Fetuin-A in Activated Liver Macrophages Is a Key Feature of Non-Alcoholic Steatohepatitis
Abstract
1. Introduction
2. Results
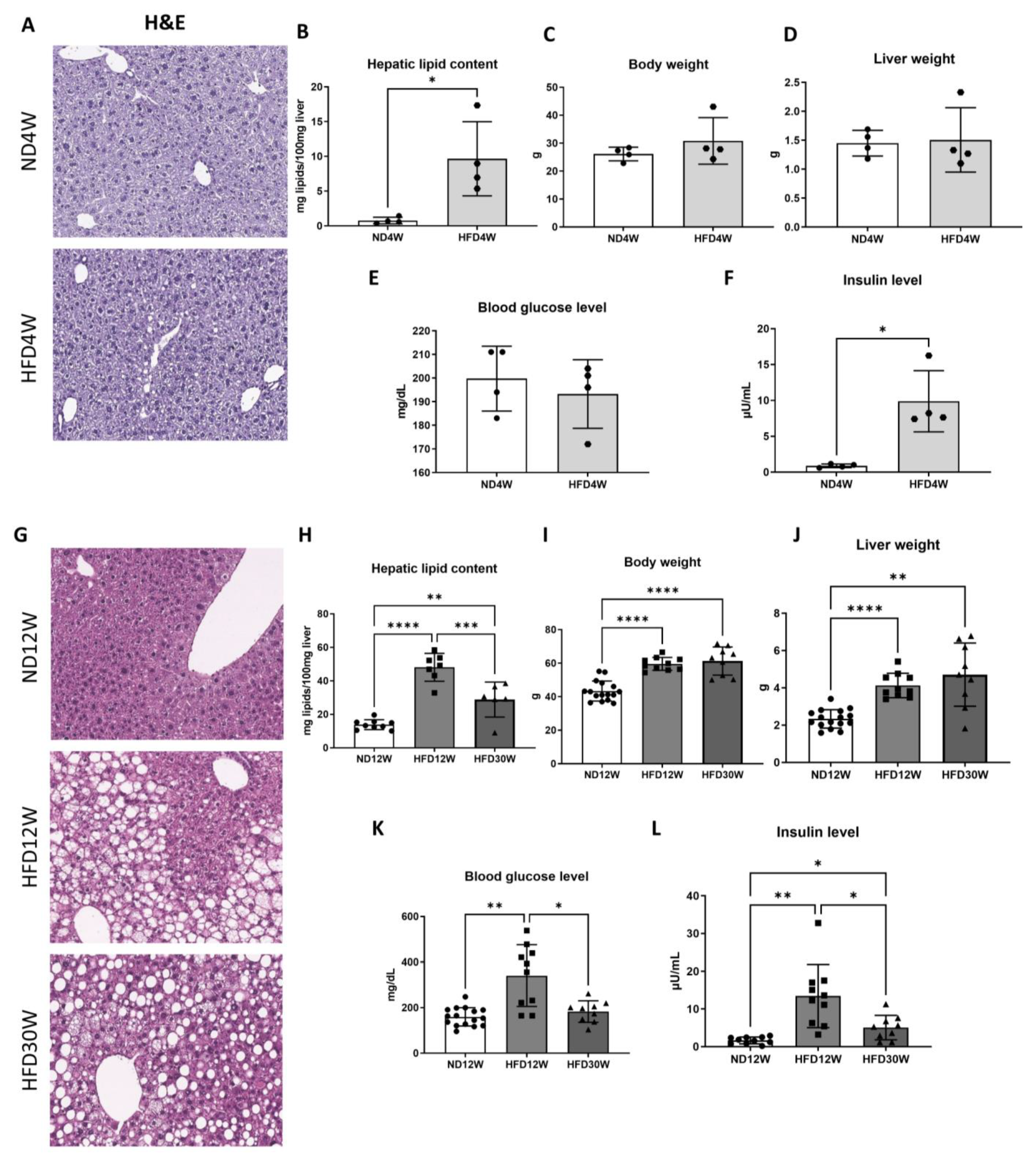
2.1. Liver Steatosis Appears Early under HFD in Foz−/− Mice and Progresses towards NASH
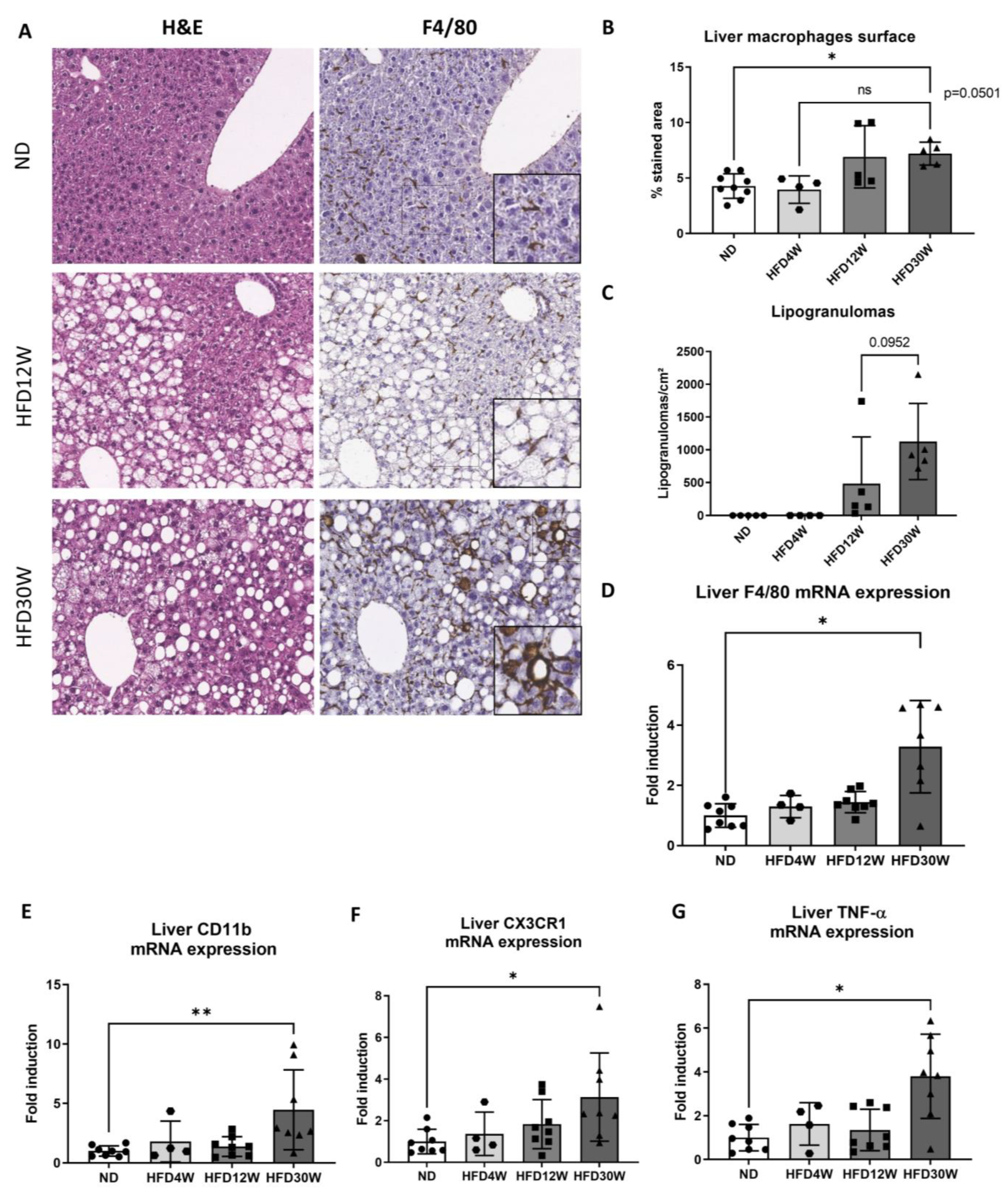
2.2. Macrophage Activation Occurs during MAFLD Progression
2.3. Serum Fetuin-A Level Increases during Steatosis Progression
2.4. Characterization of Fetuin-A: Liver Versus Adipose Tissue Content and Production
2.5. Histological Pattern of Fetuin-A during MAFLD/NASH Development
2.6. Validation of Results in Human MAFLD-NASH
3. Discussion
4. Materials and Methods
4.1. Animals and Diet
4.2. Human Data
4.3. Cell Culture Experiments
4.4. Evaluation of Liver Lipid Content
4.5. Histology, Immunohistochemistry, and Morphometrical Analysis
4.6. Fetuin-A Serum Concentration
4.7. Protein Studies: Western Blotting
4.8. RNA Extraction and PCR Analysis
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lanthier, N.; Leclercq, I.A. Adipose Tissues as Endocrine Target Organs. Best Pract. Res. Clin. Gastroenterol. 2014, 28, 545–558. [Google Scholar] [CrossRef]
- Samuel, V.T.; Liu, Z.X.; Qu, X.; Elder, B.D.; Bilz, S.; Befroy, D.; Romanelli, A.J.; Shulman, G.I. Mechanism of Hepatic Insulin Resistance in Non-Alcoholic Fatty Liver Disease. J. Biol. Chem. 2004, 279, 32345–32353. [Google Scholar] [CrossRef]
- Lanthier, N.; Molendi-Coste, O.; Horsmans, Y.; van Rooijen, N.; Cani, P.D.; Leclercq, I.A. Kupffer Cell Activation Is a Causal Factor for Hepatic Insulin Resistance. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 298, G107–G116. [Google Scholar] [CrossRef]
- Brøns, C.; Jensen, C.B.; Storgaard, H.; Hiscock, N.J.; White, A.; Appel, J.S.; Jacobsen, S.; Nilsson, E.; Larsen, C.M.; Astrup, A.; et al. Impact of Short-Term High-Fat Feeding on Glucose and Insulin Metabolism in Young Healthy Men. J. Physiol. 2009, 587, 2387–2397. [Google Scholar] [CrossRef]
- Francque, S.M.; Lanthier, N.; Verbeke, L.; Reynaert, H.; Van Steenkiste, C.; Vonghia, L.; Kwanten, W.J.; Weyler, J.; Trépo, E.; Cassiman, D.; et al. The Belgian Association for Study of the Liver Guidance Document on the Management of Adult and Paediatric Non-Alcoholic Fatty Liver Disease. Acta Gastro-Enterol. Belg. 2018, 81, 55–81. [Google Scholar]
