Application of Mass Spectrometry for Determining the Geographic Production Area of Wagyu Beef
Abstract
:1. Introduction
2. Results
2.1. Comparison of Single-Nucleotide Polymorphism Profiles in Cattle
2.2. Characterization of TG Molecular Species in Wagyu
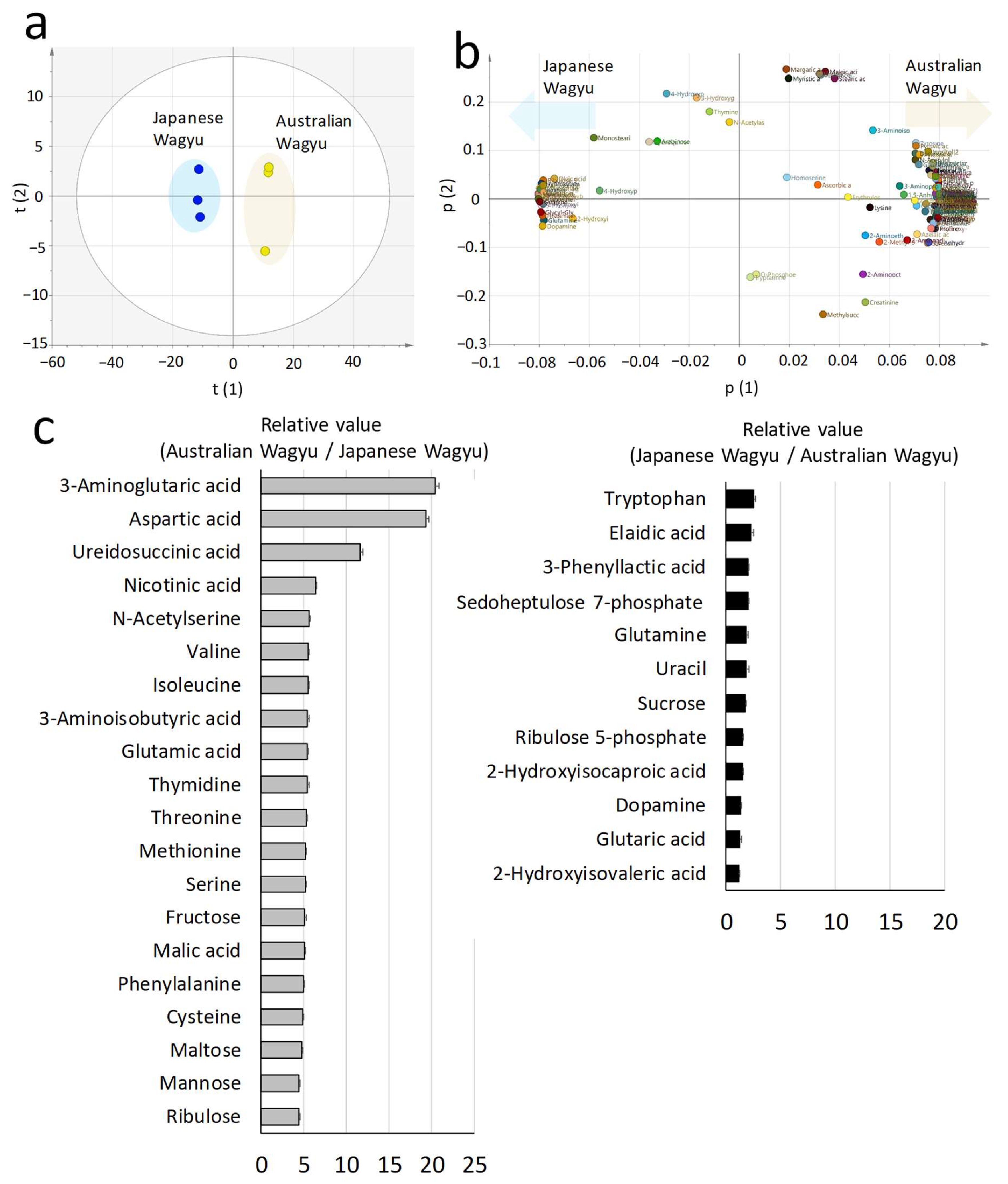
2.3. Comparison of the Composition of Elements and Metabolites in Wagyu Beef
3. Discussion
4. Materials and Methods
4.1. Sample Collection
4.2. SNP Analysis Using the Illumina Bovine Chip
4.3. LC–MS/MS Analysis of TGs
4.4. Analysis of the Fatty Acid Composition of TGs
4.5. Multielement Analysis by ICP–MS and ICP–OES
4.6. Metabolomics Analysis by GC–MS
4.7. Validated Methods for GC–MS and LC–MS/MS
4.8. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LC–MS/MS | Liquid chromatography–tandem mass spectrometry |
| GC–MS | Gas chromatography–mass spectrometry |
| HPLC | High-performance liquid chromatography |
| SNP | Single-nucleotide polymorphism |
| OPLS-DA | Orthogonal part least squares discrimination analysis |
| PCA | Principal component analysis |
| SD | Standard deviation |
| TG | Triacylglyceride |
| DG | Diglyceride |
| MG | Monoglyceride |
| LPC | Lysophosphatidylcholine |
| LPE | Lysophosphatidylethanolamine |
| PC | Phosphatidylcholine |
| PE | Phosphatidylethanolamine |
| PI | Phosphatidylinositol |
| PS | Phosphatidylserine |
| PG | Phosphatidylglycerol |
| SM | Sphingomyelin |
| CL | Cardiolipin |
| Cer | Ceramide |
References
- Henchion, M.; Moloney, A.P.; Hyland, J.; Zimmermann, J.; McCarthy, S. Review: Trends for meat, milk and egg consumption for the next decades and the role played by livestock systems in the global production of proteins. Animal 2021, 15, 100287. [Google Scholar] [CrossRef] [PubMed]
- Colgrave, M.L.; Dominik, S.; Tobin, A.B.; Stockmann, R.; Simon, C.; Howitt, C.A.; Belobrajdic, D.P.; Paull, C.; Vanhercke, T. Perspectives on Future Protein Production. J. Agric. Food Chem. 2021, 69, 15076–15083. [Google Scholar] [CrossRef] [PubMed]
- Belk, K.E.; Woerner, D.R.; Delmore, R.J.; Tatum, J.D.; Yang, H.; Sofos, J.N. The meat industry: Do we think and behave globally or locally? Meat Sci. 2014, 98, 556–560. [Google Scholar] [CrossRef] [PubMed]
- Motoyama, M.; Sasaki, K.; Watanabe, A. Wagyu and the factors contributing to its beef quality: A Japanese industry overview. Meat Sci. 2016, 120, 10–18. [Google Scholar] [CrossRef]
- Ambele, M.A.; Dhanraj, P.; Giles, R.; Pepper, M.S. Adipogenesis: A Complex Interplay of Multiple Molecular Determinants and Pathways. Int. J. Mol. Sci. 2020, 21, 4283. [Google Scholar] [CrossRef]
