Intrinsic Exercise Capacity Affects Glycine and Angiotensin-Converting Enzyme 2 (ACE2) Levels in Sedentary and Exercise Trained Rats
Abstract
1. Introduction
2. Results
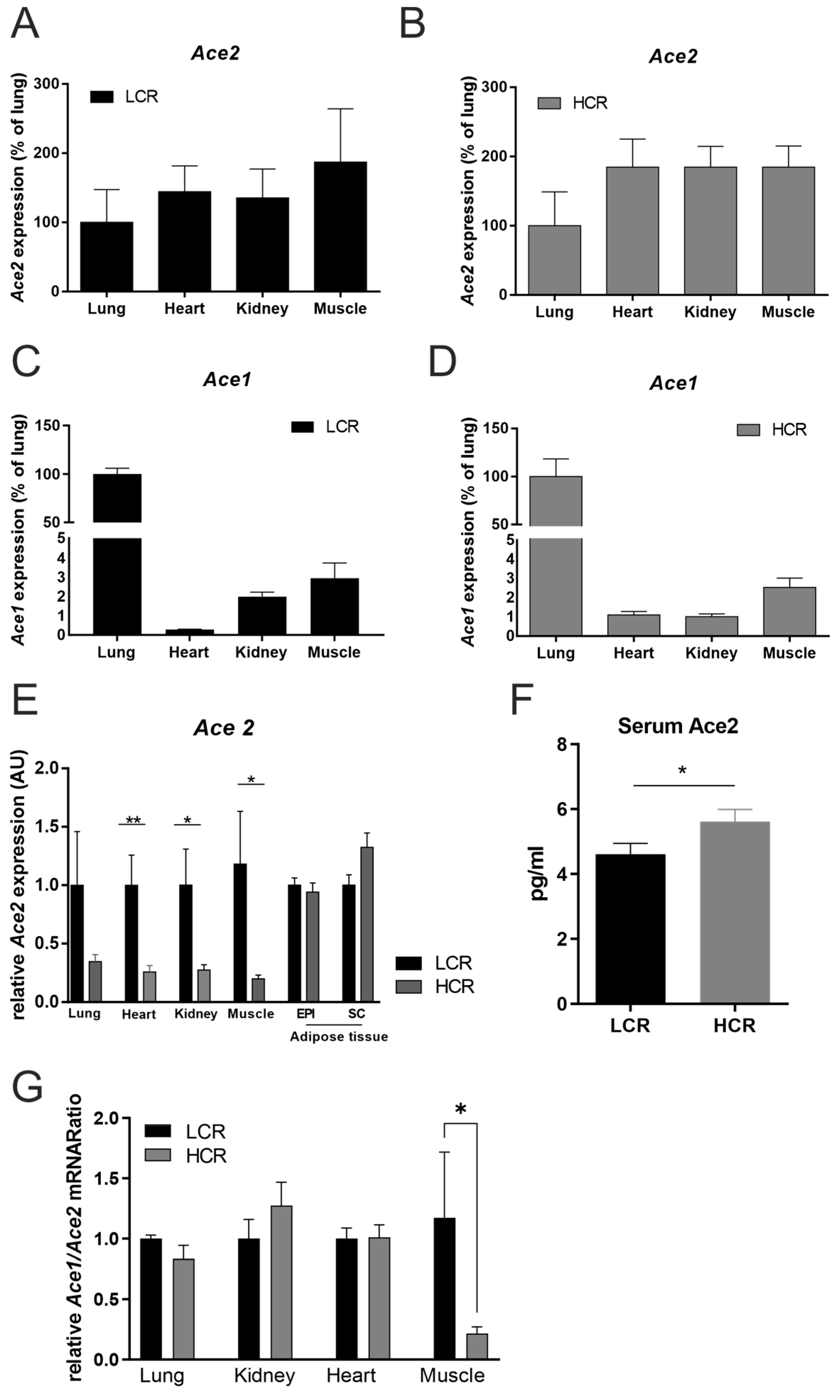
2.1. HCR Rats Are Leaner and Have Less ACE2 mRNA Tissue Expression
2.2. Metabolome Analysis
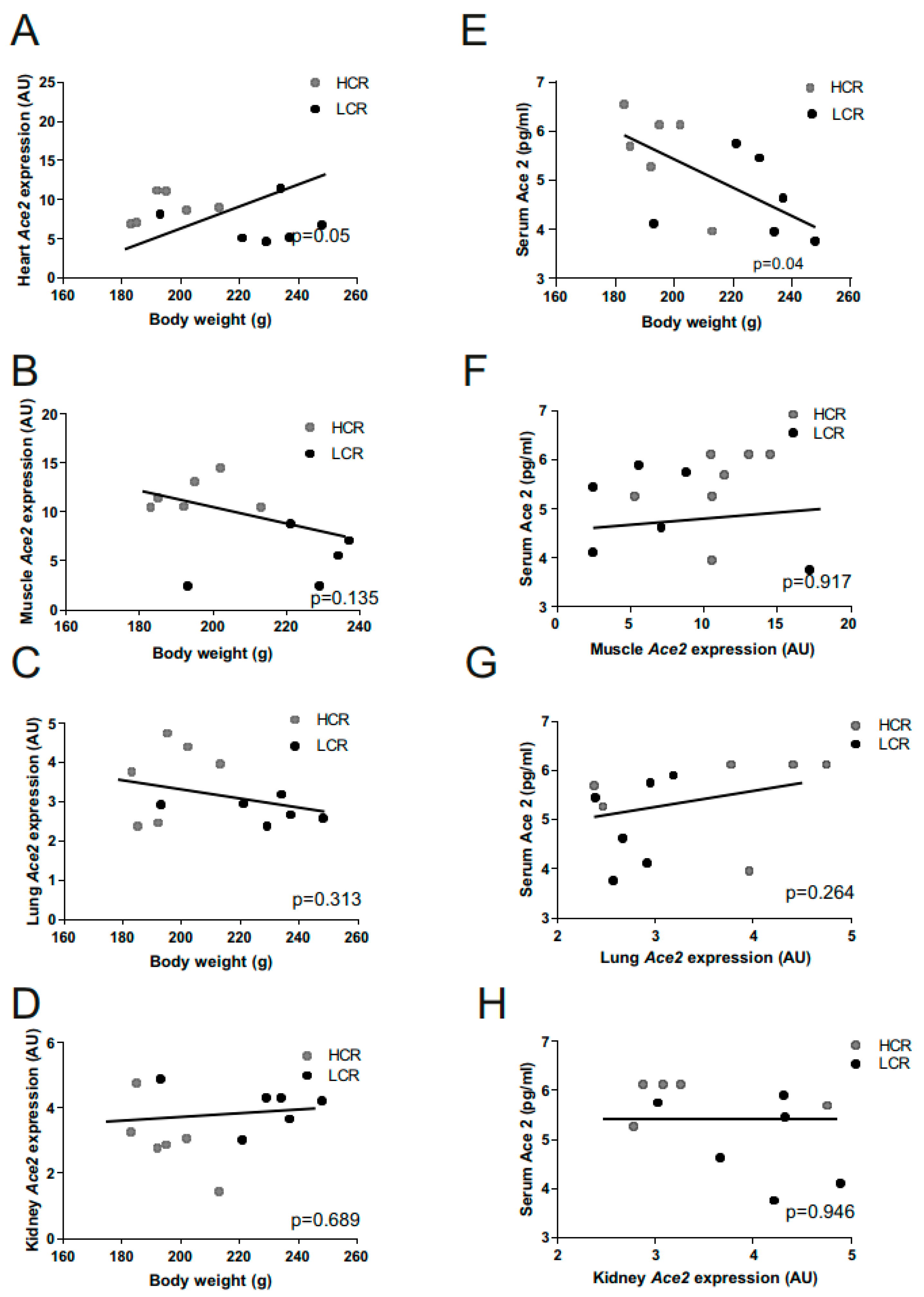
2.3. Ace2 and Body Weight Correlations
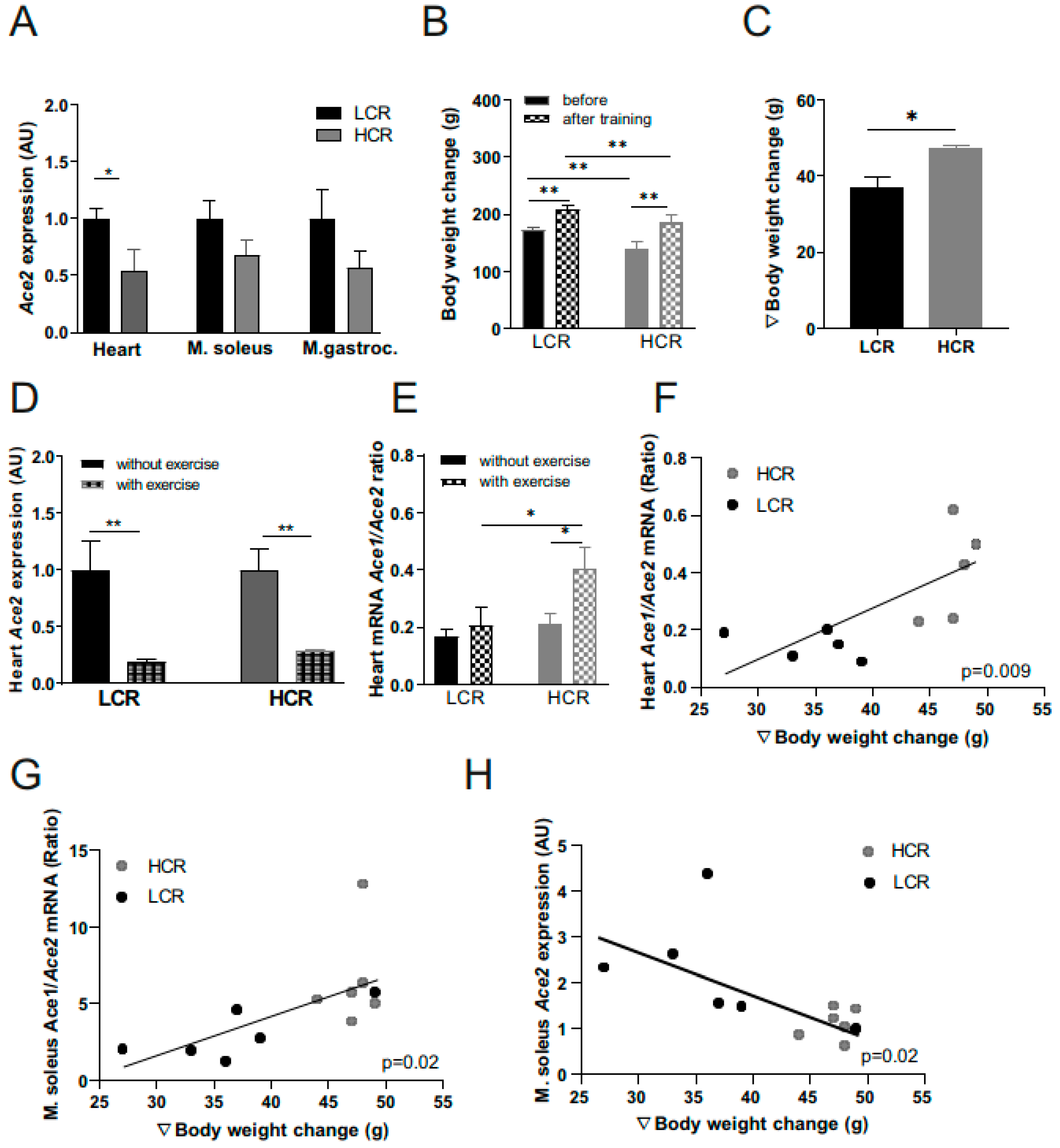
2.4. Effects of Exercise Training on Ace2 Muscle Expression
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Exercise Training Intervention
4.3. RNA Isolation, Ace 1 and Ace 2 Gene Expression, and Ace2 Serum Concentrations
4.4. Metabolome Analysis, Data Processing, and Normalization
4.5. Data analyses and Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salinas-Aguirre, J.E.; Sánchez-García, C.; Rodríguez-Sanchez, R.; Rodríguez-Muñoz, L.; Díaz-Castaño, A.; Bernal-Gómez, R. Características clínicas y comorbilidades asociadas a mortalidad en pacientes con COVID-19 en Coahuila (México). Rev. Clin. Esp. 2022, 222, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Kim, C.W.; Aronow, W.S.; Frishman, W.H. Coronavirus Disease 2019 and Cardiometabolic Disease. Cardiol. Rev. 2022, 30, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Penninger, J.M.; Li, Y.; Zhong, N.; Slutsky, A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: Molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020, 46, 586–590. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Benthin, C.; Zeno, B.; Albertson, T.E.; Boyd, J.; Christie, J.D.; Hall, R.; Poirier, G.; Ronco, J.J.; Tidswell, M.; et al. A pilot clinical trial of recombinant human angiotensin-converting enzyme 2 in acute respiratory distress syndrome. Crit. Care 2017, 21, 234. [Google Scholar] [CrossRef]
