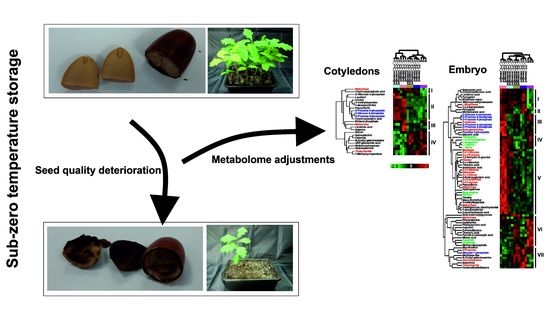
Deterioration in the Quality of Recalcitrant Quercus robur Seeds during Six Months of Storage at Subzero Temperatures: Ineffective Activation of Prosurvival Mechanisms and Evidence of Freezing Stress from an Untargeted Metabolomic Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Seed Pretreatment and Experimental Design
2.2. Seed Germination and Seedling Establishment
2.3. Preparation of Samples for Metabolomic Study
2.4. Metabolomic Study–Compound Extraction and High-Throughput GC MS/MS Analysis
2.5. Carbohydrates, C and N
2.6. Statistical and Bioinformatics Analysis
3. Results
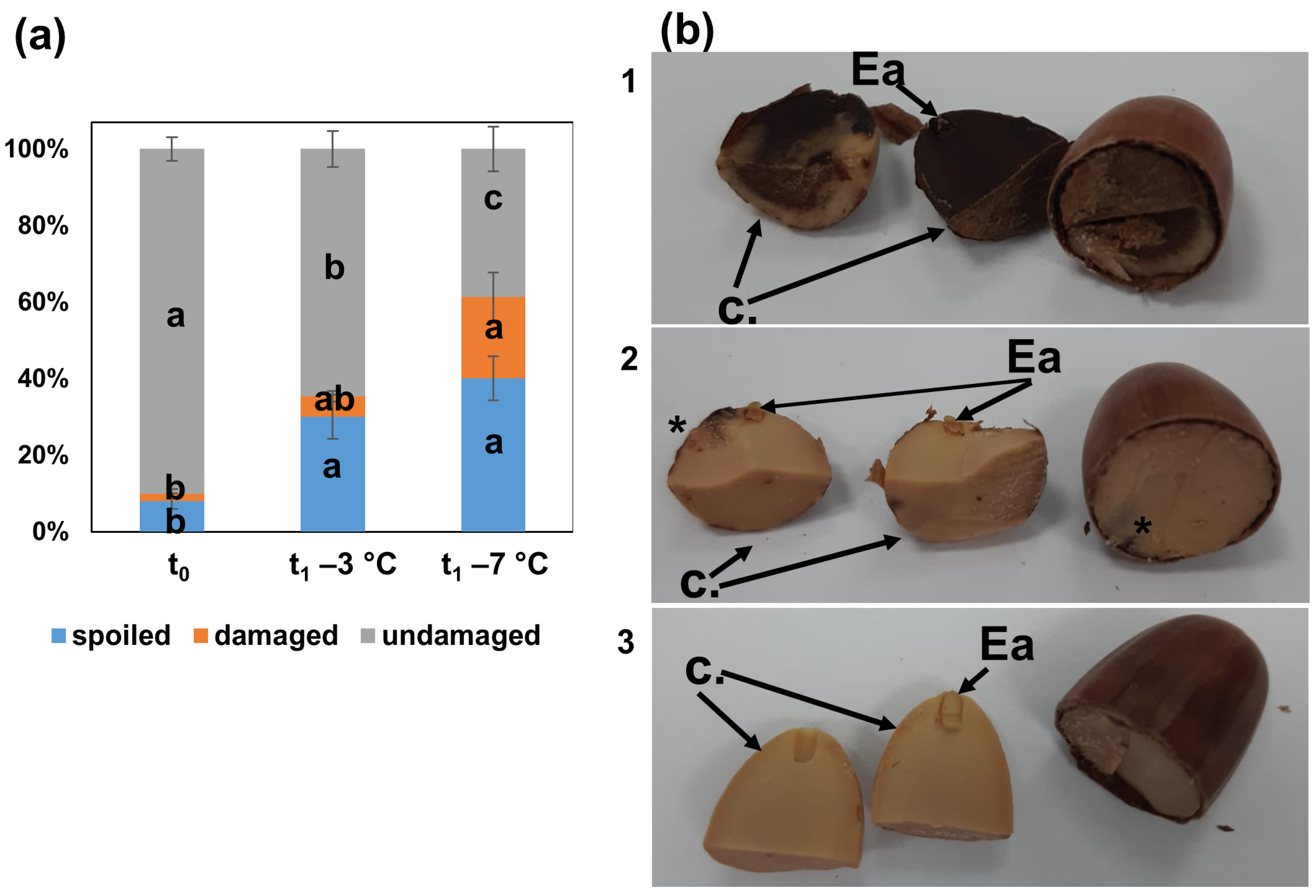
3.1. Seed Quality
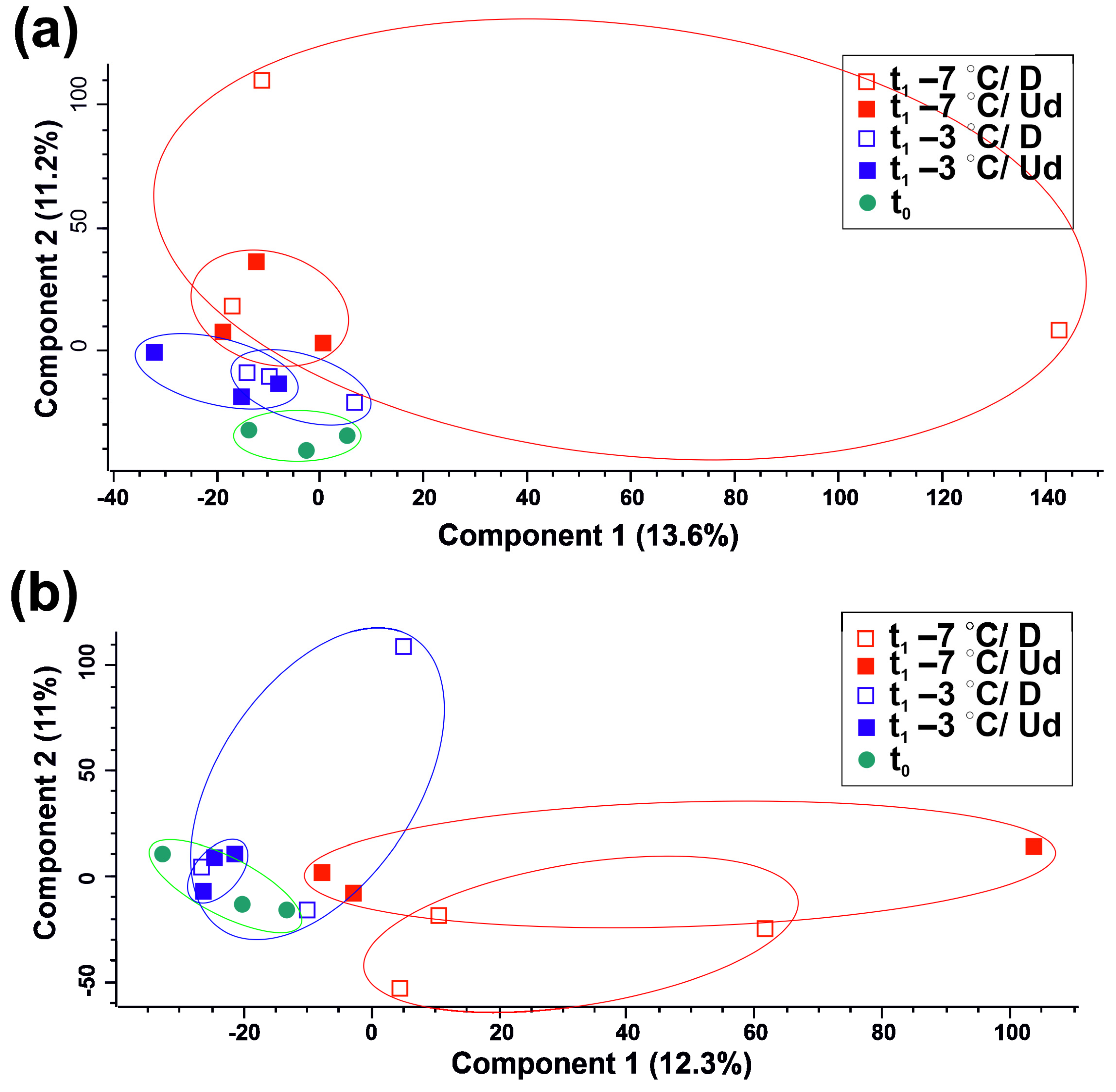
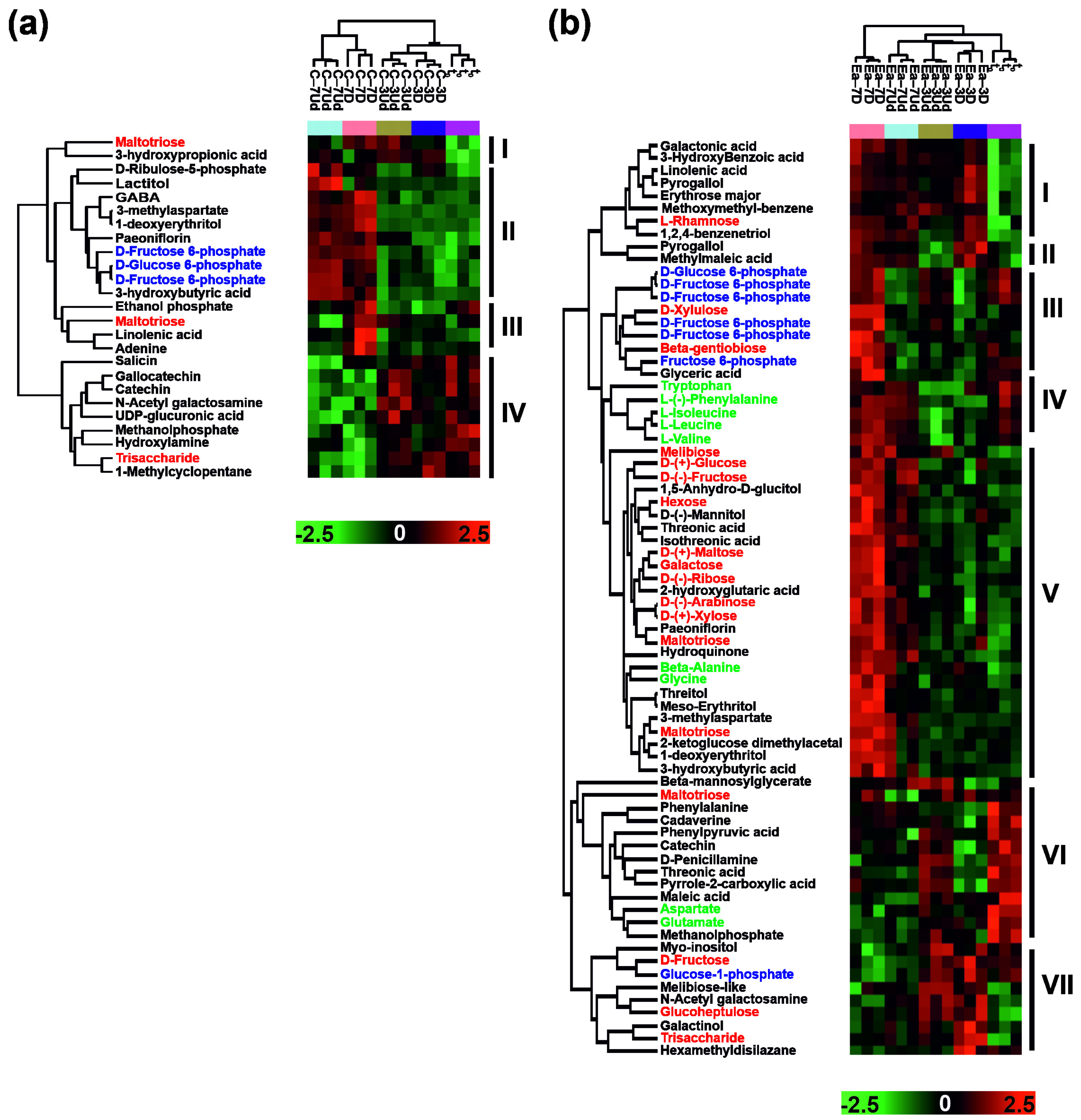
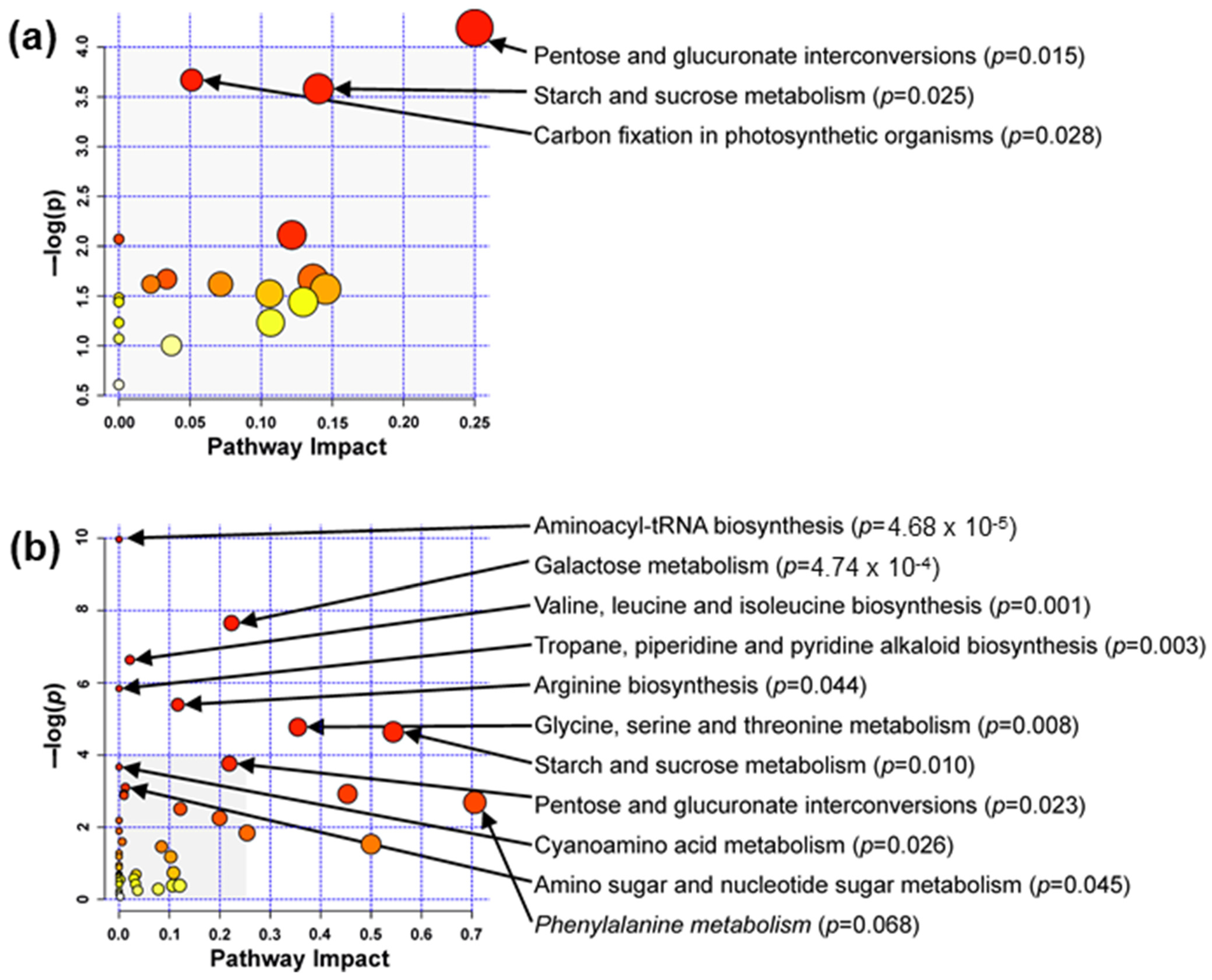
3.2. Metabolites Affected by Storage at Subzero Temperatures
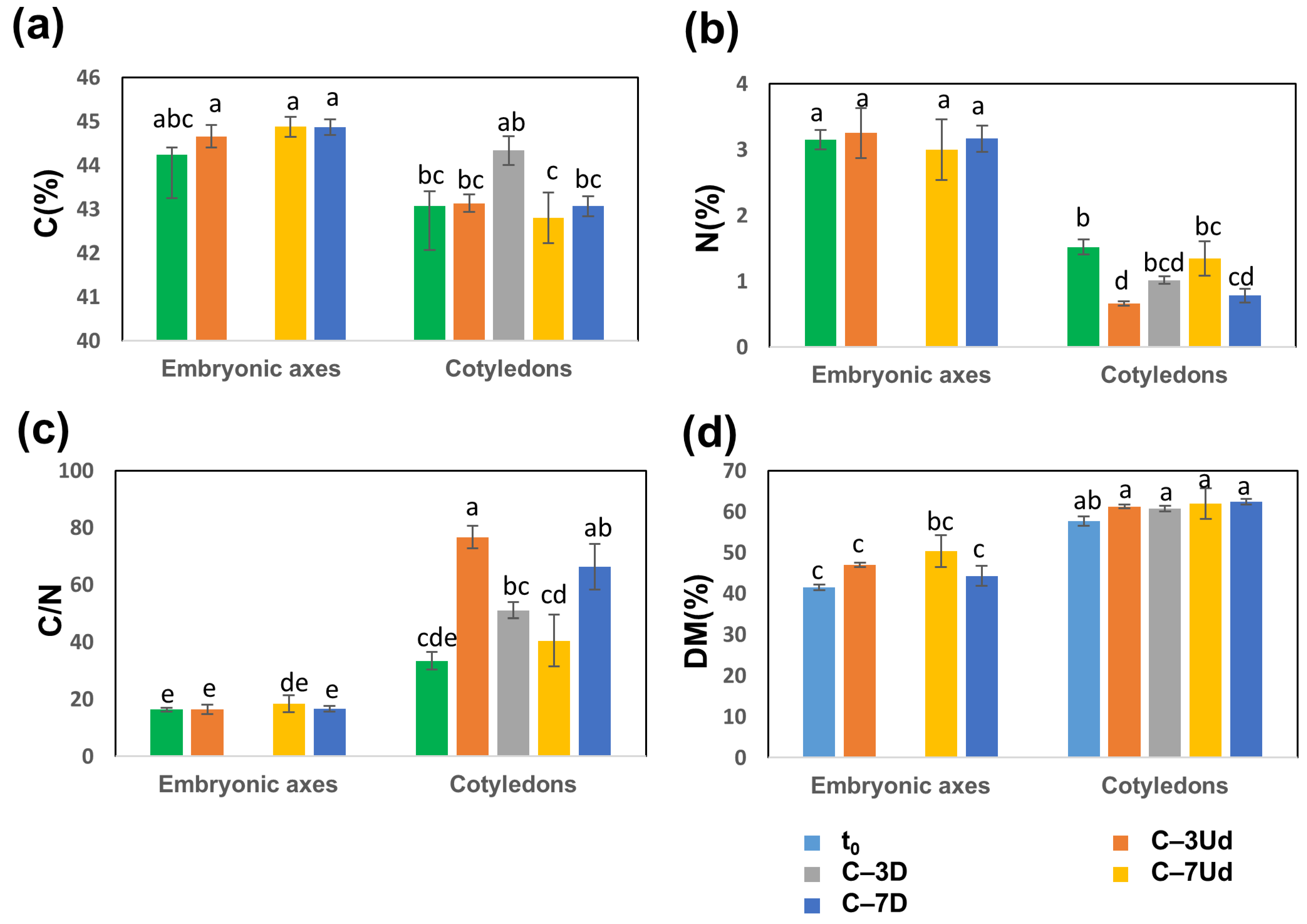
3.3. Changes in Carbon, Nitrogen and Carbohydrate Levels
4. Discussion
4.1. Embryonic Axes and Cotyledons Are Differentially Regulated Seed Tissues
4.2. Metabolic Causes of Failure in Germination and Seedling Establishment from Stored Acorns
4.2.1. Freezing Stress Is Evident at −7 °C
4.2.2. Energy Is Deficient
4.3. Metabolomic Indicators of Stress
4.3.1. Is BTO a New Player in Oxidative-Stress-Derived Acorn Deterioration?
4.3.2. Phenolic Compounds
4.3.3. Osmoprotectants
4.3.4. Membranes Are Damaged at Low Temperature
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Levanic, T.; Cater, M.; McDowell, N.G. Associations between Growth, Wood Anatomy, Carbon Isotope Discrimination and Mortality in a Quercus robur Forest. Tree Physiol. 2011, 31, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Annighöfer, P.; Beckschäfer, P.; Vor, T.; Ammer, C. Regeneration Patterns of European Oak Species (Quercus petraea (Matt.) Liebl., Quercus robur L.) in Dependence of Environment and Neighborhood. PLoS ONE 2015, 10, e0134935. [Google Scholar] [CrossRef] [PubMed]
- Jankowiak, R.; Stępniewska, H.; Bilański, P.; Taerum, S.J. Fungi as Potential Factors Limiting Natural Regeneration of Pedunculate Oak (Quercus robur) in Mixed-Species Forest Stands in Poland. Plant Pathol. 2022, 71, 805–817. [Google Scholar] [CrossRef]
- Suszka, B.; Muller, C.; Bonnet-Masimbert, M.; Gordon, A. Seeds of Forest Broadleaves: From Harvest to Sowing; Institute National de la Recherche Agronomique: Paris, France, 1996; ISBN 978-2-7380-0659-2. [Google Scholar]
- Hanley, M.E.; Cook, B.I.; Fenner, M. Climate Variation, Reproductive Frequency and Acorn Yield in English Oaks. J. Plant Ecol. 2019, 12, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Bogdziewicz, M.; Szymkowiak, J.; Kasprzyk, I.; Grewling, Ł.; Borowski, Z.; Borycka, K.; Kantorowicz, W.; Myszkowska, D.; Piotrowicz, K.; Ziemianin, M.; et al. Masting in Wind-Pollinated Trees: System-Specific Roles of Weather and Pollination Dynamics in Driving Seed Production. Ecology 2017, 98, 2615–2625. [Google Scholar] [CrossRef] [PubMed]
