Segmental Bronchial Allergen Challenge Elicits Distinct Metabolic Phenotypes in Allergic Asthma
Abstract
1. Introduction
2. Results
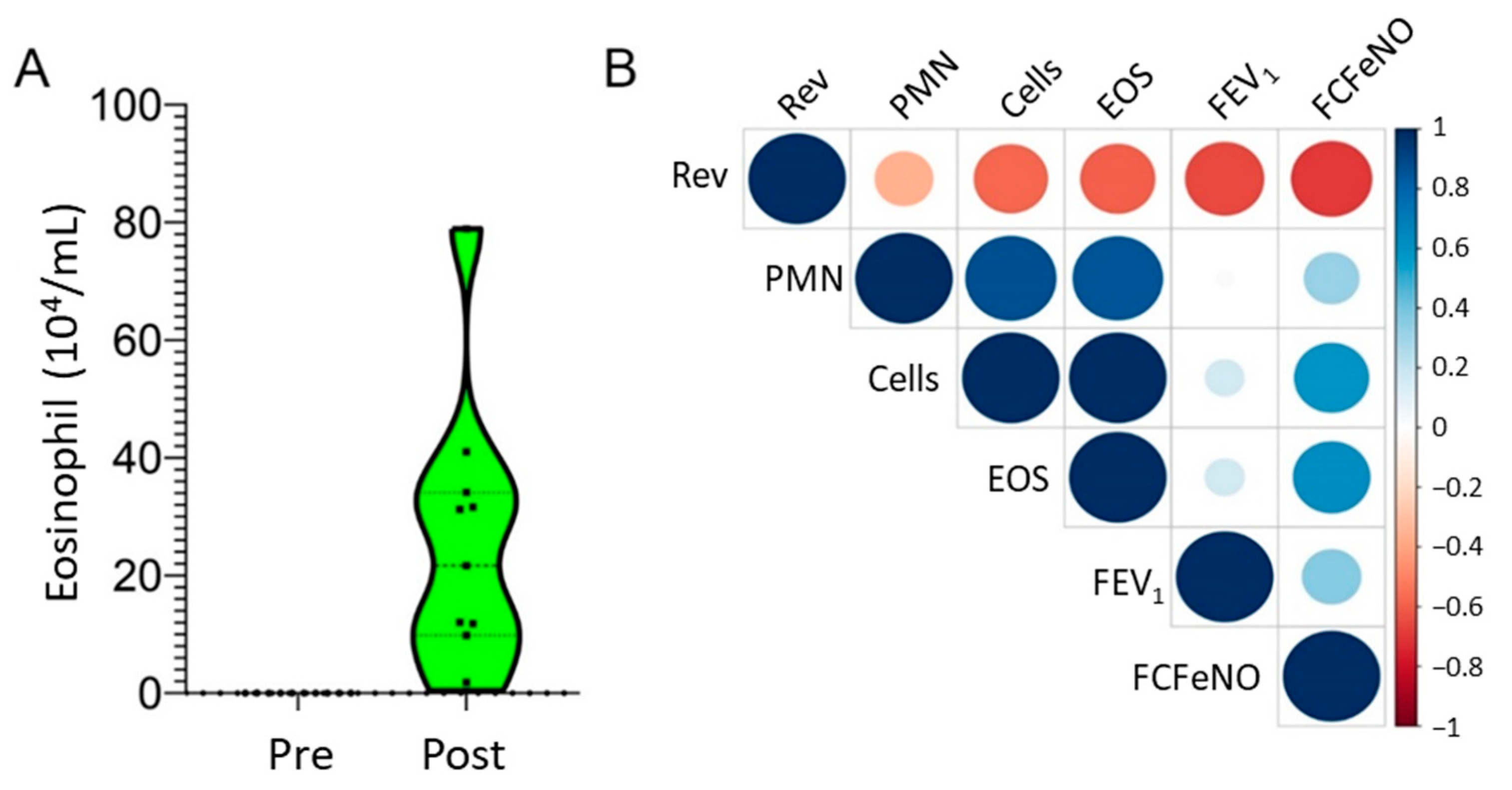
2.1. Demographics
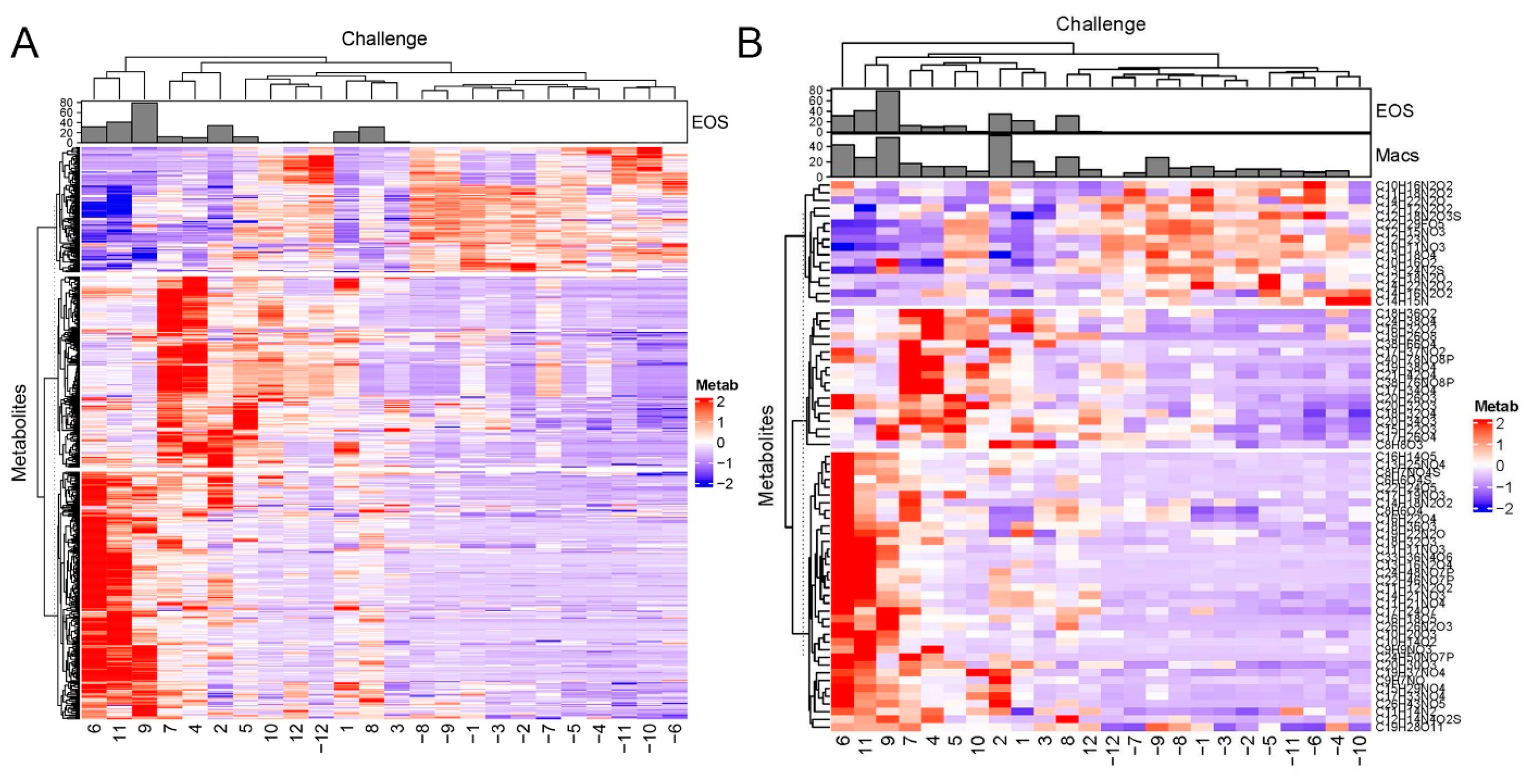
2.2. Differentially Regulated Metabolites
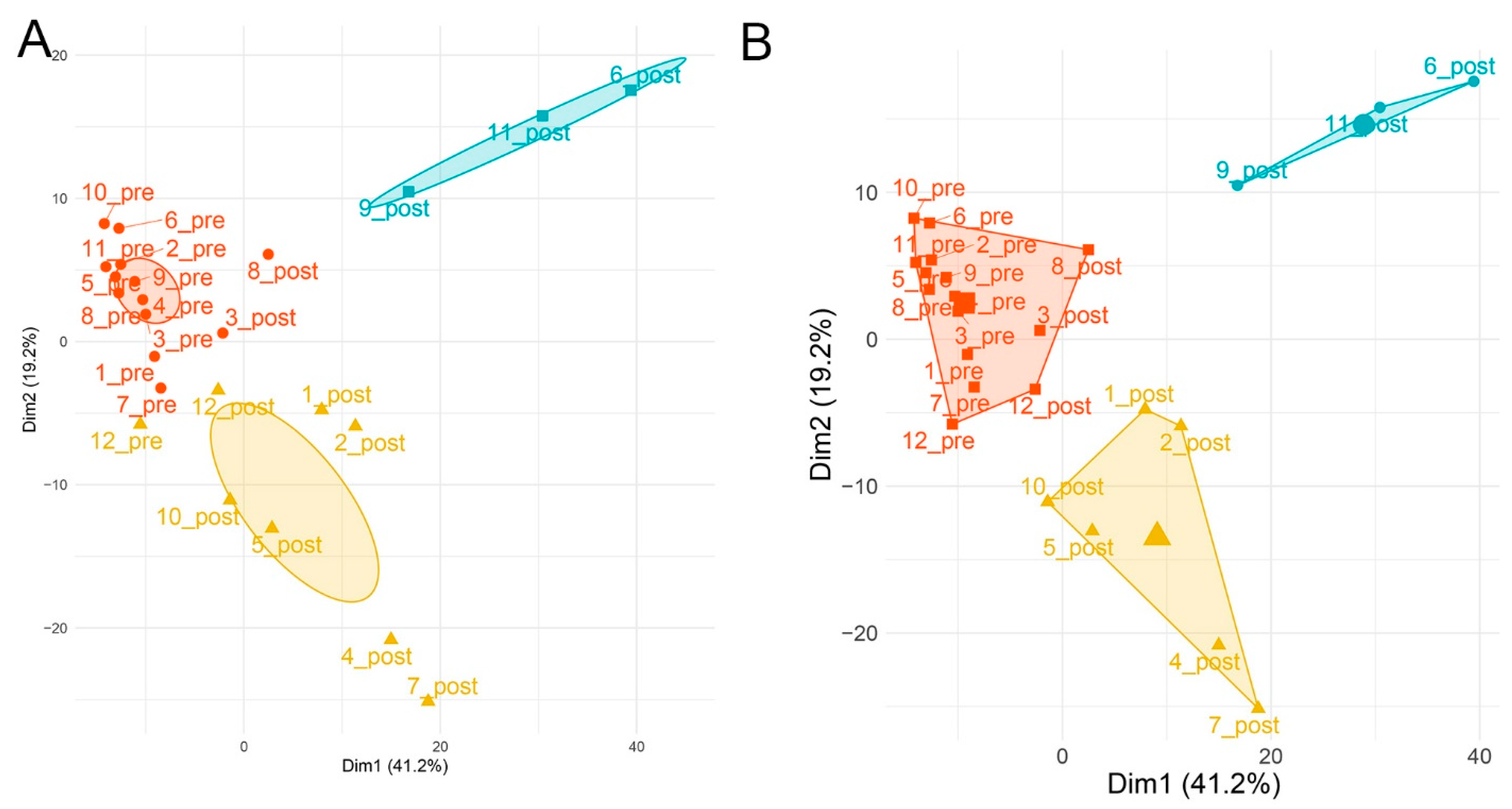
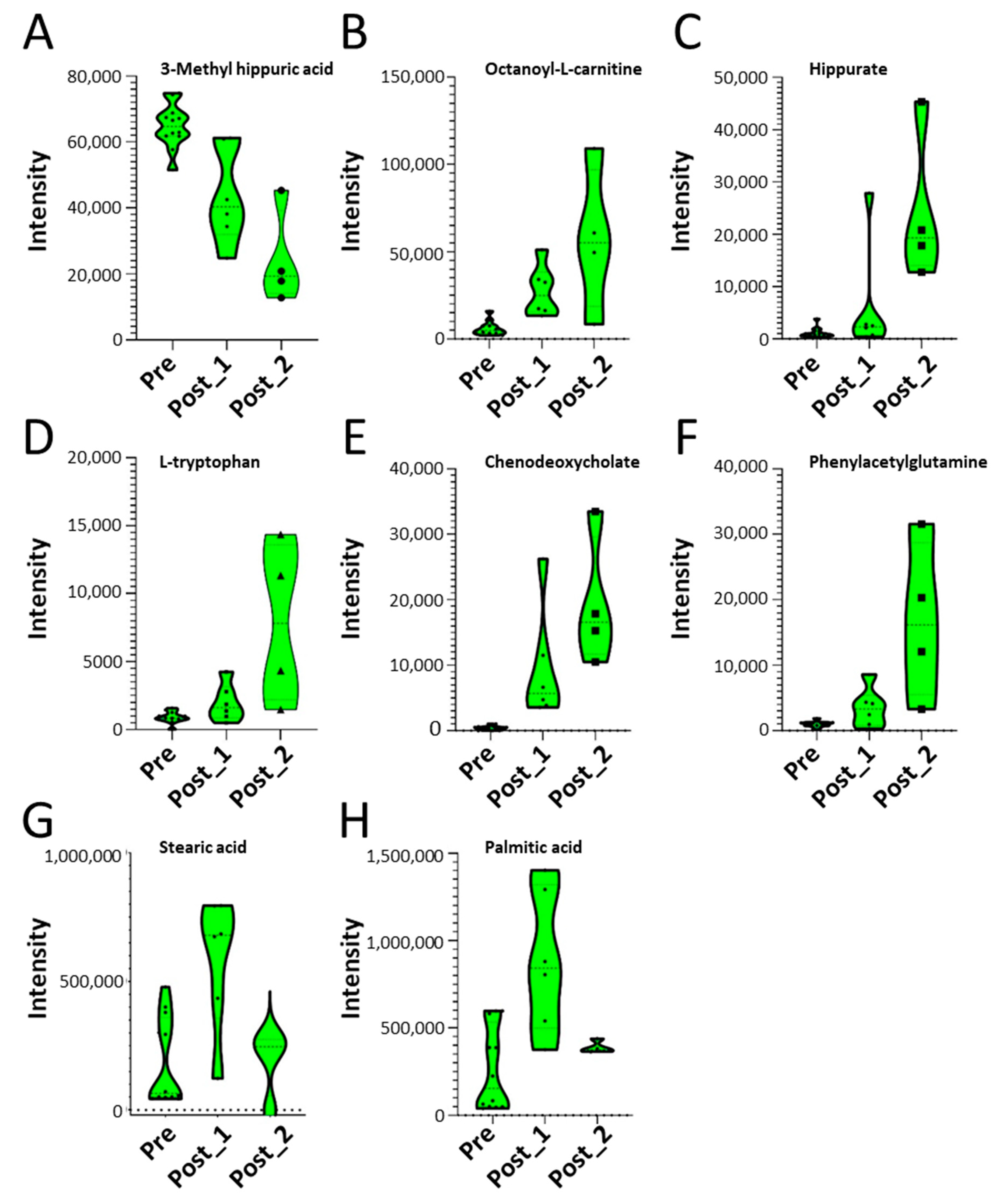
2.3. Global Metabolomics Identify Distinct Metabolite Responses
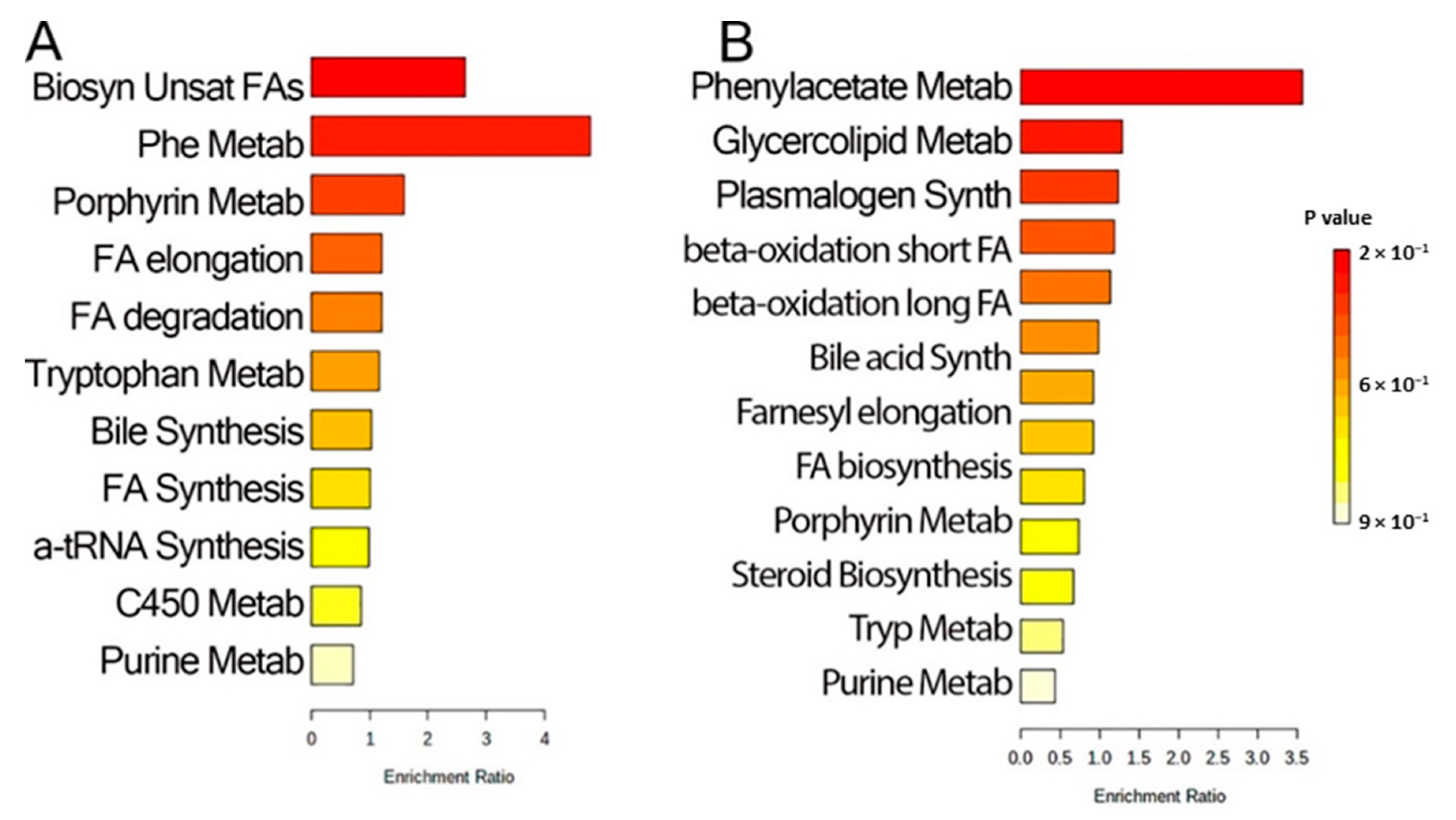
2.4. Pathway Analysis
2.5. SBP-Ag Induced Changes in Metabolite Expression Produces Two Classes of Response
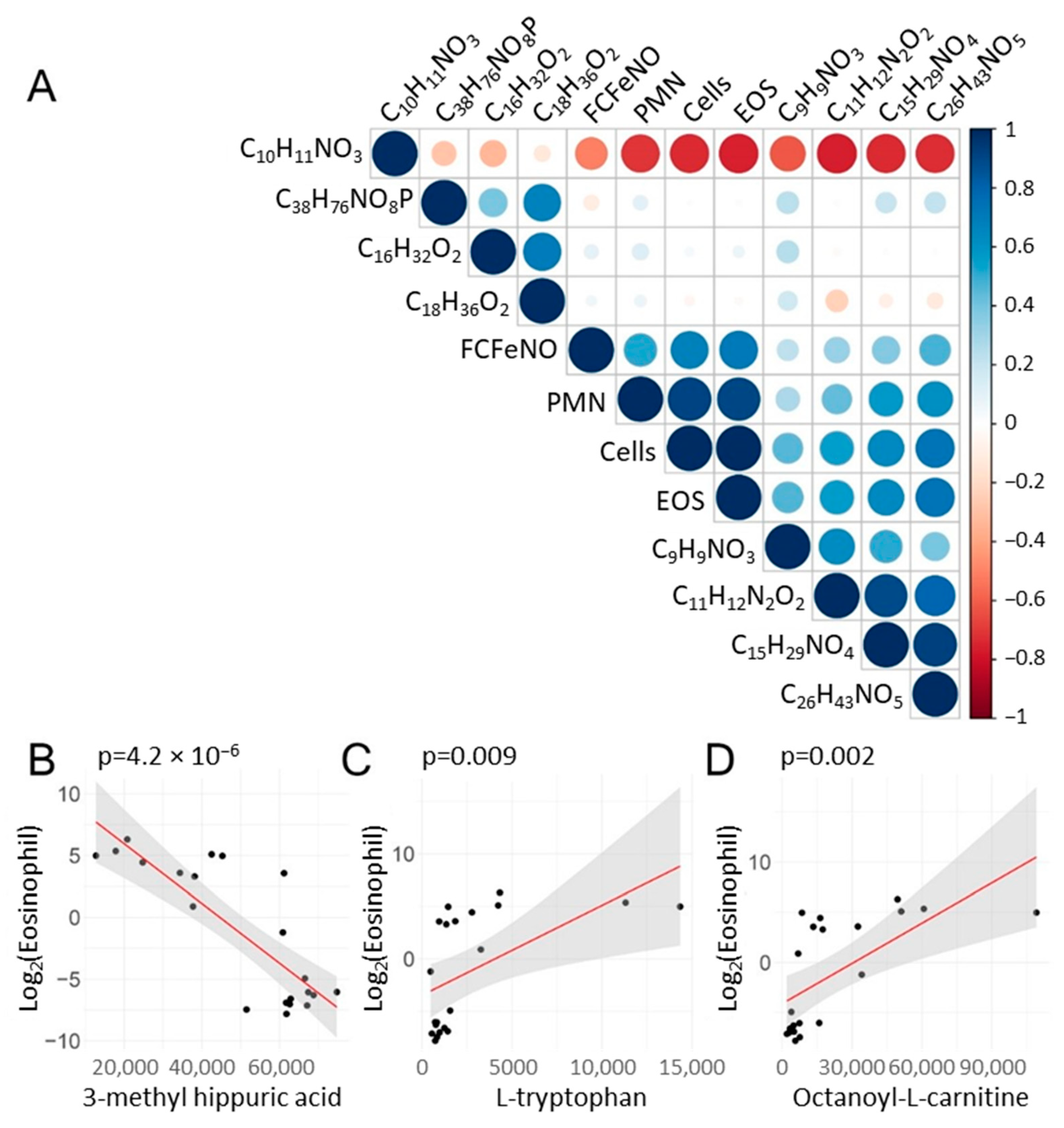
2.6. Correlations of Metabolite Abundance with Cellular Inflammation
2.7. Validation of Presence of Fatty Acid-Related Proteins in Blood Eosinophils
3. Discussion
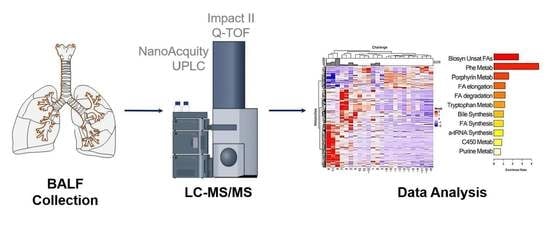
4. Materials and Methods
4.1. Segmental Bronchoprovocation with Allergen (SBP-Ag)
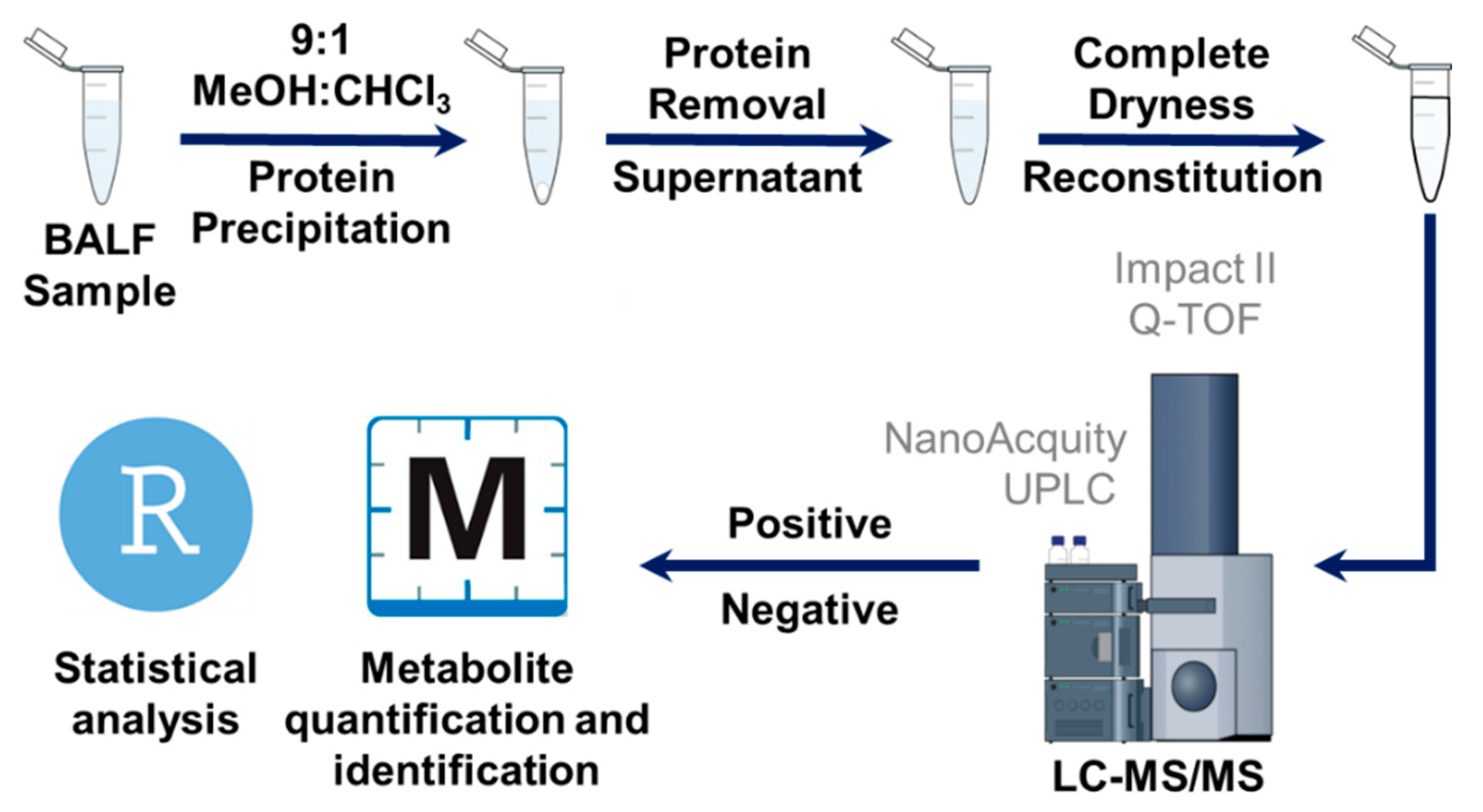
4.2. LC–MS/MS Analysis of BALF Samples
4.3. Data Analysis
4.4. Statistical Analysis
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Busse, W.W.; Lemanske, R.F. Asthma. N. Engl. J. Med. 2001, 344, 350–362. [Google Scholar] [CrossRef] [PubMed]
- Briggs, A.; Nasser, S.; Hammerby, E.; Buchs, S.; Virchow, J.C. The impact of moderate and severe asthma exacerbations on quality of life: A post hoc analysis of randomised controlled trial data. J. Patient-Rep. Outcomes 2021, 5, 6. [Google Scholar] [CrossRef] [PubMed]
