Abstract
Due to the numerous adverse effects of synthetic drugs, researchers are currently studying traditional medicinal plants to find alternatives for diabetes treatment. Eucalyptus citriodora is known to be used as a remedy for various illnesses, including diabetes. This study aimed to explore the effects of ethanol extract of Eucalyptus citriodora (EEEC) on in vitro and in vivo systems, including the mechanism/s of action. The methodology used involved the measurement of insulin secretion from clonal pancreatic β-cells, BRIN BD11, and mouse islets. Other in vitro systems further examined EEEC’s glucose-lowering properties. Obese rats fed a high-fat-fed diet (HFF) were selected for in vivo evaluation, and phytoconstituents were detected via RP-HPLC followed by LC-MS. EEEC induced insulin secretion in a concentration-dependent manner with modulatory effects, similar to 1 µM glucagon-like peptide 1 (GLP-1), which were partly declined in the presence of Ca2+-channel blocker (Verapamil), KATP-channel opener (Diazoxide), and Ca2+ chelation. The insulin secretory effects of EEEC were augmented by isobutyl methylxanthine (IBMX), which persisted in the context of tolbutamide or a depolarizing concentration of KCl. EEEC enhanced insulin action in 3T3-L1 cells and reduced glucose absorption, and protein glycation in vitro. In HFF rats, it improved glucose tolerance and plasma insulin, attenuated plasma DPP-IV, and induced active GLP-1 (7-36) levels in circulation. Rhodomyrtosone B, Quercetin-3-O-β-D-glucopyranoside, rhodomyrtosone E, and quercitroside were identified as possible phytoconstituents that may be responsible for EEEC effects. Thus, these findings revealed that E. citriodora could be used as an adjunct nutritional supplement to manage type 2 diabetes.
1. Introduction
Diabetes mellitus (DM) is a progressive group of metabolic illnesses that has become a major global concern [1] since it is currently the 7th leading cause of mortality [2]. Nearly 10.5% of the global population is suffering from DM [3] and approximately 90% of these individuals are type 2 diabetes mellitus (T2DM) patients [4]. The major pathophysiology of T2DM includes insulin resistance, obesity, chronic inflammation, oxidative stress, and mitochondrial dysfunction. Other than these genetic factors, lifestyle habits are also attributed to diabetes prevalence and incidence [5]. Obesity, generally known as an overabundance of body fat that adversely affects human health, is often measured in BMI (body mass index). The presence of nonesterified fatty acids (NEFAs) released from adipose tissue in obese patients may raise concerns of insulin resistance and β-cell dysfunction that leads to type 2 diabetes [6]. Obesity may also increase the risk of hypertension and dyslipidaemia that lead to cardiovascular disease (CVD) (such as coronary artery disease, angina, atrial fibrillation, myocardial infarction, and sudden cardiac arrest) in type 2 diabetes patients [7]. However, there is no complete cure for DM, except for symptomatic treatment [8].
One of the current treatments for DM is DPP-IV inhibitors, which work by suppressing the activity of the dipeptidyl peptidase IV (DPP-IV) enzyme. DPP-IV enzyme is responsible for inactivating the incretin hormones, Glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) by cleaving in N-terminal, resulting in GIP (3–42) and GLP-1 (9–36) [9]. These hormones are released from the intestine following nutrient intake and bind to specific receptors in pancreatic β-cells. This binding enhances the cyclic adenosine monophosphate (cAMP) pathway and thus stimulates insulin release from the clonal β-cells. Hence, the incretin hormones play a vital role in regulating post-prandial hyperglycaemia [10]. As it is known, the DPP-IV enzyme degrades the incretin hormones and reduces the life span of GLP-1 and GIP [9]. Therefore, DPP-IV inhibitors are an effective measure for controlling diabetes mellitus. However, DPP-IV inhibitors and other synthetic current therapeutics are raising a wide concern of adverse effects such as gastrointestinal disturbances, weight gain, diarrhea, renal failure, and hypoglycemia [11]. As a result, alternative medicines, particularly plant-based medicines, are frequently desired [5].
Several reports support that herbal medicine might be one of the successful approaches to maintaining T2DM globally [12], without provoking adverse effects or secondary complications. Phytochemicals including tannins, flavonoids, vitamin C, and E are potent antioxidants and have been indicated to ameliorate pancreatic β-cell functions, enhancing insulin action and glucose transport and modulating glucagon-like peptide-1 (GLP-1) homeostasis [13,14]. Several phytochemicals such as rutin, isoquercitrin, and catechin have been observed to improve insulin secretion via DPP-IV inhibition, thus protecting the active forms of the incretin hormones, GIP (1–42) and GLP-1 (7–36), from degradation [15].
Eucalyptus (Family- Myrtaceae) is a large genus of evergreen aromatic trees. This genus is native to Australia, India, Bangladesh, and the neighboring islands. Among other species of this genus, Eucalyptus citriodora Hook, commonly called the lemon- or citron-scented gum [16], is a well-known medicinal plant with bluish green leaves [17] and composed of volatile (essential oil and sterols) [16] and non-volatile chemical constituents (triterpenes, tannins, flavonoids, anthocyanins, and phenolic compounds) [18]. Traditionally, the leaf extract of E. citriodora was used as a remedy to treat various diseases, including diabetes, whooping cough, liver and gallbladder disorders, ulcer, neuralgia, stomatitis, pain, gonorrhea, rheumatism, and gastrointestinal disturbances [18]. Furthermore, volatile essential oils isolated from its leaves were found to have ethnomedicinal use against cold, fever, and bronchitis along with suppurative and general respiratory tract infections such as asthma and chronic obstructive pulmonary disease (COPD) [19]. Eucalyptus citriodora has a wide range of pharmacological indications with low or no toxicity reports [18], and some of these properties include antidiabetic, antifungal, antispasmodic, antibacterial, antiseptic, anti-inflammatory, analgesic, and diuretic activity, inhibition of bone resorption, and natural repellency [20,21]. Similarly, hot water extract of E. citriodora augmented insulin release from pancreatic β-cells, improved glucose tolerance and β-cell function, and reduced plasma DPP-IV concentration in high-fat fed (HFF) obese rats [22]. The present study aims to evaluate the basic mechanism of action of the antidiabetic activity exerted by ethanol extract of E. citriodora leaves both in vitro and in vivo.
2. Materials and Methods
2.1. Collection and Preparation of Plant Extracts
The leaves of E. citriodora were obtained from Bandarban, a tropical and hilly area of Bangladesh. The collection was performed during monsoon season when the average temperature was between 26–30 °C. A taxonomist identified the plant’s identity and assigned the accession number 43755. The leaves of E. citriodora were properly cleaned and air-dried in an oven at 40 °C before being extracted with ethanol. Plant Powder (200 g) was dissolved in 1 L of 80% (v/v) ethanol and kept on shaker at 900 g for 48–72 h at room temperature. The mixture was filtered using Whatman no. 1 filter paper, and the extract was allowed to dry under decreased pressure at <40 °C. The oily filtrate was vacuum-dried (Savant Speed vac; New York, NY, USA) using a rotary evaporator machine (Bibby RE-200, Sterilin Ltd., Newport, UK) and the ultimate product, a gummy, semi-solid crude extract of E. citriodora was achieved (~6 g) and stored at 4 °C for experimental studies [23]. EEEC was not soluble in water, therefore the extract was dissolved in 60 μL/10 mL dimethyl sulfoxide (0.6% DMSO) and was not toxic to the cells for further investigations.
2.2. In Vitro Insulin-Releasing Studies
The insulin-releasing effects of ethanol extract of E. citriodora (EEEC) was examined in clonal BRIN-BD11 cells (ECACC 10033003) [24] and isolated mouse islets [25]. Islets were isolated from the pancreas of mice via collagenase P obtained from Clostridium histolyticum and cultured for 24–48 h in an incubator at 37 °C [26]. Leaf extract of E. citriodora with or without known insulin secretagogues at various glucose concentrations (1.1, 5.6, or 16.7 mM) were incubated at 37 °C for 20 and 60 min, respectively [25]. The samples were collected, centrifuged, and stored at –20 °C for insulin radioimmunoassay [27]. For the measurement of insulin content in the islet cells, an acid-ethanol extraction method was employed [26].