- Finch-Savage, W.E. Seed Development in the Recalcitrant Species Quercus robur L.: Germinability and Desiccation Tolerance. Seed Sci. Res. 1992, 2, 17–22. [Google Scholar] [CrossRef]
- Poulsen, K.M.; Eriksen, E.N. Physiological Aspects of Recalcitrance in Embryonic Axes of Quercus robur L. Seed Sci. Res. 1992, 2, 215–221. [Google Scholar] [CrossRef]
- Berjak, P.; Pammenter, N.W. Implications of the Lack of Desiccation Tolerance in Recalcitrant Seeds. Front. Plant Sci. 2013, 4, 478. [Google Scholar] [CrossRef]
- Gosling, P.G. The Effect of Drying Quercus robur Acorns to Different Moisture Contents, Followed by Storage, Either with or without Imbibition. For. Int. J. For. Res. 1989, 62, 41–50. [Google Scholar] [CrossRef]
- Suszka, B.; Tylkowski, T. Storage of Acorns of the English Oak (Quercus robur L.) over 1-5 Winters. Arbor. Korn. 1980, 25, 199–229. [Google Scholar]
- Bonner, F.T. Storage of Seeds: Potential and Limitations for Germplasm Conservation. For. Ecol. Manag. 1990, 35, 35–43. [Google Scholar] [CrossRef]
- Pritchard, H.W.; Moat, J.F.; Ferraz, J.B.S.; Marks, T.R.; Camargo, J.L.C.; Nadarajan, J.; Ferraz, I.D.K. Innovative Approaches to the Preservation of Forest Trees. For. Ecol. Manag. 2014, 333, 88–98. [Google Scholar] [CrossRef]
- Özbingöl, N.; O’reilly, C. Increasing Acorn Moisture Content Followed by Freezing-Storage Enhances Germination in Pedunculate Oak. For. Int. J. For. Res. 2005, 78, 73–81. [Google Scholar] [CrossRef]
- Chmielarz, P.; Grenier-de March, G.; Boucaud, M.-T. Cryopreservation of Quercus robur L. Embryogenic Calli. Cryo Lett. 2005, 26, 349–356. [Google Scholar]
- Chmielarz, P.; Michalak, M.; Pałucka, M.; Wasileńczyk, U. Successful Cryopreservation of Quercus robur Plumules. Plant Cell Rep. 2011, 30, 1405–1414. [Google Scholar] [CrossRef]
- Bondarenko, A.S.; Butenko, O.Y. The Results of Experiments on the Storage of Quercus robur L. Acorns at Natural Low Temperatures. Proc. St. Petersburg For. Res. Inst. 2017, 47–56. [Google Scholar] [CrossRef]
- Chmielarz, P.; Suszka, J.; Wawrzyniak, M.K. Desiccation Does Not Increase Frost Resistance of Pedunculate Oak (Quercus robur L.) Seeds. Ann. For. Sci. 2022, 79, 3. [Google Scholar] [CrossRef]
- Chmielarz, P. Frost Resistance of Quercus robur L. Acorns. In Treatment and Storage of Oak Seeds. Present Situation and Research; Mitt.Biol.Bundesanst, Land-Forstwirtsch: Berlin, Germany, 1997; pp. 76–81. [Google Scholar]
- Tikkanen, O.-P.; Kilpeläinen, J.; Mellado, A.; Hämäläinen, A.; Hódar, J.A.; Jaroszewicz, B.; Luoto, M.; Repo, T.; Rigling, A.; Wang, A.; et al. Freezing Tolerance of Seeds Can Explain Differences in the Distribution of Two Widespread Mistletoe Subspecies in Europe. For. Ecol. Manag. 2021, 482, 118806. [Google Scholar] [CrossRef]
- Kalemba, E.M.; Wawrzyniak, M.K.; Suszka, J.; Chmielarz, P. Thermotherapy and Storage Temperature Manipulations Limit the Production of Reactive Oxygen Species in Stored Pedunculate Oak Acorns. Forests 2021, 12, 1338. [Google Scholar] [CrossRef]
- Löf, M.; Castro, J.; Engman, M.; Leverkus, A.B.; Madsen, P.; Reque, J.A.; Villalobos, A.; Gardiner, E.S. Tamm Review: Direct Seeding to Restore Oak (Quercus Spp.) Forests and Woodlands. For. Ecol. Manag. 2019, 448, 474–489. [Google Scholar] [CrossRef]
