Protective Effects of Bee Pollen on Multiple Propionic Acid-Induced Biochemical Autistic Features in a Rat Model
Abstract
:1. Introduction
2. Results
2.1. Neurotransmitters, GABA, Glutamine, Glutamate, and Caspase-3
2.2. Neuroinflammation and Oxidative Stress Related Markers
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Experimental Design
4.3. Ethics Approval
4.4. Collection of Brain Samples
4.5. Quantification of Neurotransmitters (Norepinephrine, Dopamine, and Serotonin)
4.6. Quantification of GABA, Glutamine, Glutamate, and Caspase-3
4.7. Quantification of Cytokines
4.8. Quantification of Oxidative Stress Markers
4.9. Bacterial Counts in Fecal Samples
4.10. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Volkmar, F.; Wiesner, L. Essential Clinical Guide to Understanding and Treating Autism; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017. [Google Scholar]
- Bradshaw, J.; Steiner, A.M.; Gengoux, G.; Koegel, L. Feasibility and effectiveness of very early intervention for infants at-risk for autism spectrum disorder: A systematic review. J. Autism Dev. Disord. 2014, 45, 778–794. [Google Scholar] [CrossRef] [PubMed]
- Daniels, A.M.; Halladay, A.K.; Shih, A.; Elder, L.M.; Dawson, G. Approaches to enhancing the early detection of autism spectrum disorders: A systematic review of the literature. J. Am. Acad. Child Adolesc. Psychiatry 2014, 53, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Pijl, M.K.; Buitelaar, J.K.; de Korte, M.W.; Rommelse, N.N.; Oosterling, I.J. Sustainability of an early detection program for autism spectrum disorder over the course of 8 years. Autism 2018, 22, 1018–1024. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.T.; Taur, Y.; Walkup, J.T. Gut microbiota and autism: Key concepts and findings. Autism Dev. Disord. 2017, 47, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Vuong, H.E.; Hsiao, E.Y. Emerging Roles for the Gut Microbiome in Autism Spectrum Disorder. Biol. Psychiatry 2017, 81, 411–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kandeel, W.A.; Meguid, N.A.; Bjørklund, G.; Eid, E.M.; Farid, M.; Mohamed, S.K.; Wakeel, K.E.; Chirumbolo, S.; Elsaeid, A.; Hammad, D.Y. Impact of Clostridium Bacteria in Children with Autism Spectrum Disorder and Their Anthropometric Measurements. J. Mol. Neurosci. 2020, 70, 897–907. [Google Scholar] [CrossRef]
- Abuaish, S.; Al-Otaibi, N.M.; Abujamel, T.S.; Alzahrani, S.A.; Alotaibi, S.M.; AlShawakir, Y.A.; Aabed, K.; El-Ansary, A. Fecal Transplant and Bifidobacterium Treatments Modulate Gut Clostridium Bacteria and Rescue Social Impairment and Hippocampal BDNF Expression in a Rodent Model of Autism. Brain Sci. 2020, 11, 1038. [Google Scholar] [CrossRef]
- Mohammad, F.K.; Palukuri, M.V.; Shivakumar, S.; Rengaswamy, R.; Sahoo, S. A Computational Framework for Studying Gut-Brain Axis in Autism Spectrum Disorder. Front. Physiol. 2022, 13, 760753. [Google Scholar] [CrossRef]
- Chapman, N.H.; Nato, A.Q.; Bernier, R.; Ankenman, K.; Sohi, H.; Munson, J.; Patowary, A.; Archer, M.; Blue, E.M.; Webb, S.J.; et al. Whole exome sequencing in extended families with autism spectrum disorder implicates four candidate genes. Hum. Genet. 2015, 134, 1055–1068. [Google Scholar] [CrossRef] [Green Version]
- Abdelli, L.S.; Samsam, A.; Naser, S.A. Propionic Acid Induces Gliosis and Neuro-inflammation through Modulation of PTEN/AKT Pathway in Autism Spectrum Disorder. Sci. Rep. 2019, 9, 8824. [Google Scholar] [CrossRef] [Green Version]
