Age Worsens the Cognitive Phenotype in Mice Carrying the Thr92Ala-DIO2 Polymorphism
Abstract
:1. Introduction
2. Results
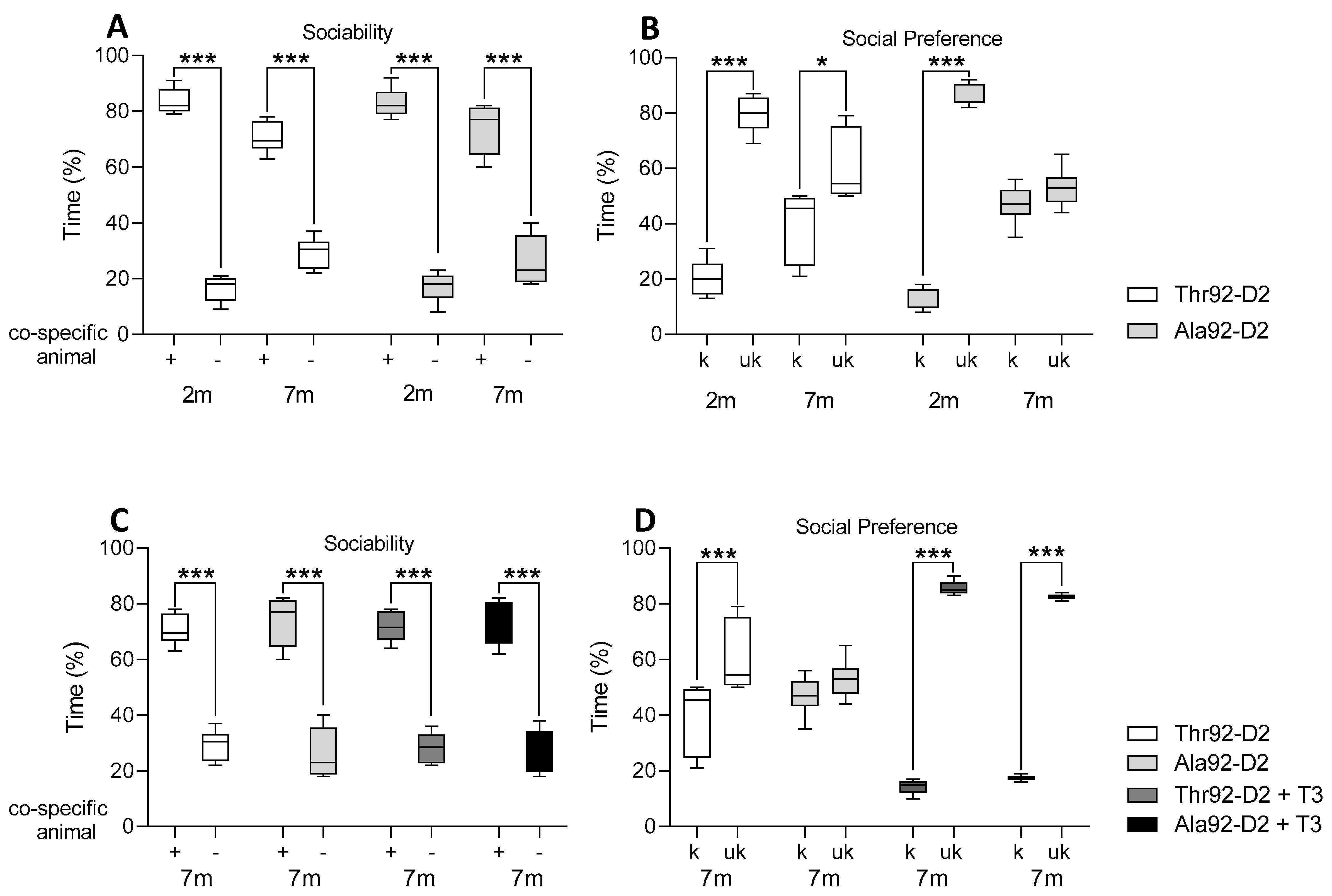
2.1. Older Age Intensifies the Cognitive Phenotype in Male Ala92-Dio2 Mice
2.2. The Phenotype Associated with Ala92-Dio2 Polymorphism Is Modified in Female Mice
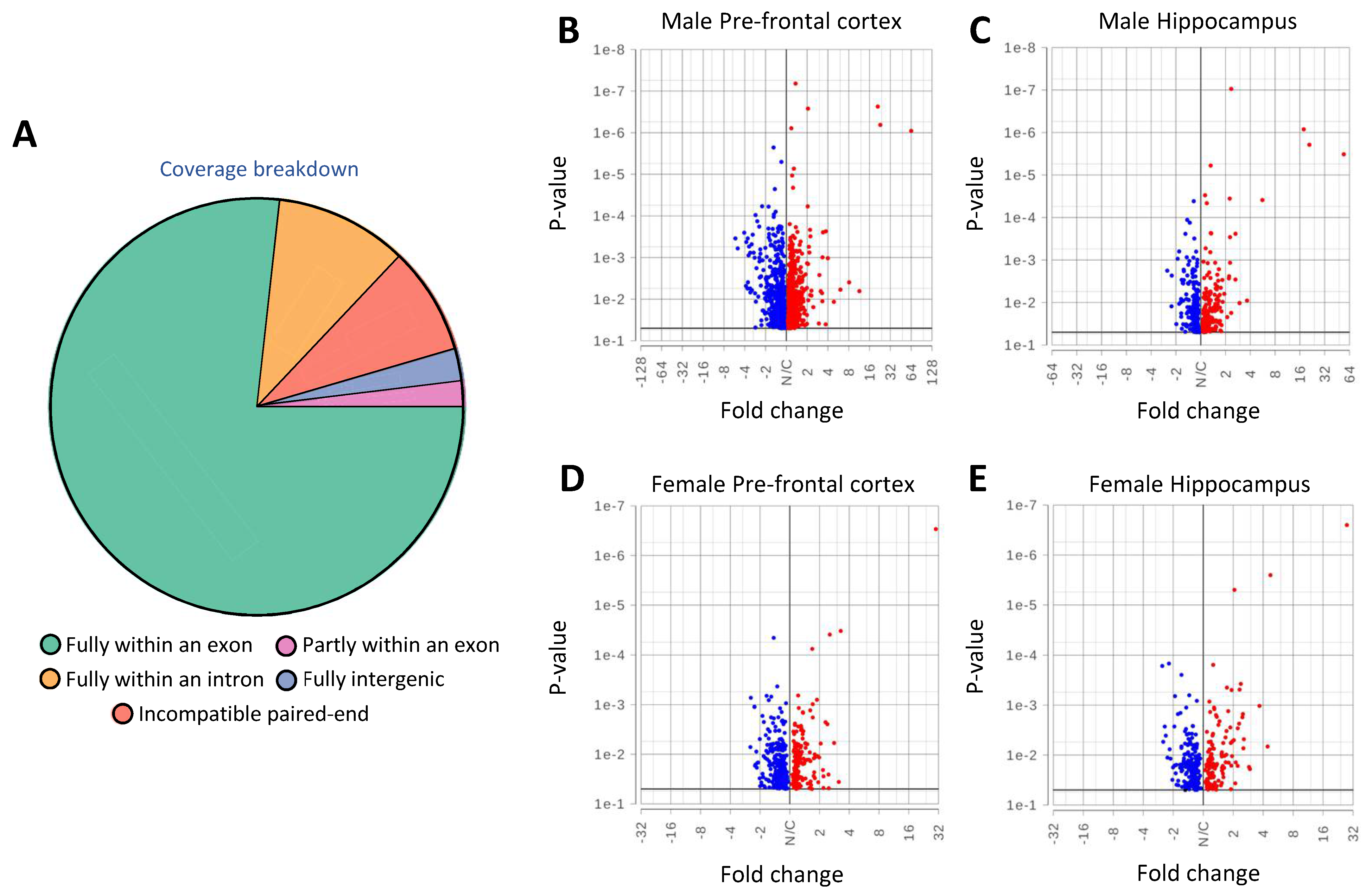
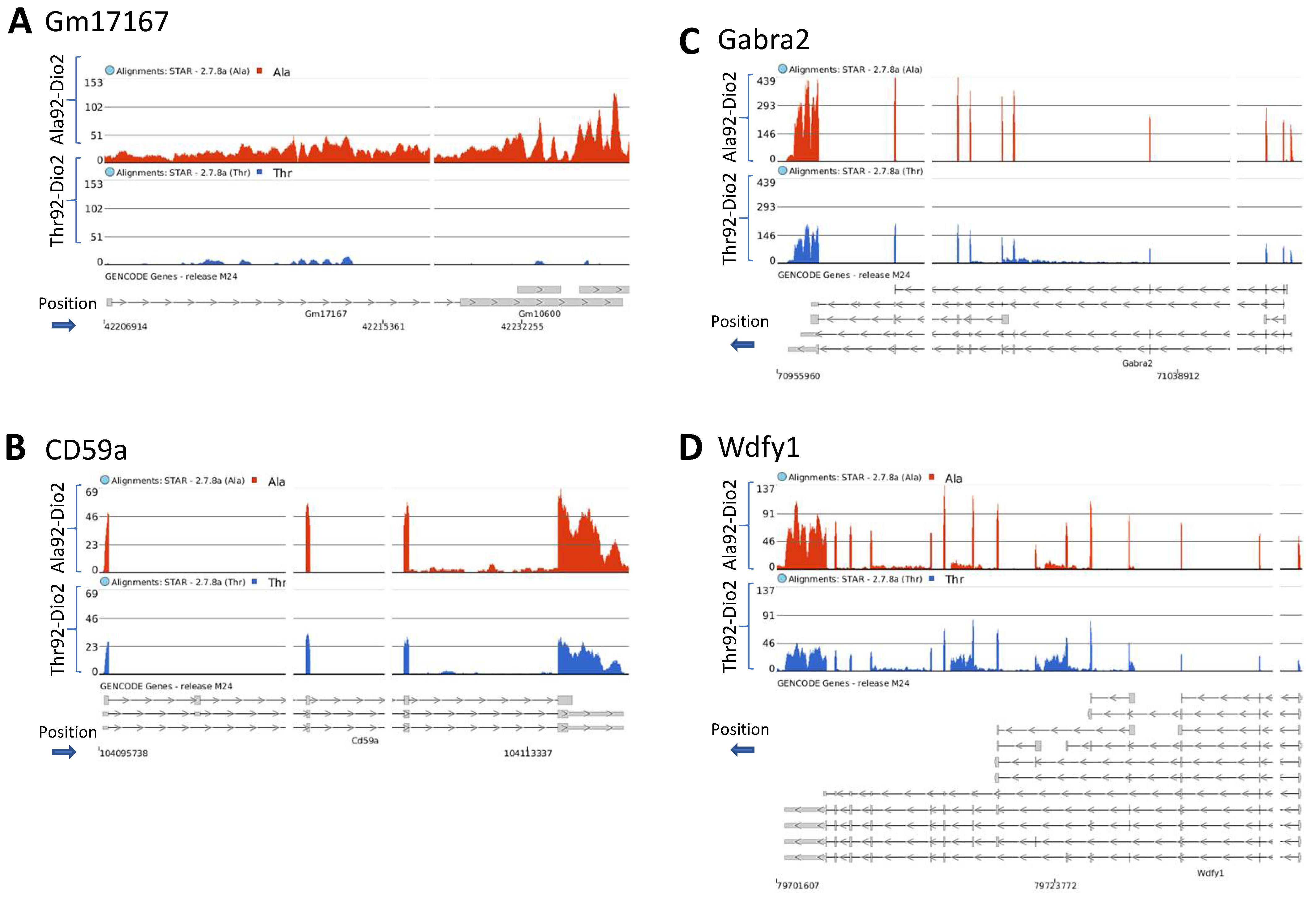
2.3. Transcriptome Analysis
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Behavior and Cognitive Assessment
4.3. RNA Sequencing and Analyses
4.4. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chaker, L.; Bianco, A.C.; Jonklaas, J.; Peeters, R.P. Hypothyroidism. Lancet 2017, 390, 1550–1562. [Google Scholar] [CrossRef]
- Jonklaas, J.; Bianco, A.C.; Bauer, A.J.; Burman, K.D.; Cappola, A.R.; Celi, F.S.; Cooper, D.S.; Kim, B.W.; Peeters, R.P.; Rosenthal, M.S.; et al. Guidelines for the treatment of hypothyroidism: Prepared by the american thyroid association task force on thyroid hormone replacement. Thyroid. Off. J. Am. Thyroid. Assoc. 2014, 24, 1670–1751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gereben, B.; McAninch, E.A.; Ribeiro, M.O.; Bianco, A.C. Scope and limitations of iodothyronine deiodinases in hypothyroidism. Nat. Rev. Endocrinol. 2015, 11, 642–652. [Google Scholar] [CrossRef] [Green Version]
- Bianco, A.C.; Dumitrescu, A.; Gereben, B.; Ribeiro, M.O.; Fonseca, T.L.; Fernandes, G.W.; Bocco, B. Paradigms of Dynamic Control of Thyroid Hormone Signaling. Endocr. Rev. 2019, 40, 1000–1047. [Google Scholar] [CrossRef] [PubMed]
- Castagna, M.G.; Dentice, M.; Cantara, S.; Ambrosio, R.; Maino, F.; Porcelli, T.; Marzocchi, C.; Garbi, C.; Pacini, F.; Salvatore, D. DIO2 Thr92Ala Reduces Deiodinase-2 Activity and Serum-T3 Levels in Thyroid-Deficient Patients. J. Clin. Endocrinol. Metab. 2017, 102, 1623–1630. [Google Scholar] [CrossRef]