- Trevor, J.; Lugogo, N.; Carr, W.; Moore, W.C.; Soong, W.; Panettieri, R.A., Jr.; Desai, P.; Trudo, F.; Ambrose, C.S. Severe asthma exacerbations in the United States:: Incidence, characteristics, predictors, and effects of biologic treatments. Ann. Allergy Asthma Immunol. 2021, 127, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Calhoun, W.J.; Haselkorn, T.; Miller, D.P.; Omachi, T.A. Asthma exacerbations and lung function in patients with severe or difficult-to-treat asthma. J. Allergy Clin. Immunol. 2015, 136, 1125–1127.e4. [Google Scholar] [CrossRef]
- Tian, B.; Hosoki, K.; Liu, Z.; Yang, J.; Zhao, Y.; Sun, H.; Zhou, J.; Rytting, E.; Kaphalia, L.; Calhoun, W.J.; et al. Mucosal bromodomain-containing protein 4 mediates aeroallergen-induced inflammation and remodeling. J. Allergy Clin. Immunol. 2019, 143, 1380–1394.e9. [Google Scholar] [CrossRef]
- Hosoki, K.; Redding, D.; Itazawa, T.; Chakraborty, A.; Tapryal, N.; Qian, S.; Qi, H.; Aguilera-Aguirre, L.; Brasier, A.R.; Phani, V.S.; et al. Innate mechanism of pollen- and cat dander-induced oxidative stress and DNA damage in the airways. J. Allergy Clin. Immunol. 2017, 140, 1436–1439. [Google Scholar] [CrossRef]
- Platts-Mills, T.A.E. The Role of Immunoglobulin E in Allergy and Asthma. Am. J. Respir. Crit. Care Med. 2001, 164 (Suppl. 1), S1–S5. [Google Scholar] [CrossRef]
- Pradalier, A. Late-phase reaction in asthma: Basic mechanisms. Int. Arch. Allergy Immunol. 1993, 101, 322–325. [Google Scholar] [CrossRef]
- Wark, P.A.B.; Gibson, P.G. Asthma exacerbations. 3: Pathogenesis. Thorax 2006, 61, 909–915. [Google Scholar] [CrossRef]
- Brasier, A.R. Identification of innate immune response endotypes in asthma: Implications for personalized medicine. Curr. Allergy Asthma Rep. 2013, 13, 462–468. [Google Scholar] [CrossRef][Green Version]
