Metabolic Profile and Metabolite Analyses in Extreme Weight Responders to Gastric Bypass Surgery
Abstract
:1. Introduction
2. Results
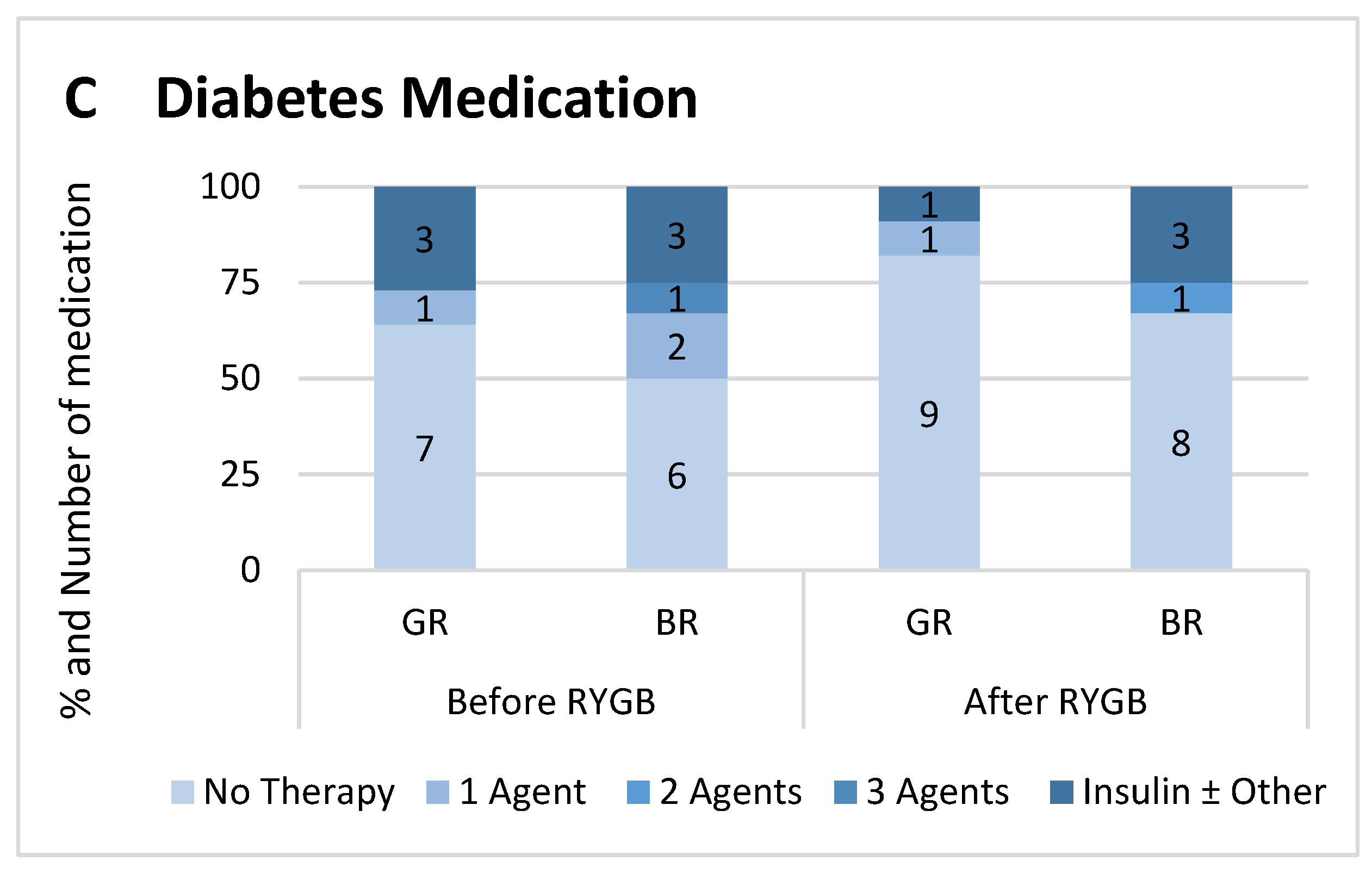
2.1. Study Cohort and Metabolic Profile
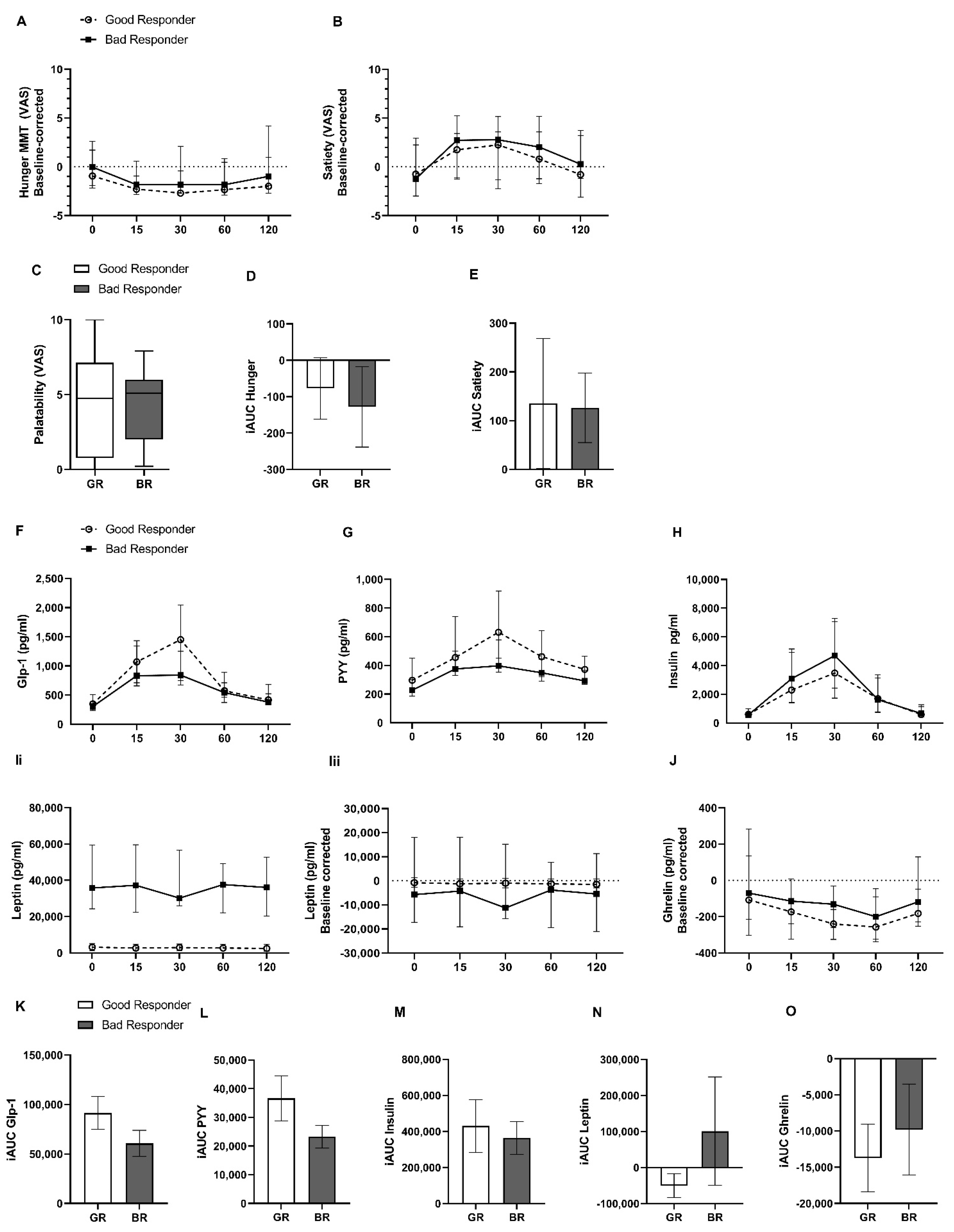
2.2. Hunger and Satiety Rating and Gut Hormone Release during Standardized Mixed-Meal Test
2.3. Fecal Microbiota Composition and Metabolomics
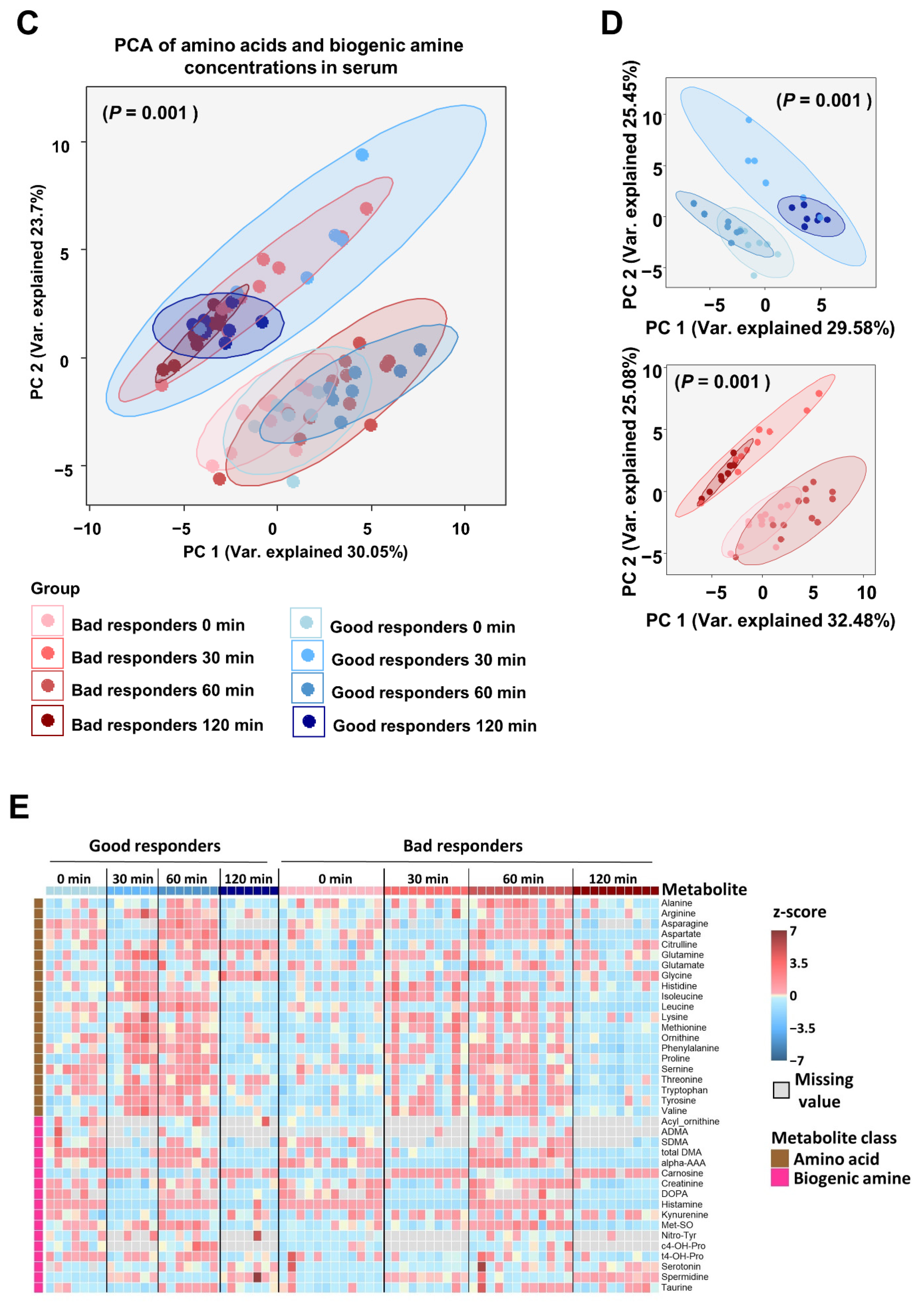
2.3.1. Fecal Microbiota Composition and Bacterial Metabolites
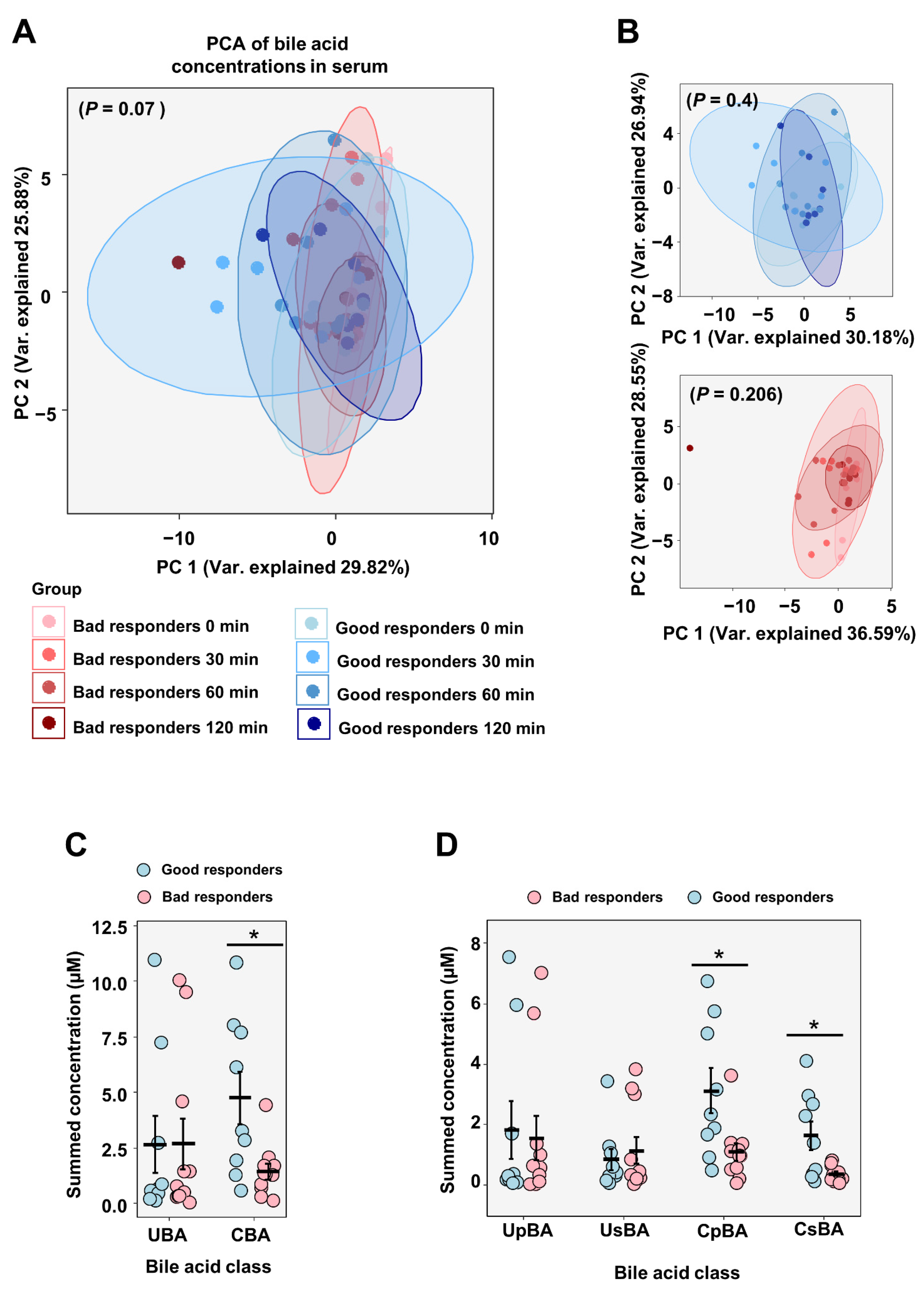
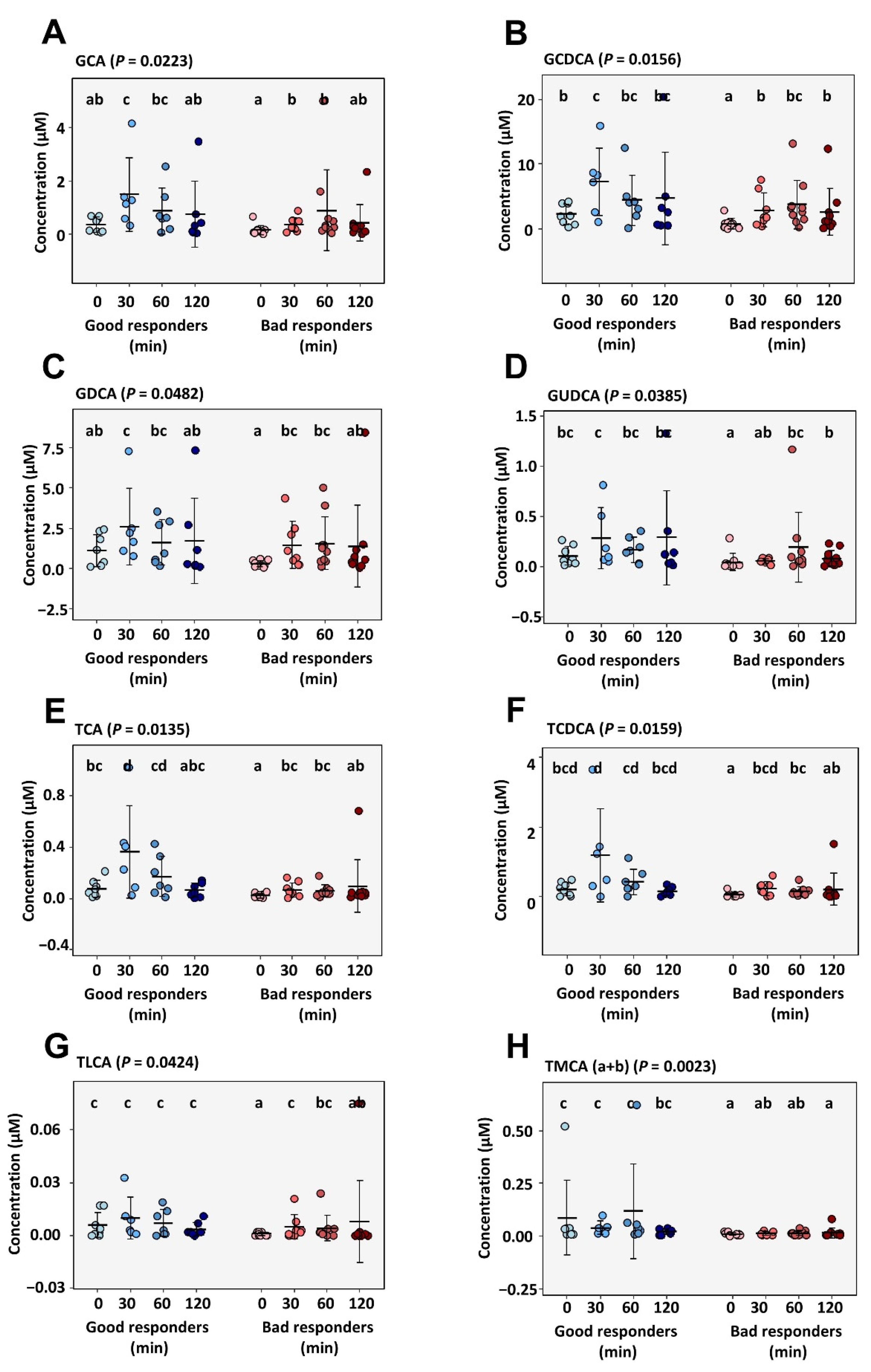
2.3.2. Circulating Metabolites and Bile Acids
3. Discussion
4. Materials and Methods
4.1. Study Cohort and Test Date
4.2. Bariatric Surgery
4.3. Microbiome Analyses
4.4. Mass Spectrometric Measurements
4.5. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wing, R.R.; Bolin, P.; Brancati, F.L.; Bray, G.A.; Clark, J.M.; Coday, M.; Crow, R.S.; Curtis, J.M.; Egan, C.M.; Espeland, M.A.; et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N. Engl. J. Med. 2013, 369, 145–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schauer, P.R.; Mingrone, G.; Ikramuddin, S.; Wolfe, B. Clinical Outcomes of Metabolic Surgery: Efficacy of Glycemic Control, Weight Loss, and Remission of Diabetes. Diabetes Care 2016, 39, 902–911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sjöström, L.; Peltonen, M.; Jacobson, P.; Ahlin, S.; Andersson-Assarsson, J.; Anveden, Å.; Bouchard, C.; Carlsson, B.; Karason, K.; Lönroth, H.; et al. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA 2014, 311, 2297–2304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gloy, V.L.; Briel, M.; Bhatt, D.L.; Kashyap, S.R.; Schauer, P.R.; Mingrone, G.; Bucher, H.C.; Nordmann, A.J. Bariatric surgery versus non-surgical treatment for obesity: A systematic review and meta-analysis of randomised controlled trials. BMJ 2013, 347, f5934. [Google Scholar] [CrossRef] [Green Version]
- Schauer, P.R.; Bhatt, D.L.; Kirwan, J.P.; Wolski, K.; Aminian, A.; Brethauer, S.A.; Navaneethan, S.D.; Singh, R.P.; Pothier, C.E.; Nissen, S.E.; et al. Bariatric Surgery versus Intensive Medical Therapy for Diabetes—5-Year Outcomes. N. Engl. J. Med. 2017, 376, 641–651. [Google Scholar] [CrossRef] [Green Version]
- Fakhry, T.K.; Mhaskar, R.; Schwitalla, T.; Muradova, E.; Gonzalvo, J.P.; Murr, M.M. Bariatric surgery improves nonalcoholic fatty liver disease: A contemporary systematic review and meta-analysis. Surg. Obes. Relat. Dis. 2019, 15, 502–511. [Google Scholar] [CrossRef]
- Dogan, K.; Betzel, B.; Homan, J.; Aarts, E.O.; Ploeger, N.; de Boer, H.; Aufenacker, T.J.; van Laarhoven, C.J.H.M.; Janssen, I.M.C.; Berends, F.J. Long-term effects of laparoscopic Roux-en-Y gastric bypass on diabetes mellitus, hypertension and dyslipidaemia in morbidly obese patients. Obes. Surg. 2014, 24, 1835–1842. [Google Scholar] [CrossRef]
- Welbourn, R.; Hollyman, M.; Kinsman, R.; Dixon, J.; Liem, R.; Ottosson, J.; Ramos, A.; Våge, V.; Al-Sabah, S.; Brown, W.; et al. Bariatric Surgery Worldwide: Baseline Demographic Description and One-Year Outcomes from the Fourth IFSO Global Registry Report 2018. Obes. Surg. 2019, 29, 782–795. [Google Scholar] [CrossRef] [Green Version]
- Adams, T.D.; Davidson, L.E.; Litwin, S.E.; Kim, J.; Kolotkin, R.L.; Nanjee, M.N.; Gutierrez, J.M.; Frogley, S.J.; Ibele, A.R.; Brinton, E.A.; et al. Weight and Metabolic Outcomes 12 Years after Gastric Bypass. N. Engl. J. Med. 2017, 377, 1143–1155. [Google Scholar] [CrossRef]