- Jo, S.; Fonseca, T.L.; Bocco, B.; Fernandes, G.W.; McAninch, E.A.; Bolin, A.P.; Da Conceição, R.R.; Werneck-de-Castro, J.P.; Ignacio, D.L.; Egri, P.; et al. Type 2 deiodinase polymorphism causes ER stress and hypothyroidism in the brain. J. Clin. Investig. 2019, 129, 230–245. [Google Scholar] [CrossRef]
- McAninch, E.A.; Rajan, K.B.; Evans, D.A.; Jo, S.; Chaker, L.; Peeters, R.P.; Bennett, D.A.; Mash, D.C.; Bianco, A.C. A Common DIO2 Polymorphism and Alzheimer Disease Dementia in African and European Americans. J. Clin. Endocrinol. Metab. 2018, 103, 1818–1826. [Google Scholar] [CrossRef]
- McAninch, E.A.; Jo, S.; Preite, N.Z.; Farkas, E.; Mohacsik, P.; Fekete, C.; Egri, P.; Gereben, B.; Li, Y.; Deng, Y.; et al. Prevalent polymorphism in thyroid hormone-activating enzyme leaves a genetic fingerprint that underlies associated clinical syndromes. J. Clin. Endocrinol. Metab. 2015, 100, 920–933. [Google Scholar] [CrossRef] [Green Version]
- Göbel, A.; Heldmann, M.; Göttlich, M.; Dirk, A.-L.; Brabant, G.; Münte, T.F. Effect of mild thyrotoxicosis on performance and brain activations in a working memory task. PLoS ONE 2016, 11, e0161552. [Google Scholar] [CrossRef]
- Samuels, M.H. Psychiatric and cognitive manifestations of hypothyroidism. Curr. Opin. Endocrinol. Diabetes Obes. 2014, 21, 377. [Google Scholar] [CrossRef] [Green Version]
- Poplawski, S.G. The Regulation of Gene Expression during Memory Consolidation in the Hippocampus; University of Pennsylvania: Philadelphia, PA, USA, 2014. [Google Scholar]
- Stahel, P.F.; Flierl, M.A.; Morgan, B.P.; Persigehl, I.; Stoll, C.; Conrad, C.; Touban, B.M.; Smith, W.R.; Beauchamp, K.; Schmidt, O.I. Absence of the complement regulatory molecule CD59a leads to exacerbated neuropathology after traumatic brain injury in mice. J. Neuroinflam. 2009, 6, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dixon, C.; Rosahl, T.; Stephens, D. Targeted deletion of the GABRA2 gene encoding α2-subunits of GABAA receptors facilitates performance of a conditioned emotional response, and abolishes anxiolytic effects of benzodiazepines and barbiturates. Pharmacol. Biochem. Behav. 