The Plasma Oxylipidome Links Smoking Status to Peripheral Artery Disease
Abstract
:1. Summary
2. Results
2.1. Participant Characterstics
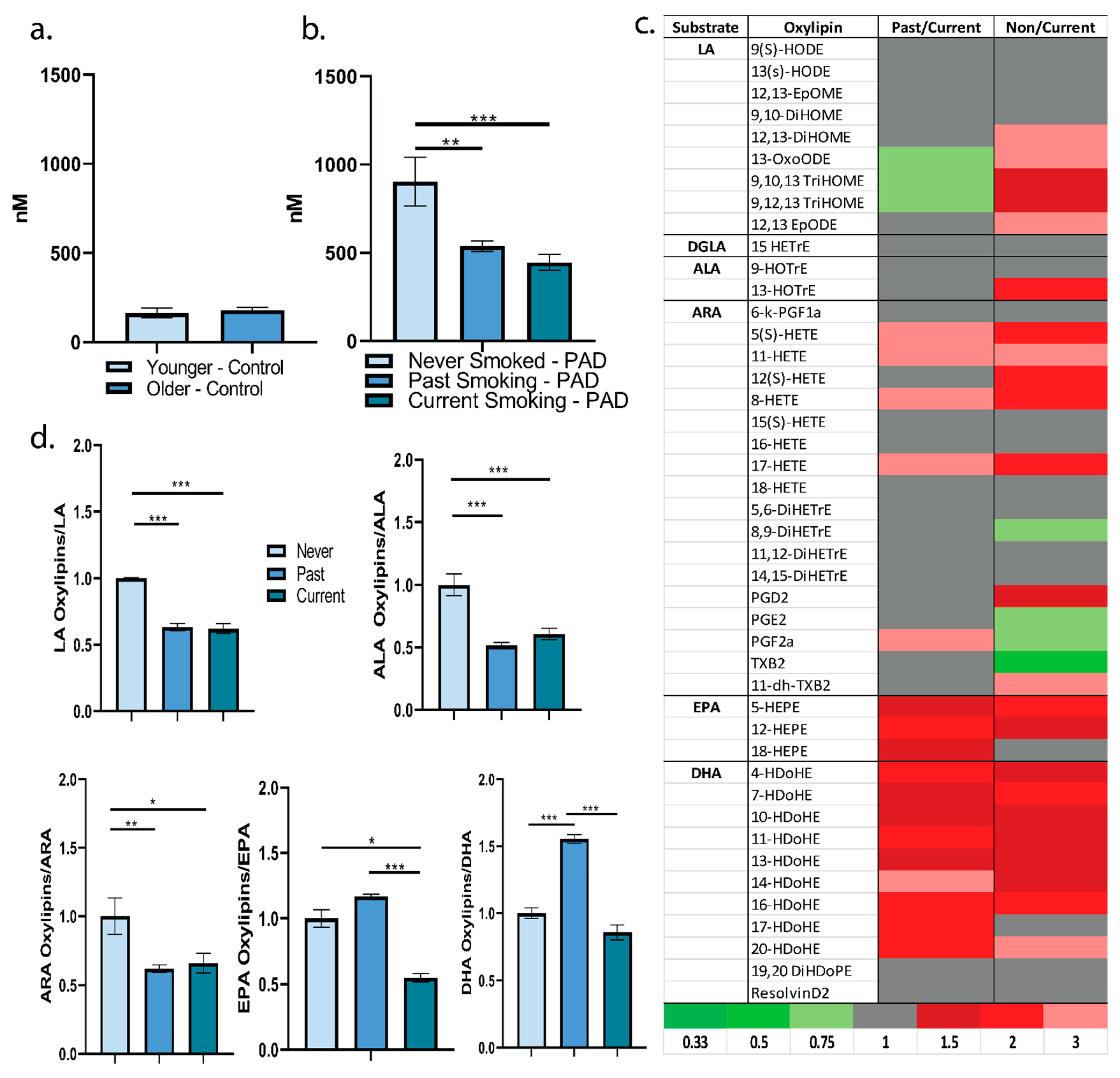
2.2. Plasma Oxylipin Profile
2.3. Oxidized Phosphatidylcholines
3. Discussion
3.1. Linking the Plasma Oxylipidome to PAD
3.2. Limitations
3.3. Conclusions
4. Methods
4.1. Clinical Trials
4.2. Oxylipidome
4.3. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Lu, L.; Mackay, D.F.; Pell, J.P. Meta-analysis of the association between cigarette smoking and peripheral arterial disease. Heart 2014, 100, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Messner, B.; Bernhard, D. Smoking and cardiovascular disease: Mechanisms of endothelial dysfunction and early atherogenesis. Arterioscler Thromb. Vasc. Biol. 2014, 34, 509–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caligiuri, S.P.B.; Parikh, M.; Stamenkovic, A.; Pierce, G.N.; Aukema, H.M. Dietary modulation of oxylipins in cardiovascular disease and aging. Am. J. Physiol. Heart Circ. Physiol. 2017, 313, H903–H918. [Google Scholar] [CrossRef] [Green Version]
- Gabbs, M.; Leng, S.; Devassy, J.G.; Monirujjaman, M.; Aukema, H.M. Advances in Our Understanding of Oxylipins Derived from Dietary PUFAs. Adv. Nutr. 2015, 6, 513–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawahara, K.; Hohjoh, H.; Inazumi, T.; Tsuchiya, S.; Sugimoto, Y. Prostaglandin E2-induced inflammation: Relevance of prostaglandin E receptors. Biochim. Biophys. Acta 2015, 1851, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Simmons, D.L.; Botting, R.M.; Hla, T. Cyclooxygenase isozymes: The biology of prostaglandin synthesis and inhibition. Pharmacol. Rev. 2004, 56, 387–437. [Google Scholar] [CrossRef] [Green Version]
- Solati, Z.; Surendran, A.; Edel, A.; Roznik, M.; Allen, D.; Ravandi, A. Increase in Plasma Oxidized Phosphatidylcholines (OxPCs) in Patients Presenting With ST-Elevation Myocardial Infarction (STEMI). Front. Med. (Lausanne) 2021, 8, 716944. [Google Scholar] [CrossRef]
- Caligiuri, S.P.B.; Aukema, H.M.; Ravandi, A.; Lavallée, R.; Guzman, R.; Pierce, G.N. Specific plasma oxylipins increase the odds of cardiovascular and cerebrovascular events in patients with peripheral artery disease. Can. J. Physiol. Pharmacol. 2017, 95, 961–968. [Google Scholar] [CrossRef] [Green Version]
- Caligiuri, S.P.; Aukema, H.M.; Ravandi, A.; Pierce, G.N. Elevated levels of pro-inflammatory oxylipins in older subjects are normalized by flaxseed consumption. Exp. Gerontol. 2014, 59, 51–57. [Google Scholar] [CrossRef]