- Kaur, R.; Chupp, G. Phenotypes and endotypes of adult asthma: Moving toward precision medicine. J. Allergy Clin. Immunol. 2019, 144, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Moore, W.C.; Meyers, D.A.; Wenzel, S.E.; Teague, W.G.; Li, H.; Li, X.; D’Agostino, R., Jr.; Castro, M.; Curran-Everett, D.; Fitzpatrick, A.M.; et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am. J. Respir. Crit. Care Med. 2010, 181, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Brasier, A.R.; Victor, S.; Boetticher, G.; Ju, H.; Lee, C.; Bleecker, E.R.; Castro, M.; Busse, W.W.; Calhoun, W.J. Molecular Phenotyping Of Severe Asthma Using Pattern Recognition Of Bronchoalveolar Lavage-Derived Cytokines. J. Allergy Clin. Immunol. 2008, 121, 30–37. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shaw, D.E.; Sousa, A.R.; Fowler, S.J.; Fleming, L.J.; Roberts, G.; Corfield, J.; Pandis, I.; Bansal, A.T.; Bel, E.H.; Auffray, C.; et al. Clinical and inflammatory characteristics of the European U-BIOPRED adult severe asthma cohort. Eur. Respir. J. 2015, 46, 1308–1321. [Google Scholar] [CrossRef]
- Anderson, G.P. Endotyping asthma: New insights into key pathogenic mechanisms in a complex, heterogeneous disease. Lancet 2008, 372, 1107–1119. [Google Scholar] [CrossRef]
- Esnault, S.; Kelly, E.A.; Sorkness, R.L.; Evans, M.D.; Busse, W.W.; Jarjour, N.N. Airway factor XIII associates with type 2 inflammation and airway obstruction in asthmatic patients. J. Allergy Clin. Immunol. 2016, 137, 767–773.e6. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Agache, I.; Antolin-Amerigo, D.; de Blay, F.; Boccabella, C.; Caruso, C.; Chanez, P.; Couto, M.; Covar, R.; Doan, S.; Fauquert, J.L.; et al. EAACI position paper on the clinical use of the bronchial allergen challenge: Unmet needs and research priorities. Allergy 2022. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Skibba, M.; Xu, X.; Wiess, K.; Huisken, J.; Brasier, A.R. Role of Secretoglobin+ (Club cell) NFκB/RelA-TGFβ signaling in Aero-Allergen-Induced Epithelial Plasticity and Subepithelial Myofibroblast Transdifferentiation. BMC Respir. Res. 2021, 22, 315. [Google Scholar] [CrossRef]
- Dumitru, C.; Kabat, A.M.; Maloy, K.J. Metabolic Adaptations of CD4+ T Cells in Inflammatory Disease. Front. Immunol. 2018, 9, 540. [Google Scholar] [CrossRef]
- Porter, L.; Toepfner, N.; Bashant, K.R.; Guck, J.; Ashcroft, M.; Farahi, N.; Chilvers, E.R. Metabolic Profiling of Human Eosinophils. Front. Immunol. 2018, 9, 1404. [Google Scholar] [CrossRef]
- Tusher, V.G.; Tibshirani, R.; Chu, G. Significance analysis of microarrays applied to the ionizing radiation response. Proc. Natl. Acad. Sci. USA 2001, 98, 5116–5121. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, L.; Rousseau, P.J. Finding Groups in Data: An Introduction to Cluster Analysis; Wiley: New York, NY, USA, 1990. [Google Scholar]
- Hastie, T.; Tibshirani, R.; Friedman, A. The Elements of Statistical Learning: Data Mining, Inference, and Prediction; Springer: New York, NY, USA, 2009; Volume 2. [Google Scholar]
