. Biol. Pharm. Bull. 2006, 29, 1538–1541. [Google Scholar] [CrossRef][Green Version]
- Patterson, P.H.; Acar, N.; Ferguson, A.D.; Trimble, L.D.; Sciubba, H.B.; Koutsos, E.A. The impact of dietary black soldier fly larvae oil and meal on laying hen performance and egg quality. Poult. Sci. 2021, 100, 101272. [Google Scholar] [CrossRef]
- Navrotskaya, V.; Wnorowski, A.; Turski, W.; Oxenkrug, G. Effect of lynurenic acid on pupae viability of Drosophila melanogaster cinnabar and cardinal eye color mutants with altered tryptophan-kynurenine metabolism. Neurotox. Res. 2018, 34, 324–331. [Google Scholar] [CrossRef]
- Koch, P.B.; Behnecke, B.; Weigmann-Lenz, M.; Ffrench-Constant, R.H. Insect pigmentation: Activities of beta-alanyldopamine synthase in wing color patterns of wild-type and melanic mutant swallowtail butterfly Papilio glaucus. Pigment Cell. Res. 2000, 13, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Zornik, E.; Paisley, K.; Nichols, R. Neural transmitters and a peptide modulate Drosophila heart rate. Peptide 1999, 20, 45–51. [Google Scholar] [CrossRef]
- Kita, T.; Ozoem, F.; Azuma, M.; Ozoe, Y. Differential distribution of glutamate- and GABA-gated chloride channels in the housefly Musca domestica. J. Insect Physiol. 2013, 59, 887–893. [Google Scholar] [CrossRef] [PubMed]
- Sellin, J.; Fülle, J.B.; Thiele, C.; Bauer, R.; Bülow, M.H. Free fatty acid determination as a tool for modeling metabolic diseases in Drosophila. J. Insect Physiol. 2020, 126, 104090. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, A.; Wrońska, A.K.; Kazek, M.; Boguś, M.I. Metamorphosis-related changes in the free fatty acid profiles of Sarcophaga (Liopygia) argyrostoma (Robineau-Desvoidy, 1830). Sci. Rep. 2020, 10, 17337. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.L.; Dong, H.; Han, Y.; Yang, L.; Zhang, A.H.; Sun, H.; Li, Y.; Yan, G.; Wang, X.J. Network pharmacology combined with metabolomics approach to investigate the protective role and detoxification mechanism of Yunnan Baiyao formulation. Phytomedicine 2020, 77, 153266. [Google Scholar] [CrossRef]
- Daniels, R.C.; Tiba, M.H.; Cummings, B.C.; Yap, Y.R.; Ansari, S.; McCracken, B.M.; Sun, Y.; Jennaro, T.S.; Ward, K.R.; Stringer, K.A. Redox potential correlates with changes in metabolite concentrations attributable to pathways active in oxidative stress response in swine traumatic shock. Shock 2022, 57, 282–290. [Google Scholar] [CrossRef]
- Fazio, F.; Lionetto, L.; Molinaro, G.; Bertrand, H.O.; Acher, F.; Ngomba, R.T.; Notartomaso, S.; Curini, M.; Rosati, O.; Scarselli, P.; et al. Cinnabarinic acid, an endogenous metabolite of the kynurenine pathway, activates type 4 metabotropic glutamate receptors. Mol. Pharmacol. 2012, 81, 643–656. [Google Scholar] [CrossRef]
- Ze, L.J.; Xu, P.; Kang, W.N.; Wu, J.J.; Jin, L.; Anjum, A.A.; Li, G.Q. Disruption of kynurenine pathway reveals physiological importance of tryptophan catabolism in Henosepilachna vigintioctopunctata. Amino Acids 2021, 53, 1091–1104. [Google Scholar] [CrossRef]
- Liu, J.; Chen, Z.; Xiao, Y.; Asano, T.; Li, S.; Peng, L.; Chen, E.; Zhang, J.; Li, W.; Zhang, Y.; et al. Lepidopteran wing scales contain abundant cross-linked film-forming histidine-rich cuticular proteins. Commun. Biol. 2021, 4, 491. [Google Scholar] [CrossRef]