- Meeking, M.M.; MacFabe, D.F.; Mepham, J.R.; Foley, K.A.; Tichenoff, L.J.; Boon, F.H.; Kavaliers, M.; Ossenkopp, K.P. Propionic acid induced behavioural effects of relevance to autism spectrum disorder evaluated in the hole board test with rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 2020, 97, 109794. [Google Scholar] [CrossRef] [PubMed]
- El-Ansary, A.; Bacha, A.B.; Bjørklund, G.; Al-Orf, N.; Bhat, R.S.; Moubayed, N.; Abed, K. Probiotic treatment reduces the autistic-like excitation/inhibition imbalance in juvenile hamsters induced by orally administered propionic acid and clindamycin. Metab. Brain Dis. 2018, 33, 1155–1164. [Google Scholar] [CrossRef] [PubMed]
- MacFabe, D. Autism: Metabolism, mitochondria, and the microbiome. Glob. Adv. Health Med. 2013, 2, 52–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shultz, S.R.; Aziz, N.A.; Yang, L.; Sun, M.; MacFabe, D.F.; O’Brien, T.J. Intracerebroventricular injection of propionic acid, an enteric metabolite implicated in autism, induces social abnormalities that do not differ between seizure-prone (FAST) and seizure-resistant (SLOW) rats. Behav. Brain Res. 2015, 278, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Kocot, J.; Kiełczykowska, M.; Luchowska-Kocot, D.; Kurzepa, J.; Musik, I. Antioxidant Potential of Propolis, Bee Pollen, and Royal Jelly: Possible Medical Application. Oxidative Med. Cell. Longev. 2018, 2018, 7074209. [Google Scholar] [CrossRef] [PubMed]
- Oyarzún, J.E.; Andia, M.E.; Uribe, S.; Núñez Pizarro, P.; Núñez, G.; Montenegro, G.; Bridi, R. Honeybee pollen extracts reduce oxidative stress and steatosis in Hepatic cells. Molecules 2020, 26, 6. [Google Scholar] [CrossRef]
- Rzepecka-Stojko, A.; Stojko, J.; Kurek-Górecka, A.; Górecki, M.; Kabała-Dzik, A.; Kubina, R.; Moździerz, A.; Buszman, E. Polyphenols from Bee Pollen: Structure, Absorption, Metabolism and Biological Activity. Molecules 2015, 20, 21732–21749. [Google Scholar] [CrossRef] [Green Version]
- Spulber, R.; Doğaroğlu, M.; Băbeanu, N.; Popa, O. Physicochemical characteristics of fresh bee pollen from different botanical origins. Rom. Biotechnol. Lett. 2018, 23, 13357–13365. [Google Scholar]
- Taha, E.A.; Al-Kahtani, S. Macro- and trace elements content in honeybee pollen loads in relation to the harvest season. Saudi J. Biol. Sci. 2020, 27, 1797–1800. [Google Scholar] [CrossRef]
- Kieliszek, M.; Piwowarek, K.; Kot, A.M.; Błażejak, S.; Chlebowska-Śmigiel, A.; Wolska, I. Pollen and bee bread as new health-oriented products: A review. Trends Food Sci. Technol. 2018, 71, 170–180. [Google Scholar] [CrossRef]
- Prđun, S.; Svečnjak, L.; Valentić, M.; Marijanović, Z.; Jerković, I. Characterization of Bee Pollen: Physico-Chemical Properties, Headspace Composition and FTIR Spectral Profiles. Foods 2021, 10, 2103. [Google Scholar] [CrossRef] [PubMed]
- Szczesna, T. Long chain fatty acids composition of honeybee-collected pollen. J. Apic. Sci. 2006, 50, 65–79. [Google Scholar]
- Khider, M.; Elbanna, K.; Mahmoud, A.; Owayss, A.A. Egyptian honeybee pollen as antimicrobial, antioxidant agents, and dietary food supplements. Food Sci. Biotechnol. 2013, 22, 1–9. [Google Scholar] [CrossRef]
- Solgajová, M.; Ivanišová, E.; Nôžkova, J.; Frančáková, H.; Tóth, Ž.; Dráb, Š. Antioxidant activity and polyphenol content of malt beverages enriched with bee pollen. J. Microbiol. Biotechnol. Food Sci. 2014, 3, 281–284. [Google Scholar]