- Whitehouse, K.J.; Hay, F.R.; Lusty, C. Why Seed Physiology Is Important for Genebanking. Plants 2020, 9, 584. [Google Scholar] [CrossRef] [PubMed]
- FAO/IPGRI. Genebank Standards for Plant Genetic Resources for Food and Agriculture-World|ReliefWeb. Available online: https://reliefweb.int/report/world/genebank-standards-plant-genetic-resources-food-and-agriculture (accessed on 24 May 2022).
- Solberg, S.Ø.; Yndgaard, F.; Andreasen, C.; von Bothmer, R.; Loskutov, I.G.; Asdal, Å. Long-Term Storage and Longevity of Orthodox Seeds: A Systematic Review. Front. Plant Sci. 2020, 11, 1007. [Google Scholar] [CrossRef] [PubMed]
- Zinsmeister, J.; Leprince, O.; Buitink, J. Molecular and Environmental Factors Regulating Seed Longevity. Biochem. J. 2020, 477, 305–323. [Google Scholar] [CrossRef] [PubMed]
- Probert, R.J.; Daws, M.I.; Hay, F.R. Ecological Correlates of Ex Situ Seed Longevity: A Comparative Study on 195 Species. Ann. Bot. 2009, 104, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Rajjou, L.; Debeaujon, I. Seed Longevity: Survival and Maintenance of High Germination Ability of Dry Seeds. Comptes Rendus Biol. 2008, 331, 796–805. [Google Scholar] [CrossRef]
- Guo, Y. The Power of Omics for Improving Seed Oil Content. Nat. Food 2021, 2, 5. [Google Scholar] [CrossRef]
- Rajjou, L.; Lovigny, Y.; Groot, S.P.C.; Belghazi, M.; Job, C.; Job, D. Proteome-Wide Characterization of Seed Aging in Arabidopsis: A Comparison between Artificial and Natural Aging Protocols. Plant Physiol. 2008, 148, 620–641. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, W.-Q.; Liu, S.-J.; Møller, I.M.; Song, S.-Q. Proteome Analysis of Poplar Seed Vigor. PLoS ONE 2015, 10, e0132509. [Google Scholar] [CrossRef]
- Pawłowski, T.A.; Klupczyńska, E.A.; Staszak, A.M.; Suszka, J. Proteomic Analysis of Black Poplar (Populus nigra L.) Seed Storability. Ann. For. Sci. 2019, 76, 104. [Google Scholar] [CrossRef]
- Galland, M.; He, D.; Lounifi, I.; Arc, E.; Clément, G.; Balzergue, S.; Huguet, S.; Cueff, G.; Godin, B.; Collet, B.; et al. An Integrated “Multi-Omics” Comparison of Embryo and Endosperm Tissue-Specific Features and Their Impact on Rice Seed Quality. Front. Plant Sci. 2017, 8, 1984. [Google Scholar] [CrossRef]
- López-Hidalgo, C.; Trigueros, M.; Menéndez, M.; Jorrin-Novo, J.V. Phytochemical Composition and Variability in Quercus ilex Acorn Morphotypes as Determined by NIRS and MS-Based Approaches. Food Chem. 2021, 338, 127803. [Google Scholar] [CrossRef] [PubMed]
- Romero-Rodríguez, M.C.; Archidona-Yuste, A.; Abril, N.; Gil-Serrano, A.M.; Meijón, M.; Jorrín-Novo, J.V. Germination and Early Seedling Development in Quercus ilex Recalcitrant and Non-Dormant Seeds: Targeted Transcriptional, Hormonal, and Sugar Analysis. Front. Plant Sci. 2018, 9, 1508. [Google Scholar] [CrossRef] [PubMed]