- Mentel, M.; Ahuja, E.G.; Mavrodi, D.V.; Breinbauer, R.; Thomashow, L.S.; Blankenfeldt, W. Of two make one: The biosynthesis of phenazines. ChemBioChem 2009, 10, 2295–2304. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, Y.; Monteiro, A. Melanin pathway fenes regulate color and morphology of butterfly wing scales. Cell Rep. 2018, 24, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Tomcala, A.; Tollarová, M.; Overgaard, J.; Simek, P.; Kostál, V. Seasonal acquisition of chill tolerance and restructuring of membrane glycerophospholipids in an overwintering insect: Triggering by low temperature, desiccation and diapause progression. J. Exp. Biol. 2006, 209, 4102–4114. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ran, L.L.; Li, Y.; Liu, Y.H. Comparative proteomics provides insights into diapause program of Bactrocera minax (Diptera: Tephritidae). PLoS ONE 2020, 15, e0244493. [Google Scholar] [CrossRef] [PubMed]
- Didion, E.M.; Sabree, Z.L.; Kenyon, L.; Nine, G.; Hagan, R.W.; Osman, S.; Benoit, J.B. Microbiome reduction prevents lipid accumulation during early diapause in the northern house mosquito, Culex pipiens pipiens. J. Insect Physiol. 2021, 134, 104295. [Google Scholar] [CrossRef]
- Khanmohamadi, F.; Khajehali, J.; Izadi, H. Diapause and cold hardiness of the almond wasp, Eurytoma amygdali (Hymenoptera: Eurytomidae), two independent phenomena. J. Econ. Entomol. 2016, 109, 1646–1650. [Google Scholar] [CrossRef]
- Santos, J.L.; Ebert, D. Trehalose provisioning in Daphnia resting stages reflects local adaptation to the harshness of diapause conditions. Biol. Lett. 2022, 18, 20210615. [Google Scholar] [CrossRef]
- Huang, Q.; Zhang, G.; Nan, J.; Cheng, W.; Zhu-Salzman, K. Characterization of trehalose metabolic genes and corresponding enzymatic activities during diapause of Sitodiplosis mosellana. J. Insect Physiol. 2021, 135, 104324. [Google Scholar] [CrossRef]
- Song, Y.; Huang, W.W.; Zhou, Y.; Li, Z.W.; Ji, R.; Ye, X.F. Physiological characteristics and cold tolerance of overwintering eggs in Gomphocerus sibiricus L. (Orthoptera: Acrididae). Arch. Insect Biochem. Physiol. 2021, 108, e21846. [Google Scholar] [CrossRef]
- Tanwar, A.K.; Kirti, J.S.; Kumar, S.; Dhillon, M.K. The amino acid and lipophilic profiles of Chilo partellus (Swinhoe) larvae fluctuate with diapause. J. Exp. Zool. A Ecol. Integr. Physiol. 2021, 335, 595–601. [Google Scholar] [CrossRef]
- Koštál, V.; Renault, D.; Rozsypal, J. Seasonal changes of free amino acids and thermal hysteresis in overwintering heteropteran insect, Pyrrhocoris apterus. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2011, 160, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Van der Krieken, S.E.; van-der Pijl, P.C.; Lin, Y.; Popeijus, H.E.; Mensink, R.P.; Plat, J. Search for natural compounds that increase apolipoprotein A-I transcription in HepG2 cells: Specific attention for BRD4 inhibitors. Lipids 2019, 54, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cao, F.; Yin, H.L.; Huang, Z.J.; Lin, Z.T.; Mao, N.; Sun, B.; Wang, G. Ferroptosis: Past, present and future. Cell Death Dis. 2020, 11, 88. [Google Scholar] [CrossRef] [PubMed]