- Ueda, S.; Hosoda, M.; Yoshino, K.-I.; Yamanoue, M.; Shirai, Y. Gene Expression Analysis Provides New Insights into the Mechanism of Intramuscular Fat Formation in Japanese Black Cattle. Genes 2021, 12, 1107. [Google Scholar] [CrossRef]
- Matsuishi, M.; Fujimori, M.; Okitani, A. Wagyu Beef Aroma in Wagyu (Japanese Black Cattle) Beef Preferred by the Japanese over Imported Beef. Anim. Sci. J. 2001, 72, 498–504. [Google Scholar] [CrossRef]
- Ueda, S.; Yamanoue, M.; Sirai, Y.; Iwamoto, E. Exploring the Characteristic Aroma of Beef from Japanese Black Cattle (Japanese Wagyu) via Sensory Evaluation and Gas Chromatography-Olfactometry. Metabolites 2021, 11, 56. [Google Scholar] [CrossRef]
- Ueda, S.; Hosoda, M.; Kasamatsu, K.; Horiuchi, M.; Nakabayashi, R.; Kang, B.; Shinohara, M.; Nakanishi, H.; Ohto-Nakanishi, T.; Yamanoue, M.; et al. Production of Hydroxy Fatty Acids, Precursors of γ-Hexalactone, Contributes to the Characteristic Sweet Aroma of Beef. Metabolites 2022, 12, 332. [Google Scholar] [CrossRef]
- Ministry of Agriculture, Forestry and Fisheries. Statistical Survey of Livestock Production; Ministry of Agriculture, Forestry and Fisheries: Tokyo, Japan, 2020. Available online: https://www.maff.go.jp/e/index.html (accessed on 23 March 2022).
- O’Quinn, T.G.; Brooks, J.C.; Polkinghorne, R.J.; Garmyn, A.J.; Johnson, B.J.; Starkey, J.D.; Rathmann, R.J.; Miller, M.F. Consumer assessment of beef strip loin steaks of varying fat levels. J. Anim. Sci. 2012, 90, 626–634. [Google Scholar] [CrossRef]
- Kerth, C.R.; Miller, R.K. Beef flavor: A review from chemistry to consumer. J. Sci. Food Agric. 2015, 95, 2783–2798. [Google Scholar] [CrossRef]
- Sasaki, K. Diversity of Japanese consumers’ requirements, sensory perceptions, and eating preferences for meat. Anim. Sci. J. 2022, 93, e13705. [Google Scholar] [CrossRef]
- Bermingham, E.N.; Reis, M.G.; Subbaraj, A.K.; Cameron-Smith, D.; Fraser, K.; Jonker, A.; Craigie, C.R. Distribution of fatty acids and phospholipids in different table cuts and co-products from New Zealand pasture-fed Wagyu-dairy cross beef cattle. Meat Sci. 2018, 140, 26–37. [Google Scholar] [CrossRef]
- Hastie, M.; Ashman, H.; Torrico, D.; Ha, M.; Warner, R. A Mixed Method Approach for the Investigation of Consumer Responses to Sheepmeat and Beef. Foods 2020, 9, 126. [Google Scholar] [CrossRef]