- Haro, A.; López-Aliaga, I.; Lisbona, F.; Barrionuevo, M.; Alférez, M.J.; Campos, M.S. Beneficial effect of pollen and/or propolis on the metabolism of iron, calcium, phosphorus, and magnesium in rats with nutritional ferropenic anemia. J. Agric. Food Chem. 2000, 48, 5715–5722. [Google Scholar] [CrossRef]
- Denisow, B.; Denisow-Pietrzyk, M. Biological and therapeutic properties of bee pollen: A review. J. Sci. Food Agric. 2016, 96, 4303–4309. [Google Scholar] [CrossRef]
- Komosinska-Vassev, K.; Olczyk, P.; Kázmierczak, J.; Mencner, L.; Olczyk, K. Bee pollen chemical composition and therapeutic application. J. Evid. Based Complement. Altern. Med. 2015, 2015, 297425. [Google Scholar] [CrossRef] [Green Version]
- Salem, H.; Al-Youssef, H.; Ashour, A.; Ahmed, A.; Amina, M.; Issa, I.; Bhat, R. Antioxidant and hepatorenal protective effects of bee pollen fractions against propionic acid-induced autistic feature in rats. Food Sci. Nutr. 2020, 8, 5114–5127. [Google Scholar] [CrossRef]
- Al-Yousef, H.M.; Alkhulaifi, M.M.; Al-Salem, H.S.; Syed, R.A. Clostridium perfringens induced autism disorders counteract by using natural BP in vitro. J. Biol. Med. Res. 2018, 2, 8. [Google Scholar]
- El-Ansary, A.K.; Bacha, A.B.; Kotb, M. Etiology of autistic features: The persisting neurotoxic effects of propionic acid. J. Neuroinflamm. 2012, 9, 74. [Google Scholar] [CrossRef] [Green Version]
- Daghestani, M.H.; Selim, M.E.; Abd-Elhakim, Y.M.; Said, E.N.; El-Hameed, N.E.A.; Khalil, S.R.; El-Tawil, O.S. The role of apitoxin in alleviating propionic acid-induced neurobehavioral impairments in rat pups: The expression pattern of Reelin gene. Biomed. Pharmacother. 2017, 93, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Olloquequi, J.; Cornejo-Córdova, E.; Verdaguer, E.; Soriano, F.X.; Binvignat, O.; Auladell, C.; Camins, A. Excitotoxicity in the pathogenesis of neurological and psychiatric disorders: Therapeutic implications. J. Psychopharmacol. 2018, 32, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.; Song, H.L.; Zhou, Y.; Li, L.X.; Cui, W.; Wang, W.; Liu, P. Tumour necrosis factor-alpha affects blood-brain barrier permeability and tight junction-associated occludin in acute liver failure. Liver Int. 2010, 30, 1198–1210. [Google Scholar] [CrossRef] [PubMed]
- Mirza, R.; Sharma, B. A selective peroxisome proliferator-activated receptor-γ agonist benefited propionic acid induced autism-like behavioral phenotypes in rats by attenuation of neuroinflammation and oxidative stress. Chem. Biol. Interact. 2019, 311, 108758. [Google Scholar] [CrossRef] [PubMed]
- Dolga, A.M.; Granic, I.; Blank, T.; Knaus, H.G.; Spiess, J.; Luiten, P.G.; Eisel, U.L.; Nijholt, I.M. TNF-alpha-mediates neuroprotection against glutamate-induced excitotoxicity via NF-kappaB-dependent up-regulation of K2.2 channels. J. Neurochem. 2008, 107, 1158–1167. [Google Scholar]
- Macfabe, D.F. Short-chain fatty acid fermentation products of the gut microbiome: Implications in autism spectrum disorders. Microb. Ecol. Health Dis. 2012, 23, 19260. [Google Scholar] [CrossRef]
- El-Ansary, A.; Al-Ayadhi, L. GABAergic/glutamatergic imbalance relative to excessive neuroinflammation in autism spectrum disorders. J Neuroinflammation. 2014, 1, 189. [Google Scholar] [CrossRef]
- Rörig, B.; Klausa, G.; Sutor, B. Intracellular acidification reduced gap junction coupling between immature rat neocortical pyramidal neurones. J. Physiol. 1996, 490 Pt 1, 31–34. [Google Scholar] [CrossRef] [Green Version]