- Volf, M.; Weinhold, A.; Seifert, C.L.; Holicová, T.; Uthe, H.; Alander, E.; Richter, R.; Salminen, J.-P.; Wirth, C.; van Dam, N.M. Branch-Localized Induction Promotes Efficacy of Volatile Defences and Herbivore Predation in Trees. J. Chem. Ecol. 2021, 47, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Huang, W.; Gao, J.; Fu, H.; Liu, J. Comparative Metabolomic Analysis of Seed Metabolites Associated with Seed Storability in Rice (Oryza sativa L.) during Natural Aging. Plant Physiol. Biochem. 2018, 127, 590–598. [Google Scholar] [CrossRef]
- De Vitis, M.; Hay, F.R.; Dickie, J.B.; Trivedi, C.; Choi, J.; Fiegener, R. Seed Storage: Maintaining Seed Viability and Vigor for Restoration Use. Restor. Ecol. 2020, 28, S249–S255. [Google Scholar] [CrossRef]
- Kranjec Orlović, J.; Drvodelić, D.; Vukelić, M.; Rukavina, M.; Diminić, D.; Oršanić, M. Impact of Thermotherapy and Short-Term Storage on Quercus robur L. Acorn Mycobiota and Germination. Forests 2021, 12, 528. [Google Scholar] [CrossRef]
- Giertych, M.J.; Suszka, J. Consequences of Cutting off Distal Ends of Cotyledons of Quercus robur Acorns before Sowing. Ann. For. Sci. 2011, 68, 433–442. [Google Scholar] [CrossRef]
- Szuba, A.; Marczak, Ł.; Ratajczak, I.; Kasprowicz-Maluśki, A.; Mucha, J. Integrated Proteomic and Metabolomic Analyses Revealed Molecular Adjustments in Populus × canescens Colonized with the Ectomycorrhizal Fungus Paxillus involutus, Which Limited Plant Host Growth. Environ. Microbiol. 2020, 22, 3754–3771. [Google Scholar] [CrossRef]
- Szuba, A.; Marczak, Ł.; Karliński, L.; Mucha, J.; Tomaszewski, D. Regulation of the Leaf Proteome by Inoculation of Populus × canescens with Two Paxillus involutus Isolates Differing in Root Colonization Rates. Mycorrhiza 2019, 29, 503–517. [Google Scholar] [CrossRef]
- Valero Galván, J.; Valledor, L.; Navarro Cerrillo, R.M.; Gil Pelegrín, E.; Jorrín-Novo, J.V. Studies of Variability in Holm Oak (Quercus ilex Subsp. Ballota [Desf.] Samp.) through Acorn Protein Profile Analysis. J. Proteom. 2011, 74, 1244–1255. [Google Scholar] [CrossRef]
- Sghaier-Hammami, B.; Redondo-López, I.; Valero-Galvàn, J.; Jorrín-Novo, J.V. Protein Profile of Cotyledon, Tegument, and Embryonic Axis of Mature Acorns from a Non-Orthodox Plant Species: Quercus ilex. Planta 2016, 243, 369–396. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Gao, X. Effects of Low Temperature and Nitrogen Addition during Cold Stratification on Seed Germination of Korean Pine (Pinus koraiensis). Can. J. For. Res. 2021, 51, 1698–1706. [Google Scholar] [CrossRef]
- Kuznetsova, I.M.; Turoverov, K.K.; Uversky, V.N. What Macromolecular Crowding Can Do to a Protein. Int. J. Mol. Sci. 2014, 15, 23090–23140. [Google Scholar] [CrossRef]
- Sghaier-Hammami, B.; Hammami, B.M.S.; Baazaoui, N.; Gómez-Díaz, C.; Jorrín-Novo, J.V. Dissecting the Seed Maturation and Germination Processes in the Non-Orthodox Quercus ilex Species Based on Protein Signatures as Revealed by 2-DE Coupled to MALDI-TOF/TOF Proteomics Strategy. Int. J. Mol. Sci. 2020, 21, 4870. [Google Scholar] [CrossRef] [PubMed]
- Jancewicz, A.L.; Gibbs, N.M.; Masson, P.H. Cadaverine’s Functional Role in Plant Development and Environmental Response. Front. Plant Sci. 2016, 7, 870. [Google Scholar] [CrossRef] [PubMed]