2008, 90, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yeo, I.J.; Park, M.H.; Son, D.J.; Kim, J.Y.; Nam, K.T.; Hyun, B.K.; Kim, S.Y.; Jung, M.H.; Song, M.J.; Chun, H.O. PRDX6 inhibits neurogenesis through downregulation of WDFY1-mediated TLR4 signal. Mol. Neurobiol. 2019, 56, 3132–3144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, P.; Xu, Y.; Zhang, Z.; Gao, C.; Zhu, J.; Li, H.; Wan, Q. Decreased plasma neuregulin 4 levels are associated with peripheral neuropathy in Chinese patients with newly diagnosed type 2 diabetes: A cross-sectional study. Cytokine 2019, 113, 356–364. [Google Scholar] [CrossRef]
- Yuan, S. Expression of BEX1 and MSK1 in Motor Neurons with Differential Susceptibility to Degeneration in Amyotrophic Lateral Sclerosis in Transgenic Rats and Human Patients. Bachelor’s Thesis, Princeton University, Princeton, NJ, USA, 2014. [Google Scholar]
- Remaud, S.; Gothié, J.-D.; Morvan-Dubois, G.; Demeneix, B.A. Thyroid hormone signaling and adult neurogenesis in mammals. Front. Endocrinol. 2014, 5, 62. [Google Scholar] [CrossRef]
- Shacham, T.; Sharma, N.; Lederkremer, G.Z. Protein misfolding and ER stress in Huntington’s disease. Front. Mol. Biosci. 2019, 6, 20. [Google Scholar] [CrossRef] [Green Version]
- Gomez-Nicola, D.; Boche, D. Post-mortem analysis of neuroinflammatory changes in human Alzheimer’s disease. Alzheimers Res. Ther. 2015, 7, 42. [Google Scholar] [CrossRef] [Green Version]
- Salminen, A.; Kauppinen, A.; Suuronen, T.; Kaarniranta, K.; Ojala, J. ER stress in Alzheimer’s disease: A novel neuronal trigger for inflammation and Alzheimer’s pathology. J. Neuroinflam. 2009, 6, 41. [Google Scholar] [CrossRef] [Green Version]
- Uddin, M.S.; Yu, W.S.; Lim, L.W. Exploring ER stress response in cellular aging and neuroinflammation in Alzheimer’s disease. Aging Res. Rev. 2021, 70, 101417. [Google Scholar] [CrossRef]
