Effect of Different Climatic Regions and Seasonal Variation on the Antibacterial and Antifungal Activity, and Chemical Profile of Helichrysum aureonitens Sch. Bip
Abstract
:1. Introduction
2. Results
2.1. Antibacterial Activity of H. aureonitens Extracts
2.2. Antifungal Activity of H. aureonitens Extracts
2.3. Rainfall and Temperature Data for Collection Locations
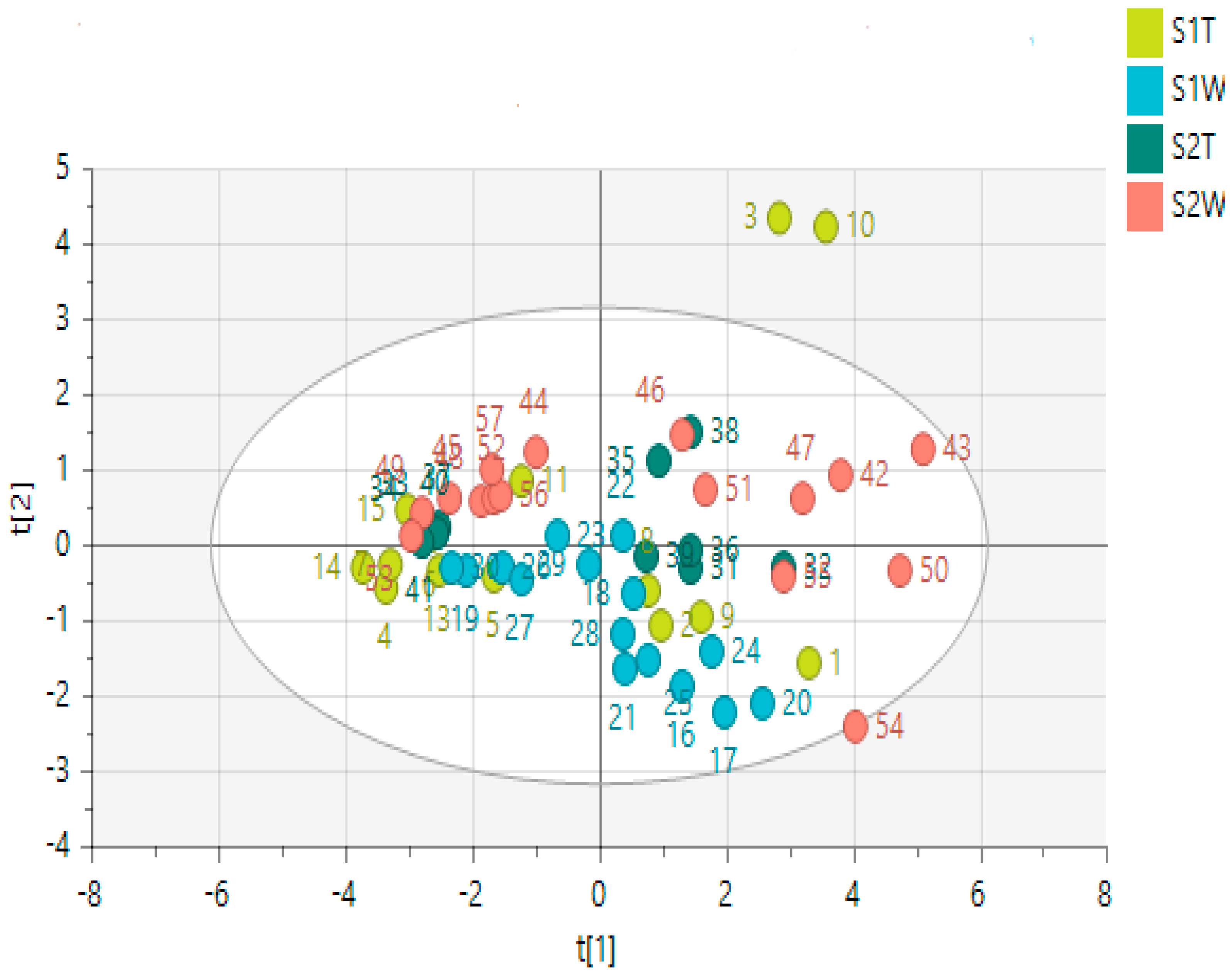
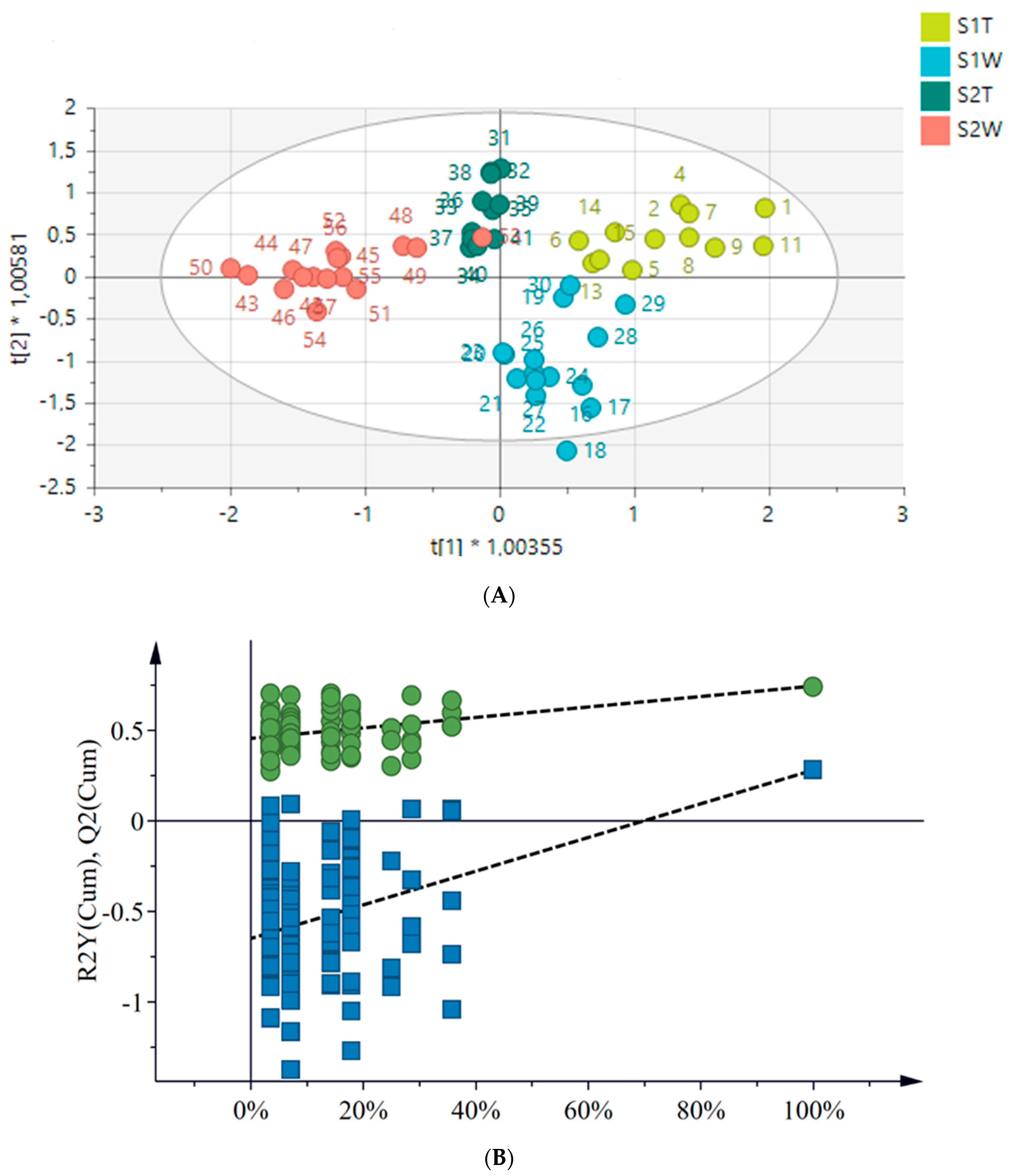
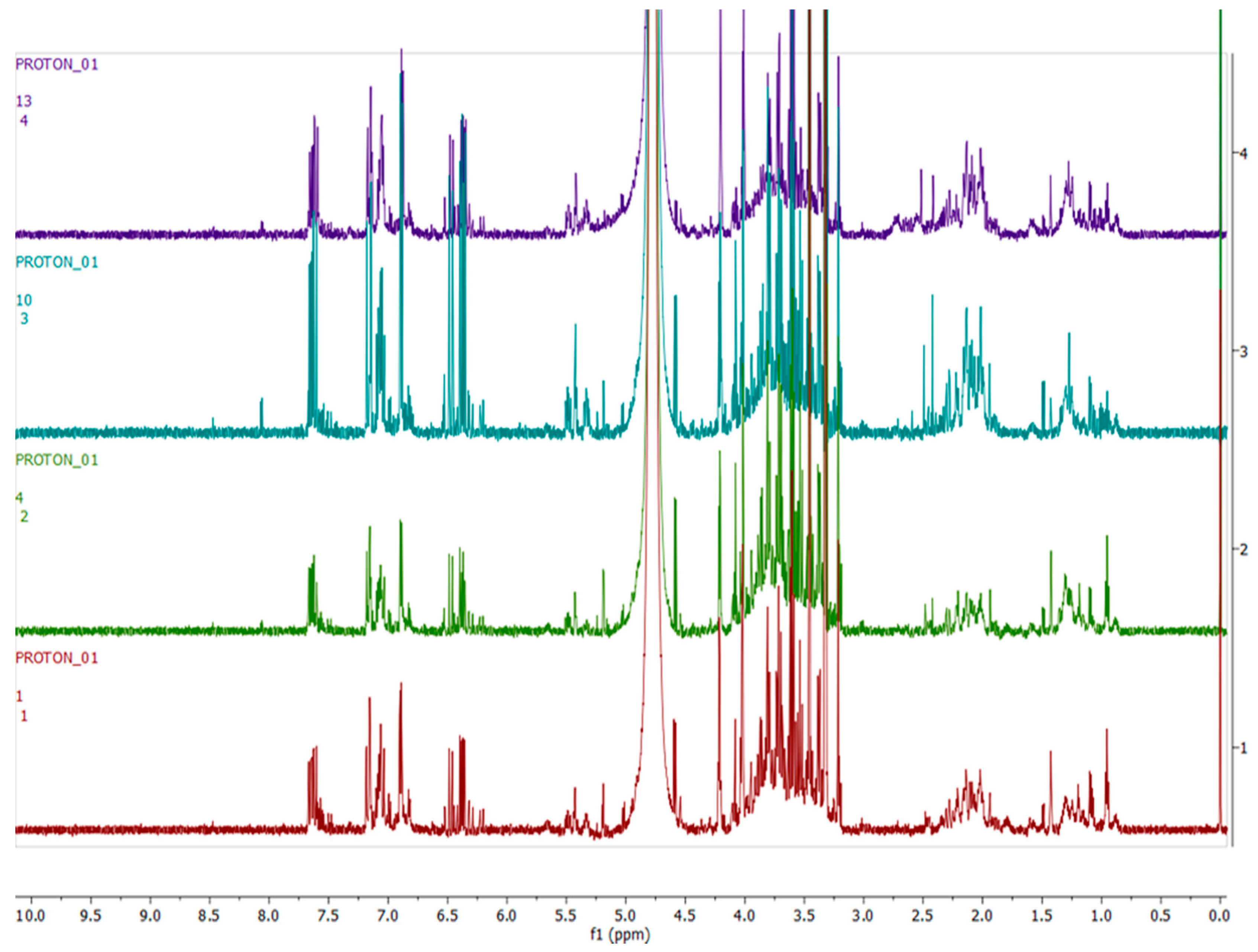
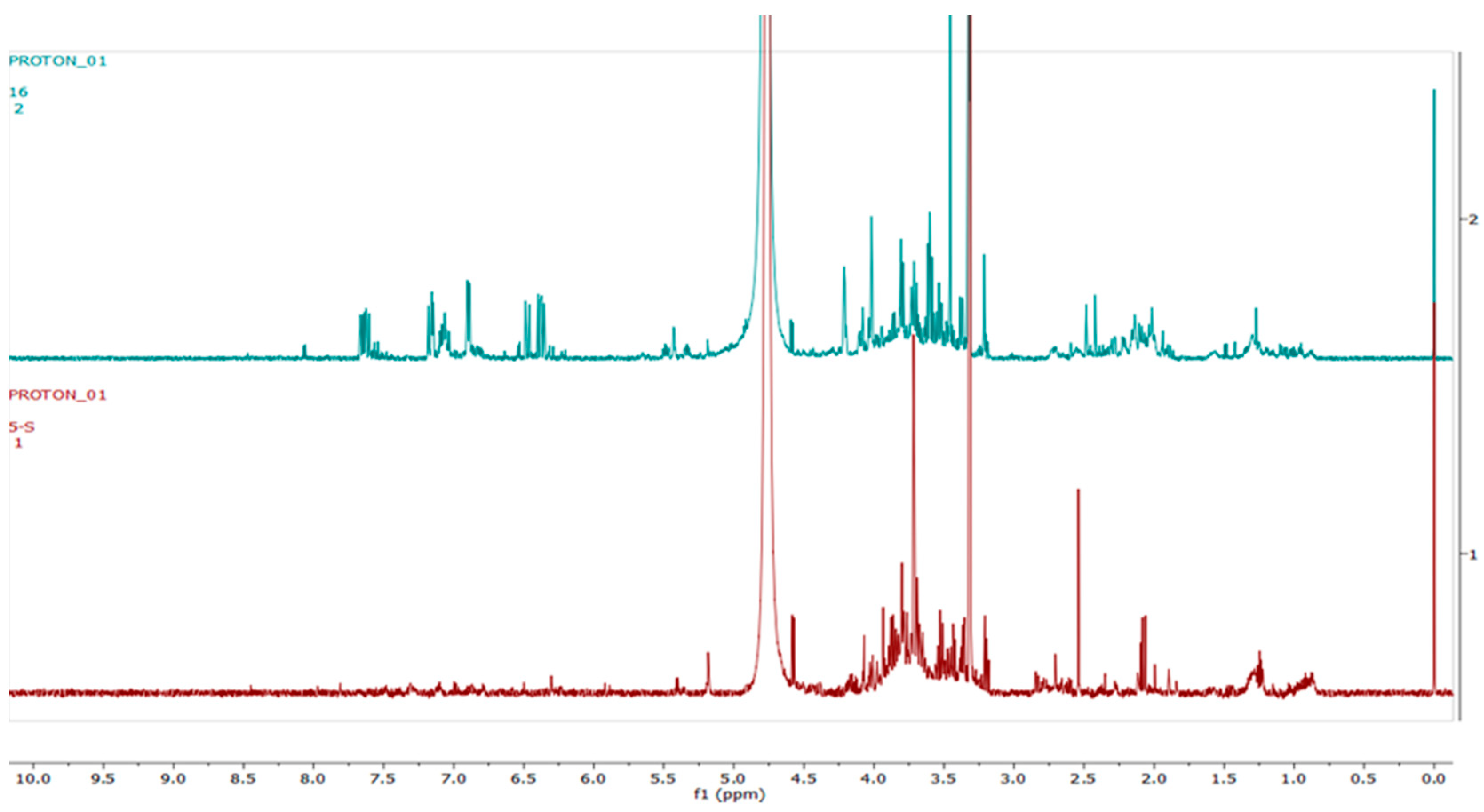
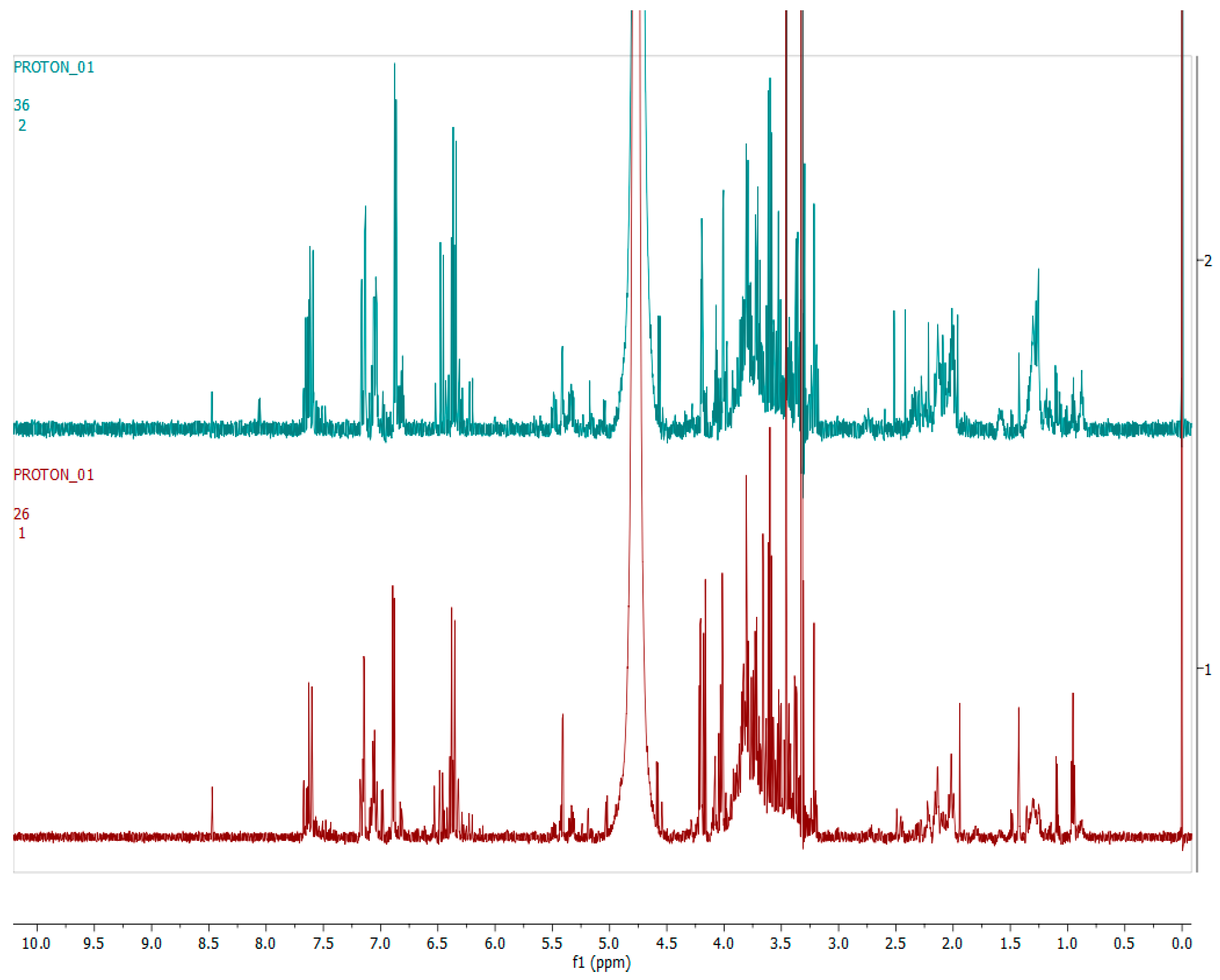
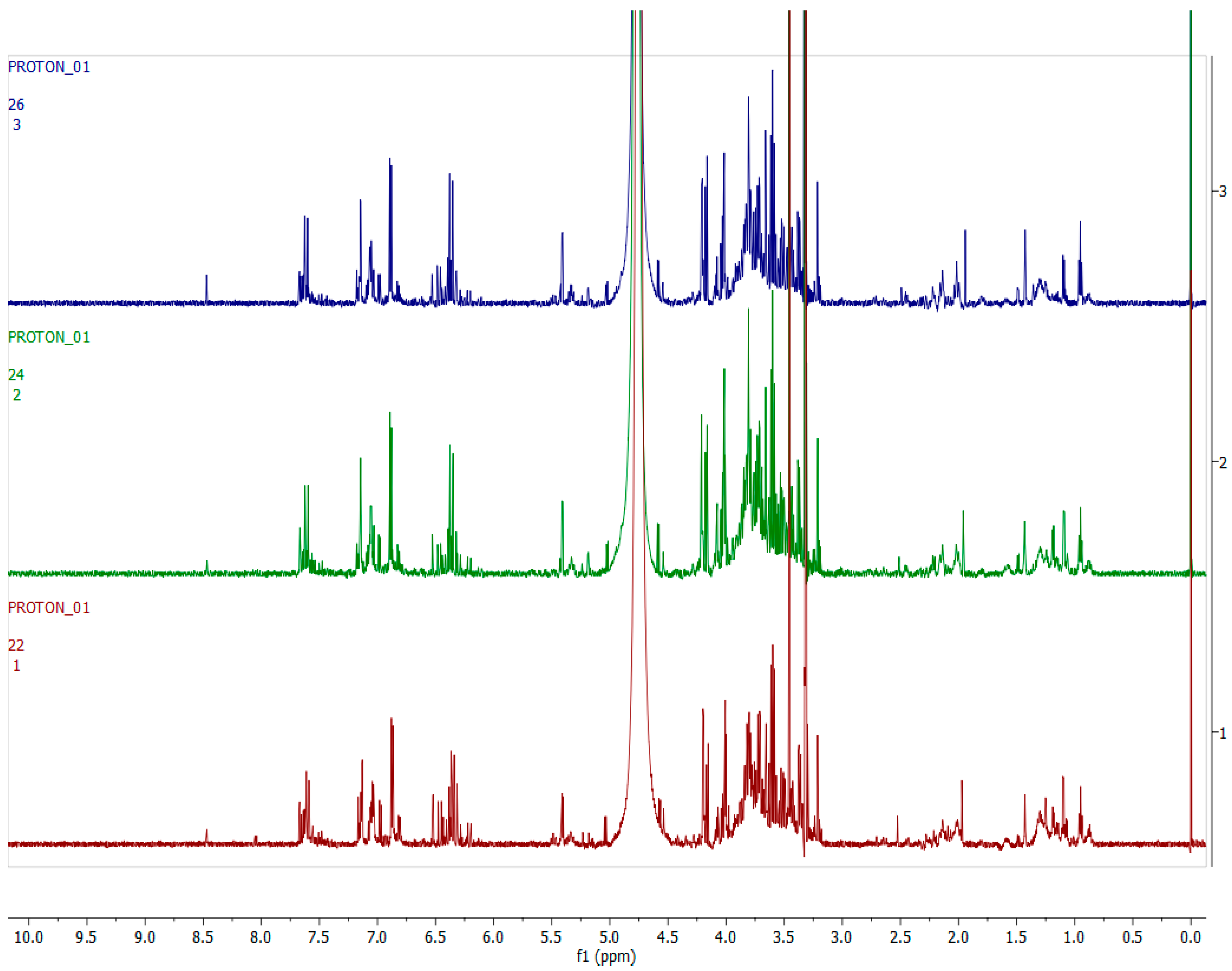
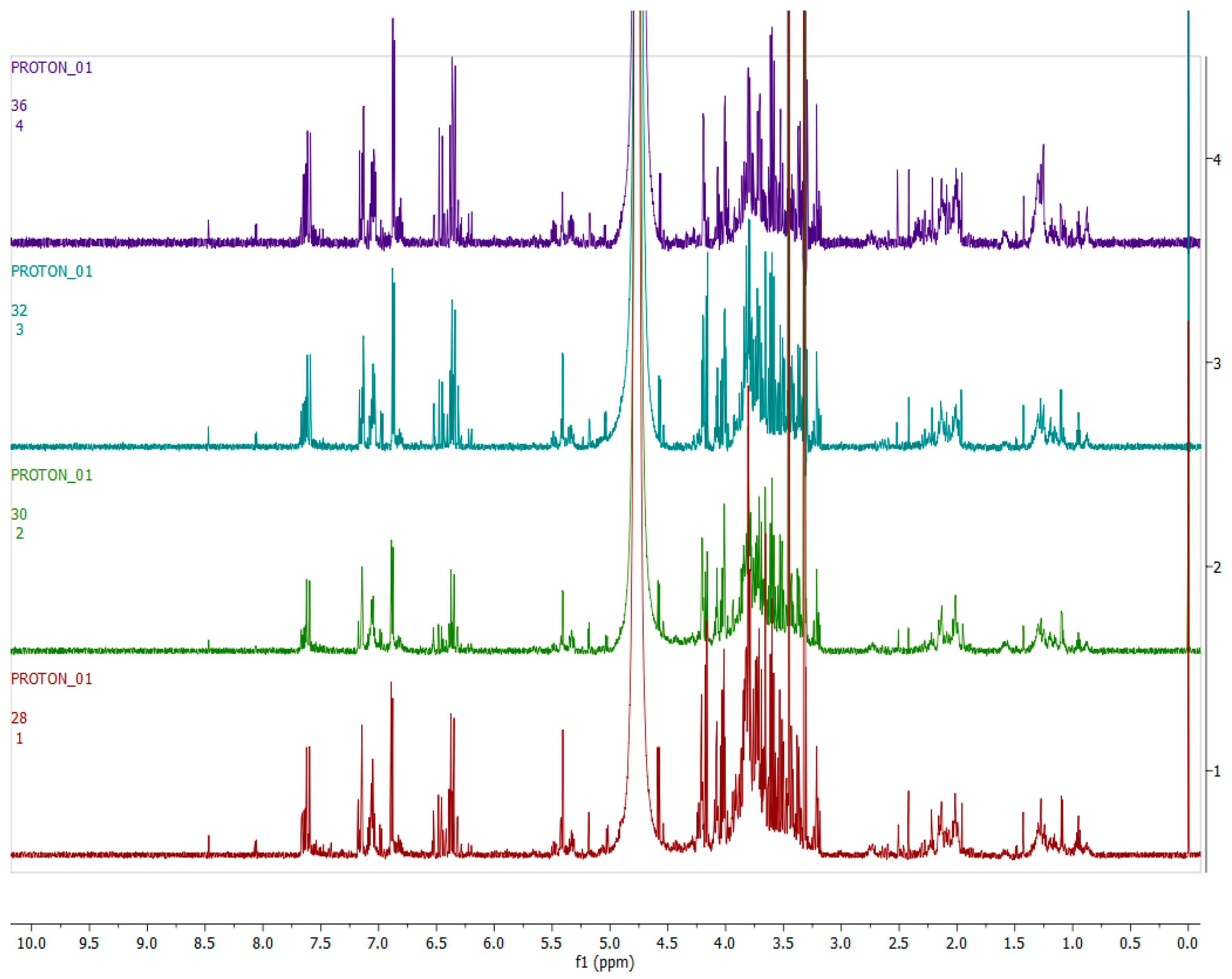
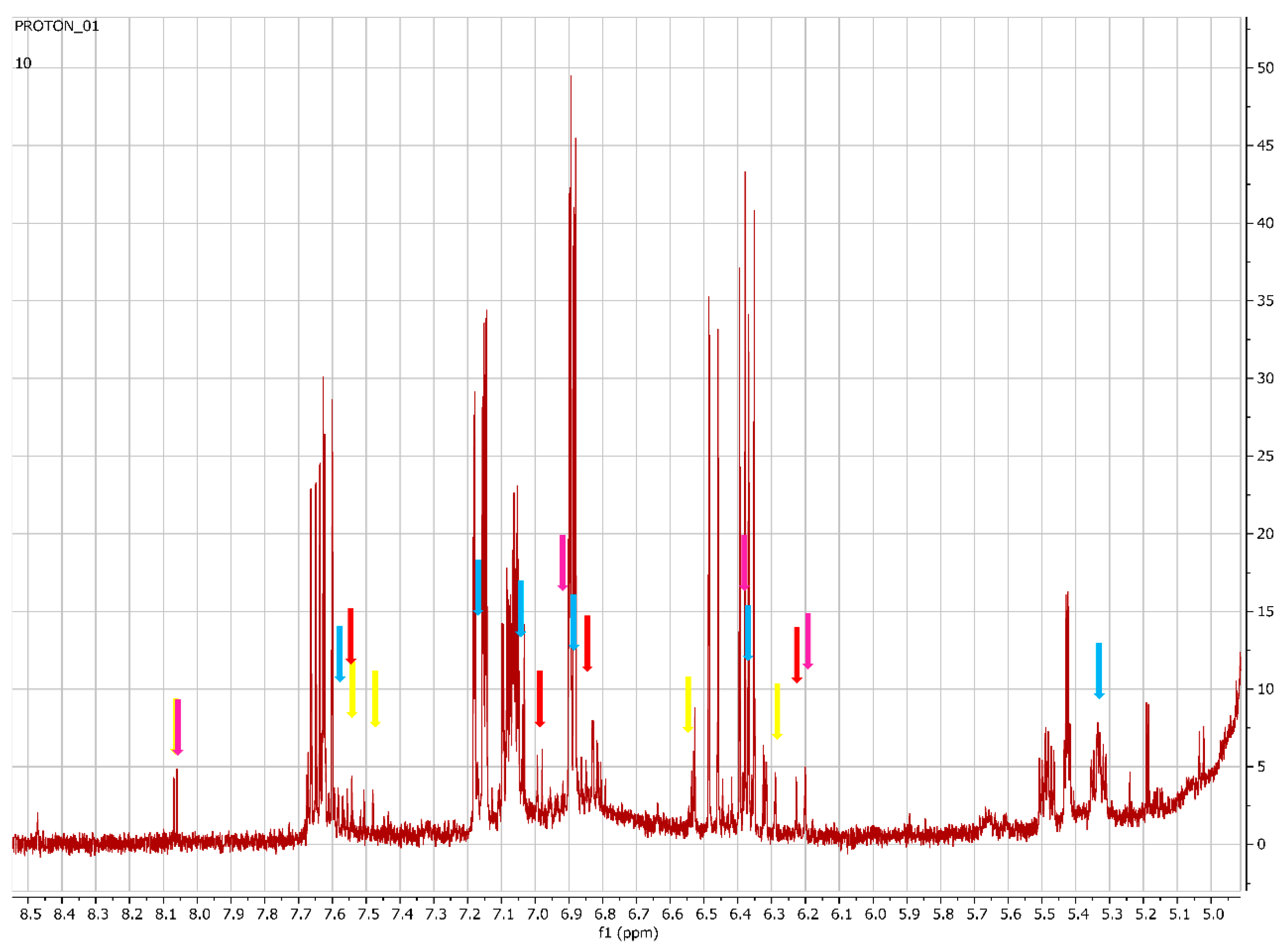
2.4. Metabolomics
2.4.1. Chemical Profile of H. aureonitens Collected from Telperion and Wakefield during Spring and Autumn Seasons
2.4.2. Chemical Profile of H. aureonitens Extracts Collected from Wet and Dry Sites at Both Telperion and Wakefield during the Spring and Autumn Seasons
3. Discussion
3.1. Antibacterial Activity
3.2. Antifungal Activity
3.3. Metabolomics
4. Materials and Methods
4.1. Plant Material Collection
4.2. Preparation of Plant Extracts and Antimicrobial Testing
4.2.1. Preparation of Plant Extracts
4.2.2. Antibacterial Activity
4.2.3. Antifungal Activity
4.3. Metabolomics Analysis
4.4. Compound Annotation
4.5. Rainfall and Temperature Data
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Department of Agriculture, Forestry and Fisheries of the Republic of South Africa; Directorate of Plant Production. Medicinal Plants of South Africa 2013; Compiled by Directorate Plant Production; Design and Layout by Directorate Communication Services Content; Department of Agriculture, Forestry and Fisheries of the Republic of South Africa: Pretoria, South Afica, 2013.