- Tomar, P.C.; Lakra, N.; Mishra, S.N. Effect of Cadaverine on Brassica juncea (L.) under Multiple Stress. Indian J. Exp. Biol. 2013, 51, 758–763. [Google Scholar]
- Ilyas, M.; Khan, W.A.; Ali, T.; Ahmad, N.; Khan, Z.; Fazal, H.; Zaman, N.; Ualiyeva, D.; Ali, M.; Amissah, O.B.; et al. Cold Stress-Induced Seed Germination and Biosynthesis of Polyphenolics Content in Medicinally Important Brassica rapa. Phytomedicine Plus 2022, 2, 100185. [Google Scholar] [CrossRef]
- Chen, H.; Chen, S.; Chen, H.; Wu, Y.; Shu, G. Effects of Sugar Alcohol and Proteins on the Survival of Lactobacillus bulgaricus LB6 during Freeze Drying. Acta Sci. Pol. Technol. Aliment. 2015, 14, 117–124. [Google Scholar] [CrossRef]
- Scanga, S.E.; Hasanspahič, B.; Zvorničanin, E.; Koženjić, J.S.; Rahme, A.K.; Shinn-Thomas, J.H. Erythritol, at Insecticidal Doses, Has Harmful Effects on Two Common Agricultural Crop Plants. PLoS ONE 2018, 13, e0192749. [Google Scholar] [CrossRef]
- Kamran, M.; Khan, A.L.; Ali, L.; Hussain, J.; Waqas, M.; Al-Harrasi, A.; Imran, Q.M.; Kim, Y.-H.; Kang, S.-M.; Yun, B.-W.; et al. Hydroquinone; A Novel Bioactive Compound from Plant-Derived Smoke Can Cue Seed Germination of Lettuce. Front. Chem. 2017, 5, 30. [Google Scholar] [CrossRef]
- Yue, Y.; Chen, C.; Zhong, K.; Wu, Y.; Gao, H. Purification, Fermentation Optimization, and Antibacterial Activity of Pyrrole-2-Carboxylic Acid Produced by an Endophytic Bacterium, Bacillus cereus ZBE, Isolated from Zanthoxylum bungeanum. Ind. Eng. Chem. Res. 2022, 61, 1267–1276. [Google Scholar] [CrossRef]
- Qu, C.; Zuo, Z.; Cao, L.; Huang, J.; Sun, X.; Zhang, P.; Yang, C.; Li, L.; Xu, Z.; Liu, G. Comprehensive Dissection of Transcript and Metabolite Shifts during Seed Germination and Post-Germination Stages in Poplar. BMC Plant Biol. 2019, 19, 279. [Google Scholar] [CrossRef] [PubMed]
- Sabbatinelli, J.; Prattichizzo, F.; Olivieri, F.; Procopio, A.D.; Rippo, M.R.; Giuliani, A. Where Metabolism Meets Senescence: Focus on Endothelial Cells. Front. Physiol. 2019, 10, 1523. [Google Scholar] [CrossRef] [PubMed]
- Sipari, N.; Lihavainen, J.; Shapiguzov, A.; Kangasjärvi, J.; Keinänen, M. Primary Metabolite Responses to Oxidative Stress in Early-Senescing and Paraquat Resistant Arabidopsis thaliana Rcd1 (Radical-Induced Cell Death1). Front. Plant Sci. 2020, 11, 194. [Google Scholar] [CrossRef]
- Stein, O.; Avin-Wittenberg, T.; Krahnert, I.; Zemach, H.; Bogol, V.; Daron, O.; Aloni, R.; Fernie, A.R.; Granot, D. Arabidopsis Fructokinases Are Important for Seed Oil Accumulation and Vascular Development. Front. Plant Sci. 2016, 7, 2047. [Google Scholar] [CrossRef] [PubMed]
- Stein, O.; Granot, D. Plant Fructokinases: Evolutionary, Developmental, and Metabolic Aspects in Sink Tissues. Front. Plant Sci. 2018, 9, 339. [Google Scholar] [CrossRef]
- Toro, G.; Pinto, M. Plant Respiration under Low Oxygen. Chil. J. Agric. Res. 2015, 75, 57–70. [Google Scholar] [CrossRef]
- Michaeli, S.; Fromm, H. Closing the Loop on the GABA Shunt in Plants: Are GABA Metabolism and Signaling Entwined? Front. Plant Sci. 2015, 6, 419. [Google Scholar] [CrossRef]