- Manaboon, M.; Yasanga, T.; Sakurai, S.; Singtripop, T. Programmed cell death of larval tissues induced by juvenile hormone in the bamboo borer, Omphisa fuscidentalis. J. Insect Physiol. 2012, 58, 1202–1208. [Google Scholar] [CrossRef]
- Galles, C.; Prez, G.M.; Penkov, S.; Boland, S.; Porta, E.; Altabe, S.G.; Labadie, G.R.; Schmidt, U.; Knölker, H.J.; Kurzchalia, T.V. Endocannabinoids in Caenorhabditis elegans are essential for the mobilization of cholesterol from internal reserves. Sci. Rep. 2018, 8, 6398. [Google Scholar] [CrossRef]


| Group | Members | Treatment | Collection Time | State |
|---|---|---|---|---|
| LA | LA01 (Ki084), LA02 (Ki022), LA03 (Ki025), LA04 (Ki050), LA05 (Ki084), LA06 (Ki113) | liquid nitrogen | 2020.5.30–2020.6.14 | first day of the fifth instar |
| PU | PU01 (Ki007), PU02 (Ki012), PU03 (Ki129), PU04 (Ki067), PU05 (Ki047), PU06 (Ki081) | liquid nitrogen | 2020.6.17–2020.6.28 | fifth day after pupation |
| KM | KM01 (Ki040), KM02 (Ki018), KM03 (Ki080), KM04 (Ki035), KM05 (Ki189), KM06 (Ki159) | liquid nitrogen | 2020.7.03–2020.7.22 | tenth day after eclosion (males) |
| KF | KF01 (Ki026), KF02 (Ki129), KF03 (Ki022), KF04 (Ki015), KF05 (Ki031), KF06 (Ki078) | liquid nitrogen | 2020.7.06–2020.7.29 | tenth day after eclosion (females) |
| dKF | dKF01 (Ki032), dKF02 (Ki116), dKF03 (Ki115), dKF04 (Ki058), dKF05 (Ki063), dKF06 (Ki066) | liquid nitrogen | 2021.2.23–2021.2.24 | fifth day after entering of diapause (females) |
| Name | Formula | Primary Category | VIP | p-Value | Log2FC | Pathway |
|---|---|---|---|---|---|---|
| LA vs. PU (up) | ||||||
| 2-Keto-6-acetamidocaproate | C8H13NO4 | - | 1.25 | 9.21 × 10−3 | 17.25 | Lysine degradation |
| Carnitine C13:1 | C20H37NO4 | Lipids | 1.26 | 8.67 × 10−5 | 13.49 | - |
| N-phenylacetylglycine | C10H11NO3 | AAD | 1.06 | 2.31 × 10−2 | 12.14 | Phenylalanine metabolism |
| 3-Chloro-L-tyrosine | C9H10ClNO3 | AAD | 1.26 | 1.46 × 10−4 | 11.59 | - |
| Carnitine C14:1-OH | C21H39NO5 | Lipids | 1.26 | 8.83 × 10−5 | 11.30 | - |
| LA vs. PU (down) | ||||||
| Cinnabarinic acid | C14H8N2O6 | AAD | 1.25 | 7.10 × 10−3 | −18.84 | Tryptophan metabolism |
| Trans,cis-3,6-Nonadien-1-ol | C9H16O | Alcohol | 1.25 | 1.60 × 10−2 | −18.68 | - |
| 2-(Formylamino) benzoic acid | C8H7NO3 | BD | 1.26 | 1.24 × 10−4 | −18.20 | Tryptophan metabolism |
| 13,14-Dihydro-15-keto-PGD2 | C20H32O5 | Lipids | 1.26 | 4.04 × 10−5 | −18.07 | - |
| (Z)-6-octadecenoic acid | C18H34O2 | Lipids | 1.26 | 7.51 × 10−3 | −18.05 | - |
| PU vs. KF (up) | ||||||
| DL-O-tyrosine | C9H11NO2 | AAD | 1.08 | 1.73 × 10−3 | 24.21 | - |
| Tauropine | C5H11NO5S | AAD | 1.09 | 3.48 × 10−6 | 23.03 | - |
| L-palmitoylcarnitine | C25H49NO4 | FA | 1.09 | 1.87 × 10−4 | 22.96 | Fatty acid metabolism |
| Carnitine C16:0 | C23H45NO4 | FA | 1.08 | 1.26 × 10−3 | 22.74 | Fatty acid metabolism |
| L-histidine | C6H9N3O2 | Amino acids | 1.09 | 1.31 × 10−5 | 22.52 | Histidine metabolism |
| PU vs. KF (down) | ||||||
| L-glutamate | C5H9NO4 | Amino acids | 1.08 | 1.41 × 10−5 | −23.91 | Histidine metabolism |
| ®(R)-(-)-2-phenylglycine | C8H11NO | ACD | 1.08 | 9.26 × 10−4 | −23.37 | - |
| L-isoisoleucine | C6H13NO2 | ACD | 1.08 | 1.71 × 10−2 | −22.48 | - |
| L-tryptophan | C11H12N2O2 | ACD | 1.08 | 1.89 × 10−2 | −22.36 | Tryptophan metabolism |
| KM vs. KF (up) | ||||||