- Kikushima, R.; Nakajima, S.; Takano, M.; Ito, N. Hong Kong consumer preferences for Japanese beef: Label knowledge and reference point effects. Anim. Sci. J. 2018, 89, 1519–1529. [Google Scholar] [CrossRef]
- Scraggs, E.; Zanella, R.; Wojtowicz, A.; Taylor, J.F.; Gaskins, C.T.; Reeves, J.J.; de Avila, J.M.; Neibergs, H.L. Estimation of inbreeding and effective population size of full-blood Wagyu cattle registered with the American Wagyu Cattle Association. J. Anim Breed. Genet. 2014, 131, 3–10. [Google Scholar] [CrossRef]
- Gotoh, T.; Nishimura, T.; Kuchida, K.; Mannen, H. The Japanese Wagyu beef industry: Current situation and future prospects—A review. Asian-Australas. J. Anim. Sci. 2018, 31, 933–950. [Google Scholar] [CrossRef]
- Menozzi, D.; Giraud, G.; Saïdi, M.; Yeh, C.H. Choice Drivers for Quality-Labelled Food: A Cross-Cultural Comparison on PDO Cheese. Foods 2021, 10, 1176. [Google Scholar] [CrossRef]
- Ríos-Reina, R.; Segura-Borrego, M.P.; García-González, D.L.; Morales, M.L.; Callejón, R.M. A comparative study of the volatile profile of wine vinegars with protected designation of origin by headspace stir bar sorptive extraction. Food Res. Int. 2019, 123, 298–310. [Google Scholar] [CrossRef]
- Sardi, L.; Gastaldo, A.; Borciani, M.; Bertolini, A.; Musi, V.; Garavaldi, A.; Martelli, G.; Cavallini, D.; Nannoni, E. Pre-Slaughter Sources of Fresh Meat Quality Variation: The Case of Heavy Pigs Intended for Protected Designation of Origin Products. Animals 2020, 10, 2386. [Google Scholar] [CrossRef]
- Mendes, E.; Duarte, N. Mid-Infrared Spectroscopy as a Valuable Tool to Tackle Food Analysis: A Literature Review on Coffee, Dairies, Honey, Olive Oil and Wine. Foods 2021, 10, 477. [Google Scholar] [CrossRef]
- Visciano, P.; Schirone, M. Food frauds: Global incidents and misleading situations. Trends Food Sci. Technol. 2021, 114, 424–442. [Google Scholar] [CrossRef]
- Sajali, N.; Wong, S.C.; Abu Bakar, S.; Khairil Mokhtar, N.F.; Manaf, Y.N.; Yuswan, M.H.; Mohd Desa, M.N. Analytical approaches of meat authentication in food. Int. J. Food Sci. Technol. 2021, 56, 1535–1543. [Google Scholar] [CrossRef]
- Cifuentes, A. Food Analysis: Present, Future, and Foodomics. ISRN Anal. Chem. 2012, 2012, 801607. [Google Scholar] [CrossRef]
- Kawaguchi, F.; Kitamura, Y.; Nakajima, R.; Takahashi, M.; Goto, H.; Washida, Y.; Yamamoto, Y.; Sasazaki, S.; Mannen, H. Application of DNA markers for discrimination between Japanese and Australian Wagyu beef. Anim. Sci. J. 2018, 89, 257–258. [Google Scholar] [CrossRef] [PubMed]