- Jewison, T.; Su, Y.; Disfany, F.M.; Liang, Y.; Knox, C.; Maciejewski, A.; Poelzer, J.; Huynh, J.; Zhou, Y.; Arndt, D.; et al. SMPDB 2.0: Big improvements to the Small Molecule Pathway Database. Nucleic Acids Res. 2014, 42, D478–D484. [Google Scholar] [CrossRef] [PubMed]
- Esnault, S.; Hebert, A.S.; Jarjour, N.N.; Coon, J.J.; Mosher, D.F. Proteomic and Phosphoproteomic Changes Induced by Prolonged Activation of Human Eosinophils with IL-3. J. Proteome Res. 2018, 17, 2102–2111. [Google Scholar] [CrossRef] [PubMed]
- Da Huang, W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]
- Kelly, R.S.; Dahlin, A.; McGeachie, M.J.; Qiu, W.; Sordillo, J.; Wan, E.S.; Wu, A.C.; Lasky-Su, J. Asthma Metabolomics and the Potential for Integrative Omics in Research and the Clinic. Chest 2017, 151, 262–277. [Google Scholar] [CrossRef]
- Kuo, C.H.S.; Pavlidis, S.; Loza, M.; Baribaud, F.; Rowe, A.; Pandis, I.; Sousa, A.; Corfield, J.; Djukanovic, R.; Lutter, R.; et al. T-helper cell type 2 (Th2) and non-Th2 molecular phenotypes of asthma using sputum transcriptomics in U-BIOPRED. Eur. Respir. J. 2017, 49, 2. [Google Scholar] [CrossRef]
- Singh, A.; Shannon, C.P.; Kim, Y.W.; Yang, C.X.; Balshaw, R.; Freue, G.V.C.; Gauvreau, G.M.; FitzGerald, J.M.; Boulet, L.P.; O’Byrne, P.M.; et al. Novel Blood-based Transcriptional Biomarker Panels Predict the Late-Phase Asthmatic Response. Am. J. Respir. Crit. Care Med. 2018, 197, 450–462. [Google Scholar] [CrossRef]
- Al-Khami, A.A.; Ghonim, M.A.; Del Valle, L.; Ibba, S.V.; Zheng, L.; Pyakurel, K.; Okpechi, S.C.; Garay, J.; Wyczechowska, D.; Sanchez-Pino, M.D.; et al. Fuelling the mechanisms of asthma: Increased fatty acid oxidation in inflammatory immune cells may represent a novel therapeutic target. Clin. Exp. Allergy 2017, 47, 1170–1184. [Google Scholar] [CrossRef]
