Extraction and Identification of Volatile Organic Compounds in Scentless Flowers of 14 Tillandsia Species Using HS-SPME/GC-MS
Abstract
1. Introduction
2. Results
2.1. Selection of the SPME Fiber
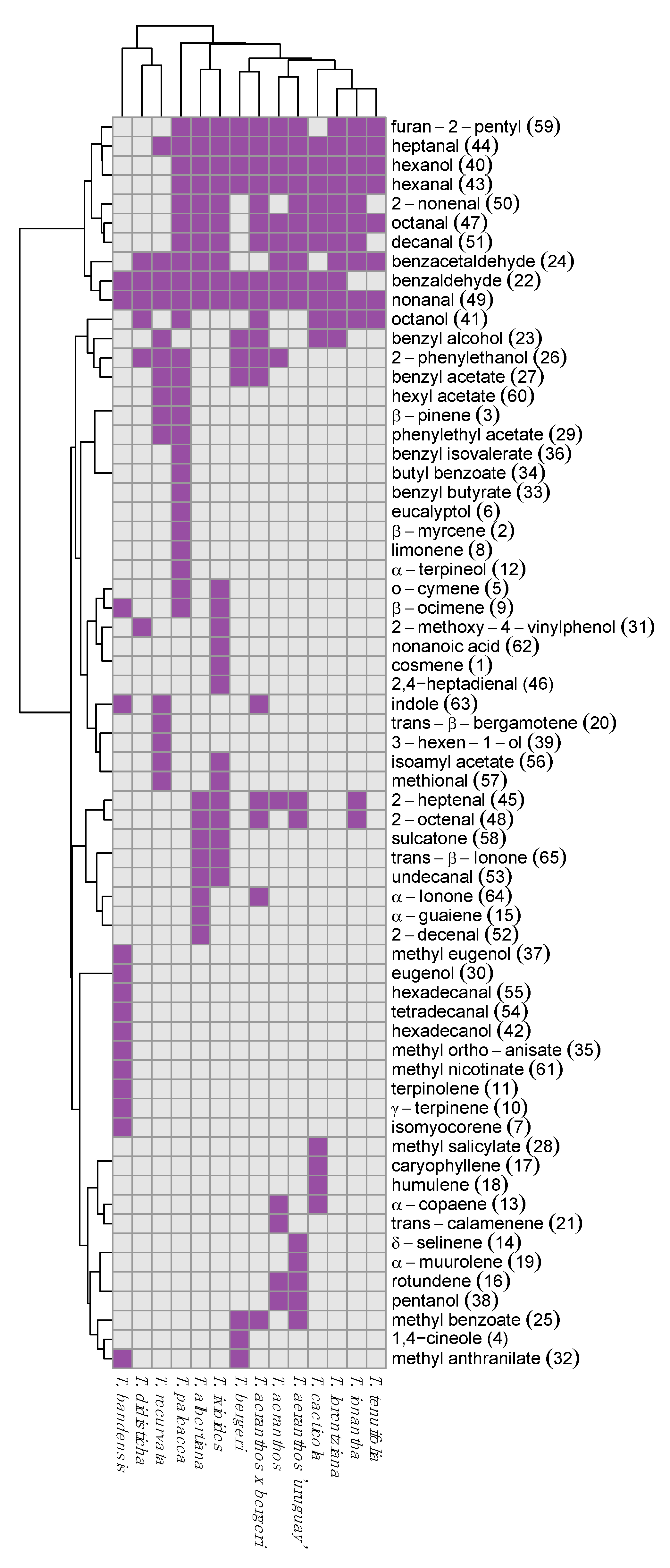
2.2. Identification
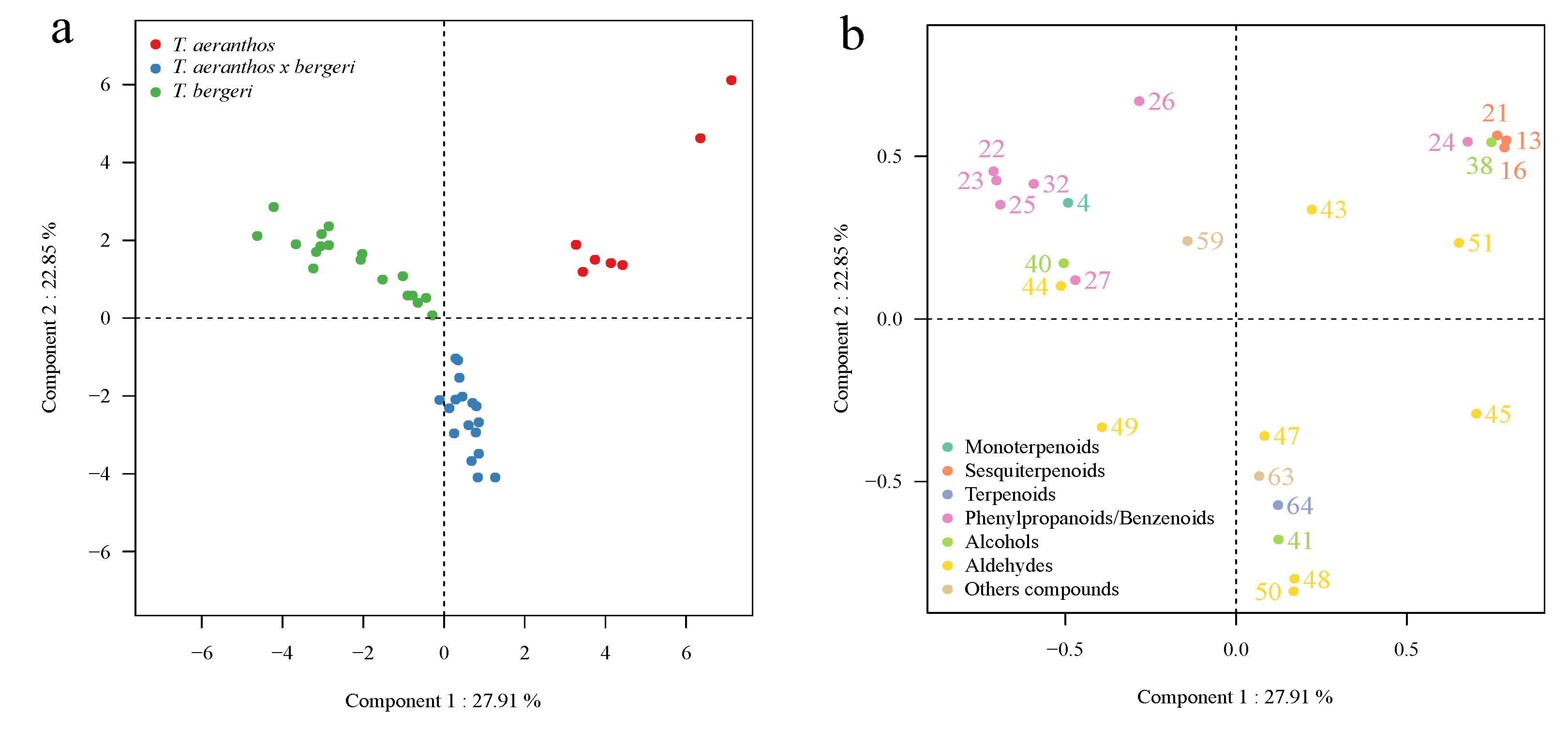
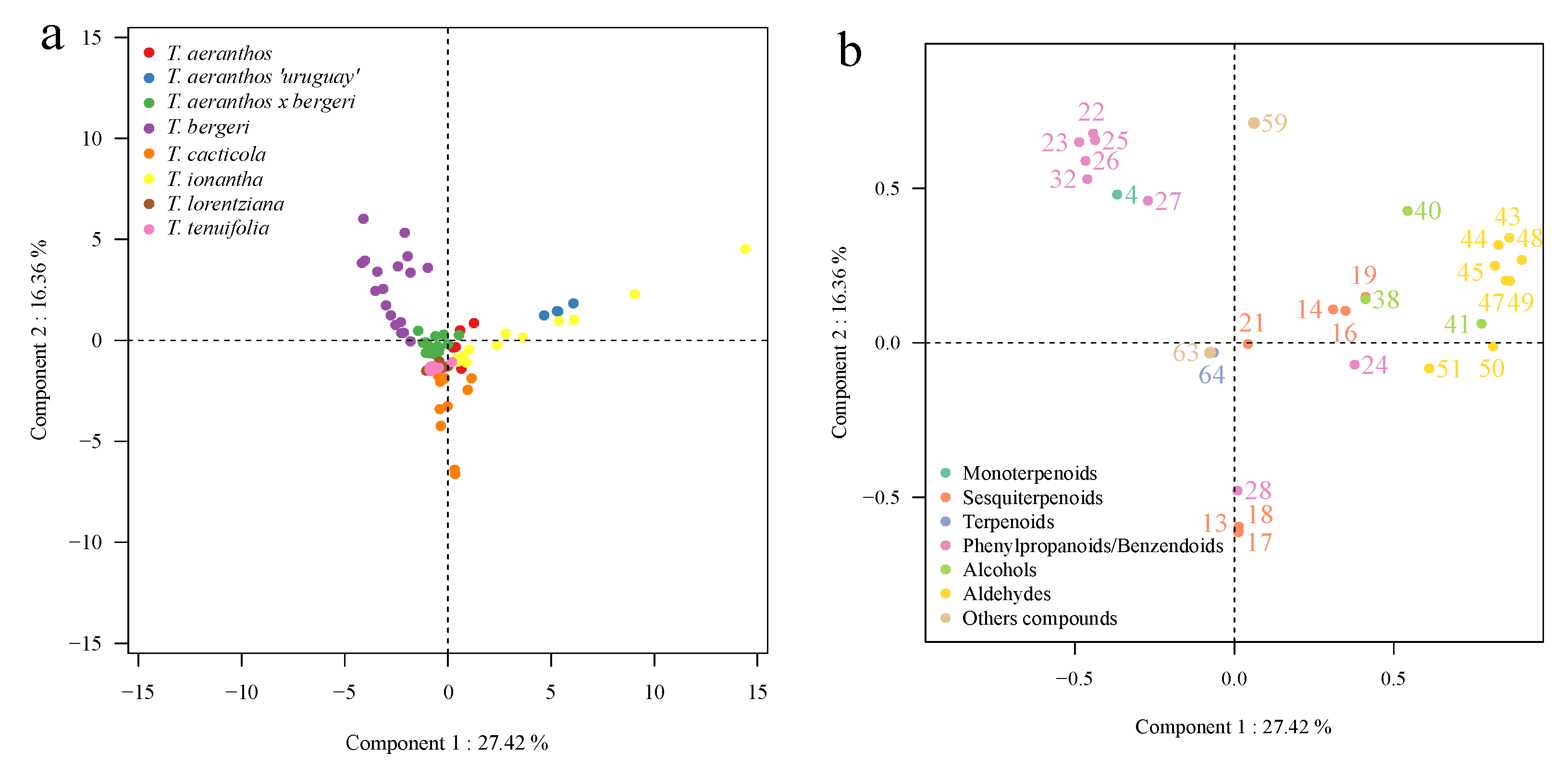
2.3. Chemometric Analysis of Volatile Organic Compounds
3. Discussion
4. Materials and Methods
4.1. Plant Material and Chemicals
4.2. HS-SPME Conditions
4.3. Instrumentation and GC-MS Conditions
4.4. Identification
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Estrella-Parra, E.; Flores-Cruz, M.; Blancas-Flores, G.; Koch, S.D.; Alarcón-Aguilar, F.J. The Tillandsia Genus: History, Uses, Chemistry, and Biological Activity. Bol. Latinoam. Caribe Pl. 2019, 18, 239–264. [Google Scholar]
- Dudareva, N.; Negre, F.; Nagegowda, D.A.; Orlova, I. Plant Volatiles: Recent Advances and Future Perspectives. CRC. Crit. Rev. Plant Sci. 2006, 25, 417–440. [Google Scholar] [CrossRef]
- Vivaldo, G.; Masi, E.; Taiti, C.; Caldarelli, G.; Mancuso, S. The Network of Plants Volatile Organic Compounds. Sci. Rep. 2017, 7, 11050. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Yu, C.; Cheng, B.; Wan, H.; Luo, L.; Pan, H.; Zhang, Q. Volatile Compound Analysis and Aroma Evaluation of Tea-Scented Roses in China. Ind. Crops Prod. 2020, 155, 112735. [Google Scholar] [CrossRef]
- Eggli, U.; Gouda, E.J. Monocotyledons, Tillandsia Bromeliaceae. In Illustrated Handbook of Succulent Plants; Springer: Berlin, Germany, 2020; pp. 1107–1164. ISBN 9783662564868. [Google Scholar]
- Eggli, U.; Gouda, E.J. Monocotyledons, Bromeliaceae. In Illustrated Handbook of Succulent Plants; Springer: Berlin, Germany, 2020; pp. 835–847. ISBN 9783662564868. [Google Scholar]
- De Queiroga, M.A.; De Andrade, L.M.; Florêncio, K.C.; Agra, M.D.F.; Da Silva, M.S.; Barbosa-Filho, J.M.; Leitão Da-Cunha, E.V. Chemical Constituents from Tillandsia recurvata. Fitoterapia 2004, 75, 423–425. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Chardi, A. Biomonitoring Potential of Five Sympatric Tillandsia Species for Evaluating Urban Metal Pollution (Cd, Hg and Pb). Atmos. Environ. 2016, 131, 352–359. [Google Scholar] [CrossRef]
- Wannaz, E.D.; Pignata, M.L. Calibration of Four Species of Tillandsia as Air Pollution Biomonitors. J. Atmos. Chem. 2006, 53, 185–209. [Google Scholar] [CrossRef]
- Zheng, G.; Zhang, R.; Zhou, F.; Li, P. Foliar Uptake and Transport of Atmospheric Trace Metals Bounded on Particulate Matters in Epiphytic Tillandsia brachycaulos. Int. J. Phytoremediat. 2020, 23, 400–406. [Google Scholar] [CrossRef]