- El Ansari, W.; Elhag, W. Weight Regain and Insufficient Weight Loss After Bariatric Surgery: Definitions, Prevalence, Mechanisms, Predictors, Prevention and Management Strategies, and Knowledge Gaps-a Scoping Review. Obes. Surg. 2021, 31, 1755–1766. [Google Scholar] [CrossRef]
- Magro, D.O.; Geloneze, B.; Delfini, R.; Pareja, B.C.; Callejas, F.; Pareja, J.C. Long-term Weight Regain after Gastric Bypass: A 5-year Prospective Study. Obes. Surg. 2008, 18, 648–651. [Google Scholar] [CrossRef]
- Robinson, A.H.; Adler, S.; Stevens, H.B.; Darcy, A.M.; Morton, J.M.; Safer, D.L. What variables are associated with successful weight loss outcomes for bariatric surgery after 1 year? Surg. Obes. Relat. Dis. 2014, 10, 697–704. [Google Scholar] [CrossRef] [Green Version]
- Courcoulas, A.P.; King, W.C.; Belle, S.H.; Berk, P.; Flum, D.R.; Garcia, L.; Gourash, W.; Horlick, M.; Mitchell, J.E.; Pomp, A.; et al. Seven-Year Weight Trajectories and Health Outcomes in the Longitudinal Assessment of Bariatric Surgery (LABS) Study. JAMA Surg. 2018, 153, 427–434. [Google Scholar] [CrossRef]
- Monpellier, V.M.; Antoniou, E.E.; Aarts, E.O.; Janssen, I.M.C.; Jansen, A.T.M. Improvement of Health-Related Quality of Life After Roux-en-Y Gastric Bypass Related to Weight Loss. Obes. Surg. 2017, 27, 1168–1173. [Google Scholar] [CrossRef] [Green Version]
- Sundbom, M.; Hedberg, J.; Marsk, R.; Boman, L.; Bylund, A.; Hedenbro, J.; Laurenius, A.; Lundegårdh, G.; Möller, P.; Olbers, T.; et al. Substantial Decrease in Comorbidity 5 Years After Gastric Bypass: A Population-based Study from the Scandinavian Obesity Surgery Registry. Ann. Surg. 2017, 265, 1166–1171. [Google Scholar] [CrossRef] [Green Version]
- Kolotkin, R.L.; Andersen, J.R. A systematic review of reviews: Exploring the relationship between obesity, weight loss and health-related quality of life. Clin. Obes. 2017, 7, 273–289. [Google Scholar] [CrossRef] [Green Version]
- Andalib, A.; Alamri, H.; Almuhanna, Y.; Bouchard, P.; Demyttenaere, S.; Court, O. Short-term outcomes of revisional surgery after sleeve gastrectomy: A comparative analysis of re-sleeve, Roux en-Y gastric bypass, duodenal switch (Roux en-Y and single-anastomosis). Surg. Endosc. 2021, 35, 4644–4652. [Google Scholar] [CrossRef]
- Bonouvrie, D.S.; Uittenbogaart, M.; Luijten, A.A.P.M.; van Dielen, F.M.H.; Leclercq, W.K.G. Lack of Standard Definitions of Primary and Secondary (Non)responders After Primary Gastric Bypass and Gastric Sleeve: A Systematic Review. Obes. Surg. 2019, 29, 691–697. [Google Scholar] [CrossRef]
- Mann, J.P.; Jakes, A.D.; Hayden, J.D.; Barth, J.H. Systematic Review of Definitions of Failure in Revisional Bariatric Surgery. Obes. Surg. 2015, 25, 571–574. [Google Scholar] [CrossRef]
- Bittner, J.G.; Clingempeel, N.L.; Wolf, L.G. Weight Loss Failure and Reoperation After Laparoscopic Adjustable Gastric Banding and Gastric Bypass: A Case-Matched Cohort Study. Obes. Surg. 2017, 27, 2885–2889. [Google Scholar] [CrossRef]
- Melton, G.B.; Steele, K.E.; Schweitzer, M.A.; Lidor, A.O.; Magnuson, T.H. Suboptimal Weight Loss after Gastric Bypass Surgery: Correlation of Demographics, Comorbidities, and Insurance Status with Outcomes. J. Gastrointest. Surg. 2008, 12, 250–255. [Google Scholar] [CrossRef]
- Pinto-Bastos, A.; Conceição, E.M.; Machado, P.P.P. Reoperative Bariatric Surgery: A Systematic Review of the Reasons for Surgery, Medical and Weight Loss Outcomes, Relevant Behavioral Factors. Obes. Surg. 2017, 27, 2707–2715. [Google Scholar] [CrossRef]