- Reynolds, H.R.; Adhikari, S.; Pulgarin, C.; Troxel, A.B.; Iturrate, E.; Johnson, S.B.; Hausvater, A.; Newman, J.D.; Berger, J.S.; Bangalore, S.; et al. Renin-Angiotensin-Aldosterone System Inhibitors and Risk of COVID-19. N. Engl. J. Med. 2020, 382, 2441–2448. [Google Scholar] [CrossRef]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; Barnaby, D.P.; Becker, L.B.; Chelico, J.D.; Cohen, S.L.; et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA 2020, 323, 2052–2059. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Chen, J.; Zhang, H.; Deng, A. Association of Renin-Angiotensin System Inhibitors With Severity or Risk of Death in Patients With Hypertension Hospitalized for Coronavirus Disease 2019 (COVID-19) Infection in Wuhan, China. JAMA Cardiol. 2020, 5, 825–830. [Google Scholar] [CrossRef]
- Magalhães, D.M.; Nunes-Silva, A.; Rocha, G.C.; Vaz, L.N.; de Faria, M.H.S.; Vieira, E.L.M.; Rocha, N.P.; e Silva, A.C.S. Two protocols of aerobic exercise modulate the counter-regulatory axis of the renin-angiotensin system. Heliyon 2020, 6, e03208. [Google Scholar] [CrossRef]
- Motta-Santos, D.; Dos Santos, R.A.S.; Oliveira, M.; Qadri, F.; Poglitsch, M.; Mosienko, V.; Becker, L.K.; Campagnole-Santos, M.; Penninger, J.; Alenina, N.; et al. Effects of ACE2 deficiency on physical performance and physiological adaptations of cardiac and skeletal muscle to exercise. Hypertens. Res. 2016, 39, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Singer, D.; Camargo, S.M.R.; Ramadan, T.; Schäfer, M.; Mariotta, L.; Herzog, B.; Huggel, K.; Wolfer, D.; Werner, S.; Penninger, J.M.; et al. Defective intestinal amino acid absorption in Ace2 null mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G686–G695. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-Y. Can Glycine Mitigate COVID-19 Associated Tissue Damage and Cytokine Storm? Radiat. Res. 2020, 194, 199–201. [Google Scholar] [CrossRef] [PubMed]