- Bouwmeester, H.; Schuurink, R.C.; Bleeker, P.M.; Schiestl, F. The Role of Volatiles in Plant Communication. Plant J. 2019, 100, 892–907. [Google Scholar] [CrossRef]
- De Simón, B.F.; Sanz, M.; Cadahía, E.; Poveda, P.; Broto, M. Chemical Characterization of Oak Heartwood from Spanish Forests of Quercus pyrenaica (Wild.) Ellagitannins, Low Molecular Weight Phenolic, and Volatile Compounds. J. Agric. Food Chem. 2006, 54, 8314–8321. [Google Scholar] [CrossRef]
- Shi, T.; Yue, Y.; Shi, M.; Chen, M.; Yang, X.; Wang, L. Exploration of Floral Volatile Organic Compounds in Six Typical Lycoris Taxa by GC-MS. Plants 2019, 8, 422. [Google Scholar] [CrossRef] [PubMed]
- Widjaja, R.; Craske, J.D.; Wootton, M. Comparative Studies on Volatile Components of Non-Fragrant and Fragrant Rices. J. Sci. Food Agric. 1996, 70, 151–161. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, K.; Kim, N.S.; Lee, D.S. Determination of Floral Fragrances of Rosa Hybrida Using Solid-Phase Trapping-Solvent Extraction and Gas Chromatography-Mass Spectrometry. J. Chromatogr. A 2000, 902, 389–404. [Google Scholar] [CrossRef]
- Knudsen, J.; Tollsten, L. Trends in Floral Scent Chemistry in Pollination Syndromes: Floral Scent Composition in Moth-Pollinated Taxa. Bot. J. Linn. Soc. 1993, 113, 263–284. [Google Scholar] [CrossRef]
- Lawson, D.A.; Chittka, L.; Whitney, H.M.; Rands, S.A. Bumblebees Distinguish Floral Scent Patterns, and Can Transfer These to Corresponding Visual Patterns. Proc. R. Soc. B Biol. Sci. 2018, 285, 20180661. [Google Scholar] [CrossRef] [PubMed]
- Shalit, M.; Shafir, S.; Larkov, O.; Bar, E.; Kaslassi, D.; Adam, Z.; Zamir, D.; Vainstein, A.; Weiss, D.; Ravid, U.; et al. Volatile Compounds Emitted by Rose Cultivars: Fragrance Perception by Man and Honeybees. Isr. J. Plant Sci. 2004, 52, 245–255. [Google Scholar] [CrossRef]
- Ayasse, M.; Schiestl, F.P.; Paulus, H.F.; Ibarra, F.; Francke, W. Pollinator Attraction in a Sexually Deceptive Orchid by Means of Unconventional Chemicals. Proc. R. Soc. B Biol. Sci. 2003, 270, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Schiestl, F.P.; Peakall, R.; Mant, J. Chemical Communication in the Sexually Deceptive Orchid Genus Cryptostylis. Bot. J. Linn. Soc. 2004, 144, 199–205. [Google Scholar] [CrossRef]
- Aguilar-Rodríguez, P.A.; MacSwiney, G.M.C.; Krömer, T.; García-Franco, J.G.; Knauer, A.; Kessler, M. First Record of Bat-Pollination in the Species-Rich Genus Tillandsia (Bromeliaceae). Ann. Bot. 2014, 113, 1047–1055. [Google Scholar] [CrossRef]
- Du, F.; Wang, T.; Fan, J.M.; Liu, Z.Z.; Zong, J.X.; Fan, W.X.; Han, Y.H.; Grierson, D. Volatile Composition and Classification of Lilium Flower Aroma Types and Identification, Polymorphisms, and Alternative Splicing of Their Monoterpene Synthase Genes. Hortic. Res. 2019, 6, 1–15. [Google Scholar] [CrossRef]