- Lanthier, N.; Vanuytsel, T. Metabolic Dysfunction-Associated Fatty Liver Disease: A New Clearer Nomenclature with Positive Diagnostic Criteria. Acta Gastro-Enterol. Belg. 2020, 83, 513–515. [Google Scholar]
- Ortega, F.B.; Lavie, C.J.; Sui, X. Health Effects of Overweight and Obesity in 195 Countries. N. Engl. J. Med. 2017, 377, 1495–1497. [Google Scholar] [CrossRef]
- Albhaisi, S.; Chowdhury, A.; Sanyal, A.J. Non-Alcoholic Fatty Liver Disease in Lean Individuals. JHEP Rep. 2019, 1, 329–341. [Google Scholar] [CrossRef]
- Gonzalez-Cantero, J.; Martin-Rodriguez, J.L.; Gonzalez-Cantero, A.; Arrebola, J.P.; Gonzalez-Calvin, J.L. Insulin Resistance in Lean and Overweight Nondiabetic Caucasian Adults: Study of Its Relationship with Liver Triglyceride Content, Waist Circumference and BMI. PLoS ONE 2018, 13, e0192663. [Google Scholar] [CrossRef]
- Sheka, A.C.; Adeyi, O.; Thompson, J.; Hameed, B.; Crawford, P.A.; Ikramuddin, S. Nonalcoholic Steatohepatitis: A Review. JAMA 2020, 323, 1175–1183. [Google Scholar] [CrossRef]
- Parlakgül, G.; Arruda, A.P.; Pang, S.; Cagampan, E.; Min, N.; Güney, E.; Lee, G.Y.; Inouye, K.; Hess, H.F.; Xu, C.S.; et al. Regulation of Liver Subcellular Architecture Controls Metabolic Homeostasis. Nature 2022, 603, 736–742. [Google Scholar] [CrossRef]
- Kirpich, I.A.; Gobejishvili, L.N.; Homme, M.B.; Waigel, S.; Cave, M.; Arteel, G.; Barve, S.S.; McClain, C.J.; Deaciuc, I.V. Integrated Hepatic Transcriptome and Proteome Analysis of Mice with High-Fat Diet-Induced Nonalcoholic Fatty Liver Disease. J. Nutr. Biochem. 2011, 22, 38–45. [Google Scholar] [CrossRef]
- Meex, R.C.R.; Watt, M.J. Hepatokines: Linking Nonalcoholic Fatty Liver Disease and Insulin Resistance. Nat. Rev. Endocrinol. 2017, 13, 509–520. [Google Scholar] [CrossRef]
- Pal, D.; Dasgupta, S.; Kundu, R.; Maitra, S.; Das, G.; Mukhopadhyay, S.; Ray, S.; Majumdar, S.S.; Bhattacharya, S. Fetuin-A Acts as an Endogenous Ligand of TLR4 to Promote Lipid-Induced Insulin Resistance. Nat. Med. 2012, 18, 1279–1285. [Google Scholar] [CrossRef]
- Stefan, N.; Hennige, A.M.; Staiger, H.; Machann, J.; Schick, F.; Kröber, S.M.; Machicao, F.; Fritsche, A.; Häring, H.U. A2-Heremans-Schmid Glycoprotein/Fetuin—A Is Associated with Insulin Resistance and Fat Accumulation in the Liver in Humans. Diabetes Care 2006, 29, 853–857. [Google Scholar] [CrossRef]