- Caligiuri, S.P.; Rodriguez-Leyva, D.; Aukema, H.M.; Ravandi, A.; Weighell, W.; Guzman, R.; Pierce, G.N. Dietary Flaxseed Reduces Central Aortic Blood Pressure Without Cardiac Involvement but Through Changes in Plasma Oxylipins. Hypertension 2016, 68, 1031–1038. [Google Scholar] [CrossRef]
- Caligiuri, S.P.; Aukema, H.M.; Ravandi, A.; Guzman, R.; Dibrov, E.; Pierce, G.N. Flaxseed consumption reduces blood pressure in patients with hypertension by altering circulating oxylipins via an α-linolenic acid-induced inhibition of soluble epoxide hydrolase. Hypertension 2014, 64, 53–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kostelli, G.; Kourea, K.; Ikonomidis, I. Effects of combustible tobacco smoking and novel tobacco products on oxidative stress: Different sides of the same coin? Curr. Opin. Opin. Opin. Toxicol. 2020, 20–21, 41–47. [Google Scholar] [CrossRef]
- Edel, A.L.; Patenaude, A.F.; Richard, M.N.; Dibrov, E.; Austria, J.A.; Aukema, H.M.; Pierce, G.N.; Aliani, M. The effect of flaxseed dose on circulating concentrations of alpha-linolenic acid and secoisolariciresinol diglucoside derived enterolignans in young, healthy adults. Eur. J. Nutr. 2016, 55, 651–663. [Google Scholar] [CrossRef] [PubMed]
- Patenaude, A.; Rodriguez-Leyva, D.; Edel, A.L.; Dibrov, E.; Dupasquier, C.M.; Austria, J.A.; Richard, M.N.; Chahine, M.N.; Malcolmson, L.J.; Pierce, G.N. Bioavailability of alpha-linolenic acid from flaxseed diets as a function of the age of the subject. Eur. J. Clin. Nutr. 2009, 63, 1123–1129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gargalovic, P.S.; Imura, M.; Zhang, B.; Gharavi, N.M.; Clark, M.J.; Pagnon, J.; Yang, W.P.; He, A.; Truong, A.; Patel, S.; et al. Identification of inflammatory gene modules based on variations of human endothelial cell responses to oxidized lipids. Proc Natl. Acad. Sci. USA 2006, 103, 12741–12746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertoia, M.L.; Pai, J.K.; Lee, J.H.; Taleb, A.; Joosten, M.M.; Mittleman, M.A.; Yang, X.; Witztum, J.L.; Rimm, E.B.; Tsimikas, S.; et al. Oxidation-specific biomarkers and risk of peripheral artery disease. J. Am. Coll. Cardiol. 2013, 61, 2169–2179. [Google Scholar] [CrossRef] [Green Version]
- Caligiuri, S.P.; Love, K.; Winter, T.; Gauthier, J.; Taylor, C.G.; Blydt-Hansen, T.; Zahradka, P.; Aukema, H.M. Dietary linoleic acid and α-linolenic acid differentially affect renal oxylipins and phospholipid fatty acids in diet-induced obese rats. J. Nutr. 2013, 143, 1421–1431. [Google Scholar] [CrossRef] [Green Version]