- Knudsen, J.T.; Tollsten, L.; Bergstrom, L.G. Floral Scents—A Checklist of Volatile Compounds Isolated by Head-Space Techniques. Phytochemistry 1993, 33, 253–280. [Google Scholar] [CrossRef]
- Stashenko, E.E.; Martínez, J.R. Sampling Flower Scent for Chromatographic Analysis. J. Sep. Sci. 2008, 31, 2022–2031. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wang, J.; Li, D. Microextraction Techniques for the Determination of Volatile and Semivolatile Organic Compounds from Plants: A Review. Anal. Chim. Acta 2013, 799, 8–22. [Google Scholar] [CrossRef]
- Lo, M.M.; Benfodda, Z.; Bénimélis, D.; Fontaine, J.X.; Molinié, R.; Meffre, P. Development of a HS-SPME/GC-MS Method for the Extraction and Identification of the Volatile Compounds Emitted by Flowers of Tillandsia xiphioides. ACS Omega 2021, 6, 12691–12698. [Google Scholar] [CrossRef]
- Lo, M.M.; Benfodda, Z.; Bénimélis, D.; Fontaine, J.X.; Molinié, R.; Meffre, P. Extraction and Identification of Volatile Organic Compounds Emitted by Fragrant Flowers of Three Tillandsia Species by Hs-Spme/Gc-Ms. Metabolites 2021, 11, 594. [Google Scholar] [CrossRef] [PubMed]
- Gerlach, G.; Schill, R. Composition of Orchid Scents Attracting Euglossine Bees. Bot. Acta 1991, 104, 379–384. [Google Scholar] [CrossRef]
- Bicchi, C.; Drigo, S.; Rubiolo, P. Influence of Fibre Coating in Headspace Solid-Phase Microextraction-Gas Chromatographic Analysis of Aromatic and Medicinal Plants. J. Chromatogr. A 2000, 892, 469–485. [Google Scholar] [CrossRef]
- Song, G.; Xiao, J.; Deng, C.; Zhang, X.; Hu, Y. Use of Solid-Phase Microextraction as a Sampling Technique for the Characterization of Volatile Compounds Emitted from Chinese Daffodil Flowers. J. Anal. Chem. 2007, 62, 674–679. [Google Scholar] [CrossRef]
- Aline Raynal-Roques, A.R. Les Tillandsia et Les Racinaea; Belin: Paris, France, 2002; ISBN 2701128218. [Google Scholar]
- Aline Raynal-Roques, A.R. (Ed.) Les Broméliacées; Belin: Paris, France, 2016; ISBN 2701164737. [Google Scholar]
- Démares, F.; Gibert, L.; Creusot, P.; Lapeyre, B.; Proffit, M. Acute Ozone Exposure Impairs Detection of Floral Odor, Learning, and Memory of Honey Bees, through Olfactory Generalization. Sci. Total Environ. 2022, 827, 154342. [Google Scholar] [CrossRef]
- Picazo-Aragonés, J.; Terrab, A.; Balao, F. Plant Volatile Organic Compounds Evolution: Transcriptional Regulation, Epigenetics and Polyploidy. Int. J. Mol. Sci. 2020, 21, 8956. [Google Scholar] [CrossRef]