- Klockmann, S.; Reiner, E.; Cain, N.; Fischer, M. Food Targeting: Geographical Origin Determination of Hazelnuts (Corylus avellana) by LC-QqQ-MS/MS-Based Targeted Metabolomics Application. J. Agric. Food Chem. 2017, 65, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Harrieder, E.M.; Kretschmer, F.; Böcker, S.; Witting, M. Current state-of-the-art of separation methods used in LC-MS based metabolomics and lipidomics. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2022, 1188, 123069. [Google Scholar] [CrossRef] [PubMed]
- Crupi, R.; Lo Turco, V.; Gugliandolo, E.; Nava, V.; Potortì, A.G.; Cuzzocrea, S.; Di Bella, G.; Licata, P. Mineral Composition in Delactosed Dairy Products: Quality and Safety Status. Foods 2022, 11, 139. [Google Scholar] [CrossRef]
- Muroya, S.; Ueda, S.; Komatsu, T.; Miyakawa, T.; Ertbjerg, P. MEATabolomics: Muscle and Meat Metabolomics in Domestic Animals. Metabolites 2020, 10, 188. [Google Scholar] [CrossRef]
- Chien, H.-J.; Zheng, Y.-F.; Wang, W.-C.; Kuo, C.-Y.; Hsu, Y.-M.; Lai, C.-C. Determination of adulteration, geographical origins, and species of food by mass spectrometry. Mass Spectrom. Rev. 2022, e21780. [Google Scholar] [CrossRef]
- Alexandre, P.A.; Li, Y.; Hine, B.C.; Duff, C.J.; Ingham, A.B.; Porto-Neto, L.R.; Reverter, A. Bias, dispersion, and accuracy of genomic predictions for feedlot and carcase traits in Australian Angus steers. Genet. Sel. Evol. 2021, 53, 77. [Google Scholar] [CrossRef]
- Rubio Lozano, M.S.; Ngapo, T.M.; Huerta-Leidenz, N. Tropical Beef: Is There an Axiomatic Basis to Define the Concept? Foods 2021, 10, 1025. [Google Scholar] [CrossRef]
- Ueda, S.; Sasaki, R.; Nakabayashi, R.; Yamanoue, M.; Sirai, Y.; Iwamoto, E. Exploring the Lipids Involved in the Formation of Characteristic Lactones in Japanese Black Cattle. Metabolites 2021, 11, 203. [Google Scholar] [CrossRef]
- De Nadai Fernandes, E.A.; Sarriés, G.A.; Bacchi, M.A.; Mazola, Y.T.; Gonzaga, C.L.; Sarriés, S.R.V. Trace elements and machine learning for Brazilian beef traceability. Food Chem. 2020, 333, 127462. [Google Scholar] [CrossRef]
- Song, O.Y.; Islam, M.A.; Son, J.H.; Jeong, J.Y.; Kim, H.E.; Yeon, L.S.; Khan, N.; Jamila, N.; Kim, K.S. Elemental composition of pork meat from conventional and animal welfare farms by inductively coupled plasma-optical emission spectrometry (ICP-OES) and ICP-mass spectrometry (ICP-MS) and their authentication via multivariate chemometric analysis. Meat Sci. 2021, 172, 108344. [Google Scholar] [CrossRef]