- Fait, A.; Fromm, H.; Walter, D.; Galili, G.; Fernie, A.R. Highway or Byway: The Metabolic Role of the GABA Shunt in Plants. Trends Plant Sci. 2008, 13, 14–19. [Google Scholar] [CrossRef]
- Zhou, Y.; Cai, H.; Xiao, J.; Li, X.; Zhang, Q.; Lian, X. Over-Expression of Aspartate Aminotransferase Genes in Rice Resulted in Altered Nitrogen Metabolism and Increased Amino Acid Content in Seeds. Theor. Appl. Genet. 2009, 118, 1381–1390. [Google Scholar] [CrossRef]
- Delic, V.; Griffin, J.W.D.; Zivkovic, S.; Zhang, Y.; Phan, T.-A.; Gong, H.; Chaput, D.; Reynes, C.; Dinh, V.B.; Cruz, J.; et al. Individual Amino Acid Supplementation Can Improve Energy Metabolism and Decrease ROS Production in Neuronal Cells Overexpressing Alpha-Synuclein. Neuromolecular Med. 2017, 19, 322–344. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.-X.; Fu, H.; Gao, J.-D.; Zhang, Y.-X.; Huang, W.-J.; Chen, Z.-J.; Zhang, Q.; Yan, S.-J.; Liu, J. Identification of Metabolomic Biomarkers of Seed Vigor and Aging in Hybrid Rice. Rice 2022, 15, 7. [Google Scholar] [CrossRef] [PubMed]
- Randolph, C.; Lahive, C.W.; Sami, S.; Havenith, R.W.A.; Heeres, H.J.; Deuss, P.J. Biobased Chemicals: 1,2,4-Benzenetriol, Selective Deuteration and Dimerization to Bifunctional Aromatic Compounds. Org. Process. Res. Dev. 2018, 22, 1663–1671. [Google Scholar] [CrossRef] [PubMed]
- Kawanishi, S.; Inoue, S.; Kawanishi, M. Human DNA Damage Induced by 1,2,4-Benzenetriol, a Benzene Metabolite. Cancer Res. 1989, 49, 164–168. [Google Scholar]
- Debolt, S.; Melino, V.; Ford, C.M. Ascorbate as a Biosynthetic Precursor in Plants. Ann. Bot. 2007, 99, 3–8. [Google Scholar] [CrossRef]
- Zhang, L.; Robertson, M.L.; Kolachana, P.; Davison, A.J.; Smith, M.T. Benzene Metabolite, 1,2,4-Benzenetriol, Induces Micronuclei and Oxidative DNA Damage in Human Lymphocytes and HL60 Cells. Environ. Mol. Mutagenesis 1993, 21, 339–348. [Google Scholar] [CrossRef]
- Chung, H.W.; Kang, S.J.; Kim, S.Y. A Combination of the Micronucleus Assay and a FISH Technique for Evaluation of the Genotoxicity of 1,2,4-Benzenetriol. Mutat. Res. 2002, 516, 49–56. [Google Scholar] [CrossRef]
- Hou, J.-; Liu, G.-N.; Xue, W.; Fu, W.-J.; Liang, B.-C.; Liu, X.-H. Seed Germination, Root Elongation, Root-Tip Mitosis, and Micronucleus Induction of Five Crop Plants Exposed to Chromium in Fluvo-Aquic Soil. Environ. Toxicol. Chem. 2014, 33, 671–676. [Google Scholar] [CrossRef]
- Zhao, L.; Huang, Y.; Keller, A.A. Comparative Metabolic Response between Cucumber (Cucumis sativus) and Corn (Zea mays) to a Cu(OH)2 Nanopesticide. J. Agric. Food Chem. 2018, 66, 6628–6636. [Google Scholar] [CrossRef]
- Miyahara, E.; Nishikawa, T.; Takeuchi, T.; Yasuda, K.; Okamoto, Y.; Kawano, Y.; Horiuchi, M. Effect of Myeloperoxidase Inhibition on Gene Expression Profiles in HL-60 Cells Exposed to 1,2,4,-Benzenetriol. Toxicology 2014, 317, 50–57. [Google Scholar] [CrossRef]