- Lavinskiene, S.; Malakauskas, K.; Jeroch, J.; Hoppenot, D.; Sakalauskas, R. Functional activity of peripheral blood eosinophils in allergen-induced late-phase airway inflammation in asthma patients. J. Inflamm. 2015, 12, 25. [Google Scholar] [CrossRef]
- Cieslewicz, G.; Tomkinson, A.; Adler, A.; Duez, C.; Schwarze, J.; Takeda, K.; Larson, K.A.; Lee, J.J.; Irvin, C.G.; Gelfand, E.W. The late, but not early, asthmatic response is dependent on IL-5 and correlates with eosinophil infiltration. J. Clin. Investig. 1999, 104, 301–308. [Google Scholar] [CrossRef]
- Reddy, P.H. Mitochondrial Dysfunction and Oxidative Stress in Asthma: Implications for Mitochondria-Targeted Antioxidant Therapeutics. Pharmaceuticals 2011, 4, 429–456. [Google Scholar] [CrossRef] [PubMed]
- Taylor, B.; Mannino, D.; Brown, C.; Crocker, D.; Twum-Baah, N.; Holguin, F. Body mass index and asthma severity in the National Asthma Survey. Thorax 2008, 63, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Motta, A.; Paris, D.; D’Amato, M.; Melck, D.; Calabrese, C.; Vitale, C.; Stanziola, A.A.; Corso, G.; Sofia, M.; Maniscalco, M. NMR Metabolomic Analysis of Exhaled Breath Condensate of Asthmatic Patients at Two Different Temperatures. J. Proteome Res. 2014, 13, 6107–6120. [Google Scholar] [CrossRef] [PubMed]