- Ueda, S.; Iwamoto, E.; Kato, Y.; Shinohara, M.; Shirai, Y.; Yamanoue, M. Comparative metabolomics of Japanese Black cattle beef and other meats using gas chromatography–mass spectrometry. Biosci. Biotechnol. Biochem. 2019, 83, 137–147. [Google Scholar] [CrossRef]
- Wei, M.; Liu, X.; Xie, P.; Lei, Y.; Yu, H.; Han, A.; Xie, L.; Jia, H.; Lin, S.; Bai, Y.; et al. Characterization of Volatile Profiles and Correlated Contributing Compounds in Pan-Fried Steaks from Different Chinese Yellow Cattle Breeds through GC-Q-Orbitrap, E-Nose, and Sensory Evaluation. Molecules 2022, 27, 3593. [Google Scholar] [CrossRef]
- Frank, D.; Ball, A.; Hughes, J.; Krishnamurthy, R.; Piyasiri, U.; Stark, J.; Watkins, P.; Warner, R. Sensory and Flavor Chemistry Characteristics of Australian Beef: Influence of Intramuscular Fat, Feed, and Breed. J. Agric. Food Chem. 2016, 64, 4299–4311. [Google Scholar] [CrossRef]
- Kawaguchi, F.; Kakiuchi, F.; Oyama, K.; Mannen, H.; Sasazaki, S. Effect of Five Polymorphisms on Percentage of Oleic Acid in Beef and Investigation of Linkage Disequilibrium to Confirm the Locations of Quantitative Trait Loci on BTA19 in Japanese Black Cattle. Life 2021, 11, 597. [Google Scholar] [CrossRef]
- San Vito, E.; Lage, J.F.; Ribeiro, A.F.; Silva, R.A.; Berchielli, T.T. Fatty acid profile, carcass and quality traits of meat from Nellore young bulls on pasture supplemented with crude glycerin. Meat Sci. 2015, 100, 17–23. [Google Scholar] [CrossRef]
- Li, M.; Zhu, M.; Chai, W.; Wang, Y.; Song, Y.; Liu, B.; Cai, C.; Song, Y.; Sun, X.; Xue, P.; et al. Determination of the Heterogeneity of Intramuscular Fat and Visceral Adipose Tissue from Dezhou Donkey by Lipidomics and Transcriptomics Profiling. Front. Nutr. 2021, 8, 746684. [Google Scholar] [CrossRef] [PubMed]
- Mi, S.; Shang, K.; Li, X.; Zhang, C.-H.; Liu, J.-Q.; Huang, D.-Q. Characterization and discrimination of selected China’s domestic pork using an LC-MS-based lipidomics approach. Food Control 2019, 100, 305–314. [Google Scholar] [CrossRef]
- Huang, W.; Guo, Y.; Du, W.; Zhang, X.; Li, A.; Miao, X. Global transcriptome analysis identifies differentially expressed genes related to lipid metabolism in Wagyu and Holstein cattle. Sci. Rep. 2017, 7, 5278. [Google Scholar] [CrossRef] [PubMed]
- Ishak, W.M.W.; Katas, H.; Yuen, N.P.; Abdullah, M.A.; Zulfakar, M.H. Topical application of omega-3-, omega-6-, and omega-9-rich oil emulsions for cutaneous wound healing in rats. Drug Deliv. Transl. Res. 2019, 9, 418–433. [Google Scholar] [CrossRef]