- Sima, E.; Webb, D.-L.; Hellström, P.M.; Sundbom, M. Non-responders After Gastric Bypass Surgery for Morbid Obesity: Peptide Hormones and Glucose Homeostasis. Obes. Surg. 2019, 29, 4008–4017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vitolo, E.; Santini, E.; Seghieri, M.; Giannini, L.; Coppedè, F.; Rossi, C.; Dardano, A.; Solini, A. Heterozygosity for the rs696217 SNP in the Preproghrelin Gene Predicts Weight Loss After Bariatric Surgery in Severely Obese Individuals. Obes. Surg. 2017, 27, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Bandstein, M.; Voisin, S.; Nilsson, E.K.; Schultes, B.; Ernst, B.; Thurnheer, M.; Benedict, C.; Mwinyi, J.; Schiöth, H.B. A Genetic Risk Score Is Associated with Weight Loss Following Roux-en Y Gastric Bypass Surgery. Obes. Surg. 2016, 26, 2183–2189. [Google Scholar] [CrossRef] [Green Version]
- Kaouk, L.; Hsu, A.T.; Tanuseputro, P.; Jessri, M. Modifiable factors associated with weight regain after bariatric surgery: A scoping review. F1000Research 2019, 8, 615. [Google Scholar] [CrossRef]
- Karmali, S.; Brar, B.; Shi, X.; Sharma, A.M.; De Gara, C.; Birch, D.W. Weight Recidivism Post-Bariatric Surgery: A Systematic Review. Obes. Surg. 2013, 23, 1922–1933. [Google Scholar] [CrossRef]
- Herzog, K.; Berggren, J.; Al Majdoub, M.; Arroyo, C.B.; Lindqvist, A.; Hedenbro, J.; Groop, L.; Wierup, N.; Spégel, P. Metabolic Effects of Gastric Bypass Surgery: Is It All About Calories? Diabetes 2020, 69, 2027–2035. [Google Scholar] [CrossRef]
- Schlottmann, F.; Galvarini, M.M.; Dreifuss, N.H.; Laxague, F.; Buxhoeveden, R.; Gorodner, V. Metabolic Effects of Bariatric Surgery. J. Laparoendosc. Adv. Surg. Tech. 2018, 28, 944–948. [Google Scholar] [CrossRef]
- Xu, G.; Song, M. Recent advances in the mechanisms underlying the beneficial effects of bariatric and metabolic surgery. Surg. Obes. Relat. Dis. 2021, 17, 231–238. [Google Scholar] [CrossRef]
- Liu, R.; Hong, J.; Xu, X.; Feng, Q.; Zhang, D.; Gu, Y.; Shi, J.; Zhao, S.; Liu, W.; Wang, X.; et al. Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention. Nat. Med. 2017, 23, 859–868. [Google Scholar] [CrossRef]
- Haange, S.-B.; Jehmlich, N.; Krügel, U.; Hintschich, C.; Wehrmann, D.; Hankir, M.; Seyfried, F.; Froment, J.; Hübschmann, T.; Müller, S.; et al. Gastric bypass surgery in a rat model alters the community structure and functional composition of the intestinal microbiota independently of weight loss. Microbiome 2020, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Haase, N.; Haange, S.-B.; Sucher, R.; Münzker, J.; Jäger, E.; Schischke, K.; Seyfried, F.; von Bergen, M.; Hankir, M.K.; et al. Roux-en-Y gastric bypass contributes to weight loss-independent improvement in hypothalamic inflammation and leptin sensitivity through gut-microglia-neuron-crosstalk. Mol. Metab. 2021, 48, 101214. [Google Scholar] [CrossRef] [PubMed]
- Medawar, E.; Haange, S.-B.; Rolle-Kampczyk, U.; Engelmann, B.; Dietrich, A.; Thieleking, R.; Wiegank, C.; Fries, C.; Horstmann, A.; Villringer, A.; et al. Gut microbiota link dietary fiber intake and short-chain fatty acid metabolism with eating behavior. Transl. Psychiatry 2021, 11, 500. [Google Scholar] [CrossRef] [PubMed]
- Liou, A.P.; Paziuk, M.; Luevano, J.-M.; Machineni, S.; Turnbaugh, P.J.; Kaplan, L.M. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci. Transl. Med. 2013, 5, 178ra41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryan, K.K.; Tremaroli, V.; Clemmensen, C.; Kovatcheva-Datchary, P.; Myronovych, A.; Karns, R.; Wilson-Pérez, H.E.; Sandoval, D.A.; Kohli, R.; Bäckhed, F.; et al. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature 2014, 509, 183–188. [Google Scholar] [CrossRef] [Green Version]