- Lourens, A.C.U.; Viljoen, A.M.; van Heerden, F.R. South African Helichrysum Species: A Review of the Traditional Uses, Biological Activity and Phytochemistry. J. Ethnopharmacol. 2008, 119, 630–652. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.J.M.; Dilika, F. Antibacterial Activity of Helichrysum Pedunculatum Used in Circumcision Rites. J. Ethnopharmacol. 1996, 53, 51–54. [Google Scholar] [CrossRef]
- Demir, A.; Mercanoglu Taban, B.; Aslan, M.; Yesilada, E.; Aykut Aytac, S. Antimicrobial Effect of Helichrysum Plicatum Subsp. Plicatum. Pharm. Biol. 2009, 47, 289–297. [Google Scholar] [CrossRef]
- Meyer, J.J.M.J.M.; Afolayan, A.J.J. Antibacterial Activity of Helichrysum aureonitens (Asteraceae). J. Ethnopharmacol. 1995, 47, 109–111. [Google Scholar] [CrossRef]
- Afolayan, A.J.; Meyer, J.J.M. The Antimicrobial Activity of 3,5,7-Trihydroxyflavone Isolated from the Shoots of Helichrysum aureonitens. J. Ethnopharmacol. 1997, 57, 177–181. [Google Scholar] [CrossRef]
- Mathekga, A.D.M.; Meyer, J.J.M.; Horn, M.M.; Drewes, S.E. An Acylated Phloroglucinol with Antimicrobial Properties from Helichrysum caespititium. Phytochemistry 2000, 53, 93–96. [Google Scholar] [CrossRef]
- Lourens, A.C.U.; Reddy, D.; Başer, K.H.C.; Viljoen, A.M.; van Vuuren, S.F. In Vitro Biological Activity and Essential Oil Composition of Four Indigenous South African Helichrysum Species. J. Ethnopharmacol. 2004, 95, 253–258. [Google Scholar] [CrossRef]
- Mathekga, A.D.M.; Meyer, J.J.M. Antibacterial Activity of South African Helichrysum Species. S. Afr. J. Bot. 1998, 64, 293–295. [Google Scholar] [CrossRef]
- Kutluk, I.; Aslan, M.; Orhan, I.E.; Özçelik, B. Antibacterial, Antifungal and Antiviral Bioactivities of Selected Helichrysum Species. S. Afr. J. Bot. 2018, 119, 252–257. [Google Scholar] [CrossRef]
- Goyal, S.; Lambert, C.; Cluzet, S.; Mérillon, J.M.; Ramawat, K.G. Secondary Metabolites and Plant Defence. In Plant Defence: Biological Control; Springer: Dordrecht, The Netherlands, 2012; pp. 109–138. ISBN 9789400719330. [Google Scholar]
- Usano-Alemany, J.; Palá-Pául, J.; Rodríguez, M.S.C.; Herraiz-Peñalver, D. Chemical Description and Essential Oil Yield Variability of Different Accessions of Salvia lavandulifolia. Nat. Prod. Commun. 2014, 9, 273–276. [Google Scholar] [CrossRef]
- Kale, V.S. Variable Rates of Primary and Secondary Metabolites during Different Seasons and Physiological Stages in Convolvulus, Datura and Withania. Asian J. Exp. Biol. Sci. 2010, 10, 50–53. [Google Scholar]
- López-Martínez, L.M.; Santacruz-Ortega, H.; Navarro, R.-E.; Sotelo-Mundo, R.R.; González-Aguilar, G.A. A 1 H NMR Investigation of the Interaction between Phenolic Acids Found in Mango (Manguifera Indica Cv Ataulfo) and Papaya (Carica Papaya Cv Maradol) and 1,1-Diphenyl-2-Picrylhydrazyl (DPPH) Free Radicals. PLoS ONE 2015, 10, e0140242. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.Y.; Jang, W.Y.; Lee, J.Y.; Jun, D.Y.; Ko, J.Y.; Yun, Y.H.; Kim, Y.H. Kaempferol Activates G2-Checkpoint of the Cell Cycle Resulting in G2-Arrest and Mitochondria-Dependent Apoptosis in Human Acute Leukemia Jurkat T Cells. J. Microbiol. Biotechnol. 2015, 26, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Bennett, R.N.; Wallsgrove, R.M. Tansley Review No. 72 Secondary Metabolites in Plant Defence Mechanisms. New Phytol. 1994, 127, 617–633. [Google Scholar] [CrossRef]
- Pavarini, D.P.; Pavarini, S.P.; Niehues, M.; Lopes, N.P. Exogenous Influences on Plant Secondary Metabolite Levels. Anim. Feed Sci. Technol. 2012, 176, 5–16. [Google Scholar] [CrossRef]
- Ramakrishna, A.; Ravishankar, G.A. Influence of Abiotic Stress Signals on Secondary Metabolites in Plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar]
- Lennette, E.H.; Balows, A.; Hausler, J.W., Jr.; Truant, J.P. Manual of Clinical Microbiology; American Society for Microbiology: Washington, DC, USA, 1980; pp. 195–219. [Google Scholar]
- King, J.B.; Stickler, D.J. The Effect of Repeated Instillations of Antiseptics on Catheter-Associated Urinary Tract Infections: A Study in a Physical Model of the Catheterized Bladder. Urol. Res. 1992, 20, 403–407. [Google Scholar] [CrossRef]
- Afolayan, A.J.; Grierson, D.S.; Kambizi, L.; Madamombe, I.; Masika, P.J. In Vitro Antifungal Activity of Some South African Medicinal Plants. S. Afr. J. Bot. 2002, 68, 72–76. [Google Scholar] [CrossRef]
- Akinyede, K.A.; Cupido, C.N.; Hughes, G.D.; Oguntibeju, O.O.; Ekpo, O.E. Medicinal Properties and in Vitro Biological Activities of Selected Helichrysum Species from South Africa: A Review. Plants 2021, 10, 1566. [Google Scholar] [CrossRef]
- Albayrak, S.; Aksoy, A.; Saǧdiç, O.; Budak, Ü. Phenolic compounds and antioxidant and antimicrobial properties of Helichrysum species collected from eastern Anatolia, Turkey. Turk. J. Biol. 2010, 34, 463–473. [Google Scholar] [CrossRef]