- Jialal, I.; Devaraj, S.; Bettaieb, A.; Haj, F.; Adams-Huet, B. Increased Adipose Tissue Secretion of Fetuin-A, Lipopolysaccharide-Binding Protein and High-Mobility Group Box Protein 1 in Metabolic Syndrome. Atherosclerosis 2015, 241, 130–137. [Google Scholar] [CrossRef]
- Pérez-Sotelo, D.; Roca-Rivada, A.; Larrosa-García, M.; Castelao, C.; Baamonde, I.; Baltar, J.; Crujeiras, A.B.; Seoane, L.M.; Casanueva, F.F.; Pardo, M. Visceral and Subcutaneous Adipose Tissue Express and Secrete Functional Alpha2hsglycoprotein (Fetuin a) Especially in Obesity. Endocrine 2017, 55, 435–446. [Google Scholar] [CrossRef]
- Chatterjee, P.; Seal, S.; Mukherjee, S.; Kundu, R.; Mukherjee, S.; Ray, S.; Mukhopadhyay, S.; Majumdar, S.S.; Bhattacharya, S. Adipocyte Fetuin-A Contributes to Macrophage Migration into Adipose Tissue and Polarization of Macrophages. J. Biol. Chem. 2013, 288, 28324–28330. [Google Scholar] [CrossRef]
- Jirak, P.; Stechemesser, L.; Moré, E.; Franzen, M.; Topf, A.; Mirna, M.; Paar, V.; Pistulli, R.; Kretzschmar, D.; Wernly, B.; et al. Clinical Implications of Fetuin-A. Adv. Clin. Chem. 2019, 89, 79–130. [Google Scholar]
- Dadej, D.; Szczepanek-Parulska, E.; Ruchała, M. Interplay between Fatty Acid Binding Protein 4, Fetuin-A, Retinol Binding Protein 4 and Thyroid Function in Metabolic Dysregulation. Metabolites 2022, 12, 300. [Google Scholar] [CrossRef]
- Srinivas, P.R. Serum Alpha 2-HS-Glycoprotein Is an Inhibitor of the Human Insulin Receptor at the Tyrosine Kinase Level. Mol. Endocrinol. 1993, 7, 1445–1455. [Google Scholar] [CrossRef] [PubMed]
- Goustin, A.S.; Derar, N.; Abou-Samra, A.B. Ahsg-Fetuin Blocks the Metabolic Arm of Insulin Action through Its Interaction with the 95-KD β-Subunit of the Insulin Receptor. Cell Signal. 2013, 25, 981–988. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, S.; Bhattacharya, S.; Biswas, A.; Majumdar, S.S.; Mukhopadhyay, S.; Ray, S.; Bhattacharya, S. NF-ΚB Mediates Lipid-Induced Fetuin-A Expression in Hepatocytes That Impairs Adipocyte Function Effecting Insulin Resistance. Biochem. J. 2010, 429, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Mathews, S.T.; Singh, G.P.; Ranalletta, M.; Cintron, V.J.; Qiang, X.; Goustin, A.S.; Jen, K.L.C.; Charron, M.J.; Jahnen-Dechent, W.; Grunberger, G. Improved Insulin Sensitivity and Resistance to Weight Gain in Mice Null for the Ahsg Gene. Diabetes 2002, 51, 2450–2458. [Google Scholar] [CrossRef]