- Panigrahy, D.; Gilligan, M.M.; Serhan, C.N.; Kashfi, K. Resolution of inflammation: An organizing principle in biology and medicine. Pharmacol. Ther. 2021, 227, 107879. [Google Scholar] [CrossRef]
- Serhan, C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature 2014, 510, 92–101. [Google Scholar] [CrossRef] [Green Version]
- Hu, S.; Luo, J.; Fu, M.; Luo, L.; Cai, Y.; Li, W.; Li, Y.; Dong, R.; Yang, Y.; Tu, L.; et al. Soluble epoxide hydrolase deletion attenuated nicotine-induced arterial stiffness via limiting the loss of SIRT1. Am. J. Physiol. Heart Circ. Physiol. 2021, 321, H353–H368. [Google Scholar] [CrossRef]
- Smith, K.R.; Pinkerton, K.E.; Watanabe, T.; Pedersen, T.L.; Ma, S.J.; Hammock, B.D. Attenuation of tobacco smoke-induced lung inflammation by treatment with a soluble epoxide hydrolase inhibitor. Proc. Natl. Acad. Sci. USA 2005, 102, 2186–2191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gentzke, A.S.; Wang, T.W.; Cornelius, M.; Park-Lee, E.; Ren, C.; Sawdey, M.D.; Cullen, K.A.; Loretan, C.; Jamal, A.; Homa, D.M. Tobacco Product Use and Associated Factors Among Middle and High School Students-National Youth Tobacco Survey, United States, 2021. MMWR Surveill Summ 2022, 71, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, Y.; Sisler, J.D.; Shaffer, J.; Leonard, S.S.; Morris, A.M.; Qian, Y.; Bello, D.; Demokritou, P. Assessment of reactive oxygen species generated by electronic cigarettes using acellular and cellular approaches. J. Hazard Mater. 2018, 344, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Haddad, C.; Salman, R.; El-Hellani, A.; Talih, S.; Shihadeh, A.; Saliba, N.A. Reactive Oxygen Species Emissions from Supra- and Sub-Ohm Electronic Cigarettes. J. Anal. Toxicol. 2019, 43, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Lin, Y.; Luna, K.; Logue, A.; Yoon, A.J.; Haptonstall, K.P.; Moheimani, R.; Choroomi, Y.; Nguyen, K.; Tran, E.; et al. Electronic and Tobacco Cigarettes Alter Polyunsaturated Fatty Acids and Oxidative Biomarkers. Circ. Res. 2021, 129, 514–526. [Google Scholar] [CrossRef]
- Duncan, A.; Heyer, M.P.; Ishikawa, M.; Caligiuri, S.P.B.; Liu, X.A.; Chen, Z.; Micioni Di Bonaventura, M.V.; Elayouby, K.S.; Ables, J.L.; Howe, W.M.; et al. Habenular TCF7L2 links nicotine addiction to diabetes. Nature 2019, 574, 372–377. [Google Scholar] [CrossRef]