- Semiz, S. SIT1 transporter as a potential novel target in treatment of COVID-19. Biomol. Concepts 2021, 12, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Schwarzer, M.; Britton, S.L.; Koch, L.G.; Wisloff, U.; Doenst, T. Low intrinsic aerobic exercise capacity and systemic insulin resistance are not associated with changes in myocardial substrate oxidation or insulin sensitivity. Basic Res. Cardiol. 2010, 105, 357–364. [Google Scholar] [CrossRef][Green Version]
- Koch, L.G.; Britton, S.L. Artificial selection for intrinsic aerobic endurance running capacity in rats. Physiol. Genom. 2001, 5, 45–52. [Google Scholar] [CrossRef]
- Guan, W.-J.; Ni, Z.-Y.; Hu, Y.; Liang, W.-H.; Ou, C.-Q.; He, J.-X.; Liu, L.; Shan, H.; Lei, C.-L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Garg, S.; Kim, L.; Whitaker, M.; O’Halloran, A.; Cummings, C.; Holstein, R.; Prill, M.; Chai, S.J.; Kirley, P.D.; Alden, N.B.; et al. Hospitalization Rates and Characteristics of Patients Hospitalized with Laboratory-Confirmed Coronavirus Disease 2019—COVID-NET, 14 States, 1–30 March 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 458–464. [Google Scholar] [CrossRef]
- Rahmati-Ahmadabad, S.; Hosseini, F. Exercise against SARS-CoV-2 (COVID-19): Does workout intensity matter? (A mini review of some indirect evidence related to obesity). Obes. Med. 2020, 19, 100245. [Google Scholar] [CrossRef]
- Simpson, R.J.; Campbell, J.P.; Gleeson, M.; Krüger, K.; Nieman, D.C.; Pyne, D.B.; Turner, J.E.; Walsh, N.P. Can exercise affect immune function to increase susceptibility to infection? Exerc. Immunol. Rev. 2020, 26, 8–22. [Google Scholar]
- Zbinden-Foncea, H.; Francaux, M.; Deldicque, L.; Hawley, J.A. Does High Cardiorespiratory Fitness Confer Some Protection Against Proinflammatory Responses After Infection by SARS-CoV-2? Obesity 2020, 28, 1378–1381. [Google Scholar] [CrossRef] [PubMed]
- Kruglikov, I.L.; Scherer, P.E. The Role of Adipocytes and Adipocyte-Like Cells in the Severity of COVID-19 Infections. Obesity 2020, 28, 1187–1190. [Google Scholar] [CrossRef] [PubMed]