- Dringen, R.; Pawlowski, P.G.; Hirrlinger, J. Peroxide detoxification by brain cells. J. Neurosci. Res. 2005, 79, 157–165. [Google Scholar] [CrossRef]
- Pawate, S.; Shen, Q.; Fan, F.; Bhat, N.R. Redox regulation of glial inflammatory response to lipopolysaccharide and interferon gamma. J. Neurosci. Res. 2004, 77, 540–551. [Google Scholar] [CrossRef]
- Zhang, B.; Yin, X.-R.; Li, X.; Yang, S.-L.; Ferguson, I.; Chen, K.-S. Lipoxygenase Gene Expression in Ripening Kiwifruit in Relation to Ethylene and Aroma Production. J. Agric. Food Chem. 2009, 57, 2875–2881. [Google Scholar] [CrossRef] [PubMed]
- Chopra, K.; Kumar, B.; Kuhad, A. Pathobiological targets of depression. Expert Opin Ther. Targets 2011, 15, 379. [Google Scholar] [CrossRef] [PubMed]
- Erten, F. Lycopene ameliorates propionic acid-induced autism spectrum disorders by inhibiting inflammation and oxidative stress in rats. J. Food Biochem. 2021, 45, e13922. [Google Scholar] [CrossRef] [PubMed]
- Alabdali, A.; Al-Ayadhi, L.; El-Ansary, A. Association of social and cognitive impairment and biomarkers in autism spectrum disorders. J. Neuroinflam. 2014, 11, 4. [Google Scholar] [CrossRef] [Green Version]
- Kalinichenko, L.S.; Kornhuber, J.; Müller, C.P. Individual differences in inflammatory and oxidative mechanisms of stress-related mood disorders. Front. Neuroendocrinol. 2019, 55, 100783. [Google Scholar] [CrossRef]
- Ambrogini, P.; Torquato, P.; Bartolini, D.; Albertini, M.C.; Lattanzi, D.; Di Palma, M.; Marinelli, R.; Betti, M.; Minelli, A.; Cuppini, R.; et al. Excitotoxicity, neuroinflammation and oxidant stress as molecular bases of epileptogenesis and epilepsy-derived neurodegeneration: The role of vitamin E. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 1098–1112. [Google Scholar] [CrossRef]
- Kostić, A.Ž.; Milinčić, D.D.; Barać, M.B.; Ali Shariati, M.; Tešić, Ž.L.; Pešić, M.B. The Application of Pollen as a Functional Food and Feed Ingredient-The Present and Perspectives. Biomolecules 2020, 10, 84. [Google Scholar] [CrossRef] [Green Version]
- Melo, I.L.P.D.; Almeida-Muradian, L.B.D. Stability of antioxidants vitamins in bee pollen samples. Química Nova 2010, 33, 514–518. [Google Scholar] [CrossRef]
- Alfawaz, H.; Al-Onazi, M.; Bukhar.i, S.I.; Binobead, M.; Othman, N.; Algahtani, N.; Bhat, R.S.; Moubayed, N.M.S.; Alzeer, H.S.; El-Ansary, A. The independent and combined effects of omega-3 and vitamin B12 in ameliorating propionic acid induced biochemical features in juvenile rats as rodent model of autism. J. Mol. Neurosci. 2018, 66, 403–413. [Google Scholar] [CrossRef]
- Catania, A. Neuroprotective actions of melanocortins: A therapeutic opportunity. Trends Neurosci. 2008, 31, 353–360. [Google Scholar] [CrossRef]
- Li, Q.; Liang, X.; Guo, N.; Hu, L.; Prasad, E.M.; Wu, Y.; Xue, X.; Wu, L.; Wang, K. Protective effects of Bee pollen extract on the Caco-2 intestinal barrier dysfunctions induced by dextran sulfate sodium. Biomed. Pharmacother. 2019, 117, 109200. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, M.; Piccolo, M.; Vannini, L.; Siragusa, S.; De Giacomo, A.; Serrazzanetti, D.I.; Cristofori, F.; Guerzoni, M.E.; Gobbetti, M.; Francavilla, R. Fecal microbiota and metabolome of children with autism and pervasive developmental disorder not otherwise specified. PLoS ONE 2013, 8, e76993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.; Park, J.Y.; Lee, E.H.; Yang, J.; Jeong, B.R.; Kim, Y.K.; Seoh, J.Y.; Lee, S.; Han, P.L.; Kim, E.J. Rapid Assessment of Microbiota Changes in Individuals with Autism Spectrum Disorder Using Bacteria-derived Membrane Vesicles in Urine. Exp. Neurobiol. 2017, 26, 307–317. [Google Scholar] [CrossRef] [Green Version]