- Filardi, T.; Panimolle, F.; Tiberti, C.; Crescioli, C.; Lenzi, A.; Pallotta, N.; Morano, S. Circulating Levels of Fetuin-A Are Associated with Moderate–Severe Hepatic Steatosis in Young Adults. J. Endocrinol. Investig. 2021, 44, 105–110. [Google Scholar] [CrossRef]
- Peter, A.; Kovarova, M.; Staiger, H.; Machann, J.; Schick, F.; Königsrainer, A.; Königsrainer, I.; Schleicher, E.; Fritsche, A.; Häring, H.U.; et al. The Hepatokines Fetuin-A and Fetuin-B Are Upregulated in the State of Hepatic Steatosis and May Differently Impact on Glucose Homeostasis in Humans. Am. J. Physiol. Endocrinol. Metab. 2018, 314, E266–E273. [Google Scholar] [CrossRef]
- Sun, Q.; Cornelis, M.C.; Manson, J.A.E.; Hu, F.B. Plasma Levels of Fetuin-A and Hepatic Enzymes and Risk of Type 2 Diabetes in Women in the U.S. Diabetes 2013, 62, 49–55. [Google Scholar] [CrossRef][Green Version]
- Lumeng, C.N.; DeYoung, S.M.; Bodzin, J.L.; Saltiel, A.R. Increased Inflammatory Properties of Adipose Tissue Macrophages Recruited during Diet-Induced Obesity. Diabetes 2007, 56, 16–23. [Google Scholar] [CrossRef]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W. Obesity Is Associated with Macrophage Accumulation in Adipose Tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef]
- Lefere, S.; Tacke, F. Macrophages in Obesity and Non-Alcoholic Fatty Liver Disease: Crosstalk with Metabolism. JHEP Rep. 2019, 1, 30–43. [Google Scholar] [CrossRef]
- Lumeng, C.N.; Bodzin, J.L.; Saltiel, A.R. Obesity Induces a Phenotypic Switch in Adipose Tissue Macrophage Polarization. J. Clin. Investig. 2007, 117, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Yang, L.; van Rooijen, N.; Ohnishi, H.; Seki, E. Hepatic Recruitment of Macrophages Promotes Nonalcoholic Steatohepatitis through CCR2. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G1310–G1321. [Google Scholar] [CrossRef] [PubMed]
- Reid, D.T.; Reyes, J.L.; McDonald, B.A.; Vo, T.; Reimer, R.A.; Eksteen, B. Kupffer Cells Undergo Fundamental Changes during the Development of Experimental NASH and Are Critical in Initiating Liver Damage and Inflammation. PLoS ONE 2016, 11, e0159524. [Google Scholar] [CrossRef] [PubMed]