| 4-O-Dimethylallyl-L-tyrosine | C14H19NO3 | AAD | 1.33 | 1.76 × 10−2 | 3.10 | - |
| N-lactoyl-phenylalanine | C12H15NO4 | AAD | 1.28 | 4.04 × 10−2 | 2.63 | - |
| Salicin-6P | C13H19O10P | Glycosides | 1.35 | 4.04 × 10−2 | 2.62 | Gly/Glu |
| Uridine triphosphate | C9H15N2O15P3 | ND | 2.71 | 5.04 × 10−3 | 2.33 | Pyrimidine metabolism |
| 3-hydroxypicolinic acid | C6H5NO3 | HC | 1.03 | 4.19 × 10−2 | 2.06 | - |
| KM vs. KF (down) | ||||||
| 5’-deoxyadenosine | C10H13N5O3 | ND | 1.72 | 3.22 × 10−2 | −1.64 | - |
| 2’-deoxyadenosine | C10H13N5O3 | ND | 1.72 | 2.09 × 10−2 | −1.63 | Purine metabolism |
| S-Sulfo-L-xysteine | C3H7NO5S2 | AAD | 1.14 | 2.88 × 10−2 | −1.41 | Cys and Met metabolism |
| Protocatechuic aldehyde | C7H6O3 | BD | 2.51 | 1.41 × 10−3 | −1.21 | - |
| Carnitine C9:2-OH | C16H27NO5 | FA | 2.17 | 3.62 × 10−2 | −1.08 | - |
| Name | Formula | Second Category | In Diapause Adult Females | Reliability |
|---|---|---|---|---|
| Glycerophospholipids | ||||
| LPC(13:0/0:0) | C21H44NO7P | lysophosphatidylcholine | Increase | High |
| PC(8:0/8:0) | C24H48NO8P | phosphatidylcholine | Increase | Relatively high |
| LPE(17:1/0:0) | C22H44NO7P | Lysophosphatidylethanolamine | Increase | High |
| LPA(16:0/0:0) | C19H39O7P | lysophosphatidic acid | Increase | High |
| LPS(22:6/0:0) | C28H44NO9P | Lysophosphatidylserine | Increase | Relatively high |
| PysoPS 18:2 | C24H44NO9P | Lysophosphatidylserine | Decrease | High |
| Lysopg 18:1 | C24H47O9P | Lysophosphatidyl glycerol | Decrease | Moderate |
| PI 18:3 | C27H47O12P | phosphatidyl inositol | Decrease | Relatively high |
| Amino acids and AADs | ||||
| L-alanine | C3H7NO2 | Amino acids | Increase | Moderate |
| L-cysteine | C3H7NO2S | Amino acids | Increase | Relatively high |
| L-proline | C5H9NO2 | Amino acids | Increase | Relatively high |
| L-theanine | C7H14N2O3 | Amino acids | Increase | High |
| L-arginine | C6H14N4O2 | Amino acids | Decrease | Relatively high |
| Cis-L-3-hydroxyproline | C5H9NO3 | Amino acid derivatives | Increase | High |
| L-threo-3-methylaspartate | C5H9NO4 | Amino acid derivatives | Increase | High |
| L-pyroglutamic acid | C5H7NO3 | Amino acid derivatives | Increase | Relatively high |
| Glutathione | C20H32N6O12S2 | Small peptides | Decrease | High |
| Glutathione reducedform | C10H17N3O6S | Small peptides | Decrease | High |
| N-Acetyl-Asp-Glu | C11H16N2O8 | Small peptides | Increase | Moderate |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.-J.; Jiang, G.-F.; Wang, W. Metabolite Changes in Orange Dead Leaf Butterfly Kallima inachus during Ontogeny and Diapause. Metabolites 2022, 12, 804. https://doi.org/10.3390/metabo12090804
Li M-J, Jiang G-F, Wang W. Metabolite Changes in Orange Dead Leaf Butterfly Kallima inachus during Ontogeny and Diapause. Metabolites. 2022; 12(9):804. https://doi.org/10.3390/metabo12090804
Chicago/Turabian StyleLi, Ming-Jie, Guo-Fang Jiang, and Wei Wang. 2022. "Metabolite Changes in Orange Dead Leaf Butterfly Kallima inachus during Ontogeny and Diapause" Metabolites 12, no. 9: 804. https://doi.org/10.3390/metabo12090804
APA StyleLi, M.-J., Jiang, G.-F., & Wang, W. (2022). Metabolite Changes in Orange Dead Leaf Butterfly Kallima inachus during Ontogeny and Diapause. Metabolites, 12(9), 804. https://doi.org/10.3390/metabo12090804