- Abujamel, T.S.; Al-Otaibi, N.M.; Abuaish, S.; AlHarbi, R.H.; Assas, M.B.; Alzahrani, S.A.; Alotaibi, S.M.; El-Ansary, A.; Aabed, K. Different Alterations in Gut Microbiota between Bifidobacterium longum and Fecal Microbiota Transplantation Treatments in Propionic Acid Rat Model of Autism. Nutrients 2022, 14, 608. [Google Scholar] [CrossRef] [PubMed]
- Mărgăoan, R.; Mărghitaş, L.A.; Dezmirean, D.S.; Gherman, B.; Chirilă, F.; Zacharias, I.; Bobiș, O. Antimicrobial Activity of Bee Pollen Ethanolic and Methanolic Extracts on Staphylococcus aureus Bacterial Strain. Bulletin of University of Agricultural Sciences and Veterinary Medicine Cluj-Napoca. Anim. Sci. Biotechnol. 2015, 72, 78–80. [Google Scholar] [CrossRef] [Green Version]
- Santos, A.C.D.; Biluca, F.C.; Braghini, F.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Phenolic composition and biological activities of stingless bee honey: An overview based on its aglycone and glycoside compounds. Food Res. Int. 2021, 147, 110553. [Google Scholar] [CrossRef]
- Aabed, K.; Bhat, R.S.; Al-Dbass, A.; Moubayed, N.; Algahtani, N.; Merghani, N.M.; Alanazi, A.; Zayed, N.; El-Ansary, A. Bee Pollen and Propolis Improve Neuroinflammation and Dysbiosis Induced by Propionic Acid, a Short Chain Fatty Acid in a Rodent Model of Autism. Lipids Health Dis. 2019, 18, 200. [Google Scholar] [CrossRef] [Green Version]
- Beutler, E.; Duran, O.; Kelly, B.M. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 1963, 61, 882–888. [Google Scholar]
- Ruiz-Larrea, M.B.; Leal, A.M.; Liza, M.; Lacort, M.; de Groot, H. Antioxidant effects of estradiol and 2-hydroxyestradiol on iron-induced lipid peroxidation of rat liver microsomes. Steroids 1994, 59, 383–388. [Google Scholar]
- Jagota, S.K.; Dani, H.M. A New colorimetric technique for the estimation of Vitamin C using Folin phenol reagent. Anal. Biochem. 1982, 15, 178. [Google Scholar] [CrossRef]
- Zhang, F.M.; Wang, H.G.; Wang, M. Fecal microbiota transplantation for severe enterocolonic fistulizing Crohn’s disease. World J. Gastroenterol. 2013, 19, 7213–7216. [Google Scholar] [CrossRef] [PubMed]
- Li, S.S.; Zhu, A.; Benes, V.; Costea, P.I.; Hercog, R.; Hildebrand, F.; Huerta-Cepas, J.; Nieuwdorp, M.; Salojärvi, J.; Voigt, A.Y.; et al. Durable coexistenceof donor and recipient strains after fecal microbiota transplantation. Science 2016, 352, 586–589. [Google Scholar] [CrossRef] [PubMed]
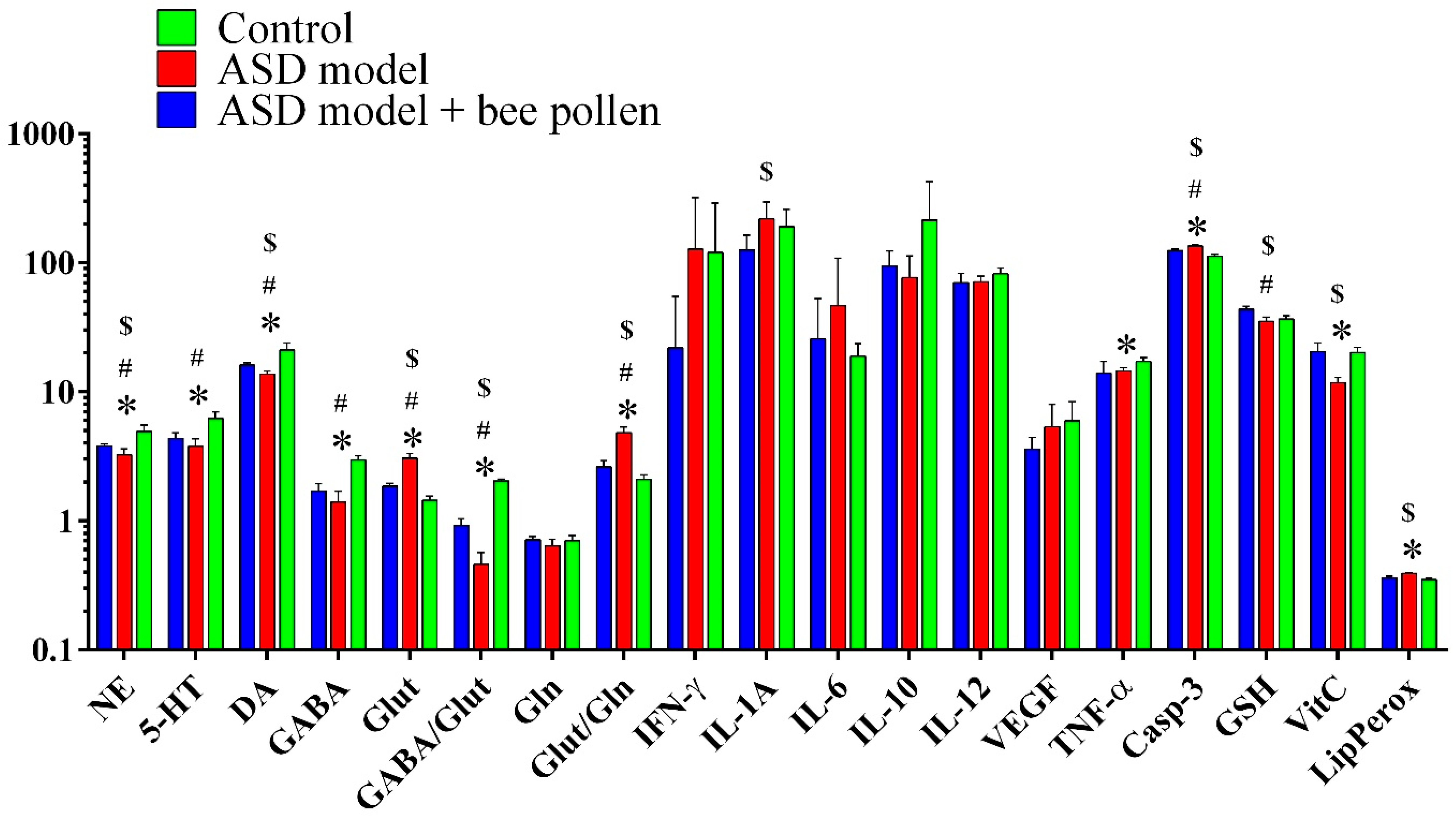

| Variables | Control | ASD Model | Bee Pollen-Protected ASD Model |
|---|---|---|---|
| Norepinephrine (ng/100 mg) | 4.92 ± 0.618 | 3.25 ± 0.36 *,# | 3.79 ± 0.15 *,# |
| Serotonin (ng/100 mg) | 6.20 ± 0.78 | 3.78 ± 0.56 *,# | 4.36 ± 0.42 *,# |
| Dopamine (ng/100 mg) | 21.12 ± 2.72 | 13.69 ± 0.80 *,# | 16.09 ± 0.71 *,# |
| GABA (ng/100 mg) | 2.95 ± 0.24 | 1.40 ± 0.29 *,# | 1.70 ± 0.24 *,# |
| Glutamate (µg/mg) | 1.44 ± 0.11 | 3.05 ± 0.27 *,# | 1.85 ± 0.10 *,# |
| GABA/Glutamine | 2.04 ± 0.06 | 0.46 ± 0.11 *,# | 0.92 ± 0.12 *,# |
| Glutamine (µg/mg) | 0.70 ± 0.07 | 0.64 ± 0.08 *,# | 0.71 ± 0.05 *,# |
| Glutamate/Glutamine | 2.09 ± 0.19 | 4.80 ± 0.52 *,# | 2.62 ± 0.30 *,# |