- Sáyago, R.; Quesada, M.; Aguilar, R.; Ashworth, L.; Lopezaraiza-Mikel, M.; Martén-Rodríguez, S. Consequences of Habitat Fragmentation on the Reproductive Success of Two Tillandsia Species with Contrasting Life History Strategies. AoB Plants 2018, 10, ply038. [Google Scholar] [CrossRef] [PubMed]
- Ramírez Morillo, I.M.; May, F.C.; Carnevali, G.; Pat, F.M. It Takes Two to Tango: Self Incompatibility in the Bromeliad Tillandsia streptophylla (Bromeliaceae) in Mexico. Rev. Biol. Trop. 2009, 57, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Neves, B.; Kessous, I.M.; Moura, R.L.; Couto, D.R.; Zanella, C.M.; Antonelli, A.; Bacon, C.D.; Salgueiro, F.; Costa, A.F. Pollinators Drive Floral Evolution in an Atlantic Forest Genus. AoB Plants 2021, 12, plaa046. [Google Scholar] [CrossRef] [PubMed]
- Burkle, L.A.; Runyon, J.B. The Smell of Environmental Change: Using Floral Scent to Explain Shifts in Pollinator Attraction. Appl. Plant Sci. 2017, 5, 1600123. [Google Scholar] [CrossRef]
- Krömer, T.; Kessler, M.; Lohaus, G.; Schmidt-Lebuhn, A.N. Nectar Sugar Composition and Concentration in Relation to Pollination Syndromes in Bromeliaceae. Plant Biol. 2008, 10, 502–511. [Google Scholar] [CrossRef]
- Mosti, S.; Friedman, C.R.; Pacini, E.; Brighigna, L.; Papini, A. Nectary Ultrastructure and Secretory Modes in Three Species of Tillandsia (Bromeliaceae) That Have Different Pollinators. Botany 2013, 91, 786–798. [Google Scholar] [CrossRef]
- Knudsen, J.T.; Tollsten, L.; Groth, I.; Bergström, G.; Raguso, R.A. Trends in Floral Scent Chemistry in Pollination Syndromes: Floral Scent Composition in Hummingbird-Pollinated Taxa. Bot. J. Linn. Soc. 2004, 146, 191–199. [Google Scholar] [CrossRef]
- Zavala-Sánchez, M.A.; Pérez-Gutiérrez, S.; Pérez-González, C.; Sánchez-Saldivar, D.; Arias-García, L. Antidiarrhoeal Activity of Nonanal, an Aldehyde Isolated from Artemisia ludoviciana. Pharm. Biol. 2002, 40, 263–268. [Google Scholar] [CrossRef]
- Zhang, J.H.; Sun, H.L.; Chen, S.Y.; Zeng, L.I.; Wang, T.T. Anti-Fungal Activity, Mechanism Studies on α-Phellandrene and Nonanal against Penicillium cyclopium. Bot. Stud. 2017, 58, 13. [Google Scholar] [CrossRef]
- Bisignano, G.; Laganà, M.G.; Trombetta, D.; Arena, S.; Nostro, A.; Uccella, N.; Mazzanti, G.; Saija, A. In Vitro Antibacterial Activity of Some Aliphatic Aldehydes from Olea europaea L. FEMS Microbiol. Lett. 2001, 198, 9–13. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, Y.; Wu, K.; Xi, W.G.; Yu, Y.G. Field-Testing of Synthetic Herbivore-Induced Plant Volatiles as Attractants for Beneficial Insects. Environ. Entomol. 2008, 37, 1410–1415. [Google Scholar] [CrossRef] [PubMed]