2.3. Membrane Potential and Intracellular Calcium ([Ca2+]i)
A FLIPR Membrane Potential and Calcium Assay Kit (Molecular Devices, Sunnyvale, CA, USA) were used to determine the influence of EEEC on membrane potential and intracellular calcium [Ca2+] in BRIN-BD11 cells [28]. At first, microplates with ninety-six wells were used to seed BRIN-BD11 cells and allowed to stand for 18 h at 37 °C. After the removal of the medium, cells in 100 μL KRB buffer solution were incubated at 37 °C for 10 min. The positive controls were a depolarizing concentration of 30 mM KCl and 10 mM alanine. The changes in signal intensity caused by EEEC were observed in a fluorometer [5].
2.4. Cellular Glucose Uptake
The insulin action of EEEC and its impact on the cellular glucose uptake were evaluated using 3T3L1 differentiated cells as discussed previously [29]. The cells were first incubated with 50 µL EEEC (200 µg/mL) at 37 °C for 30 min in the presence/absence of 100 nM insulin followed by the addition of 2-NBDG (50 nM). The resultant mixture was allowed to stand for 5 min and then rinsed with ice-cold PBS. The slides were fitted with coverslips and the corners were tightly sealed with nail polish. A microscope (10× magnification) was used to capture images of the coverslips and the fluorescence intensity was assessed as previously stated [21].
2.5. Glycation of Insulin
The impact of EEEC on in vitro glycation of insulin was measured as described [30]. D-Glucose (246·5 mM), Human insulin (1 mg/mL), sodium phosphate buffer (10 Mm, pH 7.4) and NaBH3CN (0.0853 g/mL), with (treatment)/without (control) plant extract were mixed to create a volume of 1 mL. The resultant mixture was incubated at 37 °C for 24 h and the reaction was ended by adding 30 µL of 0.5 M acetic acid. Aminoguanidine, a known inhibitor of protein glycation, was used as the positive control, and glycated and non-glycated insulin were detected via RP-HPLC [31].
2.6. DPP-IV Enzyme Activity In Vitro
DPP-IV enzyme activity in vitro was analyzed using a fluorometer following the processes stated earlier [32]. The test reagents, DPP-IV (8 mU/mL) enzyme and Gly-Pro-AMC (200 µM) with (treatment) or without (control) treatment, were incubated in 96-well black-walled, clear-bottomed microplates (Greiner). The fluorescence intensity was calculated using a Flex Station 3 (Molecular Devices, Sunnyvale, CA, USA) consisting of a 2.5 nm slit width with excitation and emission at 370 nm and 440 nm, respectively. The drug sitagliptin was considered a positive control [22].
2.7. Starch Digestion
An assay for in vitro starch digestion under the influence of EEEC was performed on the previous study [33]. A mixture of starch solution (2 mg/mL; 100 mg in 50 mL water), with (treatment) or without (control) treatment and heat stable α-amylase (40 µL of 0.01%) (from Bacillus leicheniformis, Sigma-Aldrich, St. Louis, MO, USA) were incubated at 80 °C for 20 min. The resultant diluted mixture was again incubated with amyloglucosidase (30 µL of 0.1%, Sigma-Aldrich) from Rhizopus mold at 60 °C for 30 min. Samples were collected and stored at 4 °C until further analysis for the glucose release using liquid GOD/PAP method (Randox GL 2623) [22]. As a positive control, α-glucosidase inhibitor, acarbose was used.
2.8. Glucose Diffusion In Vitro
A cellulose ester dialysis tube (CEDT) filled with 2 mL volume of 0.9% NaCl and 220 mM glucose in the presence (treatment) or absence (control) of plant extract was used to assess the in vitro glucose diffusion and absorption [34]. CEDT was placed inside a 50 mL centrifuge tube (Orange Scientific, Orange, CA, USA) consisting of 0.9% NaCl (45 mL) solution after being tightly sealed from both ends. Samples were removed after 24 h at 37 °C from the orbital shaker to analyze the glucose diffusion [22].
2.9. Animals
At about 6–8 weeks before the start of the experiments, 8–9-week-old Sprague–Dawley male rats (Envigo UK, approximately 200–250 g) were fed a high-fat diet [20% protein, 45% fat, and 35% carbohydrate: 26.15 KJ/g total energy percent (Special Diet Service, Essex, UK)]. The age-matched rats were fed a standard rodent diet [10% fat, 30% protein, and 60% carbohydrate making 12.99 KJ/g total energy (Trouw Nutrition, Cheshire, UK)] and these rats were used as controls. Animals were housed under a maintained temperature and humidity (25 ± 0.5 °C and 65–70%) conditions. Animal housing had a 12 h automatic light on-off facility that ensured a day-night circadian rhythm. All experiments were approved by the Animal Welfare and Ethical Review Board (AWERB) (14 May 2018) at Ulster University and were conducted under the PIL1822 and PPL 2804 UK Home Office Animal project/personal license numbers, which were authorized on 6 May 2016 and 5 February 2017, respectively. The experiments were performed in compliance with the UK Act 1986 and EU Directive 2010/63EU. Measures were performed to ensure that no animals would be harmed during the duration of the study. Figure 1 illustrates a summary of the experimental design for animal studies.
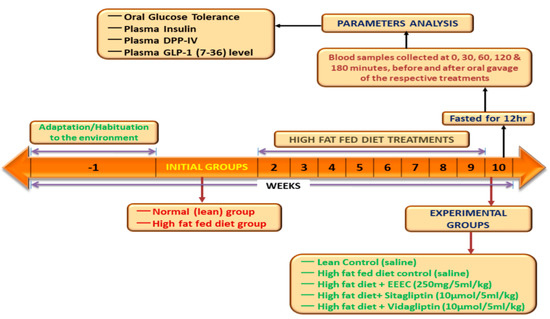
Figure 1.
Schematic diagram represents the experimental design for animal studies.
2.10. Oral Glucose Tolerance Test
High-fat-fed rats (380–400 g body wt.) were used to assess the effects of EEEC on blood glucose. All the blood samples were collected at specific times after oral administration of glucose (18 mmol/kg body weight) with and without EEEC (250 mg/5 mL/kg), as indicated in Figure 4B. Plasma was obtained by centrifuging the blood for 5 min at 12,000 rpm at 4 °C and then storing it at 20 °C for insulin assay. Blood glucose was measured using an Ascencia Contour glucose meter (Bayer, Newbury, UK), and insulin was assessed by dextran-charcoal radioimmunoassay [34].
2.11. DPP-IV Enzyme Activity
The effects of EEEC on the DPP-IV enzyme were measured in plasma using a fluorometric assay, as reported previously [25]. After oral gavage of EEEC (250 mg/5 mL/kg), DPP-IV inhibitors sitagliptin (10 mol/5 mL/kg) and vildagliptin (10 mol/5 mL/kg), or saline control, blood samples were collected from rats fed a high-fat diet at certain times, as indicated in Figure 4D. Using a GLP-1 (Active) ELISA Kit (EGLP-35K, Merck Millipore, Dorset, UK), active GLP-1 (7–36) was measured in plasma samples collected at 30 min.
2.12. Purification of Crude Extract
The possible active phytoconstituents from the EEEC leaf were isolated using the techniques described previously [34]. Initially, crude leaf extract was dissolved in 0.12 percent (v/v; TFA/water) for further evaluation by RP-HPLC, where separation of the components was performed based on their affinity. The filtered extract was injected into the stainless-steel column of the RP-HPLC, which was then equilibrated with 0.12 percent (v/v; TFA/water) at a flow rate of 1.0 mL/min. Acetonitrile was utilized as an eluent and was retained at a gradient ratio of 20% over 10 min and 70% over a 40 min cycle. The peak fractions were collected at 254 nm, and the individual peak retention times on the HPLC were recorded [25].
2.13. Mass Spectroscopy
Molecular weights of peak fractions of EEEC from the RP-HPLC were determined by means of liquid chromatography-mass spectrometry (LC-MS) through electrospray ionization mass spectrometry (ESI-MS) method [25]. Peak fractions of the samples were separated and identified via Spectra System LC (Thermo Separation Products) using a Kinetex 5m F5 LC column (150 × 4.6 mm, Phenomenex) and a UV detection system (220–256 nm) following the detailed methodology discussed previously [34].