| IFN-γ (pg/mL) | 120.71 ± 167.85 | 127.09 ± 192.57 $ | 21.86 ± 32.80 $ |
| IL-1A (pg/mL) | 190.54 ± 68.50 | 217.85 ± 76.89 *,# | 125.94 ± 37.27 *,# |
| IL-6 (pg/mL) | 18.87 ± 4.69 | 46.44 ± 61.93 $ | 25.64 ± 27.15 $ |
| IL-10 (pg/mL) | 213.86 ± 212.98 | 76.76 ± 36.44 $ | 94.08 ± 28.87 $ |
| IL-12 (pg/mL) | 82.07 ± 8.65 | 71.03 ± 7.57 $ | 70.11 ± 13.10 $ |
| VEGF (pg/mL) | 5.95 ± 2.43 | 5.36 ± 2.64 $,# | 3.58 ± 0.85 *,# |
| TNF-α (pg/mL) | 17.11 ± 1.39 | 14.50 ± 0.91 $,# | 13.95 ± 3.27 *,# |
| Caspase-3 (u/100 mg) | 112.66 ± 4.20 | 134.79 ± 3.27 *,# | 124.23 ± 2.89 *,# |
| Glutathione (µg/mL) | 36.44 ± 2.43 | 35.07 ± 2.83 *,$ | 43.65 ± 2.09 *,# |
| Vitamin C (µg/mL) | 20.16 ± 2.00 | 11.74 ± 1.16 *,# | 20.42 ± 3.42 $,# |
| Lipid peroxides | 0.35 ± 0.01 | 0.39 ± 0.01 *,# | 0.36 ± 0.01 $,# |
| Variable | Correlated Variables | Spearman Correlation (R) | p Value | Variable | Correlated Variables | Spearman Correlation (R) | p Value |
|---|---|---|---|---|---|---|---|
| Interferonϒ | NE | −0.829 | 0.000 | Norepinephrine | 5-HT | 0.82 | 0.000 |
| 5-HT | −0.69 | 0.002 | DA | 0.888 | 0.000 | ||
| DA | −0.818 | 0.000 | Caspase 3 | −0.865 | 0.000 | ||
| Caspase 3 | 0.771 | 0.000 | GABA | 0.919 | 0.000 | ||
| GABA | −0.686 | 0.002 | Glut | −0.91 | 0.000 | ||
| Glut | 0.756 | 0.000 | GABA/Glut | 0.95 | 0.000 | ||
| GABA/Glut | −0.748 | 0.000 | Glut/Gln | −0.85 | 0.000 | ||
| Glut/Gln | 0.735 | 0.001 | IL-12 | 0.473 | 0.047 | ||
| VEGF | 0.652 | 0.006 | TNF-α | 0.557 | 0.016 | ||
| TNF-α | 0.649 | 0.007 | VitC | 0.634 | 0.005 | ||
| VitC | −0.646 | 0.004 | MDA | −0.702 | 0.001 | ||
| MDA | 0.717 | 0.001 | |||||
| Caspase 3 | GABA | −0.846 | 0.000 | Dopamine | Caspase 3 | −0.913 | 0.000 |
| Glut | 0.856 | 0.000 | GABA | 0.839 | 0.000 | ||
| GABA/Glut | −0.874 | 0.000 | Glut | −0.851 | 0.000 | ||
| Glut/Gln | 0.872 | 0.000 | GABA/Glut | 0.89 | 0.000 | ||
| IL-12 | −0.527 | 0.024 | Glut/Gln | −0.86 | 0.000 | ||
| TNF-α | −0.499 | 0.035 | TNF-α | 0.508 | 0.031 | ||
| VitC | −0.633 | 0.005 | VitC | 0.596 | 0.009 | ||
| MDA | 0.71 | 0.001 | MDA | −0.778 | 0.000 | ||
| Serotonin | DA | 0.827 | 0.000 | GABA | Glut | −0.781 | 0.000 |
| Caspase 3 | −0.839 | 0.000 | GABA/Glut | 0.926 | 0.000 | ||
| GABA | 0.859 | 0.000 | Glut/Gln | −0.778 | 0.000 | ||
| GABA/Glut | 0.853 | 0.000 | VitC | 0.521 | 0.027 | ||
| Glut/Gln | −0.824 | 0.000 | MDA | −0.605 | 0.008 | ||
| TNF-α | 0.547 | 0.019 | IL-1A | GSH | −0.75 | 0.001 | |
| VitC | −0.658 | 0.003 | MDA | 0.528 | 0.035 | ||
| GABA/Glut | Glut/Gln | −0.862 | 0.000 | TNF-α | 0.746 | 0.000 | |
| TNF-α | 0.486 | 0.041 | GSH | VitC | 0.552 | 0.018 | |
| VitC | 0.622 | 0.006 | |||||
| Lipid peroxides | −0.775 | 0.000 | VitC | MDA | −0.553 | 0.017 |
| Dependent Variable | Predictor Variable | Coefficient | S.E. | p Value | Adjusted R2 | 95% CI | Dependent Variable | Predictor Variable | Coefficient | S.E. | p Value | Adjusted R2 | 95% CI | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | Lower | Upper | ||||||||||||
| Caspase 3 | Glut | 10.044 | 1.612 | 0.000 | 0.791 | 6.398 | 13.690 | VEGF | IL-10 | 0.011 | 0.001 | 0.000 | 0.853 | 0.008 | 0.014 |
| Glut | 8.641 | 1.397 | 0.000 | 0.868 | 5.419 | 11.862 | IL-10 | 0.011 | 0.001 | 0.214 | 0.912 | 0.009 | 0.014 | ||
| IL-12 | −0.309 | 0.123 | 0.000 | −0.594 | −0.025 | IL-1A | 0.012 | 0.005 | 0.000 | 0.002 | 0.023 | ||||
| Glut | 6.986 | 0.712 | 0.000 | 0.972 | 5.303 | 8.668 | IL-10 | 0.011 | 0.001 | 0.000 | 0.946 | 0.009 | 0.013 | ||