- Albaugh, V.L.; Banan, B.; Antoun, J.; Xiong, Y.; Guo, Y.; Ping, J.; Alikhan, M.; Clements, B.A.; Abumrad, N.N.; Flynn, C.R. Role of Bile Acids and GLP-1 in Mediating the Metabolic Improvements of Bariatric Surgery. Gastroenterology 2019, 156, 1041–1051.e4. [Google Scholar] [CrossRef] [Green Version]
- Flynn, C.R.; Albaugh, V.L.; Abumrad, N.N. Metabolic Effects of Bile Acids: Potential Role in Bariatric Surgery. Cell. Mol. Gastroenterol. Hepatol. 2019, 8, 235–246. [Google Scholar] [CrossRef] [Green Version]
- Flynn, C.R.; Albaugh, V.L.; Cai, S.; Cheung-Flynn, J.; Williams, P.E.; Brucker, R.M.; Bordenstein, S.R.; Guo, Y.; Wasserman, D.H.; Abumrad, N.N. Bile diversion to the distal small intestine has comparable metabolic benefits to bariatric surgery. Nat. Commun. 2015, 6, 7715. [Google Scholar] [CrossRef]
- Riddle, M.C.; Cefalu, W.T.; Evans, P.H.; Gerstein, H.C.; Nauck, M.A.; Oh, W.K.; Rothberg, A.E.; Le Roux, C.W.; Rubino, F.; Schauer, P.; et al. Consensus Report: Definition and Interpretation of Remission in Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2022, 107, 1–9. [Google Scholar] [CrossRef]
- Al-Khyatt, W.; Ryall, R.; Leeder, P.; Ahmed, J.; Awad, S. Predictors of Inadequate Weight Loss After Laparoscopic Gastric Bypass for Morbid Obesity. Obes. Surg. 2017, 27, 1446–1452. [Google Scholar] [CrossRef]
- Colles, S.L.; Dixon, J.B.; O’Brien, P.E. Grazing and loss of control related to eating: Two high-risk factors following bariatric surgery. Obesity 2008, 16, 615–622. [Google Scholar] [CrossRef]
- Freire, R.H.; Borges, M.C.; Alvarez-Leite, J.I.; Correia, M.I.T.D. Food quality, physical activity, and nutritional follow-up as determinant of weight regain after Roux-en-Y gastric bypass. Nutrition 2012, 28, 53–58. [Google Scholar] [CrossRef]
- Kalarchian, M.A.; Marcus, M.D.; Wilson, G.T.; Labouvie, E.W.; Brolin, R.E.; LaMarca, L.B. Binge eating among gastric bypass patients at long-term follow-up. Obes. Surg. 2002, 12, 270–275. [Google Scholar] [CrossRef]
- Hilbert, A.; de Zwaan, M.; Braehler, E. How frequent are eating disturbances in the population? Norms of the eating disorder examination-questionnaire. PLoS ONE 2012, 7, e29125. [Google Scholar] [CrossRef] [Green Version]
- Yoshino, M.; Kayser, B.D.; Yoshino, J.; Stein, R.I.; Reeds, D.; Eagon, J.C.; Eckhouse, S.R.; Watrous, J.D.; Jain, M.; Knight, R.; et al. Effects of Diet versus Gastric Bypass on Metabolic Function in Diabetes. N. Engl. J. Med. 2020, 383, 721–732. [Google Scholar] [CrossRef]
- Haskins, I.N.; Corcelles, R.; Froylich, D.; Boules, M.; Hag, A.; Burguera, B.; Schauer, P.R.; Kroh, M.; Brethauer, S.A. Primary Inadequate Weight Loss After Roux-en-Y Gastric Bypass Is not Associated with Poor Cardiovascular or Metabolic Outcomes: Experience from a Single Institution. Obes. Surg. 2017, 27, 676–680. [Google Scholar] [CrossRef]
- Gil, S.; Goessler, K.; Dantas, W.S.; Murai, I.H.; Merege-Filho, C.A.A.; Pereira, R.M.R.; de Cleva, R.; Santo, M.A.; Kirwan, J.P.; Roschel, H.; et al. Constraints of Weight Loss as a Marker of Bariatric Surgery Success: An Exploratory Study. Front. Physiol. 2021, 12, 640191. [Google Scholar] [CrossRef]
- Ryan, D.H.; Yockey, S.R. Weight Loss and Improvement in Comorbidity: Differences at 5%, 10%, 15%, and over. Curr. Obes. Rep. 2017, 6, 187–194. [Google Scholar] [CrossRef]
- Magkos, F.; Fraterrigo, G.; Yoshino, J.; Luecking, C.; Kirbach, K.; Kelly, S.C.; de Las Fuentes, L.; He, S.; Okunade, A.L.; Patterson, B.W.; et al. Effects of Moderate and Subsequent Progressive Weight Loss on Metabolic Function and Adipose Tissue Biology in Humans with Obesity. Cell Metab. 2016, 23, 591–601. [Google Scholar] [CrossRef] [Green Version]