- Farag, M.A.; Gad, M.Z. Omega-9 fatty acids: Potential roles in inflammation and cancer management. J. Genet. Eng. Biotechnol. 2022, 20, 48. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar] [CrossRef]
- Yamada, T.; Kamiya, M.; Higuchi, M. Gas Chromatography-Mass Spectrometry-Based Metabolomic Analysis of Wagyu and Holstein Beef. Metabolites 2020, 10, 95. [Google Scholar] [CrossRef]
- Muroya, S.; Oe, M.; Ojima, K.; Watanabe, A. Metabolomic approach to key metabolites characterizing postmortem aged loin muscle of Japanese Black (Wagyu) cattle. Asian-Australas. J. Anim. Sci. 2019, 32, 1172–1185. [Google Scholar] [CrossRef]
- Chang, C.C.; Chow, C.C.; Tellier, L.C.A.M.; Vattikuti, S.; Purcell, S.M.; Lee, J.J. Second-generation PLINK: Rising to the challenge of larger and richer datasets. GigaScience 2015, 4, s13742-015. [Google Scholar] [CrossRef]
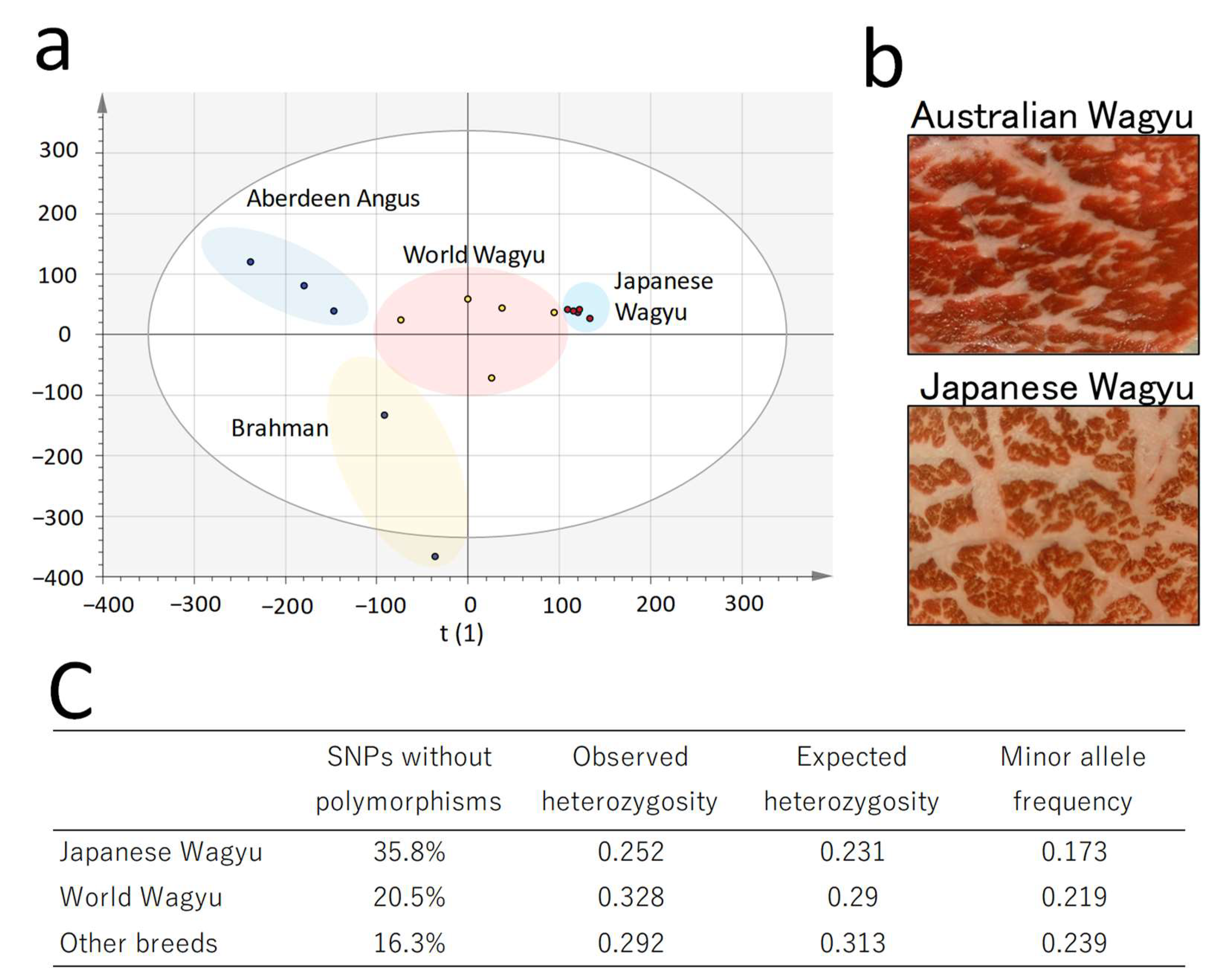
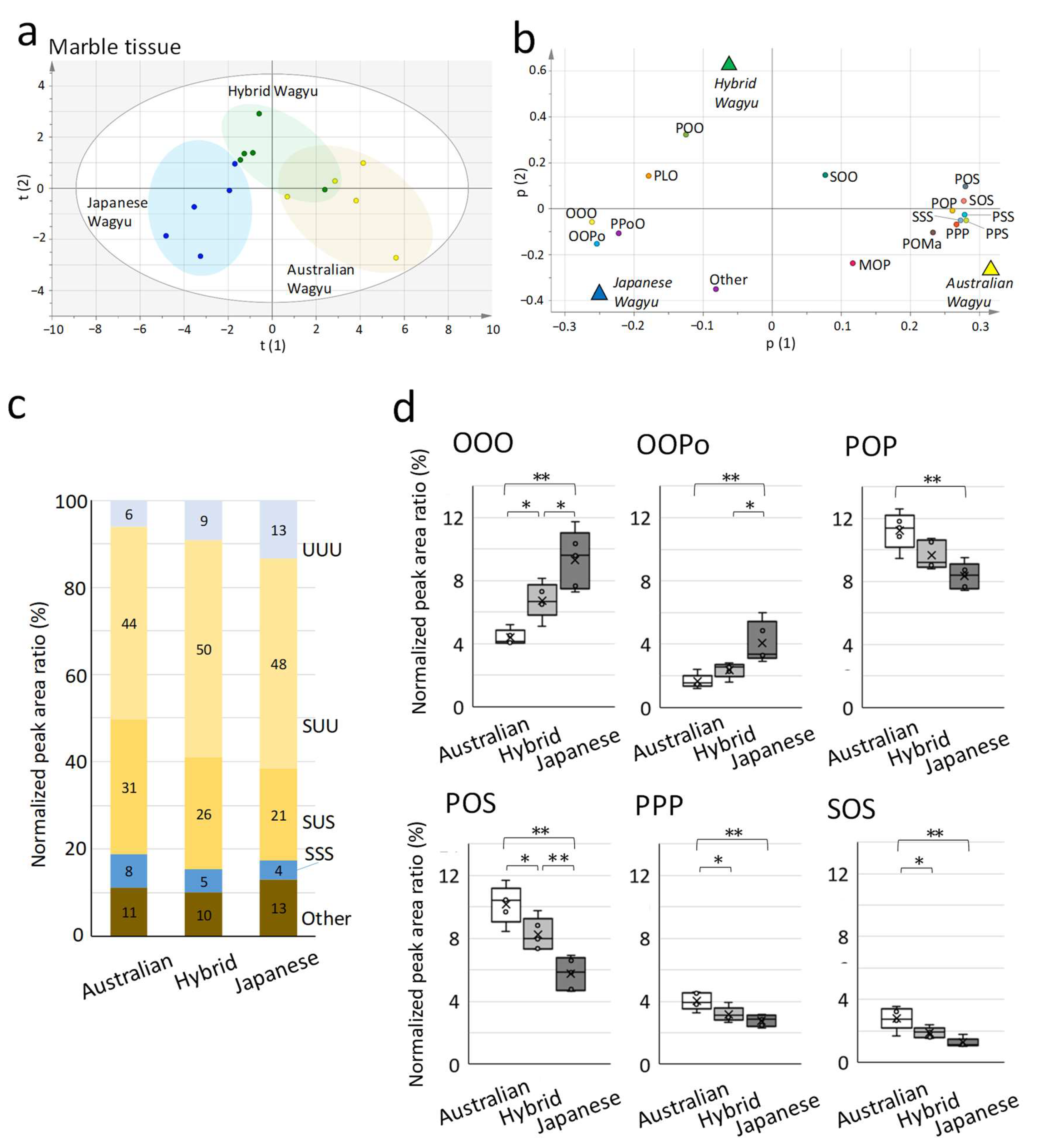
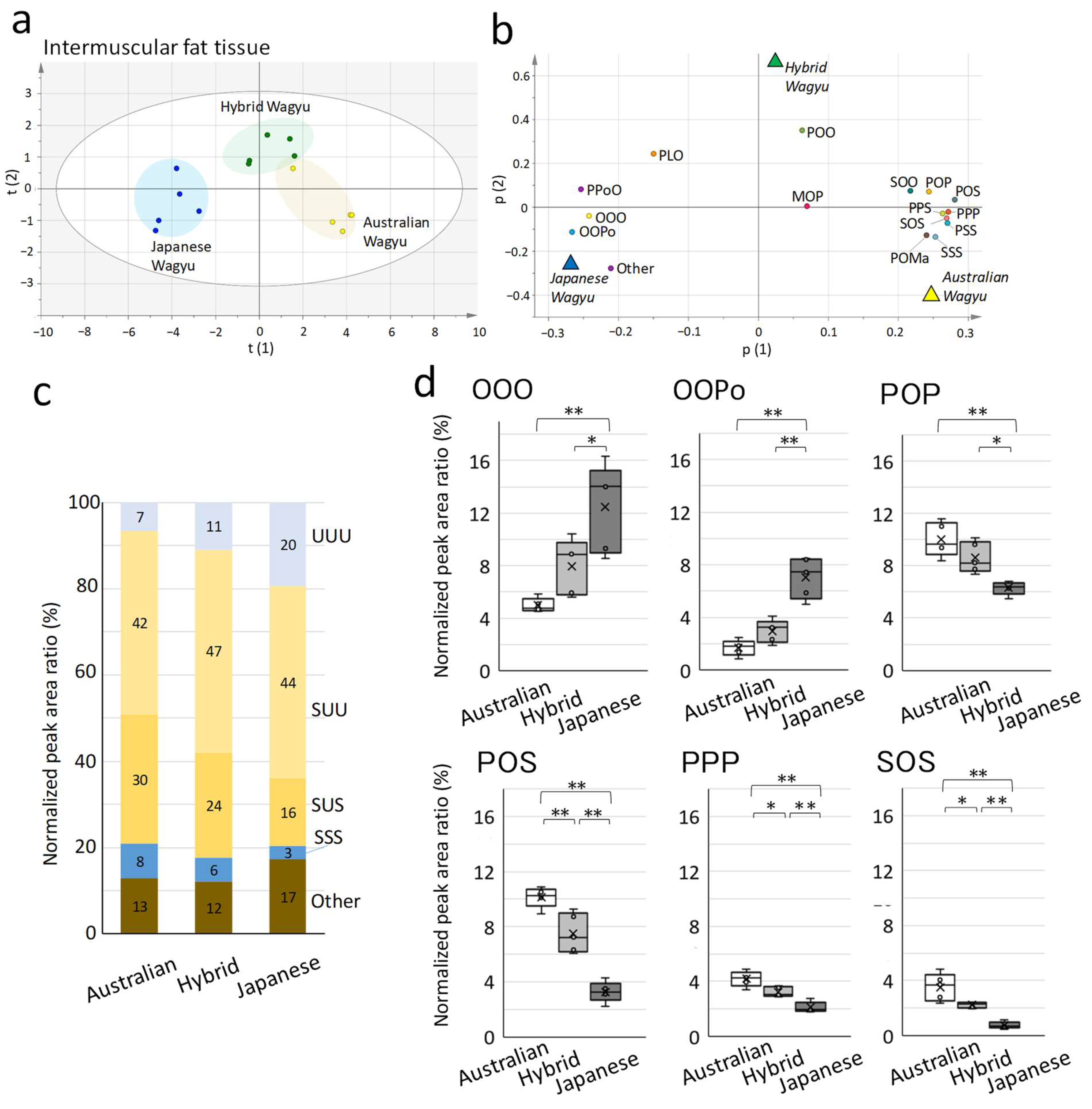
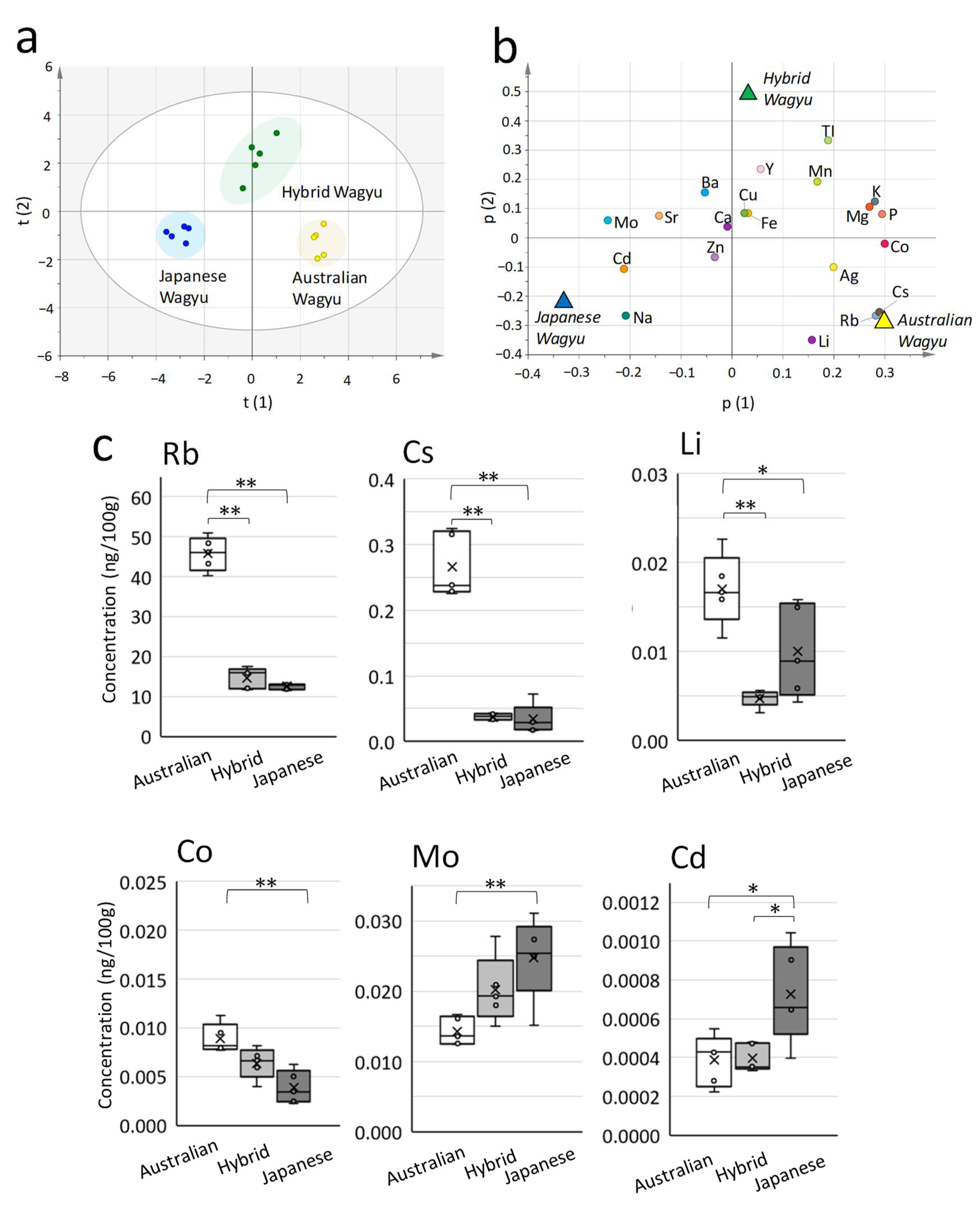
| (a) Total Amount of Each Lipid | Abbreviation | Mean ± SD (pmol/mg) | Number of Detections |
|---|---|---|---|
| Triglyceride | TG | 18,797 ± 7,753 | 128 |
| Phosphatidylcholine | PC | 3,216 ± 590.3 | 31 |
| Lysophosphatidylcholine | LPC | 1,356 ± 360.3 | 10 |
| Diglyceride | DG | 1,205 ± 2023.2 | 25 |
| Lysophosphatidylethanolamine | LPE | 629 ± 175.6 | 10 |
| Phosphatidylethanolamine | PE | 513 ± 200.4 | 25 |
| Phosphatidylinositol | PI | 301 ± 34.4 | 9 |
| Phosphatidylserine | PS | 101 ± 7.6 | 4 |
| Sphingomyeline | SM | 91 ± 25.7 | 4 |
| Monoglyceride | MG | 77 ± 72.9 | 4 |
| Cardiolipin | CL | 74 ± 23 | 2 |
| Ceramides | Cer | 16 ± 1.6 | 5 |
| Lysophosphatidylinositol | LPI | 13 ± 4.6 | 1 |
| Phosphatidylglycerol | PG | 7 ± 1.6 | 1 |
| (b) Triacylglyceride | Symbol | Mean ± SD (pmol/mg) | % of all TGs |
| TG(C16:0/C18:1/C18:1) | POO | 3,471 ± 1231 | 18.5 |
| TG(C16:0/C16:1/C18:1) | PPoO | 1,564 ± 586.4 | 8.3 |
| TG(C18:0/C16:0/C18:1) | POS | 1,347 ± 602.9 | 7.2 |
| TG(C16:0/C16:0/C18:1) | POP | 1,259 ± 524.2 | 6.7 |
| TG(C18:0/C18:1/C18:1) | SOO | 1,183 ± 549.4 | 6.3 |
| TG(C16:0/C18:1/C18:2) | PLO | 1,086 ± 489 | 5.8 |
| TG(C16:1/C18:1/C18:1) | OOPo | 1,086 ± 488.7 | 5.8 |
| TG(C16:0/C14:0/C18:1) | MOP | 918 ± 415.4 | 4.9 |
| TG(C18:1/C18:1/C18:1) | OOO | 867 ± 452.7 | 4.6 |
| TG(C16:0/C14:1/C18:1) | POMo | 661 ± 289.7 | 3.5 |
| TG(C16:0/C17:1/C18:1) | POHe | 395 ± 192.7 | 2.1 |
| TG(C18:0/C18:0/C18:1) | SOS | 384 ± 242.4 | 2.0 |