- Klöting, N.; Ristow, M.; Blüher, M. Effects of Exercise on ACE2. Obesity 2020, 28, 2266–2267. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Sá, G.; Dos Santos Neves, V.; de Oliveira Fraga, S.R.; Souza-Mello, V.; Barbosa-da-Silva, S. High-intensity interval training has beneficial effects on cardiac remodeling through local renin-angiotensin system modulation in mice fed high-fat or high-fructose diets. Life Sci. 2017, 189, 8–17. [Google Scholar] [CrossRef]
- Parthimos, T.P.; Schulpis, K.H.; Karousi, A.D.; Loukas, Y.L.; Dotsikas, Y. The relationship between neurotransmission-related amino acid blood concentrations and neuropsychological performance following acute exercise. Appl. Neuropsychol. Adult 2022, 1–15. [Google Scholar] [CrossRef]
- Irving, B.A.; Carter, R.E.; Soop, M.; Weymiller, A.; Syed, H.; Karakelides, H.; Bhagra, S.; Short, K.R.; Tatpati, L.; Barazzoni, R.; et al. Effect of insulin sensitizer therapy on amino acids and their metabolites. Metabolism 2015, 64, 720–728. [Google Scholar] [CrossRef]
- Felig, P.; Marliss, E.; Cahill, G.F. Plasma amino acid levels and insulin secretion in obesity. N. Engl. J. Med. 1969, 281, 811–816. [Google Scholar] [CrossRef]
- White, P.J.; McGarrah, R.W.; Herman, M.A.; Bain, J.R.; Shah, S.H.; Newgard, C.B. Insulin action, type 2 diabetes, and branched-chain amino acids: A two-way street. Mol. Metab. 2021, 52, 101261. [Google Scholar] [CrossRef]
- Vaduganathan, M.; Vardeny, O.; Michel, T.; McMurray, J.J.V.; Pfeffer, M.A.; Solomon, S.D. Renin-Angiotensin-Aldosterone System Inhibitors in Patients with COVID-19. N. Engl. J. Med. 2020, 382, 1653–1659. [Google Scholar] [CrossRef]
- Brojakowska, A.; Narula, J.; Shimony, R.; Bander, J. Clinical Implications of SARS-CoV-2 Interaction With Renin Angiotensin System: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2020, 75, 3085–3095. [Google Scholar] [CrossRef]
- Chen, J.; Jiang, Q.; Xia, X.; Liu, K.; Yu, Z.; Tao, W.; Gong, W.; Han, J.-D.J. Individual variation of the SARS-CoV-2 receptor ACE2 gene expression and regulation. Aging Cell 2020, 19, e13168. [Google Scholar] [CrossRef] [PubMed]