| IL-12 | −0.408 | 0.060 | 0.000 | −0.549 | −0.266 | IL-1A | 0.012 | 0.004 | 0.014 | 0.003 | 0.020 | ||||
| Gln | 64.781 | 11.733 | 0.001 | 37.038 | 92.524 | IL-6 | −0.019 | 0.008 | 0.045 | −0.038 | 0.000 | ||||
| Glut | 3.695 | 1.219 | 0.023 | 0.987 | 0.712 | 6.678 | NE | GABA | 1.205 | 0.158 | 0.000 | 0.851 | 0.848 | 1.563 | |
| IL-12 | −0.441 | 0.043 | 0.000 | −0.545 | −0.336 | GABA | 0.679 | 0.251 | 0.557 | 0.904 | 0.099 | 1.259 | |||
| Gln | 63.796 | 8.105 | 0.000 | 43.965 | 83.627 | DA | 0.138 | 0.057 | 0.027 | 0.007 | 0.269 | ||||
| IFN-ϒ | 0.143 | 0.049 | 0.026 | 0.024 | 0.262 | IFN-γ | Glut | 22.035 | 2.923 | 0.000 | 0.848 | 15.423 | 28.646 | ||
| Glut | 2.785 | 0.897 | 0.027 | 0.994 | 0.479 | 5.092 | IL-1A | IL-12 | 2.832 | 1.228 | 0.047 | 0.301 | 0.053 | 5.611 | |
| IL-12 | −0.470 | 0.031 | 0.000 | −0.550 | −0.390 | IL-12 | 4.173 | 0.967 | 0.028 | 0.650 | 1.943 | 6.404 | |||
| Gln | 67.868 | 5.745 | 0.000 | 53.101 | 82.635 | Glut/Gln | 24.766 | 7.840 | 0.003 | 6.688 | 42.844 | ||||
| IFN-ϒ | 0.188 | 0.037 | 0.004 | 0.093 | 0.283 | IL-10 | VEGF | 80.798 | 10.535 | 0.000 | 0.853 | 56.965 | 104.631 | ||
| IL-10 | 0.004 | 0.002 | 0.039 | 0.000 | 0.008 | VEGF | 83.026 | 8.106 | 0.353 | 0.914 | 64.333 | 101.719 | |||
| Glut | 2.797 | 0.356 | 0.001 | 0.999 | 1.809 | 3.784 | IL-1A | −1.052 | 0.388 | 0.000 | −1.946 | −0.158 | |||
| IL-12 | −0.516 | 0.015 | 0.000 | −0.558 | −0.474 | GSH | TNF-α | −1.134 | 0.480 | 0.042 | 0.314 | −2.220 | −0.048 | ||
| Gln | 74.889 | 2.637 | 0.000 | 67.568 | 82.211 | TNF-α | −1.288 | 0.367 | 0.000 | 0.609 | −2.133 | −0.443 | |||
| IFN-ϒ | 0.221 | 0.016 | 0.000 | 0.176 | 0.265 | IFN-ϒ | −0.130 | 0.047 | 0.008 | −0.238 | −0.023 | ||||
| IL-10 | 0.005 | 0.001 | 0.001 | 0.003 | 0.007 | Vitamin C | Glut/Gln | −3.272 | 0.864 | 0.004 | 0.571 | −5.227 | −1.317 | ||
| DA | 0.337 | 0.064 | 0.006 | 0.160 | 0.514 | Glut/Gln | −4.571 | 0.898 | 0.000 | 0.714 | −6.641 | −2.501 | |||
| Glut | Glut/Gln | 0.706 | 0.040 | 0.000 | 0.969 | 0.616 | 0.796 | 5-HT | GABA/Glut | 1.385 | 0.220 | 0.000 | 0.794 | 0.887 | 1.883 |
| Glut/Gln | 0.685 | 0.014 | 0.000 | 0.996 | 0.653 | 0.717 | DA | NE | 3.149 | 0.436 | 0.000 | 0.836 | 2.162 | 4.136 | |
| Gln | 2.869 | 0.348 | 0.000 | 2.067 | 3.670 | GABA | GABA/Glut | 0.923 | 0.069 | 0.000 | 0.947 | 0.767 | 1.080 | ||
| Glut/Gln | 0.713 | 0.009 | 0.000 | 0.999 | 0.692 | 0.735 | IL-12 | TNF-α | 2.920 | 0.786 | 0.005 | 0.561 | 1.142 | 4.698 | |
| Gln | 2.581 | 0.188 | 0.000 | 2.137 | 3.025 | TNF-α | IL-12 | 0.207 | 0.056 | 0.005 | 0.561 | 0.081 | 0.334 | ||
| IFN-γ | 0.000 | 0.000 | 0.002 | 0.000 | 0.000 | MDA | Glut | 0.018 | 0.004 | 0.002 | 0.627 | 0.009 | 0.028 | ||
| Parameters | Groups | AUC | Cut-off Value | Sensitivity | Specificity | p-Value | 95% CI |
|---|---|---|---|---|---|---|---|
| IFN-ϒ (pg/100 mg) | PPA-acute | 1 | 103.755 | 100.0% | 100.0% | 0.004 | 1.000–1.000 |
| Pollen | 0.694 | 82.67 | 100.0% | 50.0% | 0.262 | 0.369–1.020 | |
| Noradrenaline (ng/100 mg) | PPA-acute | 1 | 3.955 | 100.0% | 100.0% | 0.004 | 1.000–1.000 |
| Pollen | 1 | 4.135 | 100.0% | 100.0% | 0.004 | 1.000–1.000 | |
| Serotonin (ng/100 mg) | PPA-acute | 1 | 4.795 | 100.0% | 100.0% | 0.004 | 1.000–1.000 |
| Pollen | 1 | 5.105 | 100.0% | 100.0% | 0.004 | 1.000–1.000 | |
| Dopamine (ng/100 mg) | PPA-acute | 1 | 16.52 | 100.0% | 100.0% | 0.004 | 1.000–1.000 |
| Pollen | 1 | 17.62 | 100.0% | 100.0% | 0.004 | 1.000–1.000 | |
| Caspase-3 (u/100 mg) | PPA-acute | 1 | 124.76 | 100.0% | 100.0% | 0.004 | 1.000–1.000 |