| TG(C17:0/C18:1/C18:1) | OOMa | 364 ± 190.8 | 1.9 |
| TG(C16:1/C16:1/C18:1) | OPoPo | 284 ± 140.3 | 1.5 |
| TG(C16:0/C16:1/C18:2) | PPoL | 284 ± 139.9 | 1.5 |
| TG(C18:0/C14:0/C16:0) | MOS | 235 ± 148.1 | 1.3 |
| TG(C16:0/C14:0/C16:1) | PPoM | 231 ± 122.2 | 1.2 |
| TG(C16:0/C17:0/C18:1) | POMa | 226 ± 108.1 | 1.2 |
| TG(C18:0/C16:0/C16:0) | PPS | 217 ± 126.7 | 1.2 |
| TG(C15:0/C16:0/C18:1) | OPPe | 177 ± 76.7 | 0.9 |
| Marble Tissue | ||||
|---|---|---|---|---|
| Triacylglycerides (%) | Australian Wagyu | Hybrids Wagyu | Japanese Wagyu | |
| POO a | 27.8 ± 3.6 | 30.9 ± 1.9 | 28.9 ± 3.6 | |
| POP b | 11.2 ± 1.2 | 9.6 ± 0.9 | 8.3 ± 1.2 | |
| POS | 10.2 ± 1.2 | 8.2 ± 1.0 | 5.7 ± 1.2 | |
| PPoO c | 7.3 ± 0.9 | 7.9 ± 1.0 | 8.8 ± 0.9 | |
| SOO | 7.1 ± 0.7 | 7.2 ± 0.8 | 6.8 ± 0.7 | |
| MOP d | 5.1 ± 0.8 | 4.7 ± 0.3 | 4.6 ± 0.8 | |
| OOO | 4.4 ± 0.5 | 6.7 ± 1.1 | 9.3 ± 0.5 | |
| PPP | 4.0 ± 0.5 | 3.2 ± 0.5 | 2.8 ± 0.5 | |
| SOS | 2.8 ± 0.7 | 1.9 ± 0.3 | 1.2 ± 0.7 | |
| PPS | 2.3 ± 0.5 | 1.4 ± 0.3 | 1.1 ± 0.5 | |
| PLO | 2.1 ± 0.3 | 3.9 ± 0.8 | 3.6 ± 0.3 | |
| OOPo | 1.7 ± 0.5 | 2.4 ± 0.5 | 4.1 ± 0.5 | |
| POMa | 1.5 ± 0.2 | 1.2 ± 0.3 | 1.1 ± 0.2 | |
| PSS | 1.1 ± 0.4 | 0.7 ± 0.2 | 0.4 ± 0.4 | |
| SSS | 0.3 ± 0.1 | 0.1 ± 0.1 | 0.0 ± 0.1 | |
| Other e | 11.1 ± 1.2 | 10.0 ± 1.6 | 13.0 ± 1.2 | |
| Marble Tissue | ||||
| Fatty Acid (%) | Symbol | Australian Wagyu | Hybrids Wagyu | Japanese Wagyu |
| C18:1 | O | 40.8 ± 2.8 | 47.1 ± 2.3 | 50.3 ± 2.6 |
| C16:0 | P | 26.0 ± 1.2 | 24.3 ± 1.1 | 22.2 ± 2.3 |
| C18:0 | S | 13.6 ± 2.6 | 10.3 ± 1.0 | 8.0 ± 1.2 |
| C16:1 | Po | 3.3 ± 0.8 | 3.8 ± 0.6 | 4.9 ± 0.8 |
| C18:2 | L | 2.7 ± 0.4 | 4.4 ± 1.0 | 4.2 ± 1.0 |
| C17:0 | Ma | 1.0 ± 0.2 | 1.0 ± 0.2 | 0.7 ± 0.1 |
| C14:1 | Mo | 0.9 ± 0.3 | 0.8 ± 0.2 | 1.3 ± 0.3 |
| C15:0 | Pe | 0.4 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.1 |
| C18:3 | Al | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.0 |
| C14:0 | M | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Other e | - | 11.0 ± 1.1 | 7.7 ± 0.7 | 7.8 ± 1.5 |
| Intermuscular Fat | ||||
|---|---|---|---|---|
| Triacylglycerides (%) | Australian Wagyu | Hybrids Wagyu | Japanese Wagyu | |
| POO a | 26.0 ± 2.3 | 27.7 ± 1.0 | 25.3 ± 1.3 | |
| POS b | 10.1 ± 0.7 | 7.5 ± 1.4 | 3.3 ± 0.7 | |
| POP | 10.0 ± 1.3 | 8.6 ± 1.2 | 6.3 ± 0.5 | |
| SOO c | 8.1 ± 1.4 | 7.8 ± 0.6 | 5.5 ± 1.1 | |
| PPoO | 6.3 ± 1.5 | 8.1 ± 0.2 | 10.2 ± 1.1 | |
| OOO d | 5.0 ± 0.5 | 8.0 ± 2.1 | 12.5 ± 3.4 | |
| MOP | 4.7 ± 1.1 | 4.6 ± 0.4 | 4.3 ± 0.3 | |