- Gemmati, D.; Bramanti, B.; Serino, M.L.; Secchiero, P.; Zauli, G.; Tisato, V. COVID-19 and Individual Genetic Susceptibility/Receptivity: Role of ACE1/ACE2 Genes, Immunity, Inflammation and Coagulation. Might the Double X-chromosome in Females Be Protective against SARS-CoV-2 Compared to the Single X-Chromosome in Males? Int. J. Mol. Sci. 2020, 21, 3474. [Google Scholar] [CrossRef] [PubMed]
- Klöting, N.; Koch, L.; Wunderlich, T.; Kern, M.; Ruschke, K.; Krone, W.; Brüning, J.C.; Blüher, M. Autocrine IGF-1 action in adipocytes controls systemic IGF-1 concentrations and growth. Diabetes 2008, 57, 2074–2082. [Google Scholar] [CrossRef] [PubMed]
- Krowczynska, A.M.; Coutts, M.; Makrides, S.; Brawerman, G. The mouse homologue of the human acidic ribosomal phosphoprotein PO: A highly conserved polypeptide that is under translational control. Nucleic Acids Res. 1989, 17, 6408. [Google Scholar] [CrossRef]
- Lorenz, S.; Eszlinger, M.; Paschke, R.; Aust, G.; Weick, M.; Führer, D.; Krohn, K. Calcium signaling of thyrocytes is modulated by TSH through calcium binding protein expression. Biochim. Biophys. Acta 2010, 1803, 352–360. [Google Scholar] [CrossRef]
- Brauer, R.; Leichtle, A.B.; Fiedler, G.M.; Thiery, J.; Ceglarek, U. Preanalytical standardization of amino acid and acylcarnitine metabolite profiling in human blood using tandem mass spectrometry. Metabolomics 2011, 7, 344–352. [Google Scholar] [CrossRef]
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.-É.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef]
- Lei, C.; Qian, K.; Li, T.; Zhang, S.; Fu, W.; Ding, M.; Hu, S. Neutralization of SARS-CoV-2 spike pseudotyped virus by recombinant ACE2-Ig. Nat. Commun. 2020, 11, 2070. [Google Scholar] [CrossRef]

| Parameter | HCRs Trained (N = 5) | LCRs Trained (N = 5) |
|---|---|---|
| Body weight before training (g) | 140 ± 5 | 172 ± 5 *** |
| Body weight after training (g) | 202 ± 4 | 222 ± 7 *** |