| Pollen | 1 | 119.845 | 100.0% | 100.0% | 0.004 | 1.000–1.000 | |
| GABA (ng/100 mg) | PPA-acute | 1 | 2.275 | 100.0% | 100.0% | 0.004 | 1.000–1.000 |
| Pollen | 1 | 2.4 | 100.0% | 100.0% | 0.004 | 1.000–1.000 | |
| Glut (µg/mg) | PPA-acute | 1 | 2.19 | 100.0% | 100.0% | 0.004 | 1.000–1.000 |
| Pollen | 1 | 1.67 | 100.0% | 100.0% | 0.004 | 1.000–1.000 | |
| GABA/Glut | PPA-acute | 1 | 1.29 | 100.0% | 100.0% | 0.004 | 1.000–1.000 |
| Pollen | 1 | 1.52 | 100.0% | 100.0% | 0.004 | 1.000–1.000 | |
| Gln (µg/mg) | PPA-acute | 0.722 | 0.655 | 66.7% | 66.7% | 0.2 | 0.421–1.023 |
| Pollen | 0.569 | 0.665 | 83.3% | 50.0% | 0.689 | 0.221–0.918 | |
| Glut/Gln | PPA-acute | 1 | 3.295 | 100.0% | 100.0% | 0.004 | 1.000–1.000 |
| Pollen | 0.931 | 2.14 | 100.0% | 66.7% | 0.013 | 0.787–1.074 | |
| IFN-γ (pg/mL) | PPA-acute | 0.5 | 25.38 | 50.0% | 66.7% | 1 | 0.153–0.847 |
| Pollen | 0.833 | 8.445 | 75.0% | 100.0% | 0.088 | 0.524–1.143 | |
| IL-1A (pg/mL) | PPA-acute | 0.633 | 207.35 | 50.0% | 80.0% | 0.465 | 0.288–0.979 |
| Pollen | 0.88 | 146.235 | 80.0% | 80.0% | 0.047 | 0.662–1.098 | |
| IL-6 (pg/mL) | PPA-acute | 0.521 | 23.195 | 50.0% | 83.3% | 0.915 | 0.070–0.972 |
| Pollen | 0.6 | 16.88 | 60.0% | 83.3% | 0.584 | 0.205–0.995 | |
| IL-10 (pg/mL) | PPA-acute | 0.812 | 122.27 | 100.0% | 75.0% | 0.149 | 0.465–1.160 |
| Pollen | 0.875 | 119.215 | 100.0% | 75.0% | 0.083 | 0.608–1.142 | |
| IL-12 (pg/mL) | PPA-acute | 0.833 | 72.835 | 66.7% | 100.0% | 0.055 | 0.593–1.073 |
| Pollen | 0.861 | 72.13 | 83.3% | 100.0% | 0.037 | 0.604–1.119 | |
| VEGF (pg/mL) | PPA-acute | 0.597 | 4.5 | 66.7% | 66.7% | 0.575 | 0.253–0.941 |
| Pollen | 0.889 | 3.72 | 66.7% | 100.0% | 0.025 | 0.697–1.081 | |
| TNF-α (pg/mL) | PPA-acute | 0.944 | 15.095 | 83.3% | 100.0% | 0.01 | 0.814–1.075 |
| Pollen | 0.833 | 14.845 | 83.3% | 100.0% | 0.055 | 0.535–1.132 | |
| Pollen | 0.861 | 54.89 | 100.0% | 66.7% | 0.037 | 0.645–1.077 | |
| GSH (µg/mL) | PPA-acute | 0.667 | 37.41 | 83.3% | 50.0% | 0.337 | 0.346–0.987 |
| Pollen | 1 | 39.565 | 100.0% | 100.0% | 0.004 | 1.000–1.000 | |
| Vitamin C (µg/mL) | PPA-acute | 1 | 15.26 | 100.0% | 100.0% | 0.004 | 1.000–1.000 |
| Pollen | 0.583 | 20.485 | 66.7% | 66.7% | 0.631 | 0.234–0.933 | |
| Pollen | 0.639 | 0.355 | 66.7% | 66.7% | 0.423 | 0.301–0.977 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfawaz, H.A.; El-Ansary, A.; Al-Ayadhi, L.; Bhat, R.S.; Hassan, W.M. Protective Effects of Bee Pollen on Multiple Propionic Acid-Induced Biochemical Autistic Features in a Rat Model. Metabolites 2022, 12, 571. https://doi.org/10.3390/metabo12070571
Alfawaz HA, El-Ansary A, Al-Ayadhi L, Bhat RS, Hassan WM. Protective Effects of Bee Pollen on Multiple Propionic Acid-Induced Biochemical Autistic Features in a Rat Model. Metabolites. 2022; 12(7):571. https://doi.org/10.3390/metabo12070571
Chicago/Turabian StyleAlfawaz, Hanan A., Afaf El-Ansary, Laila Al-Ayadhi, Ramesa Shafi Bhat, and Wail M. Hassan. 2022. "Protective Effects of Bee Pollen on Multiple Propionic Acid-Induced Biochemical Autistic Features in a Rat Model" Metabolites 12, no. 7: 571. https://doi.org/10.3390/metabo12070571
APA StyleAlfawaz, H. A., El-Ansary, A., Al-Ayadhi, L., Bhat, R. S., & Hassan, W. M. (2022). Protective Effects of Bee Pollen on Multiple Propionic Acid-Induced Biochemical Autistic Features in a Rat Model. Metabolites, 12(7), 571. https://doi.org/10.3390/metabo12070571