2.14. Statistical Analysis
Analysis and interpretation of the raw data were performed using Graph Pad prism 5. Unpaired Student’s t-test (nonparametric, with two-tailed p values) in addition to one-way ANOVA with Bonferroni post hoc tests were applied for the analysis of the data and the values were stated as Mean ± SEM with a hypothetical significance limit of p < 0.05 [22,25].
3. Results
3.1. EEEC and Insulin Release
The basal rate of insulin release from BRIN-BD11 cells was 1.20 ± 0.05 ng/106 cells/20 min at 5.6 mM glucose. The rate increased to 5.97 ± 0.19 ng/106 cells/20 min (p < 0.001; Figure 2A; n = 8) in the presence of a positive control, alanine (10 mM). The effect of increasing concentrations of EEEC on insulin release from BRIN-BD11 cells are demonstrated in Figure 2A,B. EEEC produced 1.63 ± 0.10 to 5.69 ± 0.22 ng/106 cells/20 min (p < 0.05–0.001; Figure 2A; n = 8) insulin following a dose-dependent manner (1.6–5000 μg/mL) at 5.6 mM glucose. At 16.7 mM glucose, the basal concentration of insulin release from clonal pancreatic β cell line, BRIN-BD11 was 1.84 ± 0.03 ng/106 cells/20 min which reached 9.70 ± 0.40 ng/106 cells/20 min (p < 0.001; Figure 2B; n = 8) with a depolarized concentration of KCl (30 mM). EEEC significantly (p < 0.001) stimulated insulin release at 16.7 mM glucose from 2.03 ± 0.22 to 6.53 ± 0.29 ng/106 cells/20 min at 1.6-5000 μg/mL (p < 0.05–0.001; Figure 2B; n = 8). However, no remarkable effect of EEEC on lactate dehydrogenase release was observed over the concentration range of 1.6–200 μg/mL. EEEC produced a substantial increase in insulin secretion from isolated mouse islets at 16.7 mM glucose (Figure 2C). EEEC portrayed a significant increase in insulin secretion at ≥25 μg/mL (p < 0.001; Figure 2C). Insulin secretion by EEEC was comparable to that induced by GLP-1 (10−6 & 10−8 M) when tested at 200 µg/mL concentration (p < 0.001; Figure 2C).
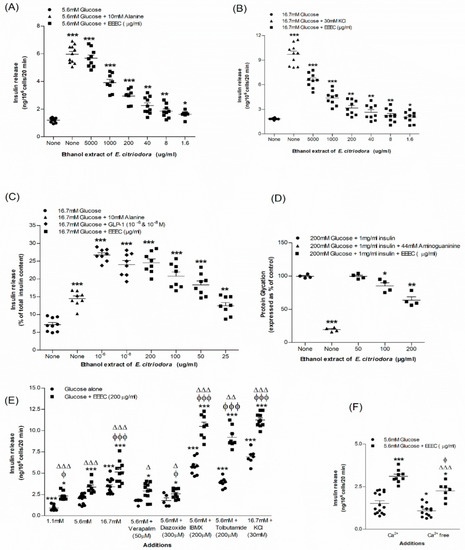
Figure 2.
Effects of EEEC on insulin secretion from (A,B) clonal pancreatic β-cells (BRIN-BD11) and (C) islets of Langerhans, (D) glycation of protein, (E) secretion of insulin with known stimulators or inhibitors and (F) plus or minus extracellular calcium from clonal β-cells. Values n = 8 and 4 for insulin secretion and n = 3 for glycation of protein are mean ± SEM. * p < 0.05, ** p < 0.01 and *** p < 0.001 compared to control. ϕ p < 0.05 and ϕϕϕ p < 0.001 compared to 5.6 mM glucose with EEEC. Δ p < 0.05, ΔΔ p < 0.01 and ΔΔΔ p < 0.001 compared to respective incubation without EEEC. EEEC, Ethanol extract of E. citriodora.
3.2. EEEC and Known Modulators of Insulin Release, Inhibitors and Free Ca2+ Conditions
EEEC at a concentration of 200 µg/mL was incubated with known stimulators or inhibitors in BRIN-BD11 cells to determine the insulin response (Figure 2E). At 5.6 mM glucose, K+ channel activator, diazoxide (300 µM), and L-type voltage-dependent Ca2+ channel blocker, verapamil (50 μM), lowered (15–24%) the insulin-releasing properties of EEEC by interfering with intracellular Ca2+ influx activity (Figure 2E). Additionally, KCl (30 mM), a depolarizing stimulus, increased insulin release of EEEC by 1.7-fold (p < 0.001; Figure 2E). Moreover, isobutyl methylxanthine (IBMX) (p < 0.001; Figure 2E) and tolbutamide (Figure 2E; p < 0.001) also increased the action of the EEEC in inducing insulin release whereas, in the absence of extracellular calcium, it was inhibited by 34% (Figure 2F).
3.3. EEEC and Membrane Depolarization and [Ca2+]i
The membrane depolarization of BRIN-BD11 cells was significant (p < 0.001) with 30 mM KCl. EEEC also depolarized the membrane (p < 0.001; Figure 3A) in the presence of 5.6 mM glucose. Both Alanine (p < 0.001) and EEEC (p < 0.001) resulted in a substantial elevation in intracellular calcium concentration at 5.6 mM glucose (Figure 3B).
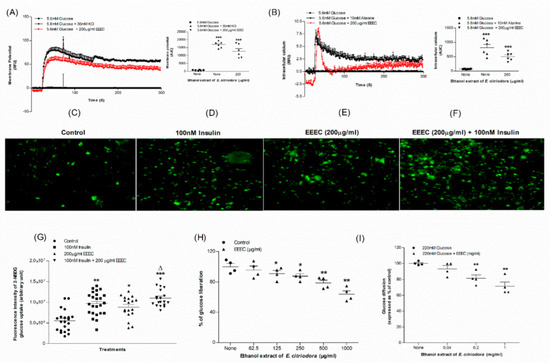
Figure 3.
Effects of EEEC on (A) membrane potential and (B) intracellular calcium in clonal pancreatic β cell (BRIN BD11) and, (C–G) glucose uptake, (H) starch digestion and (I) glucose diffusion in vitro. Changes of fluorescence intensity in differentiated 3T3L1 adipocyte incubated with EEEC (E) minus or (F) plus 100 nM insulin. The ×10 magnification was used to take the images. Values n = 6 for membrane potential and intracellular calcium, n = 4 for uptake of glucose, digestion of starch and diffusion of glucose are mean ± SEM. * p < 0.05, ** p < 0.01, and *** p < 0.001 compared to control. Δ p < 0.05 compared to insulin alone.
3.4. EEEC and Glycation of Insulin
EEEC demonstrated a significant inhibitory effect on insulin glycation. It produced a 14% inhibition at its lowest concentration of 100 µg/mL, while it produced a maximum 34% inhibition at 200 µg/mL (p < 0.001; Figure 2D). Aminoguanidine, (44 mM) used as a positive control, inhibited insulin glycation by 80.5% (p < 0.001; Figure 2D).
3.5. EEEC and Glucose Uptake and Insulin Action
EEEC was studied for effects on glucose uptake by 3T3L1 differentiated adipocyte cells using a glucose analogue, 2-NBDG (2-(N-(7-Nitrobenz-2-oxa-1,3-diazol-4-yl) Amino)-2-Deoxyglucose) fluorescent hexose (Figure 3C–G). EEEC significantly increased glucose uptake (p < 0.05; Figure 3G). Insulin at a concentration of 100 nM exacerbated this stimulatory effect (p < 0.001; Figure 3G). Glucose uptake decreased to 34%, with EEEC alone, from about 45% recorded in the presence of insulin (Figure 3G).