- Lanthier, N. Targeting Kupffer Cells in Non-Alcoholic Fatty Liver Disease/Non-Alcoholic Steatohepatitis: Why and How? World J. Hepatol. 2015, 7, 2184. [Google Scholar] [CrossRef] [PubMed]
- Kanda, H.; Tateya, S.; Tamori, Y.; Kotani, K.; Hiasa, K.I.; Kitazawa, R.; Kitazawa, S.; Miyachi, H.; Maeda, S.; Egashira, K.; et al. MCP-1 Contributes to Macrophage Infiltration into Adipose Tissue, Insulin Resistance, and Hepatic Steatosis in Obesity. J. Clin. Investig. 2006, 116, 1494–1505. [Google Scholar] [CrossRef] [PubMed]
- Gadd, V.L.; Skoien, R.; Powell, E.E.; Fagan, K.J.; Winterford, C.; Horsfall, L.; Irvine, K.; Clouston, A.D. The Portal Inflammatory Infiltrate and Ductular Reaction in Human Nonalcoholic Fatty Liver Disease. Hepatology 2014, 59, 1393–1405. [Google Scholar] [CrossRef]
- Poekes, L.; Legry, V.; Schakman, O.; Detrembleur, C.; Bol, A.; Horsmans, Y.; Farrell, G.C.; Leclercq, I.A. Defective Adaptive Thermogenesis Contributes to Metabolic Syndrome and Liver Steatosis in Obese Mice. Clin. Sci. 2017, 131, 285–296. [Google Scholar] [CrossRef]
- Arsov, T.; Larter, C.Z.; Nolan, C.J.; Petrovsky, N.; Goodnow, C.C.; Teoh, N.C.; Yeh, M.M.; Farrell, G.C. Adaptive Failure to High-Fat Diet Characterizes Steatohepatitis in Alms1 Mutant Mice. Biochem. Biophys. Res. Commun. 2006, 342, 1152–1159. [Google Scholar] [CrossRef]
- Farrell, G.C.; Mridha, A.R.; Yeh, M.M.; Arsov, T.; Van Rooyen, D.M.; Brooling, J.; Nguyen, T.; Heydet, D.; Delghingaro-Augusto, V.; Nolan, C.J.; et al. Strain Dependence of Diet-Induced NASH and Liver Fibrosis in Obese Mice Is Linked to Diabetes and Inflammatory Phenotype. Liver Int. 2014, 34, 1084–1093. [Google Scholar] [CrossRef]
- Binet, Q.; Loumaye, A.; Preumont, V.; Thissen, J.P.; Hermans, M.P.; Lanthier, N. Non-Invasive Screening, Staging and Management of Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD) in Type 2 Diabetes Mellitus Patients: What Do We Know so Far? Acta Gastroenterol. Belg. 2022, 85, 346–357. [Google Scholar] [CrossRef]
- Haczeyni, F.; Yeh, M.M.; Ioannou, G.N.; Leclercq, I.A.; Goldin, R.; Dan, Y.Y.; Yu, J.; Teoh, N.C.; Farrell, G.C. Mouse Models of Non-Alcoholic Steatohepatitis: A Reflection on Recent Literature. J. Gastroenterol. Hepatol. 2018, 33, 1312–1320. [Google Scholar] [CrossRef] [PubMed]