- Hankir, M.K.; Seyfried, F.; Miras, A.D.; Cowley, M.A. Brain Feeding Circuits after Roux-en-Y Gastric Bypass. Trends Endocrinol. Metab. 2018, 29, 218–237. [Google Scholar] [CrossRef] [Green Version]
- Hao, Z.; Townsend, R.L.; Mumphrey, M.B.; Patterson, L.M.; Ye, J.; Berthoud, H.-R. Vagal innervation of intestine contributes to weight loss After Roux-en-Y gastric bypass surgery in rats. Obes. Surg. 2014, 24, 2145–2151. [Google Scholar] [CrossRef] [Green Version]
- Mokadem, M.; Zechner, J.F.; Margolskee, R.F.; Drucker, D.J.; Aguirre, V. Effects of Roux-en-Y gastric bypass on energy and glucose homeostasis are preserved in two mouse models of functional glucagon-like peptide-1 deficiency. Mol. Metab. 2014, 3, 191–201. [Google Scholar] [CrossRef]
- Ye, J.; Hao, Z.; Mumphrey, M.B.; Townsend, R.L.; Patterson, L.M.; Stylopoulos, N.; Münzberg, H.; Morrison, C.D.; Drucker, D.J.; Berthoud, H.-R. GLP-1 receptor signaling is not required for reduced body weight after RYGB in rodents. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 306, R352–R362. [Google Scholar] [CrossRef] [Green Version]
- Salehi, M.; Gastaldelli, A.; D’Alessio, D.A. Blockade of glucagon-like peptide 1 receptor corrects postprandial hypoglycemia after gastric bypass. Gastroenterology 2014, 146, 669–680.e2. [Google Scholar] [CrossRef] [Green Version]
- Shah, M.; Law, J.H.; Micheletto, F.; Sathananthan, M.; Man, C.D.; Cobelli, C.; Rizza, R.A.; Camilleri, M.; Zinsmeister, A.R.; Vella, A. Contribution of endogenous glucagon-like peptide 1 to glucose metabolism after Roux-en-Y gastric bypass. Diabetes 2014, 63, 483–493. [Google Scholar] [CrossRef] [Green Version]
- Psichas, A.; Sleeth, M.L.; Murphy, K.G.; Brooks, L.; Bewick, G.A.; Hanyaloglu, A.C.; Ghatei, M.A.; Bloom, S.R.; Frost, G. The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents. Int. J. Obes. 2015, 39, 424–429. [Google Scholar] [CrossRef] [Green Version]
- Fouladi, F.; Brooks, A.E.; Fodor, A.A.; Carroll, I.M.; Bulik-Sullivan, E.C.; Tsilimigras, M.C.B.; Sioda, M.; Steffen, K.J. The Role of the Gut Microbiota in Sustained Weight Loss Following Roux-en-Y Gastric Bypass Surgery. Obes. Surg. 2019, 29, 1259–1267. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Fan, C.; Li, P.; Lu, Y.; Chang, X.; Qi, K. Short Chain Fatty Acids Prevent High-fat-diet-induced Obesity in Mice by Regulating G Protein-coupled Receptors and Gut Microbiota. Sci. Rep. 2016, 6, 37589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, X.; Zhang, X.; Gao, X.; Yi, Y.; Hou, Y.; Meng, X.; Jia, C.; Chao, B.; Fan, W.; Li, X.; et al. Effects of Inulin Propionate Ester on Obesity-Related Metabolic Syndrome and Intestinal Microbial Homeostasis in Diet-Induced Obese Mice. ACS Omega 2020, 5, 12865–12876. [Google Scholar] [CrossRef]
- Chambers, E.S.; Viardot, A.; Psichas, A.; Morrison, D.J.; Murphy, K.G.; Zac-Varghese, S.E.K.; MacDougall, K.; Preston, T.; Tedford, C.; Finlayson, G.S.; et al. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut 2015, 64, 1744–1754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuipers, F.; Bloks, V.W.; Groen, A.K. Beyond intestinal soap--bile acids in metabolic control. Nat. Rev. Endocrinol. 2014, 10, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.; Gioiello, A.; Noriega, L.; Strehle, A.; Oury, J.; Rizzo, G.; Macchiarulo, A.; Yamamoto, H.; Mataki, C.; Pruzanski, M.; et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009, 10, 167–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lefebvre, P.; Cariou, B.; Lien, F.; Kuipers, F.; Staels, B. Role of bile acids and bile acid receptors in metabolic regulation. Physiol. Rev. 2009, 89, 147–191. [Google Scholar] [CrossRef] [Green Version]