3.6. EEEC and Starch Digestion
EEEC at ≥125 µg/mL concentration reduced (p < 0.05) digestion of starch by 9% (Figure 3H) whereas at a higher concentration (1000 µg/mL), the reduction of glucose liberation from starch was 34%. The positive control, Acarbose, considerably (p < 0.001) inhibited enzymatic glucose liberation from starch by 85% (Data not provided).
3.7. EEEC and Glucose Diffusion In Vitro
3.8. EEEC and DPP-IV Enzyme Activity In Vitro
Results for in vitro DPP-IV enzyme inhibitory effect of EEEC are portrayed in Figure 4A. EEEC inhibited the DPP-IV enzyme by 9–52% in a concentration-dependent manner (40–5000 µg/mL) (p < 0.01; p < 0.001; Figure 4A). An established drug named Sitagliptin (10 µM) has been found to inhibit the enzymatic liberation of AMC from the DPP-IV substrate, Gly-Pro-AMC by 97.5% (Data not provided).
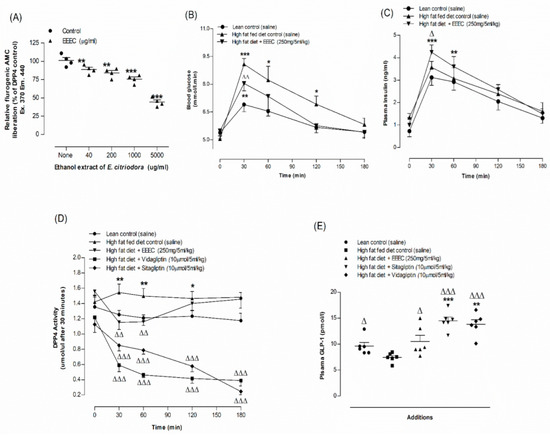
Figure 4.
Effects of EEEC on (A) DPP-IV enzyme in vitro, (B) glucose tolerance, (C) plasma insulin, (D) DPP-IV and (E) plasma active GLP-1 (7–36) in high-fat-fed rats. In vivo parameters were evaluated before and after oral gavage of glucose alone (18 mmol/kg body weight, control) or with EEEC (250 mg/5 mL/kg body weight), sitagliptin and vidagliptin (both at 10 μmol /5 mL/kg, body weight). Plasma active GLP-1 (7–36) levels was assayed at 30 min after treatments. Values n = 4 for in vitro DPP-IV enzyme activity and n = 6 for in vivo parameters are mean ± SEM. * p <0.05, ** p <0.01 and *** p < 0.001 compared to lean control and Δ p < 0.05, ΔΔ p < 0.01 and ΔΔΔ p < 0.001 compared to high-fat-fed diet control rats.
3.9. EEEC and Oral Glucose Tolerance and Plasma Insulin, DPP-IV and Active GLP-1 (7-36) Levels
An oral dose of EEEC (250 mg/5 mL/kg; b.w.) improved glucose tolerance, plasma insulin concentration, and reduced plasma DPP-IV substantially (p < 0.05–0.01; Figure 4B-D). Sitagliptin and vildagliptin (10 μmol/5 mL/kg), considered as positive controls, depicted significant (p < 0.001; Figure 4D) reduction (70–73%, Figure 4D) in DPP-IV enzyme activity. At 30 min after oral administration of EEEC, active GLP-1 (7–36) concentrations in plasma increased by 28% (p < 0.05–0.01; Figure 4E) and with sitagliptin and vidagliptin, it increased to 86–92% (p < 0.001; Figure 4E).
3.10. Identification of Purified Extract
The pharmacologically possible active molecules characterized from EEEC bark are listed in Table 1. We collected eleven major peaks from the EEEC via RP-HPLC (Figure 5A) and then were assayed for insulin secretory properties using clonal pancreatic β-cells (BRIN BD11). Peak fractions P-1, P-2, P-7, P-8, and P-9 greatly (p < 0.001; Figure 5B) produced insulin secretion, however P-1 and P-2 were associated with cell toxicity. Alanine was used as a standard control (p < 0.001; Figure 5B). Peak fractions of concern were further investigated using LC-MS (Table 1). The molecular masses of P-1, P-2, P-7, P-8, and P-9 were 442.1, 476.7, 464.2, 490.9, and 447.9 Da, respectively. Figure 6A–D depicts the chemical structures of anticipated compounds based on their molecular mass.

Table 1.
Molecular mass of peak samples of EEEC leaves obtained from the preparative RP-HPLC via LC-MS analysis.
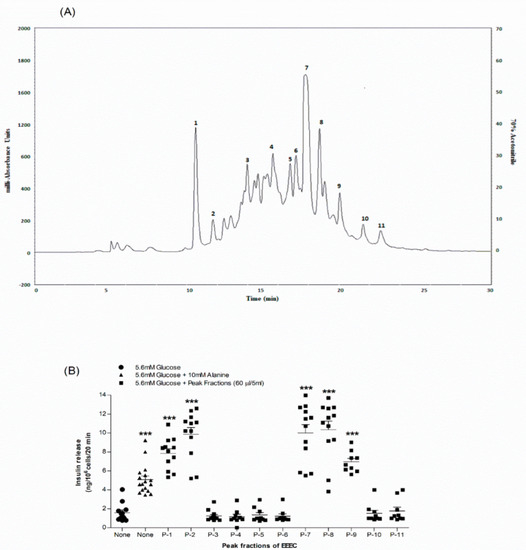
Figure 5.
Representative (A) HPLC profile and (B) insulin-releasing effects of peak fractions (1,2,7-9) of EEEC. Crude extract was chromatographed at a flow rate of 1.0 mL/min on a (10 × 250 mm) semi-preparative 5 μm C-18 column (Phenomenex, UK). Using linear gradients of acetonitrile (0–20% up to 10 min, 20–70% up to 40 min), the concentration of the eluting solvent was increased. Compounds were detected by measurement of absorbance at 254 nm. Peak fractions 1, 2 and 7–9 were collected and insulin-releasing activity assessed using BRIN-BD11 cells. Values n = 8 for insulin release are mean ± SEM. *** p < 0.001 compared to control.
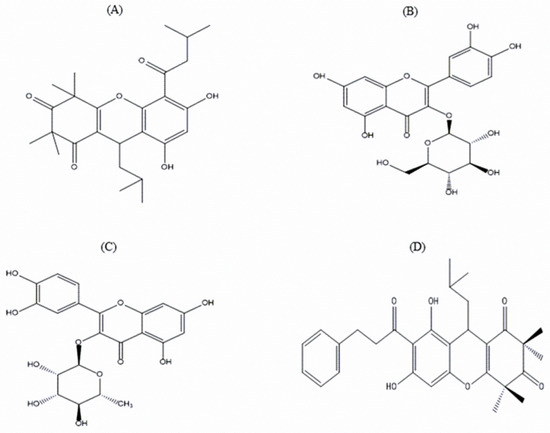
Figure 6.
Chemical structure of possible phytoconstituents extracted from the EEEC. Chemical structures of (A) Rhodomyrtosone B, (B) Quercetin-3-O-β-D-glucopyranoside, (C) Quercitroside and (D) Rhodomyrtosone E with their corresponding molecular formulae: C26H34O6, C21H20O12, C21H20O11, and C30H34O6.
4. Discussion
Diabetes mellitus (DM), a metabolic group of disorders, is currently the fastest growing chronic disease around the world and a severe threat to public health worldwide [34,35]. DM is a result of the absolute or relative deficiency of insulin secretion and/or insulin resistance and is diagnosed as a high blood glucose level that can lead to a variety of secondary acute or chronic complications [35]. For decades, medicinal plants and their phytochemicals have been found to have significant pharmacological and biological benefits. Therefore, they have been increasingly used as replacement therapy for synthetic drugs. Although E. citriodora has anti-hyperglycemic activities, the exact mechanism of action is elusive [22]. Therefore, the present study was designed to investigate the insulin secretory effects of EEEC on isolated mouse islets and BRIN-BD11 cells, including its other hypoglycemic properties.