- Heyman, H.M.; Senejoux, F.; Seibert, I.; Klimkait, T.; Maharaj, V.J.; Meyer, J.J.M. Identification of Anti-HIV Active Dicaffeoylquinic- and Tricaffeoylquinic Acids in Helichrysum Populifolium by NMR-Based Metabolomic Guided Fractionation. Fitoterapia 2015, 103, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Babotă, M.; Mocan, A.; Vlase, L.; Crișan, O.; Ielciu, I.; Gheldiu, A.-M.; Vodnar, D.C.; Crișan, G.; Păltinean, R. Phytochemical Analysis, Antioxidant and Antimicrobial Activities of Helichrysum arenarium (L.) Moench. and Antennaria dioica (L.) Gaertn. Flowers. Molecules 2018, 23, 409. [Google Scholar] [CrossRef] [PubMed]
- Wianowska, D.; Gil, M. Recent Advances in Extraction and Analysis Procedures of Natural Chlorogenic Acids. Phytochem. Rev. 2019, 18, 273–302. [Google Scholar] [CrossRef]
- Frosi, I.; Montagna, I.; Colombo, R.; Milanese, C.; Papetti, A. Recovery of Chlorogenic Acids from Agri-Food Wastes: Updates on Green Extraction Techniques. Molecules 2021, 26, 4515. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, Q.J.; Li, X.; Luo, Y.; Liu, H. Extraction and HPLC Characterization of Chlorogenic Acid from Tobacco Residuals. Sep. Sci. Technol. 2007, 42, 3481–3492. [Google Scholar] [CrossRef]
- De Macêdo, D.G.; Souza, M.M.A.; Morais-Braga, M.F.B.; Coutinho, H.D.M.; dos Santos, A.T.L.; da Cruz, R.P.; da Costa, J.G.M.; Rodrigues, F.F.G.; Quintans-Junior, L.J.; da Silva Almeida, J.R.G.; et al. Effect of Seasonality on Chemical Profile and Antifungal Activity of Essential Oil Isolated from Leaves Psidium salutare (Kunth) O. Berg. PeerJ 2018, 6, e5476. [Google Scholar] [CrossRef]
- Liu, X.; Li, F.; Zhang, Y.; Yang, Q. Effects of Deficit Irrigation on Yield and Nutritional Quality of Arabica Coffee (Coffea arabica) under Different N Rates in Dry and Hot Region of Southwest China. Agric. Water Manag. 2016, 172, 1–8. [Google Scholar] [CrossRef]
- Z Rihan, H. The Effect of Drought on Phytochemical Active Compounds Content in Chamomile and Yarrow. Agric. Res. Technol. Open Access J. 2017, 12, 555855. [Google Scholar] [CrossRef]
- Batista, E.R.; Marinho-Prado, J.S.; Mineiro, J.L.C.; Sato, M.E.; Luiz, A.J.B.; Frighetto, R.T.S. Increased Atmospheric CO2 Combined with Local Climatic Variation Affects Phenolics and Spider Mite Populations in Coffee Trees. An. Acad. Bras. Cienc. 2021, 93, e20190696. [Google Scholar] [CrossRef]
- Amaral, J.S.; Valentão, P.; Andrade, P.B.; Martins, R.C.; Seabra, R.M. Molecules Do Cultivar, Geographical Location and Crop Season Influence Phenolic Profile of Walnut Leaves? Molecules 2008, 13, 1321–1332. [Google Scholar] [CrossRef]
- Su, M.; Liu, F.; Luo, Z.; Wu, H.; Zhang, X.; Wang, D.; Zhu, Y.; Sun, Z.; Xu, W.; Miao, Y. The Antibacterial Activity and Mechanism of Chlorogenic Acid against Foodborne Pathogen Pseudomonas aeruginosa. Foodborne Pathog. Dis. 2019, 16, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.L.A.; Bhattacharya, D. Antimicrobial Activity of Quercetin: An Approach to Its Mechanistic Principle. Molecules 2022, 27, 2494. [Google Scholar] [CrossRef] [PubMed]
- Adamczak, A.; Ożarowski, M.; Karpiński, T.M. Antibacterial Activity of Some Flavonoids and Organic Acids Widely Distributed in Plants. J. Clin. Med. 2020, 9, 109. [Google Scholar] [CrossRef] [PubMed]
- Özgüven, M.; Müller, J.; Gülseren, G. Investigation of the Efficiency of Drying Conditions for Essential Oil Production from Aromatic Plants. Makara J. Sci. 2019, 23, 148–154. [Google Scholar] [CrossRef]
- Mirhosseini, F.; Rahimmalek, M.; Pirbalouti, A.G.; Taghipoor, M. Effect of Different Drying Treatments on Essential Oil Yield, Composition and Color Characteristics of Kelussia odoratissima Mozaff. J. Essent. Oil Res. 2015, 27, 204–211. [Google Scholar] [CrossRef]
- Nadia Ramlan, N.; Mohamad Azzeme, A.; Naim Md Padzil, K.; Mahmood, M. Influence Of Different Extraction Solvents On Phytochemical Content And Antioxidant Capacity Extracted From Pulp And Flower Of Dessert And Cooking Bananas. Malays. J. Biochem. Mol. Biol. 2017, 2, 10–16. [Google Scholar]
- Eloff, J.N. Which Extractant Should Be Used for the Screening and Isolation of Antimicrobial Components from Plants? J. Ethnopharmacol. 1998, 60, 1–8. [Google Scholar] [CrossRef]
- Eloff, J.N. A Sensitive and Quick Microplate Method to Determine the Minimal Inhibitory Concentration of Plant Extracts for Bacteria. Planta Med. 1998, 64, 711–713. [Google Scholar] [CrossRef]
- Zgoda, J.R.; Porter, J.R. A Convenient Microdilution Method for Screening Natural Products against Bacteria and Fungi. Pharm. Biol. 2001, 39, 221–225. [Google Scholar] [CrossRef]
- Mediani, A.; Abas, F.; Khatib, A.; Maulidiani, H.; Shaari, K.; Choi, Y.H.; Lajis, N.H. 1H-NMR-Based Metabolomics Approach to Understanding the Drying Effects on the Phytochemicals in Cosmos Caudatus. Food Res. Int. 2012, 49, 763–770. [Google Scholar] [CrossRef]




| Plant Part | Season | Location | P. vulgaris | B. subtilis |
|---|---|---|---|---|
| Leaf (Site 1) | Spring | Telperion | 62.5 | >250 |
| Stem (Site 1) | Spring | Telperion | 62.5 | >250 |
| Leaf (Site 2) | Spring | Telperion | 62.5 | >250 |
| Stem (Site 2) | Spring | Telperion | 62.5 | >250 |
| Leaf (Site 1) | Spring | Wakefield | 62.5 | >250 |
| Stem (Site 1) | Spring | Wakefield | 62.5 | >250 |
| Leaf (Site 2) | Spring | Wakefield | 62.5 | >250 |
| Stem (Site 2) | Spring | Wakefield | 62.5 | >250 |
| Leaf (Site 1) | Autumn | Telperion | 62.5 | >250 |