- Trombetta, D.; Saija, A.; Bisignano, G.; Arena, S.; Caruso, S.; Mazzanti, G.; Uccella, N.; Castelli, F. Study on the Mechanisms of the Antibacterial Action of Some Plant α,β-Unsaturated Aldehydes. Lett. Appl. Microbiol. 2002, 35, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Cha, D.H.; Roh, G.H.; Hesler, S.P.; Wallingford, A.; Stockton, D.G.; Park, S.K.; Loeb, G.M. 2-Pentylfuran: A Novel Repellent of Drosophila suzukii. Pest. Manag. Sci. 2021, 77, 1757–1764. [Google Scholar] [CrossRef]
- Lee, S.-E.; Yun, M.-S.; Yeon, B.-R.; Choi, J.-S.; Cho, N.-K.; Hwang, K.-H.; Wang, H.-Y.; Kim, S.-M. Herbicidal Activity of Benzaldehyde in Cajuput (Melaleuca cajeputi) Essential Oil. Korean J. Weed Sci. 2010, 30, 191–198. [Google Scholar] [CrossRef][Green Version]
| Name | T.aeranthos | T. bergeri | T. aeranthos x bergeri | T. aeranthos ‘uruguay’ | T. albertiana |
 |  |  |  |  | |
| Name | T. ixioides | T. ionantha | T. tenuifolia | T. paleacea | T. cacticola |
 |  |  |  |  | |
| Name | T. lorentziana | T. didisticha | T. bandensis | T. recurvata | |
 |  |  |  |
| Area (× 103) | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| # | Family | Compounds | RT (min) | RI | Aeranthos | Bergeri | Aeranthos x bergeri | Aeranthos ‘uruguay’ | Albertiana | Ixioides | Ionantha | Tenuifolia | Paleacea | Cacticola | Lorentziana | Didisticha | Bandensis | Recurvata |
| 1 | M | cosmene a,c | 17.26 | 968 | nd | nd | nd | nd | nd | 32.34 | nd | nd | nd | nd | nd | nd | nd | nd |
| 2 | M | β-myrcene a,e | 17.64 | 978 | nd | nd | nd | nd | nd | nd | nd | nd | 8.76 | nd | nd | nd | nd | nd |
| 3 | M | β-pinene a,e | 17.68 | 980 | nd | nd | nd | nd | nd | nd | nd | nd | 27.42 | nd | nd | nd | nd | 35.20 |
| 4 | M | 1,4-cineole a,f | 17.77 | 982 | nd | 110.47 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| 5 | M | o-cymene a,d | 18.67 | 1006 | nd | nd | nd | nd | nd | 44.35 | nd | nd | 60.47 | nd | nd | nd | nd | nd |
| 6 | M | eucalyptol a,e | 19.43 | 1026 | nd | nd | nd | nd | nd | nd | nd | nd | 1774.46 | nd | nd | nd | nd | nd |
| 7 | M | isomyocorene a,c | 19.09 | 1017 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 165.23 | nd |
| 8 | M | limonene a,e | 19.27 | 1022 | nd | nd | nd | nd | nd | nd | nd | nd | 192.85 | nd | nd | nd | nd | nd |
| 9 | M | β-ocimene a,f | 19.75 | 1035 | nd | nd | nd | nd | nd | 464.37 | nd | nd | 153.83 | nd | nd | nd | 4637.66 | nd |
| 10 | M | γ-terpinene a,e | 20.57 | 1056 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 174.52 | nd |
| 11 | M | terpinolene a,e | 21.48 | 1081 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 138.67 | nd |
| 12 | M | α-terpineol b,e | 26.07 | 1204 | nd | nd | nd | nd | nd | nd | nd | nd | 87.03 | nd | nd | nd | nd | nd |
| 13 | S | α-copaene b,d | 31.18 | 1374 | 90.65 | nd | nd | nd | nd | nd | nd | nd | nd | 824.63 | nd | nd | nd | nd |