EEEC significantly increased insulin secretion from clonal pancreatic β-cells and isolated islets in a dose-dependent manner. To understand the molecular mechanism of insulin secretion more accurately, the effects of a non-toxic dose of EEEC were evaluated in the presence or absence of insulin increasing/reducing modulators. A class of oral hypoglycemic medications known as sulfonylureas works by blocking KATP channels and depolarizing the plasma membrane, which stimulates Ca2+ entry via activating voltage-dependent calcium channels [36]. In the presence of both a KATP channel blocker (tolbutamide) and membrane depolarization with 30 mM KCl, EEEC stimulated insulin secretion. Thus, this indicates that the EEEC has the capability to enhance insulin secretion via several pathways, such as direct impacts on exocytosis, PI3 (phosphatidylinositol pathway), adenylate cyclase, or cAMP pathway [37]. Diazoxide partially decreased the insulin-releasing activity of the EEEC, suggesting the involvement of KATP channel blockage, which is further supported using verapamil, a voltage-dependent Ca2+ channel blocker, which suppressed insulin secretory properties of EEEC [35]. EEEC promoted membrane depolarization and raised intracellular calcium levels in BRIN BD11 cells. In the presence of the cAMP phosphodiesterase inhibitor IBMX, EEEC substantially increased insulin secretion, indicating the involvement of the cAMP pathway [22]. EEEC can cause an increase in cAMP in lung tissues, which inhibits the reproduction of bronchial smooth muscle cells and enhances airway relaxation, and this has been proven to be useful in the treatment of asthma [38,39].
Insulin predominantly modulates postprandial glucose, and anomalies in the signal transduction pathway in skeletal muscle and adipose tissue, such as decreased GLUT4 translocation, are a major contributor to insulin resistance [40,41]. Therefore, the impact of EEEC on glucose uptake in differentiated 3T3L1 adipocyte cells was examined. It was observed that the EEEC significantly enhances glucose transport with or without insulin. According to previous studies, flavonoids and other polyphenols enhance glucose uptake and insulin action by modulating AMP-activated protein kinase (AMPK), phosphoinositide 3-kinase (PI3K), and mitogen-activated protein kinase (MAPK) activity [42,43,44]. E. citriodora contains polyphenols such as flavonoids which may activate signalling pathways to promote glucose transport in adipocytes in the presence or absence of insulin [42,45].
Protein glycation is a physiological mechanism that is crucial in the pathogenesis of diabetes. Chronic hyperglycemia leads to non-enzymatic glycation of proteins such as albumin, fibrinogen, immunoglobulins, and collagen [46]. The increased production of free radicals leads to the formation of advanced glycation end products (AGE). AGEs can accumulate intracellularly and disrupt the function of plasma proteins and enhance oxidative stress, and this accelerates the severity of diabetic complications including retinopathy, neuropathy, and nephropathy [46,47]. In this study, EEEC substantially inhibited protein glycation in a concentration-dependent manner. Recent studies also reported that E. citriodora protects β-cells damaging by inhibiting protein glycation via free radical scavenging [47]. Another study with E. citriodora indicated antioxidant properties, and it might help to overcome oxidative stress-induced protein damage [20,48,49]. Plants containing flavonoids, such as procyanidin, epicatechin, and rhodomyrtosone E, have also been indicated to have anti-glycation properties in recent studies [8,22,23].
Postprandial hyperglycaemia is considered to be a risk factor for CVD, and thus treating postprandial may prevent secondary cardiovascular complications in type 2 diabetic patients [50]. Inhibition of α-amylase and α-glucosidase activities are an essential strategy for managing postprandial hyperglycemia and the inhibition of these enzymes might be beneficial to type 2 diabetes patients with impaired insulinotropic response [49]. In the present studies, EEEC significantly inhibited starch hydrolysis in a concentration-dependent manner. Furthermore, flavonoids and other phytochemicals have been observed to suppress α-amylase and α-glucosidase activities [51]. Therefore, the present findings indicate that E. citriodora contains polyphenols such as isoquercitrin, gallic acid, quercitrin and betulinic acid, which may have the potential to restrict glucose absorption [52,53].
The molecular mass and concentration of soluble dietary fibers play a vital role in glucose-lowering actions of herbs [38,54]. To mimic the gut and investigate the effects of EEEC on glucose diffusion, an in vitro dialysis method was performed. EEEC exhibited concentration-dependent suppressing effects on glucose transport across a dialysis membrane into an external solution. It has been demonstrated that dietary supplementation with E. citriodora reduces hyperglycemia in STZ-induced diabetic rats, which is believed to be due to the inhibition of glucose absorption from the gut [33]. E. citriodora has also been observed to lower blood glucose levels in alloxan-induced diabetic rats, suggesting similar effects to the anti-diabetic drug glibenclamide [18].
It is observed that a high-fat diet induces obesity and metabolic abnormalities such as insulin resistance, and the propensity to develop type 2 diabetes in healthy Sprague–Dawley rats [55]. In an acute in vivo study, EEEC improved glucose tolerance and plasma insulin levels in rats fed a high-fat diet. Recent studies also reported that hot water extract of E. citriodora ameliorates glucose tolerance, plasma insulin and β-cell function in high-fat-fed rats [22].
The unique signaling mechanism of DPP-IV inhibitors, which involves delaying the degradation of incretin hormones (GLP-1 and GIP), causing decrease in glucagon release, and improving insulin secretion, has demonstrated a significant impact on the treatment of type 2 diabetes [56]. DPP-IV enzyme deactivates incretin hormones via N-terminal cleavage, resulting in GLP-1 (9–36) and GIP (3–42). This disrupts the regulation of insulin release and increases blood glucose levels [30]. Therefore, suppression of DPP-IV enzyme activity may play a vital role in delaying the deactivation of GLP-1 and GIP, which may boost insulin secretion and positively regulate blood glucose levels [57]. In this investigation, EEEC inhibited the DPP-IV enzyme activity in vitro concentration-dependently. In vivo studies on rats fed a high-fat diet revealed a significant reduction of plasma DPP-IV enzyme activity and a substantial increase in active GLP-1 (7–36) concentration. Previous studies indicated that natural sources, such as plants and their phytochemicals including isoquercitrin, rutin, and gallic acid, have the potential to inhibit the DPP-IV enzyme [34,58,59]. These findings imply that the insulin-releasing and glucose-lowering effects of EEEC may be partly attributable to DPP-IV enzyme suppression.
RP-HPLC and LC-MS techniques were employed to explore the possible active phytoconstituents of EEEC. Based on our initial screening, we have detected several peak fractions among which P-1, P-2, P-7, P-8, and P-9 increased insulin release from BRIN BD11 cells that have been hypothesized to be active molecules contributing to antihyperglycaemic action of EEEC. The molecular mass of peak fractions P1, P-7, P-8, and P-9 were corresponded to rhodomyrtosone B [60,61], quercetin-3-O-β-D-glucopyranoside [62], rhodomyrtosone E [5], and quercitroside [63]. The results agree with previous findings of E. citriodora containing isoquercitrin, rhodomyrtosone E, and quercitrin [21,64,65]. No previous reports have proven rhodomyrtosone B as a phytomolecule of E. citriodora, while others have extracted rhodomyrtosone B from Rhodomyrtus tomentosa, a plant known to have blood glucose lowering, antioxidant, and antihyperlipidemic properties [60,66]. Recent findings also depicted that quercetin-3-O-β-D-glucopyranoside improves glucose tolerance and increases plasma GLP-1 levels by reducing DPP-4 enzyme activity in STZ-induced diabetic rats [67]. Additionally, it has been observed that quercitrin attenuates oxidative stress by scavenging free radicals and reducing lipid peroxidation, thus increasing insulin production from pancreatic β-cells in STZ-induced diabetic rats [68]. Another compound, rhodomyrtosone E, enhanced GLUT4 translocation in L6 skeletal muscle cells by AMPK pathway [69]. All these predicted compounds may be responsible for these activities of EEEC. Further investigations using other parameters such as NMR are warranted to elucidate the chemical structure of the potential phytoconstituents.