- Shaik, F.B.; Panati, K.; Narasimha, V.R.; Narala, V.R. Chenodeoxycholic acid attenuates ovalbumin-induced airway inflammation in murine model of asthma by inhibiting the TH2 cytokines. Biochem. Biophys. Res. Commun. 2015, 463, 600–605. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-D.; Chen, W.-D.; Wang, M.; Yu, D.; Forman, B.M.; Huang, W. Farnesoid X receptor antagonizes nuclear factor κB in hepatic inflammatory response. Hepatology 2008, 48, 1632–1643. [Google Scholar] [CrossRef] [PubMed]
- Kelly, E.A.; Esnault, S.; Liu, L.Y.; Evans, M.D.; Johansson, M.W.; Mathur, S.; Mosher, D.F.; Denlinger, L.C.; Jarjour, N.N. Mepolizumab Attenuates Airway Eosinophil Numbers, but Not Their Functional Phenotype, in Asthma. Am. J. Respir. Crit. Care Med. 2017, 196, 1385–1395. [Google Scholar] [CrossRef]
| Subject | Age | SBP-Ag | FEV1 (%) | Total BAL Cells (×104 Cells/mL) | EOS (%) | PMN (%) | LYM (%) | MAC (%) | FeNO | Blood EOS/µL |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 23 | Pre | 75 | 14.8 | 1.1 | 1 | 3.6 | 94.3 | 56 | 229 |
| Post | 145.4 | 74.5 | 4.8 | 6.9 | 13.8 | 82 | 510 | |||
| 2 | 27 | Pre | 102 | 10.4 | 0.4 | 0.2 | 2.5 | 96.9 | 19.9 | 387 |
| Post | 233.7 | 73 | 1.9 | 2 | 23.1 | 30.1 | 493 | |||
| 3 | 31 | Pre | 135 | 7.7 | 0.5 | 1 | 4.8 | 93.7 | 41.4 | 290 |
| Post | 17.9 | 51.7 | 5 | 7 | 36.3 | 59.8 | 290 | |||
| 4 | 36 | Pre | 87 | 8.9 | 0.4 | 0.1 | 4.8 | 94.7 | 77.1 | 185 |
| Post | 68.3 | 72 | 1.9 | 6.8 | 20.2 | 66.3 | 405 | |||
| 5 | 21 | Pre | 83 | 11.1 | 0.2 | 0.4 | 7.2 | 92.2 | 49.9 | 343 |
| Post | 76.5 | 77.2 | 1.7 | 3 | 18.1 | 66.8 | 519 | |||
| 6 | 27 | Pre | 95 | 7.1 | 0.4 | 1.2 | 8.6 | 89.8 | 40.4 | 255 |
| Post | 221.7 | 71.3 | 3.2 | 6.6 | 18.9 | 57.1 | 510 | |||
| 7 | 27 | Pre | 71 | 6.4 | 1 | 1.8 | 14.5 | 82.7 | 47.9 | 167 |
| Post | 90.7 | 66.3 | 5.4 | 8.6 | 19.7 | 57 | 132 | |||