| 14 | S | δ-selinene a,c | 31.31 | 1379 | nd | nd | nd | 22.09 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| 15 | S | α-guaiene b,d | 32.00 | 1406 | nd | nd | nd | nd | 1067.07 | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| 16 | S | rotundene b,c | 32.71 | 1439 | 424.64 | nd | nd | 671.00 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| 17 | S | caryophyllene b,d | 32.91 | 1448 | nd | nd | nd | nd | nd | nd | nd | nd | nd | 1413.36 | nd | nd | nd | nd |
| 18 | S | humulene b,d | 33.51 | 1475 | nd | nd | nd | nd | nd | nd | nd | nd | nd | 728.64 | nd | nd | nd | nd |
| 19 | S | α-muurolene b,d | 33.69 | 1484 | nd | nd | nd | 226.09 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| 20 | S | trans-β-bergamotene b,d | 32.72 | 1439 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 58.06 |
| 21 | S | trans-calamenene b,d | 33.91 | 1494 | 38.73 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| 22 | P/B | benzaldehyde b,e | 16.33 | 943 | 133.82 | 4031.24 | 385.83 | 193.67 | 400.61 | 1132.68 | nd | nd | 81.53 | 326.21 | 137.91 | 160.00 | 299.20 | 2249.88 |
| 23 | P/B | benzyl alcohol b,e | 19.09 | 1017 | nd | 13875.31 | 1743.88 | nd | nd | nd | nd | nd | nd | 1163.51 | 84.46 | nd | nd | 7269.54 |
| 24 | P/B | benzacetaldehyde b,e | 19.57 | 1029 | 193.65 | nd | nd | 258.54 | 620.21 | 9030.12 | 184.75 | 274.36 | 224.50 | nd | 220.17 | 342.89 | nd | 210.03 |
| 25 | P/B | methyl benzoate a,e | 20.70 | 1060 | nd | 18082.32 | 3736.94 | 37.51 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| 26 | P/B | 2-phenylethanol b,e | 21.72 | 1087 | 207.02 | 317.23 | 93.45 | nd | nd | nd | nd | nd | 92.60 | nd | nd | 222.72 | nd | 437.85 |
| 27 | P/B | benzyl acetate b,e | 23.37 | 1131 | nd | 123.90 | 52.76 | nd | nd | nd | nd | nd | 52.05 | nd | nd | nd | nd | 4502.82 |
| 28 | P/B | methyl salicylate b,e | 25.75 | 1195 | nd | nd | nd | nd | nd | nd | nd | nd | nd | 807.36 | nd | nd | nd | nd |
| 29 | P/B | phenylethyl acetate b,e | 27.64 | 1252 | nd | nd | nd | nd | nd | nd | nd | nd | 155.02 | nd | nd | nd | nd | 778.30 |
| 30 | P/B | Eugenol b,e | 29.70 | 1318 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 50.60 | nd |
| 31 | P/B | 2-methoxy-4-vinylphenol b,e | 29.74 | 1316 | nd | nd | nd | nd | nd | 40.77 | nd | nd | nd | nd | nd | 98.30 | nd | nd |
| 32 | P/B | methyl anthranilate b,d | 29.94 | 1327 | nd | 85.02 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 52.00 | nd |
| 33 | P/B | benzyl butyrate b,c | 29.99 | 1329 | nd | nd | nd | nd | nd | nd | nd | nd | 23.80 | nd | nd | nd | nd | nd |
| 34 | P/B | butyl benzoate b,c | 30.80 | 1360 | nd | nd | nd | nd | nd | nd | nd | nd | 111.80 | nd | nd | nd | nd | nd |