- Khadir, A.; Kavalakatt, S.; Madhu, D.; Hammad, M.; Devarajan, S.; Tuomilehto, J.; Tiss, A. Fetuin-A Levels Are Increased in the Adipose Tissue of Diabetic Obese Humans but Not in Circulation. Lipids Health Dis. 2018, 17, 291. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Xiao, J.; Zhao, Z.; Wang, M.; Wang, Y.; Xin, Y. Systematic Review and Meta-Analysis of Circulating Fetuin-A Levels in Nonalcoholic Fatty Liver Disease. J. Clin. Transl. Hepatol. 2020, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Kazankov, K.; Jørgensen, S.M.D.; Thomsen, K.L.; Møller, H.J.; Vilstrup, H.; George, J.; Schuppan, D.; Grønbæk, H. The Role of Macrophages in Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Bauche, I.B.; Ait El Mkadem, S.; Rezsohazy, R.; Funahashi, T.; Maeda, N.; Miranda, L.M.; Brichard, S.M. Adiponectin Downregulates Its Own Production and the Expression of Its AdipoR2 Receptor in Transgenic Mice. Biochem. Biophys. Res. Commun. 2006, 345, 1414–1424. [Google Scholar] [CrossRef] [PubMed]
- Lanthier, N.; Molendi-Coste, O.; Cani, P.D.; Rooijen, N.; Horsmans, Y.; Leclercq, I.A. Kupffer Cell Depletion Prevents but Has No Therapeutic Effect on Metabolic and Inflammatory Changes Induced by a High-fat Diet. FASEB J. 2011, 25, 4301–4311. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Etienne, Q.; Lebrun, V.; Komuta, M.; Navez, B.; Thissen, J.-P.; Leclercq, I.A.; Lanthier, N. Fetuin-A in Activated Liver Macrophages Is a Key Feature of Non-Alcoholic Steatohepatitis. Metabolites 2022, 12, 625. https://doi.org/10.3390/metabo12070625
Etienne Q, Lebrun V, Komuta M, Navez B, Thissen J-P, Leclercq IA, Lanthier N. Fetuin-A in Activated Liver Macrophages Is a Key Feature of Non-Alcoholic Steatohepatitis. Metabolites. 2022; 12(7):625. https://doi.org/10.3390/metabo12070625
Chicago/Turabian StyleEtienne, Quentin, Valérie Lebrun, Mina Komuta, Benoît Navez, Jean-Paul Thissen, Isabelle A. Leclercq, and Nicolas Lanthier. 2022. "Fetuin-A in Activated Liver Macrophages Is a Key Feature of Non-Alcoholic Steatohepatitis" Metabolites 12, no. 7: 625. https://doi.org/10.3390/metabo12070625
APA StyleEtienne, Q., Lebrun, V., Komuta, M., Navez, B., Thissen, J.-P., Leclercq, I. A., & Lanthier, N. (2022). Fetuin-A in Activated Liver Macrophages Is a Key Feature of Non-Alcoholic Steatohepatitis. Metabolites, 12(7), 625. https://doi.org/10.3390/metabo12070625