5. Conclusions
The current investigations indicated that EEEC has the potential to enhance insulin secretion from clonal pancreatic β-cells. EEEC substantially regulated hyperglycemia by suppressing glucose absorption, protein glycation, and DPP-IV enzyme activity in vitro, respectively. EEEC ameliorated glucose tolerance and plasma insulin and abated DPP-IV enzyme activity in rats fed a high-fat diet, thus possibly increasing the half-life of incretin hormone (GLP-1 and GIP). Insulin secretory and glucose-lowering effects of EEEC are partly due to the presence of anticipated phytoconstituents, including quercetin-3-O-β-D-glucopyranoside, rhodomyrtosone B and E, and quercitroside. Additional studies including chronic studies in animal models are needed to completely explore the role of EEEC and its phytomolecules in the retardation of diabetes mellitus progression. Further extensive research on humans is required to determine the effective dose in the management of T2DM. Thus, E. citriodora may be useful as a dietary supplement for managing diabetes mellitus and its complications.
Author Contributions
P.A. and Y.H.A.A.-W. were responsible for the conception and design of the research and contributed equally to the supervision of the study; P.A. performed the experiments, analyzed the data, interpreted the results, prepared the figures, and drafted the manuscript with S.T.C., and P.A. and Y.H.A.A.-W. edited the revised manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Ulster University Strategic Research Funding.
Institutional Review Board Statement
All experiments were approved by the Animal Welfare and Ethical Review Board (AWERB) (14/05/2018) at Ulster University and were conducted under the PIL1822 and PPL 2804 UK Home Office Animal project/personal license numbers, which were authorized on 06/05/2016 and 05/02/2017, respectively.
Informed Consent Statement
Not applicable.
Data Availability Statement
The corresponding author can provide the data presented in this study upon request. Due to restrictions, the data are not available to the public.
Acknowledgments
The authors are grateful to Ulster University Strategic Research Funding and Independent University, Bangladesh for providing access to laboratory facilities.
Conflicts of Interest
The authors declare that there is no conflict of interest in association with this manuscript.
Abbreviations
| AGE | Advanced glycation end-products |
| AMPK | AMP-activated protein kinase |
| BMI | Body mass index |
| CEDT | Cellulose ester dialysis tube |
| cAMP | Cyclic adenosine monophosphate. |
| COPD | Chronic obstructive pulmonary disease |
| CVD | Cardiovascular diseases |
| DM | Diabetes Mellitus |
| DPP-IV | Dipeptidyl peptidase IV |
| EEEC | Ethanol extract of Eucalyptus citriodora |
| GIP | Glucose-dependent insulinotropic polypeptide |
| GLP-1 | Glucagon-like peptide-1 |
| GLUT4 | Glucose transporter type 4 |
| HFF | High-fat-fed |
| IBMX | 3-isobutyl-1-methylxanthine |
| KCl | Potassium chloride |
| LC-MS | Liquid chromatography–mass spectrometry |
| MAPK | Mitogen-activated protein kinase |
| NEFA | Non-esterified fatty acids |
| NMR | Nuclear magnetic resonance |
| PI3K | Phosphoinositide 3-kinase (PI3K) |
| RP-HPLC | Reverse-phase high performance liquid chromatography |
| T2DM | Type 2 Diabetes Mellitus |
References
- Lipscombe, L.L.; Austin, P.C.; Manuel, D.G.; Shah, B.R.; Hux, J.E.; Booth, G.L. Income-related differences in mortality among people with diabetes mellitus. Can. Med. Assoc. J. 2010, 182, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, N.A.; Mazumdar, B.; Bhatt, J.D.; Hemavathi, K.G. Effect of Shilajit on blood glucose and lipid profile in alloxan-induced diabetic rats. Indian J. Pharmacol. 2004, 36, 373. [Google Scholar]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.; Mbanya, J.C. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes. Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- King, H.; Aubert, R.E.; Herman, W.H. Global burden of diabetes, 1995–2025: Prevalence, numerical estimates, and projections. Diabetes Care 1998, 21, 1414–1431. [Google Scholar] [CrossRef]
- Ansari, P.; Flatt, P.R.; Harriott, P.; Abdel-Wahab, Y.H.A. Anti-Hyperglycaemic and Insulin-Releasing Effects of Camellia Sinensis Leaves and Isolation and Characterisation of Active Compounds. Brit. J. Nutr. 2020, 126, 1149–1163. [Google Scholar] [CrossRef]
- Al-Goblan, A.S.; Al-Alfi, M.A.; Khan, M.Z. Mechanism linking diabetes mellitus and obesity. Diabetes Metab. Syndr. Obes. Targets Ther. 2014, 7, 587. [Google Scholar] [CrossRef]
- Piché, M.-E.; Tchernof, A.; Després, J.-P. Obesity Phenotypes, Diabetes, and cardiovascular diseases. Circ. Res. 2020, 126, 1477–1500. [Google Scholar] [CrossRef]
- Ansari, P.; Flatt, P.R.; Harriott, P.; Hannan, J.M.A.; Abdel-Wahab, Y.H.A. Identification of Multiple Pancreatic and Extra-Pancreatic Pathways Underlying the Glucose-Lowering Actions of Acacia Arabica Bark in Type-2 Diabetes and Isolation of Active Phytoconstituents. Plants 2021, 10, 1190. [Google Scholar] [CrossRef]
- Flatt, P.R. Dipeptidyl Peptidase IV (DPP IV) and Related Molecules in Type 2 Diabetes. Front. Biosci. 2008, 13, 3648. [Google Scholar] [CrossRef]
- Seino, Y.; Fukushima, M.; Yabe, D. GIP and GLP-1, the Two Incretin Hormones: Similarities and Differences. J. Diabetes Investig. 2010, 1, 8–23. [Google Scholar] [CrossRef]
- Inzucchi, S.E.; Bergenstal, R.M.; Buse, J.B.; Diamant, M.; Ferrannini, E.; Nauck, M.; Peters, A.L.; Tsapas, A.; Wender, R.; Matthews, D.R. Management of Hyperglycemia in Type 2 Diabetes, 2015: A Patient-Centered Approach: Update to a Position Statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015, 38, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.; Park, M.; Lee, H.C.; Kang, Y.-H.; Kang, E.S.; Kim, S.K. Antidiabetic Agents from Medicinal Plants. Curr. Med. Chem. 2006, 13, 1203–1218. [Google Scholar] [CrossRef] [PubMed]
- Zulcafli, A.S.; Lim, C.; Ling, A.P.; Chye, S.; Koh, R. Antidiabetic Potential of Syzygium Sp.: An Overview. Yale J. Biol. Med. 2020, 93, 307–325. [Google Scholar] [PubMed]
- Unuofin, J.O.; Lebelo, S.L. Antioxidant Effects and Mechanisms of Medicinal Plants and Their Bioactive Compounds for the Prevention and Treatment of Type 2 Diabetes: An Updated Review. Oxid. Med. Cell. Longev. 2020, 2020, 1356893. [Google Scholar] [CrossRef] [PubMed]
- Ansari, P.; Hannon-Fletcher, M.P.; Flatt, P.R.; Abdel-Wahab, Y.H.A. Effects of 22 Traditional Anti-Diabetic Medicinal Plants on DPP-IV Enzyme Activity and Glucose Homeostasis in High-Fat Fed Obese Diabetic Rats. Biosci. Rep. 2021, 41, BSR20203824. [Google Scholar] [CrossRef]