| PPP | 4.2 ± 0.6 | 3.2 ± 0.3 | 2.1 ± 0.4 | |
| SOS | 3.5 ± 1.0 | 2.2 ± 0.2 | 0.8 ± 0.2 | |
| PPS | 2.1 ± 0.5 | 1.4 ± 0.4 | 0.6 ± 0.1 | |
| PLO | 2.0 ± 0.4 | 3.4 ± 0.8 | 3.5 ± 1.4 | |
| OOPo | 1.7 ± 0.6 | 3.0 ± 0.9 | 7.0 ± 1.6 | |
| POMa | 1.6 ± 0.2 | 1.3 ± 0.1 | 1.1 ± 0.2 | |
| PSS | 1.3 ± 0.4 | 0.7 ± 0.2 | 0.2 ± 0.1 | |
| SSS | 0.4 ± 0.2 | 0.2 ± 0.0 | 0.1 ± 0.1 | |
| Other e | 12.9 ± 1.3 | 12.1 ± 1.2 | 17.3 ± 2.5 | |
| Intermuscular Fat | ||||
| Fatty Acid (%) | Symbol | Australian Wagyu | Hybrids Wagyu | Japanese Wagyu |
| C18:1 | O | 40.9 ± 1.7 | 47.7 ± 3.2 | 52.4 ± 4.0 |
| C16:0 | P | 23.9 ± 2.0 | 22.3 ± 2.4 | 18.6 ± 2.5 |
| C18:0 | S | 15.4 ± 1.8 | 11.0 ± 1.1 | 5.6 ± 1.3 |
| C16:1 | Po | 3.1 ± 0.9 | 4.2 ± 0.3 | 6.8 ± 1.0 |
| C18:2 | L | 2.2 ± 0.3 | 3.6 ± 0.6 | 4.7 ± 1.1 |
| C17:0 | Ma | 1.2 ± 0.2 | 1.0 ± 0.1 | 0.5 ± 0.1 |
| C14:1 | Mo | 0.9 ± 0.3 | 1.1 ± 0.3 | 2 ± 0.6 |
| C15:0 | Pe | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.3 ± 0.1 |
| C18:3 | Al | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 |
| C14:0 | M | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Other e | - | 11.7 ± 1.2 | 8.5 ± 0.9 | 8.7 ± 1.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ueda, S.; Takashima, Y.; Gotou, Y.; Sasaki, R.; Nakabayashi, R.; Suzuki, T.; Sasazaki, S.; Fukuda, I.; Kebede, B.; Kadowaki, Y.; et al. Application of Mass Spectrometry for Determining the Geographic Production Area of Wagyu Beef. Metabolites 2022, 12, 777. https://doi.org/10.3390/metabo12090777
Ueda S, Takashima Y, Gotou Y, Sasaki R, Nakabayashi R, Suzuki T, Sasazaki S, Fukuda I, Kebede B, Kadowaki Y, et al. Application of Mass Spectrometry for Determining the Geographic Production Area of Wagyu Beef. Metabolites. 2022; 12(9):777. https://doi.org/10.3390/metabo12090777
Chicago/Turabian StyleUeda, Shuji, Yasuharu Takashima, Yunosuke Gotou, Ryo Sasaki, Rio Nakabayashi, Takeshi Suzuki, Shinji Sasazaki, Ituko Fukuda, Biniam Kebede, Yuki Kadowaki, and et al. 2022. "Application of Mass Spectrometry for Determining the Geographic Production Area of Wagyu Beef" Metabolites 12, no. 9: 777. https://doi.org/10.3390/metabo12090777
APA StyleUeda, S., Takashima, Y., Gotou, Y., Sasaki, R., Nakabayashi, R., Suzuki, T., Sasazaki, S., Fukuda, I., Kebede, B., Kadowaki, Y., Tamura, M., Nakanishi, H., & Shirai, Y. (2022). Application of Mass Spectrometry for Determining the Geographic Production Area of Wagyu Beef. Metabolites, 12(9), 777. https://doi.org/10.3390/metabo12090777