- Sano, N.; Rajjou, L.; North, H.M.; Debeaujon, I.; Marion-Poll, A.; Seo, M. Staying Alive: Molecular Aspects of Seed Longevity. Plant Cell Physiol. 2016, 57, 660–674. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.-H.; Li, Y.; Zhao, X.; Yang, S.-Q.; Li, L.; Cui, N.-X.; Rong, L.; Yi, Z.-C. Benzene Metabolite 1,2,4-Benzenetriol Changes DNA Methylation and Histone Acetylation of Erythroid-Specific Genes in K562 Cells. Arch. Toxicol. 2019, 93, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Michalak, M.; Plitta-Michalak, B.P.; Naskręt-Barciszewska, M.; Barciszewski, J.; Bujarska-Borkowska, B.; Chmielarz, P. Global 5-Methylcytosine Alterations in DNA during Ageing of Quercus robur Seeds. Ann. Bot. 2015, 116, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Mierziak, J.; Burgberger, M.; Wojtasik, W. 3-Hydroxybutyrate as a Metabolite and a Signal Molecule Regulating Processes of Living Organisms. Biomolecules 2021, 11, 402. [Google Scholar] [CrossRef]
- Król, A.; Amarowicz, R.; Weidner, S. The Effects of Cold Stress on the Phenolic Compounds and Antioxidant Capacity of Grapevine (Vitis vinifera L.) Leaves. J. Plant Physiol. 2015, 189, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Hajihashemi, S.; Brestic, M.; Landi, M.; Skalicky, M. Resistance of Fritillaria Imperialis to Freezing Stress through Gene Expression, Osmotic Adjustment and Antioxidants. Sci. Rep. 2020, 10, 10427. [Google Scholar] [CrossRef] [PubMed]
- Muzaffar, S.; Ali, B.; Wani, N.A. Effect of Catechol, Gallic Acid and Pyrogallic Acid on the Germination, Seedling Growth and the Level of Endogenous Phenolics in Cucumber (Cucumis sativus L.). Int. J. Life Sci. Biotechnol. Pharma Res. 2012, 1, 50–55. [Google Scholar]
- Wu, M.; Brown, A.C. Applications of Catechins in the Treatment of Bacterial Infections. Pathogens 2021, 10, 546. [Google Scholar] [CrossRef]
- Wang, C.-M.; Li, T.-C.; Jhan, Y.-L.; Weng, J.-H.; Chou, C.-H. The Impact of Microbial Biotransformation of Catechin in Enhancing the Allelopathic Effects of Rhododendron formosanum. PLoS ONE 2013, 8, e85162. [Google Scholar] [CrossRef]
- Strimbeck, G.R.; Kjellsen, T.D.; Schaberg, P.G.; Murakami, P.F. Dynamics of Low-Temperature Acclimation in Temperate and Boreal Conifer Foliage in a Mild Winter Climate. Tree Physiol. 2008, 28, 1365–1374. [Google Scholar] [CrossRef]
- Zareei, E.; Karami, F.; Gholami, M.; Ershadi, A.; Avestan, S.; Aryal, R.; Gohari, G.; Farooq, M. Physiological and Biochemical Responses of Strawberry Crown and Leaf Tissues to Freezing Stress. BMC Plant Biol. 2021, 21, 532. [Google Scholar] [CrossRef]
- Senik, S.V.; Kolker, T.L.; Kotlova, E.R.; Vlasov, D.Y.; Shavarda, A.L.; Puzansky, R.K.; Psurtseva, N.V. Lipid and Metabolite Profiling of Serpula lacrymans Under Freezing Stress. Curr. Microbiol. 2021, 78, 961–966. [Google Scholar] [CrossRef]
- Boyd, C.S.; Lemos, J.A. Freezing Stress Influences Emergence of Germinated Perennial Grass Seeds. Rangel. Ecol. Manag. 2013, 66, 136–142. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, D.; Li, M.; Shi, L. Metabolic Profiles Reveal Changes in Wild and Cultivated Soybean Seedling Leaves under Salt Stress. PLoS ONE 2016, 11, e0159622. [Google Scholar] [CrossRef]