| 35 | P/B | methyl ortho-anisate b,c | 31.16 | 1368 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 5918.75 | nd |
| 36 | P/B | benzyl isovalerate b,c | 31.31 | 1375 | nd | nd | nd | nd | nd | nd | nd | nd | 96.73 | nd | nd | nd | nd | nd |
| 37 | P/B | methyl eugenol b,e | 31.77 | 1397 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 55.32 | nd |
| 38 | Al | pentanol b,f | 9.34 | - | 105.89 | nd | nd | 354.79 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| 39 | Al | 3-hexen-1-ol b,c | 12.42 | 839 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 173.37 |
| 40 | Al | hexanol b,e | 12.74 | 847 | 45.21 | 326.40 | 141.18 | 401.11 | 1138.06 | 312.20 | 524.56 | 140.16 | 75.12 | 306.41 | 26.78 | nd | nd | nd |
| 41 | Al | octanol b,e | 20.80 | 1063 | nd | nd | 73.07 | nd | nd | nd | 871.99 | 175.51 | 43.54 | 234.54 | 107.55 | 26.53 | nd | nd |
| 42 | Al | hexadecanol b,c | 37.26 | 1676 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 92.36 | nd |
| 43 | A | hexanal b,e | 10.15 | - | 832.91 | 394.28 | 354.29 | 1906.08 | 3379.54 | 1969.27 | 1445.37 | 326.61 | 158.25 | 456.87 | 373.34 | nd | nd | nd |
| 44 | A | heptanal b,e | 14.27 | 888 | 98.78 | 657.01 | 415.61 | 2468.93 | 6175.15 | 1620.57 | 2714.32 | 415.83 | 103.58 | 996.29 | 89.93 | nd | nd | 110.01 |
| 45 | A | 2-heptenal b,e | 15.95 | 933 | 41.32 | nd | 25.90 | 66.35 | 124.46 | 169.04 | 43.41 | nd | nd | nd | nd | nd | nd | nd |
| 46 | A | 2,4-heptadienal b,d | 17.36 | 971 | nd | nd | nd | nd | nd | 46.91 | nd | nd | nd | nd | nd | nd | nd | nd |
| 47 | A | octanal b,e | 17.95 | 987 | 10.21 | nd | 189.91 | 812.11 | 734.32 | 143.54 | 1396.31 | 77.24 | 73.62 | 239.37 | 150.42 | nd | nd | nd |
| 48 | A | 2-octenal b,e | 20.40 | 1052 | nd | nd | 26.05 | 136.31 | 180.46 | 162.36 | 118.13 | nd | nd | nd | nd | nd | nd | nd |
| 49 | A | nonanal b,e | 21.91 | 1092 | 40.12 | 240.81 | 351.16 | 2145.51 | 2451.38 | 1352.68 | 4072.88 | 484.48 | 266.99 | 1007.57 | 462.02 | 208.01 | 47.73 | 201.69 |
| 50 | A | 2-nonenal b,f | 23.62 | 1138 | nd | nd | 20.61 | 105.50 | 181.02 | 153.78 | 88.97 | nd | 5.25 | 64.20 | 27.86 | nd | nd | nd |
| 51 | A | decanal b,e | 25.58 | 1191 | 111.81 | nd | 19.33 | 76.57 | 422.97 | 140.92 | 88.81 | nd | 16.56 | 42.47 | 45.78 | nd | nd | nd |
| 52 | A | 2-decenal b,c | 26.93 | 1231 | nd | nd | nd | nd | 99.98 | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| 53 | A | undecanal b,e | 28.23 | 1270 | nd | nd | nd | nd | 62.21 | 26.70 | nd | nd | nd | nd | nd | nd | nd | nd |
| 54 | A | tetradecanal b,d | 36.17 | 1612 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 184.12 | nd |