- Hylemon, P.B.; Zhou, H.; Pandak, W.M.; Ren, S.; Gil, G.; Dent, P. Bile acids as regulatory molecules. J. Lipid Res. 2009, 50, 1509–1520. [Google Scholar] [CrossRef] [Green Version]
- McGavigan, A.K.; Garibay, D.; Henseler, Z.M.; Chen, J.; Bettaieb, A.; Haj, F.G.; Ley, R.E.; Chouinard, M.L.; Cummings, B.P. TGR5 contributes to glucoregulatory improvements after vertical sleeve gastrectomy in mice. Gut 2017, 66, 226–234. [Google Scholar] [CrossRef] [Green Version]
- Browning, M.G.; Pessoa, B.M.; Khoraki, J.; Campos, G.M. Changes in Bile Acid Metabolism, Transport, and Signaling as Central Drivers for Metabolic Improvements After Bariatric Surgery. Curr. Obes. Rep. 2019, 8, 175–184. [Google Scholar] [CrossRef]
- Ahmad, N.N.; Pfalzer, A.; Kaplan, L.M. Roux-en-Y gastric bypass normalizes the blunted postprandial bile acid excursion associated with obesity. Int. J. Obes. 2013, 37, 1553–1559. [Google Scholar] [CrossRef] [Green Version]
- Tremaroli, V.; Karlsson, F.; Werling, M.; Ståhlman, M.; Kovatcheva-Datchary, P.; Olbers, T.; Fändriks, L.; Le Roux, C.W.; Nielsen, J.; Bäckhed, F. Roux-en-Y Gastric Bypass and Vertical Banded Gastroplasty Induce Long-Term Changes on the Human Gut Microbiome Contributing to Fat Mass Regulation. Cell Metab. 2015, 22, 228–238. [Google Scholar] [CrossRef] [Green Version]
- Nakatani, H.; Kasama, K.; Oshiro, T.; Watanabe, M.; Hirose, H.; Itoh, H. Serum bile acid along with plasma incretins and serum high-molecular weight adiponectin levels are increased after bariatric surgery. Metabolism 2009, 58, 1400–1407. [Google Scholar] [CrossRef]
- Pournaras, D.J.; Glicksman, C.; Vincent, R.P.; Kuganolipava, S.; Alaghband-Zadeh, J.; Mahon, D.; Bekker, J.H.R.; Ghatei, M.A.; Bloom, S.R.; Walters, J.R.F.; et al. The role of bile after Roux-en-Y gastric bypass in promoting weight loss and improving glycaemic control. Endocrinology 2012, 153, 3613–3619. [Google Scholar] [CrossRef] [Green Version]
- Simonen, M.; Dali-Youcef, N.; Kaminska, D.; Venesmaa, S.; Käkelä, P.; Pääkkönen, M.; Hallikainen, M.; Kolehmainen, M.; Uusitupa, M.; Moilanen, L.; et al. Conjugated bile acids associate with altered rates of glucose and lipid oxidation after Roux-en-Y gastric bypass. Obes. Surg. 2012, 22, 1473–1480. [Google Scholar] [CrossRef] [Green Version]
- Higashi, T.; Watanabe, S.; Tomaru, K.; Yamazaki, W.; Yoshizawa, K.; Ogawa, S.; Nagao, H.; Minato, K.; Maekawa, M.; Mano, N. Unconjugated bile acids in rat brain: Analytical method based on LC/ESI-MS/MS with chemical derivatization and estimation of their origin by comparison to serum levels. Steroids 2017, 125, 107–113. [Google Scholar] [CrossRef]
- Castellanos-Jankiewicz, A.; Guzmán-Quevedo, O.; Fénelon, V.S.; Zizzari, P.; Quarta, C.; Bellocchio, L.; Tailleux, A.; Charton, J.; Fernandois, D.; Henricsson, M.; et al. Hypothalamic bile acid-TGR5 signaling protects from obesity. Cell Metab. 2021, 33, 1483–1492.e10. [Google Scholar] [CrossRef]
- Ockenga, J.; Valentini, L.; Schuetz, T.; Wohlgemuth, F.; Glaeser, S.; Omar, A.; Kasim, E.; duPlessis, D.; Featherstone, K.; Davis, J.R.; et al. Plasma bile acids are associated with energy expenditure and thyroid function in humans. J. Clin. Endocrinol. Metab. 2012, 97, 535–542. [Google Scholar] [CrossRef] [Green Version]
- Patti, M.-E.; Houten, S.M.; Bianco, A.C.; Bernier, R.; Larsen, P.R.; Holst, J.J.; Badman, M.K.; Maratos-Flier, E.; Mun, E.C.; Pihlajamaki, J.; et al. Serum bile acids are higher in humans with prior gastric bypass: Potential contribution to improved glucose and lipid metabolism. Obesity 2009, 17, 1671–1677. [Google Scholar] [CrossRef] [Green Version]