- Piskunov, A.; Stepanichev, M.; Tishkina, A.; Novikova, M.; Levshina, I.; Gulyaeva, N. Chronic combined stress induces selective and long-lasting inflammatory response evoked by changes in corticosterone accumulation and signaling in rat hippocampus. Metab. Brain Dis. 2016, 31, 445–454. [Google Scholar] [CrossRef]
- Gulyaeva, N.V. Functional neurochemistry of the ventral and dorsal hippocampus: Stress, depression, dementia and remote hippocampal damage. Neurochem. Res. 2019, 44, 1306–1322. [Google Scholar] [CrossRef] [PubMed]
- Belarbi, K.; Rosi, S. Modulation of adult-born neurons in the inflamed hippocampus. Front. Cell. Neurosci. 2013, 7, 145. [Google Scholar] [CrossRef] [Green Version]
- Kohman, R.A.; Rhodes, J.S. Neurogenesis, inflammation and behavior. Brain Behav. Immun. 2013, 27, 22–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mattson, M.P.; Arumugam, T.V. Hallmarks of brain aging: Adaptive and pathological modification by metabolic states. Cell Metab. 2018, 27, 1176–1199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fjell, A.M.; Walhovd, K.B. Structural brain changes in aging: Courses, causes and cognitive consequences. Rev. Neurosci. 2010, 21, 187–222. [Google Scholar] [CrossRef]
- Morrison, J.H.; Baxter, M.G. The ageing cortical synapse: Hallmarks and implications for cognitive decline. Nat. Rev. Neurosci. 2012, 13, 240–250. [Google Scholar] [CrossRef]
- Crider, A.; Nelson, T.; Davis, T.; Fagan, K.; Vaibhav, K.; Luo, M.; Kamalasanan, S.; Terry, A.V.; Pillai, A. Estrogen receptor β agonist attenuates endoplasmic reticulum stress-induced changes in social behavior and brain connectivity in mice. Mol. Neurobiol. 2018, 55, 7606–7618. [Google Scholar] [CrossRef]
- Avila, M.F.; Cabezas, R.; Torrente, D.; Gonzalez, J.; Morales, L.; Alvarez, L.; Capani, F.; Barreto, G.E. Novel interactions of GRP78: UPR and estrogen responses in the brain. Cell Biol. Int. 2013, 37, 521–532. [Google Scholar] [CrossRef]