- Pereira-da-Silva, T.; Napoleão, P.; Costa, M.C.; Gabriel, A.F.; Selas, M.; Silva, F.; Enguita, F.J.; Ferreira, R.C.; Carmo, M.M. Cigarette Smoking, miR-27b Downregulation, and Peripheral Artery Disease: Insights into the Mechanisms of Smoking Toxicity. J. Clin. Med. 2021, 10, 890. [Google Scholar] [CrossRef]
- Rodriguez-Leyva, D.; Weighell, W.; Edel, A.L.; LaVallee, R.; Dibrov, E.; Pinneker, R.; Maddaford, T.G.; Ramjiawan, B.; Aliani, M.; Guzman, R.; et al. Potent antihypertensive action of dietary flaxseed in hypertensive patients. Hypertension 2013, 62, 1081–1089. [Google Scholar] [CrossRef] [Green Version]
- Ramsden, C.E.; Ringel, A.; Feldstein, A.E.; Taha, A.Y.; MacIntosh, B.A.; Hibbeln, J.R.; Majchrzak-Hong, S.F.; Faurot, K.R.; Rapoport, S.I.; Cheon, Y.; et al. Lowering dietary linoleic acid reduces bioactive oxidized linoleic acid metabolites in humans. Prostaglandins Leukot Essent Fat. Acids 2012, 87, 135–141. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Han, Y.; Li, H.; Wang, Y.; Chen, X.; Chen, W.; Qiu, X.; Gong, J.; Li, W.; Zhu, T. Proinflammatory lipid signals trigger the health effects of air pollution in individuals with prediabetes. Environ. Pollut. 2021, 290, 118008. [Google Scholar] [CrossRef]
- Ferreiro-Vera, C.; Priego-Capote, F.; Mata-Granados, J.M.; Luque de Castro, M.D. Short-term comparative study of the influence of fried edible oils intake on the metabolism of essential fatty acids in obese individuals. Food Chem. 2013, 136, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Leyva, D.R.; Zahradka, P.; Ramjiawan, B.; Guzman, R.; Aliani, M.; Pierce, G.N. The effect of dietary flaxseed on improving symptoms of cardiovascular disease in patients with peripheral artery disease: Rationale and design of the FLAX-PAD randomized controlled trial. Contemp. Clin. Trials 2011, 32, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Hall, L.M.; Murphy, R.C. Electrospray mass spectrometric analysis of 5-hydroperoxy and 5-hydroxyeicosatetraenoic acids generated by lipid peroxidation of red blood cell ghost phospholipids. J. Am. Soc. Mass. Spectrom. 1998, 9, 527–532. [Google Scholar] [CrossRef] [Green Version]

| Never Smoked (n = 7) Average ± Standard Deviation | Quit Smoking (n = 65) Average ± Standard Deviation | Currently Smoking (n = 26) Average ± Standard Deviation | p-Value | |
|---|---|---|---|---|
| Age (years) | 71 ± 6.6 | 68 ± 8.8 | 63 ± 7.7 * | 0.0091 |
| Gender (M/F) | 5/2 | 48/17 | 19/7 | 0.29 |