- Ramezani, H.; Singh, H.P.; Batish, D.R.; Kohli, R.K. Antifungal Activity of the Volatile Oil of Eucalyptus Citriodora. Fitoterapia 2002, 73, 261–262. [Google Scholar] [CrossRef]
- Kesharwani, V.; Gupta, S.; Kushwaha, N.; Kesharwani, R.; Patel, D.K. A review on therapeutics application of Eucalyptus oil. Int. J. Herb. Med. 2018, 6, 110–115. [Google Scholar]
- Patra, A.; Jha, S.; Sahu, A.N. Antidiabetic activity of aqueous extract of Eucalyptus citriodora hook. in alloxan induced diabetic rats. Pharmacogn. Mag. 2009, 5, 51–54. [Google Scholar]
- Ho, C.-L.; Li, L.-H.; Weng, Y.-C.; Hua, K.-F.; Ju, T.-C. Eucalyptus Essential Oils Inhibit the Lipopolysaccharide-Induced Inflammatory Response in RAW264.7 Macrophages through Reducing MAPK and NF-ΚB Pathways. BMC Complement. Altern. Med. 2020, 20, 200. [Google Scholar] [CrossRef]
- Saba, I.; Iqbal, M.J.; Iqbal, M. Bioactivity of Eucalyptus citriodora leaves essential oil. J. Agrochimica. 2013, 57, 128. [Google Scholar]
- Gbenou, J.D.; Ahounou, J.F.; Akakpo, H.B.; Laleye, A.; Yayi, E.; Gbaguidi, F.; Baba-Moussa, L.; Darboux, R.; Dansou, P.; Moudachirou, M.; et al. Phytochemical Composition of Cymbopogon Citratus and Eucalyptus Citriodora Essential Oils and Their Anti-Inflammatory and Analgesic Properties on Wistar Rats. Mol. Biol. Rep. 2012, 40, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Ansari, P.; Flatt, P.R.; Harriott, P.; Abdel-Wahab, Y.H. Insulinotropic and antidiabetic properties of Eucalyptus citriodora leaves and isolation of bioactive phytomolecules. J. Pharm. Pharmacol. 2021, 73, 1049–1061. [Google Scholar] [CrossRef] [PubMed]
- Ansari, P.; Azam, S.; Seidel, V.; Abdel-Wahab, Y.H.A. In vitro and in vivo antihyperglycemic activity of the ethanol extract of Heritiera fomes bark and characterization of pharmacologically active phytomolecules. J. Pharm. Pharmacol. 2022, 3, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Hannan, J.M.A.; Ansari, P.; Haque, A.; Sanju, A.; Huzaifa, A.; Rahman, A.; Ghosh, A.; Azam, S. Nigella Sativa Stimulates Insulin Secretion from Isolated Rat Islets and Inhibits the Digestion and Absorption of (CH2O)n in the Gut. Biosci. Rep. 2019, 39, BSR20190723. [Google Scholar] [CrossRef]
- Ansari, P.; Azam, S.; Hannan, J.M.A.; Flatt, P.R.; Abdel Wahab, Y.H.A. Anti-hyperglycemic activity of H. rosa-sinensis leaves is partly mediated by inhibition of carbohydrate digestion and absorption, and enhancement of insulin secretion. J. Ethnopharmacol. 2020, 253, 112647. [Google Scholar] [CrossRef]
- Hannan, J.M.A.; Ansari, P.; Azam, S.; Flatt, P.R.; Abdel Wahab, Y.H.A. Effects of Spirulina Platensis on Insulin Secretion, Dipeptidyl Peptidase IV Activity and Both Carbohydrate Digestion and Absorption Indicate Potential as an Adjunctive Therapy for Diabetes. Brit. J. Nutr. 2020, 124, 1021–1034. [Google Scholar] [CrossRef]
- Flatt, P.R.; Bailey, C.J. Abnormal plasma glucose and insulin responses in heterozygous lean (ob/+) mice. Diabetologia 1981, 20, 573–577. [Google Scholar] [CrossRef]
- Abdel-Wahab, Y.H.A.; Marenah, L.; Flatt, P.R.; Conlon, J.M. Insulin releasing properties of the Temporin family of antimicrobial peptides. Protein Pept. Lett. 2007, 14, 702–707. [Google Scholar] [CrossRef]
- Hannan, J.M.A.; Ali, L.; Khaleque, J.; Akhter, M.; Flatt, P.R.; Abdel-Wahab, Y.H.A. Antihyperglycemic activity of Asparagus racemosus roots is partly mediated by inhibition of carbohydrate digestion and absorption, and enhancement of cellular insulin action. Brit. J. Nutri. 2012, 107, 1316–1323. [Google Scholar] [CrossRef]
- Duffy, N.A.; Green, B.D.; Irwin, N.; Gault, V.A.; McKillop, A.M.; O’Harte, F.P.M.; Flatt, P.R. Effects of Antidiabetic Drugs on Dipeptidyl Peptidase IV Activity: Nateglinide Is an Inhibitor of DPP IV and Augments the Antidiabetic Activity of Glucagon-like Peptide-1. Eur. J. Pharmacol. 2007, 568, 278–286. [Google Scholar] [CrossRef]
- Thomson, H.; Ojo, O.; Flatt, P.; AbdelWahab, Y. Antidiabetic actions of aqueous bark extract of Swertia chirayita on insulin secretion, cellular glucose uptake and protein glycation. J. Exp. Integrat. Med. 2014, 4, 268. [Google Scholar] [CrossRef]
- O’Harte, F.P.M.; Højrup, P.; Barnett, C.R.; Flatt, P.R. Identification of the Site of Glycation of Human Insulin. Peptides 1996, 17, 1323–1330. [Google Scholar] [CrossRef]
- Gallagher, A.M.; Flatt, P.R.; Duffy, G.; Abdel-Wahab, Y.H.A. The effects of traditional antidiabetic plants on in vitro glucose diffusion. Nutr. Res. 2003, 23, 413–424. [Google Scholar] [CrossRef]
- Ansari, P.; Flatt, P.R.; Harriott, P.; Abdel-Wahab, Y.H.A. Evaluation of the Antidiabetic and Insulin Releasing Effects of A. Squamosa, Including Isolation and Characterization of Active Phytochemicals. Plants 2020, 9, 1348. [Google Scholar] [CrossRef]
- Ansari, P.; Flatt, P.R.; Harriott, P.; Abdel-Wahab, Y.H.A. Insulin secretory and antidiabetic actions of Heritiera fomes bark together with isolation of active phytomolecules. PLoS ONE 2022, 17, e0264632. [Google Scholar] [CrossRef]
- Sola, D.; Rossi, L.; Schianca, G.P.C.; Maffioli, P.; Bigliocca, M.; Mella, R.; Corlianò, F.; Fra, G.P.; Bartoli, E.; Derosa, G. Sulfonylureas and Their Use in Clinical Practice. Arch. Med. Sci. 2015, 4, 840–848. [Google Scholar] [CrossRef]
- Park, J.E.; Han, J.S. A Portulaca oleracea L. extract promotes insulin secretion via a K+ATP channel dependent pathway in INS-1 pancreatic β-cells. Nutr. Res. Pract. 2018, 12, 183–190. [Google Scholar] [CrossRef]
- Dey, B.; Mitra, A. Chemo-Profiling of Eucalyptus and Study of Its Hypoglycemic Potential. World J. Diabetes 2013, 4, 170. [Google Scholar] [CrossRef]
- Billington, C.K.; Ojo, O.O.; Penn, R.B.; Ito, S. CAMP Regulation of Airway Smooth Muscle Function. Pulm. Pharmacol. Ther. 2013, 26, 112–120. [Google Scholar] [CrossRef]
- Lambadiari, V.; Triantafyllou, K.; Dimitriadis, G.D. Insulin Action in Muscle and Adipose Tissue in Type 2 Diabetes: The Significance of Blood Flow. World J. Diabetes 2015, 6, 626. [Google Scholar] [CrossRef]
- Xu, P.-T.; Song, Z.; Zhang, W.-C.; Jiao, B.; Yu, Z.-B. Impaired Translocation of GLUT4 Results in Insulin Resistance of Atrophic Soleus Muscle. BioMed Res. Int. 2015, 2015, 291987. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.-K.; Gao, J.; Zhu, D.-N. Kaempferol and Quercetin Isolated from Euonymus alatus Improve Glucose Uptake of 3T3-L1 Cells without Adipogenesis Activity. Life Sci. 2008, 82, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Telapolu, S.; Kalachavedu, M.; Punnoose, A.M.; Bilikere, D. MD-1, a Poly Herbal Formulation Indicated in Diabetes Mellitus Ameliorates Glucose Uptake and Inhibits Adipogenesis–an in Vitro Study. BMC Complement. Altern. Med. 2018, 18, 113. [Google Scholar] [CrossRef]