- Dalal, S.J.; Estep, J.S.; Valentin-Bon, I.E.; Jerse Jerse, A.E. Standardization of the Whitten Effect to induce susceptibility to Neisseria gonorrhoeae in female mice. J. Am. Assoc. Lab. Anim. Sci. 2001, 40, 13–17. [Google Scholar]
- Gangrade, B.; Dominic, C. Studies of the male-originating pheromones involved in the Whitten effect and Bruce effect in mice. Biol. Reprod. 1984, 31, 89–96. [Google Scholar] [CrossRef] [Green Version]
- Morgan, M.; Pfaff, D. Effects of estrogen on activity and fear-related behaviors in mice. Horm. Behav. 2001, 40, 472–482. [Google Scholar] [CrossRef] [PubMed]
- Gould, T.D.; Dao, D.T.; Kovacsics, C.E. The open field test. In Mood and Anxiety Related Phenotypes in Mice; Springer: Berlin/Heidelberg, Germany, 2009; pp. 1–20. [Google Scholar]
- Huckins, L.M.; Logan, D.W.; Sánchez-Andrade, G. Olfaction and olfactory-mediated behaviour in psychiatric disease models. Cell Tissue Res. 2013, 354, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Dodart, J.-C.; Bales, K.R.; Gannon, K.S.; Greene, S.J.; DeMattos, R.B.; Mathis, C.; DeLong, C.A.; Wu, S.; Wu, X.; Holtzman, D.M. Immunization reverses memory deficits without reducing brain Aβ burden in Alzheimer’s disease model. Nat. Neurosci. 2002, 5, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Kuc, K.; Gregersen, B.; Gannon, K.; Dodart, J.C. Holeboard discrimination learning in mice. Genes Brain Behav. 2006, 5, 355–363. [Google Scholar] [CrossRef]


| Test | Parameter | Thr92-Dio2 2–3 m | Ala92-Dio2 2–3 m | Thr92-Dio2 7 m | Ala92-Dio2 7 m |
|---|---|---|---|---|---|
| Open field | Distance (cm) | 5535 ± 288.8 | 4399 ± 262.7 | 4742 ± 522.7 | 3251 ± 265.5 a |
| Velocity (cm/s) | 9.8 ± 0.8 | 8.0 ± 0.5 | 10.21 ± 1.74 | 6.98 ± 1.08 | |
| Hole board | Visuospatial memory (% error) | 32.3 ± 12.4 | 54.6 ± 9.1 | 27.4 ± 5.9 | 13.58 ± 7.3 |
| Working memory (% error) | 1.63 ± 1.14 | 6.47 ± 1.69 | 3.0 ± 1.93 | 21.5.3 ± 3.17 bc |