- Chambers, E.S.; Byrne, C.S.; Aspey, K.; Chen, Y.; Khan, S.; Morrison, D.J.; Frost, G. Acute oral sodium propionate supplementation raises resting energy expenditure and lipid oxidation in fasted humans. Diabetes Obes. Metab. 2018, 20, 1034–1039. [Google Scholar] [CrossRef]
- Ben Izhak, M.; Eshel, A.; Cohen, R.; Madar-Shapiro, L.; Meiri, H.; Wachtel, C.; Leung, C.; Messick, E.; Jongkam, N.; Mavor, E.; et al. Projection of Gut Microbiome Pre- and Post-Bariatric Surgery to Predict Surgery Outcome. mSystems 2021, 6, e0136720. [Google Scholar] [CrossRef]
- Kootte, R.S.; Levin, E.; Salojärvi, J.; Smits, L.P.; Hartstra, A.V.; Udayappan, S.D.; Hermes, G.; Bouter, K.E.; Koopen, A.M.; Holst, J.J.; et al. Improvement of Insulin Sensitivity after Lean Donor Feces in Metabolic Syndrome Is Driven by Baseline Intestinal Microbiota Composition. Cell Metab. 2017, 26, 611–619.e6. [Google Scholar] [CrossRef] [Green Version]
- Olsson, L.M.; Boulund, F.; Nilsson, S.; Khan, M.T.; Gummesson, A.; Fagerberg, L.; Engstrand, L.; Perkins, R.; Uhlén, M.; Bergström, G.; et al. Dynamics of the normal gut microbiota: A longitudinal one-year population study in Sweden. Cell Host Microbe 2022, in press. [Google Scholar] [CrossRef]
- Larsen, O.F.A.; Claassen, E.; Brummer, R.J. On the importance of intraindividual variation in nutritional research. Benef. Microbes 2020, 11, 511–517. [Google Scholar] [CrossRef] [PubMed]
- de Cleva, R.; Mota, F.C.; Gadducci, A.V.; Cardia, L.; D’Andréa Greve, J.M.; Santo, M.A. Resting metabolic rate and weight loss after bariatric surgery. Surg. Obes. Relat. Dis. 2018, 14, 803–807. [Google Scholar] [CrossRef] [PubMed]
- Faria, S.L.; Kelly, E.; Faria, O.P. Energy expenditure and weight regain in patients submitted to Roux-en-Y gastric bypass. Obes. Surg. 2009, 19, 856–859. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [Green Version]
- Cole, J.R.; Wang, Q.; Fish, J.A.; Chai, B.; McGarrell, D.M.; Sun, Y.; Brown, C.T.; Porras-Alfaro, A.; Kuske, C.R.; Tiedje, J.M. Ribosomal Database Project: Data and tools for high throughput rRNA analysis. Nucleic Acids Res. 2014, 42, D633–D642. [Google Scholar] [CrossRef] [Green Version]
- Dixon, P. VEGAN, a package of R functions for community ecology. J. Veg. Sci. 2003, 14, 927–930. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2. WIREs Comp. Stat. 2011, 3, 180–185. [Google Scholar] [CrossRef]
- Han, J.; Lin, K.; Sequeira, C.; Borchers, C.H. An isotope-labeled chemical derivatization method for the quantitation of short-chain fatty acids in human feces by liquid chromatography-tandem mass spectrometry. Anal. Chim. Acta 2015, 854, 86–94. [Google Scholar] [CrossRef]



| Good Responder n = 11 | Bad Responder n = 12 | Good Responder vs. Bad Responder | |||
|---|---|---|---|---|---|
| Clinical Characteristics | |||||
| Sex (female/male)—n (%) | 7 (64)/4 (36) | 9 (75)/3 (25) | |||
| Smokers—n (%) | 4 (36) | 2 (17) | |||
| Diabetic—n (%) Before surgery (reported/A1c > 6.5%) After surgery (reported/A1c > 6.5%) | 4 (36)/4 (36) 2 (18)/1 (9) | 6 (50)/3 (25) 4 (33)/(3 (25) | |||
| mean/median * | SD/IQR * | mean/median | SD/IQR | p-value | |
| Education Years—yr | 13 | 13–15.5 | 13 | 13––13 | 0.26 |
| Age at test date—yr | 52.9 | ± 9.5 | 54.1 | ± 10.6 | 0.78 |
| Time after surgery—yr | 4.3 | ± 1.2 | 4.6 | ± 1.5 | 0.60 |
| Excess Weight Loss (EWL) at test date—% | 93.0 | ± 4.3 | 19.5 | ± 13.3 | <0.0001 |
| Body weight—kg Before surgery Nadir At test date Change from baseline | 133.4 69.9 76.6 −56.8 | ± 22.4 ± 5.8 ± 7.6 ± 18.6 | 145.7 110.4 130.3 −15.5 | ± 19.5 ± 15.9 ± 17.6 ± 10.9 | 0.17 <0.0001 <0.0001 <0.0001 |