| 8 | 19 | Pre | 103 | 12.5 | 0.6 | 0.9 | 5.3 | 93.2 | 24.5 | 387 |
| Post | 193.7 | 80.6 | 1 | 4.8 | 13.6 | 69.2 | 942 | |||
| 9 | 22 | Pre | 108 | 38 | 0.2 | 0.7 | 32.2 | 66.9 | 23 | 202 |
| Post | 542.6 | 72.7 | 3.6 | 14.2 | 9.5 | 52.5 | 396 | |||
| 10 | 27 | Pre | 83 | 11.3 | ND | ND | ND | ND | 28.7 | 70 |
| Post | 12.2 | 17.7 | 9 | 12.4 | 60.9 | 55.6 | 325 | |||
| 11 | 20 | Pre | 100 | 8.6 | 0.6 | 0.7 | 8.8 | 89.9 | 49.4 | 316 |
| Post | 263.5 | 77.8 | 1.4 | 12.2 | 9.6 | 99.3 | 941 | |||
| 12 | 33 | Pre | 99 | 9.1 | ND | ND | ND | ND | ND | 105 |
| Post | 16.3 | 29.9 | 2.1 | 10.6 | 57.4 | ND | 140 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Esnault, S.; Ge, Y.; Jarjour, N.N.; Brasier, A.R. Segmental Bronchial Allergen Challenge Elicits Distinct Metabolic Phenotypes in Allergic Asthma. Metabolites 2022, 12, 381. https://doi.org/10.3390/metabo12050381
Zhu Y, Esnault S, Ge Y, Jarjour NN, Brasier AR. Segmental Bronchial Allergen Challenge Elicits Distinct Metabolic Phenotypes in Allergic Asthma. Metabolites. 2022; 12(5):381. https://doi.org/10.3390/metabo12050381
Chicago/Turabian StyleZhu, Yanlong, Stephane Esnault, Ying Ge, Nizar N. Jarjour, and Allan R. Brasier. 2022. "Segmental Bronchial Allergen Challenge Elicits Distinct Metabolic Phenotypes in Allergic Asthma" Metabolites 12, no. 5: 381. https://doi.org/10.3390/metabo12050381
APA StyleZhu, Y., Esnault, S., Ge, Y., Jarjour, N. N., & Brasier, A. R. (2022). Segmental Bronchial Allergen Challenge Elicits Distinct Metabolic Phenotypes in Allergic Asthma. Metabolites, 12(5), 381. https://doi.org/10.3390/metabo12050381