| 55 | A | hexadecanal b,d | 39.51 | 1819 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 478.86 | nd |
| 56 | O | isoamyl acetate a,e | 13.15 | 858 | nd | nd | nd | nd | nd | 107.82 | nd | nd | nd | nd | nd | nd | nd | 78.84 |
| 57 | O | methional b,d | 14.24 | 887 | nd | nd | nd | nd | nd | 2795.63 | nd | nd | nd | nd | nd | nd | nd | 40.75 |
| 58 | O | sulcatone b,c | 16.45 | 947 | nd | nd | nd | nd | 228.81 | 284.38 | nd | nd | nd | nd | nd | nd | nd | nd |
| 59 | O | furan-2-pentyl b,e | 17.20 | 967 | 185.82 | 219.40 | 193.96 | 230.93 | 151.65 | 482.68 | 133.69 | 36.03 | 32.06 | nd | 86.61 | nd | nd | nd |
| 60 | O | hexyl acetate a,e | 18.43 | 1000 | nd | nd | nd | nd | nd | nd | nd | nd | 14.96 | nd | nd | nd | nd | 188.81 |
| 61 | O | methyl nicotinate b,f | 23.62 | 1138 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 253.44 | nd |
| 62 | O | nonanoic acid b,e | 26.64 | 1222 | nd | nd | nd | nd | nd | 162.19 | nd | nd | nd | nd | nd | nd | nd | nd |
| 63 | O | Indole b,e | 27.92 | 1261 | nd | nd | 404.59 | nd | nd | nd | nd | nd | nd | nd | nd | nd | 1393.13 | 1113.55 |
| 64 | O | α-ionone b,e | 31.45 | 1384 | nd | nd | 76.52 | nd | 101.45 | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| 65 | O | trans-β-ionone b,d | 32.76 | 1441 | nd | nd | nd | nd | 551.77 | 108.04 | nd | nd | nd | nd | nd | nd | nd | nd |
| Number of detected compounds per species | 15 | 12 | 18 | 17 | 18 | 23 | 12 | 8 | 25 | 14 | 12 | 6 | 15 | 15 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonzalez, A.; Benfodda, Z.; Bénimélis, D.; Fontaine, J.-X.; Molinié, R.; Meffre, P. Extraction and Identification of Volatile Organic Compounds in Scentless Flowers of 14 Tillandsia Species Using HS-SPME/GC-MS. Metabolites 2022, 12, 628. https://doi.org/10.3390/metabo12070628
Gonzalez A, Benfodda Z, Bénimélis D, Fontaine J-X, Molinié R, Meffre P. Extraction and Identification of Volatile Organic Compounds in Scentless Flowers of 14 Tillandsia Species Using HS-SPME/GC-MS. Metabolites. 2022; 12(7):628. https://doi.org/10.3390/metabo12070628
Chicago/Turabian StyleGonzalez, Alexandre, Zohra Benfodda, David Bénimélis, Jean-Xavier Fontaine, Roland Molinié, and Patrick Meffre. 2022. "Extraction and Identification of Volatile Organic Compounds in Scentless Flowers of 14 Tillandsia Species Using HS-SPME/GC-MS" Metabolites 12, no. 7: 628. https://doi.org/10.3390/metabo12070628
APA StyleGonzalez, A., Benfodda, Z., Bénimélis, D., Fontaine, J.-X., Molinié, R., & Meffre, P. (2022). Extraction and Identification of Volatile Organic Compounds in Scentless Flowers of 14 Tillandsia Species Using HS-SPME/GC-MS. Metabolites, 12(7), 628. https://doi.org/10.3390/metabo12070628