| BMI—kg/m2 Before surgery Nadir At test date Change from baseline | 46.5 24.4 26.6 −19.8 | ± 7.5 ± 1.8 ± 1.2 ± 6.7 | 52.7 39.9 47.2 −5.5 | ± 6.6 ± 4.4 ± 6.4 ± 3.9 | 0.04 <0.0001 <0.0001 <0.0001 |
| Questionnaire Scores at test date | |||||
| Fat and Sugar Intake (DFS-Q All) Fat Sugar Fat and Sugar | 50 23 9.8 16.3 | ± 10 ± 3.9 ± 3.4 ± 4.7 | 47 24 10.7 12.8 | ± 6.1 ± 4.6 ± 4.0 ± 4.3 | 0.43 0.71 0.59 0.09 |
| Emotional Eating (DEB-Q-EE) | 1.5 | 1.0–2.2 | 2.0 | 1.1–2.9 | 0.54 |
| Chronic Stress (TICS) | 14 | 10–19 | 15 | 7.3–18 | 0.91 |
| Metabolic Profile | |||||
| Alanine transaminase—μkat/L Before surgery At test date Change from baseline | 0.42 0.40 −0.05 | 0.33–0.92 0.29–0.47 −0.49 to 0.03 | 0.56 0.34 −0.2 | 0.38–1.1 0.31–0.66 −0.64 to −0.02 | 0.48 0.76 0.28 |
| γ-glutamyl transferase—μkat/L Before surgery At test date Change from baseline | 0.40 0.21 −0.17 | 0.28–0.70 0.13–0.42 −0.33 to −0.1 | 0.53 0.41 −0.12 | 0.360.79 0.25–1.20 −0.20 to 0.32 | 0.52 0.05 0.16 |
| Fasting Glucose—mmol/L Before surgery At test date Change from baseline | 5.7 5.0 −0.62 | 4.9–7.3 4.6–5.3 −2.3 to −0.1 | 6.5 5.4 −0.31 | 5.2–11 5.2–8.5 −2.5 to 0.03 | 0.24 0.02 0.69 |
| Hemoglobin A1c—% Before surgery At test date Change from baseline | 5.6 5.0 −0.32 | 5.0–7.0 4.8–5.6 −1.56 to −0.07 | 5.6 5.7 −0.4 | 5.3–8.5 5.2–6.6 −1.79 to 0.32 | 0.55 0.03 0.99 |
| Insulin Resistance (HOMA-IR) Before surgery At test date Change from baseline | 4.9 1.6 −2.6 | 2.4–18 1.1–2.5 −13.5 to −0.7 | 6.3 2.7 −3.3 | 3.9–11 1.8–3.1 −6.2 to −1.2 | 0.56 0.09 0.99 |
| Triglycerides—mmol/L Before surgery At test date Change from baseline | 1.3 0.94 −0.4 | 0.97–1.7 0.63–1.2 −0.64 to −0.08 | 1.4 1.3 −0.17 | 0.95–3.3 1.2–1.3 −1.78 to 0.15 | 0.85 0.02 0.69 |
| Low Density Lipoprotein—mmol/L Before surgery At test date Change from baseline | 2.5 2.2 −0.29 | ± 0.57 ± 0.54 ± 0.62 | 2.9 2.5 −0.48 | ± 1.3 ± 0.57 ± 0.93 | 0.37 0.28 0.58 |
| High Density Lipoprotein—mmol/L Before surgery At test date Change from baseline | 0.93 1.5 0.6 | ± 0.23 ± 0.33 ± 0.27 | 1.1 1.5 0.28 | 0.26 ± 0.33 ±0.3 | 0.09 0.70 0.02 |
| C-reactive Protein—mg/L Before surgery At test date Change from baseline | 5.0 0.3 −3.5 | 1.6–7.9 0.3–0.3 −7.6 to −1.3 | 9.7 2.0 −5.0 | 4.9–20 1.3–13 −9.9 to 2.0 | 0.10 <0.0001 0.79 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fries, C.M.; Haange, S.-B.; Rolle-Kampczyk, U.; Till, A.; Lammert, M.; Grasser, L.; Medawar, E.; Dietrich, A.; Horstmann, A.; von Bergen, M.; et al. Metabolic Profile and Metabolite Analyses in Extreme Weight Responders to Gastric Bypass Surgery. Metabolites 2022, 12, 417. https://doi.org/10.3390/metabo12050417
Fries CM, Haange S-B, Rolle-Kampczyk U, Till A, Lammert M, Grasser L, Medawar E, Dietrich A, Horstmann A, von Bergen M, et al. Metabolic Profile and Metabolite Analyses in Extreme Weight Responders to Gastric Bypass Surgery. Metabolites. 2022; 12(5):417. https://doi.org/10.3390/metabo12050417
Chicago/Turabian StyleFries, Charlotte M., Sven-Bastiaan Haange, Ulrike Rolle-Kampczyk, Andreas Till, Mathis Lammert, Linda Grasser, Evelyn Medawar, Arne Dietrich, Annette Horstmann, Martin von Bergen, and et al. 2022. "Metabolic Profile and Metabolite Analyses in Extreme Weight Responders to Gastric Bypass Surgery" Metabolites 12, no. 5: 417. https://doi.org/10.3390/metabo12050417
APA StyleFries, C. M., Haange, S.-B., Rolle-Kampczyk, U., Till, A., Lammert, M., Grasser, L., Medawar, E., Dietrich, A., Horstmann, A., von Bergen, M., & Fenske, W. K. (2022). Metabolic Profile and Metabolite Analyses in Extreme Weight Responders to Gastric Bypass Surgery. Metabolites, 12(5), 417. https://doi.org/10.3390/metabo12050417