- Russo, B.; Picconi, F.; Malandrucco, I.; Frontoni, S. Flavonoids and Insulin-Resistance: From Molecular Evidences to Clinical Trials. Int. J. Mol. Sci. 2019, 20, 2061. [Google Scholar] [CrossRef] [PubMed]
- Amakura, Y.; Yoshimura, M.; Sugimoto, N.; Yamazaki, T.; Yoshida, T. Marker Constituents of the Natural Antioxidant Eucalyptus Leaf Extract for the Evaluation of Food Additives. Biosci. Biotechnol. Biochem. 2009, 73, 1060–1065. [Google Scholar] [CrossRef]
- Singh, V.P.; Bali, A.; Singh, N.; Jaggi, A.S. Advanced Glycation End Products and Diabetic Complications. Korean J. Physiol. Pharmacol. 2014, 18, 1025926. [Google Scholar] [CrossRef]
- Singh, H.P.; Kaur, S.; Negi, K.; Kumari, S.; Saini, V.; Batish, D.R.; Kohli, R.K. Assessment of in Vitro Antioxidant Activity of Essential Oil of Eucalyptus Citriodora (Lemon-Scented Eucalypt; Myrtaceae) and Its Major Constituents. LWT–Food Sci. Technol. 2012, 48, 237–241. [Google Scholar] [CrossRef]
- Miguel, M.; Gago, C.; Antunes, M.; Lagoas, S.; Faleiro, M.; Megías, C.; Cortés-Giraldo, I.; Vioque, J.; Figueiredo, A. Antibacterial, Antioxidant, and Antiproliferative Activities of Corymbia citriodora and the Essential Oils of Eight Eucalyptus Species. Medicines 2018, 5, 61. [Google Scholar] [CrossRef]
- Al-Sayed, E.; El-Naga, R.N. Protective Role of Ellagitannins from Eucalyptus Citriodora against Ethanol-Induced Gastric Ulcer in Rats: Impact on Oxidative Stress, Inflammation and Calcitonin-Gene Related Peptide. Phytomedicine 2015, 22, 5–15. [Google Scholar] [CrossRef]
- Ceriello, A.; Davidson, J.; Hanefeld, M.; Leiter, L.; Monnier, L.; Owens, D.; Tajima, N.; Tuomilehto, J. Postprandial Hyperglycaemia and Cardiovascular Complications of Diabetes: An Update. Nutr. Metab. Cardiovasc. Dis. 2006, 16, 453–456. [Google Scholar] [CrossRef]
- Dey, B.; Mitra, A.; Katakam, P.; Singla, R.K. Exploration of Natural Enzyme Inhibitors with Hypoglycemic Potentials amongst Eucalyptus Spp. By in Vitro Assays. World J. Diabetes 2014, 5, 209. [Google Scholar] [CrossRef] [PubMed]
- Kidane, Y.; Bokrezion, T.; Mebrahtu, J.; Mehari, M.; Gebreab, Y.B.; Fessehaye, N.; Achila, O.O. In Vitro Inhibition of α-Amylase and α-Glucosidase by Extracts from Psiadia Punctulata and Meriandra Bengalensis. Evid. Based Complement. Altern. Med. 2018, 2018, 2164345. [Google Scholar] [CrossRef] [PubMed]
- Ansari, P.; Akther, S.; Hannan, J.M.A.; Seidel, V.; Nujat, N.J.; Abdel-Wahab, Y.H.A. Pharmacologically Active Phytomolecules Isolated from Traditional Antidiabetic Plants and Their Therapeutic Role for the Management of Diabetes Mellitus. Molecules 2022, 27, 4278. [Google Scholar] [CrossRef] [PubMed]
- Lattimer, J.M.; Haub, M.D. Effects of Dietary Fiber and Its Components on Metabolic Health. Nutrients 2010, 2, 1266–1289. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, M. The Pathobiology of Diabetic Complications: A Unifying Mechanism. Diabetes 2005, 54, 1615–1625. [Google Scholar] [CrossRef]
- Vella, A. Mechanism of Action of DPP-4 Inhibitors—New Insights. J. Clin. Endocrinol. Metab. 2012, 97, 2626–2628. [Google Scholar] [CrossRef]
- Deacon, C.F. Physiology and Pharmacology of DPP-4 in Glucose Homeostasis and the Treatment of Type 2 Diabetes. Front. Endocrinol. 2019, 10, 80. [Google Scholar] [CrossRef]
- Kim, B.-R.; Kim, H.; Choi, I.; Kim, J.-B.; Jin, C.; Han, A.-R. DPP-IV Inhibitory Potentials of Flavonol Glycosides Isolated from the Seeds of Lens Culinaris: In Vitro and Molecular Docking Analyses. Molecules 2018, 23, 1998. [Google Scholar] [CrossRef]
- Mohanty, I.R.; Borde, M.; Kumar, C.S.; Maheshwari, U. Dipeptidyl Peptidase IV Inhibitory Activity of Terminalia Arjuna Attributes to Its Cardioprotective Effects in Experimental Diabetes: In Silico, in Vitro and in Vivo Analyses. Phytomedicine 2019, 57, 158–165. [Google Scholar] [CrossRef]
- Hiranrat, A.; Mahabusarakam, W. New Acylphloroglucinols from the Leaves of Rhodomyrtus tomentosa. Tetrahedron 2008, 64, 11193–11197. [Google Scholar] [CrossRef]
- Gervais, A.; Lazarski, K.E.; Porco, J.A. Divergent Total Syntheses of Rhodomyrtosones A and B. J. Org. Chem. 2015, 80, 9584–9591. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.; Al-Amin, M.; Siddiqi, M.M.A.; Akter, S.; Haque, M.M.; Sultana, N.; Chowdhury, A.S. Isolation of Quercetin-3-O-Beta-d-Glucopyranoside from the Leaves of Azadirachta indica and Antimicrobial and Cytotoxic Screening of the Crude Extracts. Dhaka Univ. J. Sci. 2012, 60, 11–14. [Google Scholar] [CrossRef]
- He, C.-Y.; Fu, J.; Ma, J.-Y.; Feng, R.; Tan, X.-S.; Huang, M.; Shou, J.-W.; Zhao, Z.-X.; Li, X.-Y.; Zhang, X.-F.; et al. Biotransformation and in Vitro Metabolic Profile of Bioactive Extracts from a Traditional Miao-Nationality Herbal Medicine, Polygonum capitatum. Molecules 2014, 19, 10291–10308. [Google Scholar] [CrossRef] [PubMed]
- Martos, I.; Ferreres, F.; Yao, L.; D’Arcy, B.; Caffin, N.; Tomás-Barberán, F.A. Flavonoids in Monospecific Eucalyptus Honeys from Australia. J. Agric. Food Chem. 2000, 48, 4744–4748. [Google Scholar] [CrossRef] [PubMed]
- Kharwar, R.N.; Gond, S.K.; Kumar, A.; Mishra, A. A Comparative Study of Endophytic and Epiphytic Fungal Association with Leaf of Eucalyptus citriodora Hook., and Their Antimicrobial Activity. World J. Microbiol. Biotechnol. 2010, 26, 1941–1948. [Google Scholar] [CrossRef]
- Vo, T.; Ngo, D. The Health Beneficial Properties of Rhodomyrtus tomentosa as Potential Functional Food. Biomolecules 2019, 9, 76. [Google Scholar] [CrossRef]
- Huang, X.-L.; He, Y.; Ji, L.-L.; Wang, K.-Y.; Wang, Y.-L.; Chen, D.-F.; Geng, Y.; OuYang, P.; Lai, W.-M. Hepatoprotective Potential of Isoquercitrin against Type 2 Diabetes-Induced Hepatic Injury in Rats. Oncotarget 2017, 8, 101545–101559. [Google Scholar] [CrossRef]
- Babujanarthanam, R.; Kavitha, P.; Pandian, M.R. Quercitrin, a Bioflavonoid Improves Glucose Homeostasis in Streptozotocin-Induced Diabetic Tissues by Altering Glycolytic and Gluconeogenic Enzymes. Fundam. Clin. Pharmacol. 2009, 24, 357–364. [Google Scholar] [CrossRef]
- Wang, C.; Yang, J.; Zhao, P.; Zhou, Q.; Mei, Z.; Yang, G.; Yang, X.; Feng, Y. Chemical Constituents from Eucalyptus citriodora Hook Leaves and Their Glucose Transporter 4 Translocation Activities. Bioorg. Med. Chem. Lett. 2014, 24, 3096–3099. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).