| Test | Parameter | Thr92-Dio2 7 m | Ala92-Dio2 7 m | Thr92-Dio2 + T3 7 m | Ala92-Dio2 + T3 7 m |
|---|---|---|---|---|---|
| Open field | Distance (cm) | 4742 ± 522.7 | 3251 ± 265.5 | 2705 ± 141.6 a | 2096 ± 207.9 |
| Velocity (cm/s) | 10.21 ± 1.74 | 6.98 ± 1.08 | 4.45 ± 0.23 b | 3.52 ± 0.35 | |
| Hole board | Visuospatial memory (% error) | 27.4 ± 5.9 | 13.58 ± 7.3 | 25.8 ± 3.77 | 17.7 ± 4.87 |
| Working memory (% error) | 3.0 ± 1.93 | 21.5.3 ± 3.17 c | 0.00 ± 0.00 | 0.00 ± 0.00 d |
| Male | |||||
|---|---|---|---|---|---|
| Area | Parameter | Thr92-Dio2 | Ala92-Dio2 | p-Value | |
| CA1 | Area (mm2) | 117.7 ± 7.07 | 110.1 ± 6.54 | 0.464 | |
| Neuronal density (n/mm2) | 10.1 ± 0.24 | 10.1 ± 0.63 | 0.992 | ||
| CA2 | Area (mm2) | 16.6 ± 0.70 | 14.8 ± 1.69 | 0.35 | |
| Neuronal density (n/mm2) | 10.6 ± 0.51 | 9.7 ± 0.81 | 0.412 | ||
| Hippocampus | |||||
| CA3 | Area (mm2) | 100.3 ± 5.93 | 132.2 ± 16.8 | 0.124 | |
| Neuronal density (n/mm2) | 10.2 ± 0.30 | 8.5 ± 0.13 | 0.002 | ||
| DG | Area (mm2) | 144.6 ± 16.39 | 116.9 ± 11.55 | 0.216 | |
| Neuronal density (n/mm2) | 9.8 ± 0.85 | 11.5 ± 0.27 | 0.11 | ||
| Ventral | Neuronal density (n/mm2) | 7.0 ± 0.75 | 7.0 ± 0.23 | 0.957 | |
| RSC | |||||
| Dorsal | Neuronal density (n/mm2) | 7.4 ± 0.82 | 6.8 ± 0.77 | 0.769 |
| Test | Parameter | Thr92-Dio2 | Ala92-Dio2 | Thr92-Dio2 + T3 | Ala92-Dio2 + T3 |
|---|---|---|---|---|---|
| Open field | Distance (cm) | 1909 ± 305.3 | 1679 ± 200.9 | 1065 ± 37.7 | 942. ± 127.8 |
| Velocity (cm/s) | 4.74 ± 0.49 | 4.56 ± 0.51 | 5.52 ± 0.86 | 4.66 ± 0.56 | |
| Hole board | Visuospatial memory (% error) | 57.4 ± 3.5 | 50.3 ± 3.11 | 12.8 ± 7.14 a | 15.57 ± 6.16 b |
| Working memory (% error) | 0 ± 0 | 10.3 ± 1.7 c | 19.8 ± 5.49 d | 21.86 ± 5.27 e |
| Female | |||||
|---|---|---|---|---|---|
| Area | Parameter | Thr92-Dio2 | Ala92-Dio2 | p-Value | |
| CA1 | Area (mm2) | 128.5 ± 7.78 | 100.1 ± 8.22 | 0.041 | |
| Neuronal density (n/mm2) | 7.5 ± 0.46 | 9.5 ± 0.05 | 0.008 | ||
| CA2 | Area (mm2) | 19.4 ± 1.93 | 16.53 ± 1.53 | 0.327 | |