| Body Mass Index | 25 ± 4.7 | 28 ± 4.4 | 28 ± 5.0 | 0.29 |
| Duration of Smoking (years) | N/A | 31 ± 12 | 37 ± 8.2 ** | 0.044 |
| Number of Cigarettes/Day | N/A | 24 ± 9.8 | 15 ± 9.5 *** | 0.00030 |
| Time Since Quit Smoking (years) | N/A | 13 ± 13 | N/A | N/A |
| Total Cholesterol (mmol/L) | 4.2 ± 1.1 | 4.4 ± 1.1 | 4.5 ± 1.2 | 0.81 |
| LDL-C (mmol/L) | 2.1 ± 0.90 | 2.4 ± 0.92 | 2.6 ± 1.0 | 0.32 |
| HDL-C (mmol/L) | 1.3 ± 0.30 | 1.2 ± 0.33 | 1.1 ± 0.17 | 0.19 |
| Linoleic Acid (mg/mL) | 0.64 ± 0.21 | 0.71 ± 0.19 | 0.67 ± 0.21 | 0.50 |
| Alpha-Linolenic Acid (mg/mL) | 0.019 ± 0.011 | 0.020 ± 0.012 | 0.017 ± 0.011 | 0.61 |
| Arachidonic Acid (mg/mL) | 0.21 ± 0.034 | 0.21 ± 0.066 | 0.20 ± 0.046 | 0.44 |
| Eicosapentanoic Acid (mg/mL) | 0.030 ± 0.013 | 0.021 ± 0.010 | 0.017 ± 0.0090 **** | 0.0099 |
| Docosahexaenoic Acid (mg/mL) | 0.055 ± 0.017 | 0.041 ± 0.013 ***** | 0.035 ± 0.014 ***** | 0.0031 |
| Oxylipin | Intercept | Age | Smoking Status | Fatty Acid Substrate (mg/mL) | R-Squared |
|---|---|---|---|---|---|
| 12,13-EpODE | −96.0 (63.9) | 1.78 (0.721) | −4.84 (11.5) | 102 (31.0) ** | 0.176 |
| 12,13-EpOME | 0.863 (27.3) | −0.0319 (0.309) | −0.175 (4.91) | 54.5 (13.3) *** | 0.158 |
| 13-HODE | 2.08 (8.37) | −0.0314 (0.0944) | −0.653 (1.50) | 16.7 (4.06) *** | 0.161 |
| 13-OxoODE | 0.865 (20.3) | −0.159 (0.229) | −0.0505 (3.64) | 27.1 (9.84) ** | 0.0809 |
| 9-HODE | −1.03 (7.97) | 0.0184 (0.0900) | −0.425 (1.43) | 13.77 (3.87) *** | 0.127 |
| 9,10-DiHOME | 1.47 (3.75) | −0.0500 (0.0424) | −0.537 (0.672) | 9.71 (1.82) *** | 0.249 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caligiuri, S.P.B.; Pierce, G.N.; Ravandi, A.; Aukema, H.M. The Plasma Oxylipidome Links Smoking Status to Peripheral Artery Disease. Metabolites 2022, 12, 627. https://doi.org/10.3390/metabo12070627
Caligiuri SPB, Pierce GN, Ravandi A, Aukema HM. The Plasma Oxylipidome Links Smoking Status to Peripheral Artery Disease. Metabolites. 2022; 12(7):627. https://doi.org/10.3390/metabo12070627
Chicago/Turabian StyleCaligiuri, Stephanie P. B., Grant N. Pierce, Amir Ravandi, and Harold M. Aukema. 2022. "The Plasma Oxylipidome Links Smoking Status to Peripheral Artery Disease" Metabolites 12, no. 7: 627. https://doi.org/10.3390/metabo12070627
APA StyleCaligiuri, S. P. B., Pierce, G. N., Ravandi, A., & Aukema, H. M. (2022). The Plasma Oxylipidome Links Smoking Status to Peripheral Artery Disease. Metabolites, 12(7), 627. https://doi.org/10.3390/metabo12070627