| Heart weight (mg) | 728 ± 32 | 662 ± 14 *** |
| Ventricle weight (mg) | 691 ± 27 | 626 ± 14 ** |
| Lung weight (mg) | 967 ± 49 | 1004 ± 43 |
| Liver weight (g) | 9.85 ± 0.86 | 7.78 ± 0.27 *** |
| M. gastrocnemius weight (g) | 2.52 ± 0.09 | 2.82 ± 0.13 ** |
| M. soleus weight (mg) | 186 ± 16 | 177 ± 1 ** |
| Tibia length after training (mm) | 34.7 ± 0.2 | 35.0 ± 0.3 |
| Running capacity before training | ||
| Mean speed (m/min) | 52.0 ± 1.1 | 19.2 ± 0.8 *** |
| Maximal speed (m/min) | 56.8 ± 1.2 | 21.7 ± 0.7 *** |
| Running capacity after training | ||
| Mean speed (m/min) | 57.9 ± 0.9 | 29.9 ± 0.7 *** |
| Maximal speed (m/min) | 59.2 ± 0.8 | 31.9 ± 0.9 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klöting, N.; Schwarzer, M.; Heyne, E.; Ceglarek, U.; Hoffmann, A.; Krohn, K.; Doenst, T.; Blüher, M. Intrinsic Exercise Capacity Affects Glycine and Angiotensin-Converting Enzyme 2 (ACE2) Levels in Sedentary and Exercise Trained Rats. Metabolites 2022, 12, 548. https://doi.org/10.3390/metabo12060548
Klöting N, Schwarzer M, Heyne E, Ceglarek U, Hoffmann A, Krohn K, Doenst T, Blüher M. Intrinsic Exercise Capacity Affects Glycine and Angiotensin-Converting Enzyme 2 (ACE2) Levels in Sedentary and Exercise Trained Rats. Metabolites. 2022; 12(6):548. https://doi.org/10.3390/metabo12060548
Chicago/Turabian StyleKlöting, Nora, Michael Schwarzer, Estelle Heyne, Uta Ceglarek, Anne Hoffmann, Knut Krohn, Torsten Doenst, and Matthias Blüher. 2022. "Intrinsic Exercise Capacity Affects Glycine and Angiotensin-Converting Enzyme 2 (ACE2) Levels in Sedentary and Exercise Trained Rats" Metabolites 12, no. 6: 548. https://doi.org/10.3390/metabo12060548
APA StyleKlöting, N., Schwarzer, M., Heyne, E., Ceglarek, U., Hoffmann, A., Krohn, K., Doenst, T., & Blüher, M. (2022). Intrinsic Exercise Capacity Affects Glycine and Angiotensin-Converting Enzyme 2 (ACE2) Levels in Sedentary and Exercise Trained Rats. Metabolites, 12(6), 548. https://doi.org/10.3390/metabo12060548