| Neuronal density (n/mm2) | 6.9 ± 0.59 | 7.6 ± 0.29 | 0.480 | ||
| Hippocampus | |||||
| CA3 | Area (mm2) | 119.9 ± 10.1 | 95.17 ± 3.77 | 0.094 | |
| Neuronal density (n/mm2) | 7.7 ± 0.28 | 7.8 ± 0.08 | 0.657 | ||
| DG | Area (mm2) | 175 ± 12.67 | 123.2 ± 19.21 | 0.046 | |
| Neuronal density (n/mm2) | 8.2 ± 1.04 | 9.7 ± 1.26 | 0.381 | ||
| Ventral | Neuronal density (n/mm2) | 6,5 ± 0.35 | 6.4 ± 0.20 | 0.890 | |
| RSC | |||||
| Dorsal | Neuronal density (n/mm2) | 6.7 ± 0.36 | 6.2 ± 0.21 | 0.317 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorena, F.B.; Sato, J.M.; Coviello, B.M.; Arnold, A.J.T.; Batistuzzo, A.; Yamanouchi, L.M.; Dias Junior, E.; do Nascimento, B.P.P.; Fonseca, T.d.L.; Bianco, A.C.; et al. Age Worsens the Cognitive Phenotype in Mice Carrying the Thr92Ala-DIO2 Polymorphism. Metabolites 2022, 12, 629. https://doi.org/10.3390/metabo12070629
Lorena FB, Sato JM, Coviello BM, Arnold AJT, Batistuzzo A, Yamanouchi LM, Dias Junior E, do Nascimento BPP, Fonseca TdL, Bianco AC, et al. Age Worsens the Cognitive Phenotype in Mice Carrying the Thr92Ala-DIO2 Polymorphism. Metabolites. 2022; 12(7):629. https://doi.org/10.3390/metabo12070629
Chicago/Turabian StyleLorena, Fernanda B., Juliana M. Sato, Beatriz Martin Coviello, Alexandre J. T. Arnold, Alice Batistuzzo, Laís M. Yamanouchi, Eduardo Dias Junior, Bruna P. P. do Nascimento, Tatiana de L. Fonseca, Antonio C. Bianco, and et al. 2022. "Age Worsens the Cognitive Phenotype in Mice Carrying the Thr92Ala-DIO2 Polymorphism" Metabolites 12, no. 7: 629. https://doi.org/10.3390/metabo12070629
APA StyleLorena, F. B., Sato, J. M., Coviello, B. M., Arnold, A. J. T., Batistuzzo, A., Yamanouchi, L. M., Dias Junior, E., do Nascimento, B. P. P., Fonseca, T. d. L., Bianco, A. C., & Ribeiro, M. O. (2022). Age Worsens the Cognitive Phenotype in Mice Carrying the Thr92Ala-DIO2 Polymorphism. Metabolites, 12(7), 629. https://doi.org/10.3390/metabo12070629