| Stem (Site 1) | Autumn | Telperion | 62.5 | >250 |
| Leaf (Site 2) | Autumn | Telperion | 62.5 | >250 |
| Stem (Site 2) | Autumn | Telperion | 62.5 | >250 |
| Leaf (Site 3) | Autumn | Telperion | >250 | >250 |
| Stem (Site 3) | Autumn | Telperion | 250 | >250 |
| Leaf (Site 1) | Autumn | Wakefield | 250 | >250 |
| Stem (Site 1) | Autumn | Wakefield | 125 | >250 |
| Leaf (Site 2) | Autumn | Wakefield | 125 | >250 |
| Stem (Site 2) | Autumn | Wakefield | 125 | >250 |
| Leaf (Site 3) | Autumn | Wakefield | 125 | >250 |
| Stem (Site 3) | Autumn | Wakefield | 125 | >250 |
| Gentamicin | 1000 |
| Plant Part | Season | Location | A. flavus | A. nomius | C. clados | F. oxy | P. halo |
|---|---|---|---|---|---|---|---|
| Leaf (Site 1) | Spring | Telperion | 0.39 | >250 | 0.78 | 0.78 | 6.25 |
| Stem (Site 1) | Spring | Telperion | 0.39 | >250 | 0.78 | 0.78 | 6.25 |
| Leaf (Site 2) | Spring | Telperion | 0.39 | >250 | 0.39 | 0.78 | 6.25 |
| Stem (Site 2) | Spring | Telperion | 0.39 | >250 | 0.78 | 1.56 | 3.125 |
| Leaf (Site 1) | Spring | Wakefield | 0.78 | >250 | 0.78 | 3.125 | 3.125 |
| Stem (Site 1) | Spring | Wakefield | 0.78 | >250 | 0.78 | 3.125 | 6.25 |
| Leaf (Site 2) | Spring | Wakefield | 0.78 | >250 | 1.56 | 3.125 | 6.25 |
| Stem (Site 2) | Spring | Wakefield | 0.78 | >250 | 0.78 | 6.25 | 3.125 |
| Leaf (Site 3) | Spring | Wakefield | 1.56 | >250 | 0.78 | 6.25 | 3.125 |
| Stem (Site 3) | Spring | Wakefield | 1.56 | >250 | 0.78 | 3.125 | 3.125 |
| Leaf (Site 4) | Spring | Wakefield | 1.56 | >250 | 0.78 | 3.125 | 3.125 |
| Stem (Site 4) | Spring | Wakefield | 1.56 | >250 | 1.56 | 6.25 | 3.125 |
| Leaf (Site 1) | Autumn | Telperion | 0.39 | >250 | 0.78 | 3.125 | 1.56 |
| Stem (Site 1) | Autumn | Telperion | 0.39 | >250 | 0.78 | 3.125 | 1.56 |
| Leaf (Site 2) | Autumn | Telperion | 0.39 | >250 | 0.78 | 3.125 | 1.56 |
| Stem (Site 2) | Autumn | Telperion | 0.39 | >250 | 0.78 | 3.125 | 1.56 |
| Leaf (Site 3) | Autumn | Telperion | 0.39 | >250 | 0.39 | 3.125 | 1.56 |
| Stem (Site 3) | Autumn | Telperion | 0.39 | >250 | 0.78 | 6.25 | 1.56 |
| Leaf (Site 1) | Autumn | Wakefield | 0.78 | >250 | 1.56 | 1.56 | 1.56 |
| Stem (Site 1) | Autumn | Wakefield | 0.78 | >250 | 0.78 | 1.56 | 1.56 |
| Leaf (Site 2) | Autumn | Wakefield | 0.78 | >250 | 0.39 | 1.56 | 1.56 |
| Stem (Site 2) | Autumn | Wakefield | 0.78 | >250 | 1.56 | 1.56 | 1.56 |
| Leaf (Site 3) | Autumn | Wakefield | 1.56 | >250 | 0.78 | 1.56 | 3.125 |
| Stem (Site 3) | Autumn | Wakefield | 1.56 | >250 | 1.56 | 1.56 | 3.125 |
| Leaf (Site 4) | Autumn | Wakefield | 3.125 | >250 | 1.56 | 6.25 | 3.125 |
| Stem (Site 4) | Autumn | Wakefield | 6.25 | >250 | 1.56 | 6.25 | 3.125 |
| Amphotericin B | 1000 |
| Wakefield (Cedara Data) | Telperion (Witbank Data) | |||||
|---|---|---|---|---|---|---|
| Month | Avg Daily Max T (°C) | Avg Daily Min T (°C) | Total Monthly Rainfall (mm) | Avg Daily Max T (°C) | Avg Daily Min T (°C) | Total Monthly Rainfall (mm) |
| August | 20.8 | 5.2 | 3.6 | 21.3 | 5.6 | 3.8 |
| September | 24.5 | 8.9 | 9.6 | 26.8 | 9.8 | 27.2 |
| October | 22.3 | 9 | 110 | 24.5 | 10.7 | 83.8 |
| November | 23.7 | 10.7 | 105.8 | 27 | 11.9 | 109.2 |
| December | 23.8 | 12.6 | 79 | 26.5 | 14.1 | 153.2 |
| January | 27.3 | 14.2 | 79.4 | 27.9 | 13.9 | 71.8 |
| February | 26.5 | 15.3 | 170.2 | 26.3 | 15 | 75.8 |
| March | 25.7 | 13.7 | 124.2 | 26.0 | 13.4 | 148.4 |
| April | 23.9 | 12.2 | 52.4 | 23.9 | 12.0 | 26.0 |
| May | 21 | 6.7 | 31.8 | 21.4 | 7.0 | 24.6 |
| June | 20.2 | 3.4 | 1 | 20.1 | 4.5 | 0.2 |
| Compound | 1H-NMR Chemical Shift | Referenced ppm | Reference |
|---|---|---|---|
| Galangin | 8.06, 7.58, 7.49, 6.54, 6.28 | 8.25, 7.49–7.57, 6.55, 6.28 | [6] |
| Chlorogenic acid | 7.56, 7.06, 7.03, 6.83, 6.22, 5.17, 3.95, 2.14, 1.97 | 7.66, 7.18, 7.0, 6.9, 6.4, 5.3, 3.86, 1.74–2.04 | [14] |
| Kaempferol | 8.06, 6.94, 6.44, 6.20 | 8.03, 6.93, 6.43, 6.18, | [15] |
| Quercetin | 7.66, 7.54, 6.88, 6.20 | 7.67, 7.53, 6.88, 6.40, 6.18 | [15] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adeosun, W.B.; Bodede, O.; Prinsloo, G. Effect of Different Climatic Regions and Seasonal Variation on the Antibacterial and Antifungal Activity, and Chemical Profile of Helichrysum aureonitens Sch. Bip. Metabolites 2022, 12, 758. https://doi.org/10.3390/metabo12080758
Adeosun WB, Bodede O, Prinsloo G. Effect of Different Climatic Regions and Seasonal Variation on the Antibacterial and Antifungal Activity, and Chemical Profile of Helichrysum aureonitens Sch. Bip. Metabolites. 2022; 12(8):758. https://doi.org/10.3390/metabo12080758
Chicago/Turabian StyleAdeosun, Wilson Bamise, Olusola Bodede, and Gerhard Prinsloo. 2022. "Effect of Different Climatic Regions and Seasonal Variation on the Antibacterial and Antifungal Activity, and Chemical Profile of Helichrysum aureonitens Sch. Bip" Metabolites 12, no. 8: 758. https://doi.org/10.3390/metabo12080758
APA StyleAdeosun, W. B., Bodede, O., & Prinsloo, G. (2022). Effect of Different Climatic Regions and Seasonal Variation on the Antibacterial and Antifungal Activity, and Chemical Profile of Helichrysum aureonitens Sch. Bip. Metabolites, 12(8), 758. https://doi.org/10.3390/metabo12080758