- Rikin, A.; Dillwith, J.W.; Bergman, D.K. Correlation between the Circadian Rhythm of Resistance to Extreme Temperatures and Changes in Fatty Acid Composition in Cotton Seedlings. Plant Physiol. 1993, 101, 31–36. [Google Scholar] [CrossRef]
- Campos, P.S.; Quartin, V.n.; Ramalho, J.c.; Nunes, M.A. Electrolyte Leakage and Lipid Degradation Account for Cold Sensitivity in Leaves Of Coffea Sp. Plants. J. Plant Physiol. 2003, 160, 283–292. [Google Scholar] [CrossRef]
- Walters, K.R.; Pan, Q.; Serianni, A.S.; Duman, J.G. Cryoprotectant Biosynthesis and the Selective Accumulation of Threitol in the Freeze-Tolerant Alaskan Beetle, Upis ceramboides. J. Biol. Chem. 2009, 284, 16822–16831. [Google Scholar] [CrossRef]
- Storey, K.B.; Storey, J.M. Molecular Biology of Freezing Tolerance. Compr. Physiol. 2013, 3, 1283–1308. [Google Scholar] [CrossRef]
- Sych, J.; Lacroix, C.; Adambounou, L.; Castaigne, F. Cryoprotective Effects of Lactitol, Palatinit and Polydextrose® on Cod Surimi Proteins during Frozen Storage. J. Food Sci. 2006, 55, 356–360. [Google Scholar] [CrossRef]




| Abbreviation | Explanation |
|---|---|
| Ea–3Ud | Embryonic axes of seeds stored at −3 °C and classified as undamaged |
| Ea–3D | Embryonic axes of seeds stored at −3 °C and classified as damaged |
| Ea–7Ud | Embryonic axes of seeds stored at −7 °C and classified as undamaged |
| Ea–7D | Embryonic axes of seeds stored at −7 °C and classified as damaged |
| C–7Ud | Cotyledons of seeds stored at −7 °C and classified as undamaged |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szuba, A.; Kalemba, E.M.; Wawrzyniak, M.K.; Suszka, J.; Chmielarz, P. Deterioration in the Quality of Recalcitrant Quercus robur Seeds during Six Months of Storage at Subzero Temperatures: Ineffective Activation of Prosurvival Mechanisms and Evidence of Freezing Stress from an Untargeted Metabolomic Study. Metabolites 2022, 12, 756. https://doi.org/10.3390/metabo12080756
Szuba A, Kalemba EM, Wawrzyniak MK, Suszka J, Chmielarz P. Deterioration in the Quality of Recalcitrant Quercus robur Seeds during Six Months of Storage at Subzero Temperatures: Ineffective Activation of Prosurvival Mechanisms and Evidence of Freezing Stress from an Untargeted Metabolomic Study. Metabolites. 2022; 12(8):756. https://doi.org/10.3390/metabo12080756
Chicago/Turabian StyleSzuba, Agnieszka, Ewa Marzena Kalemba, Mikołaj Krzysztof Wawrzyniak, Jan Suszka, and Paweł Chmielarz. 2022. "Deterioration in the Quality of Recalcitrant Quercus robur Seeds during Six Months of Storage at Subzero Temperatures: Ineffective Activation of Prosurvival Mechanisms and Evidence of Freezing Stress from an Untargeted Metabolomic Study" Metabolites 12, no. 8: 756. https://doi.org/10.3390/metabo12080756
APA StyleSzuba, A., Kalemba, E. M., Wawrzyniak, M. K., Suszka, J., & Chmielarz, P. (2022). Deterioration in the Quality of Recalcitrant Quercus robur Seeds during Six Months of Storage at Subzero Temperatures: Ineffective Activation of Prosurvival Mechanisms and Evidence of Freezing Stress from an Untargeted Metabolomic Study. Metabolites, 12(8), 756. https://doi.org/10.3390/metabo12080756