Diversion of Acetyl CoA to 3-Methylglutaconic Acid Caused by Discrete Inborn Errors of Metabolism
Abstract
1. Introduction
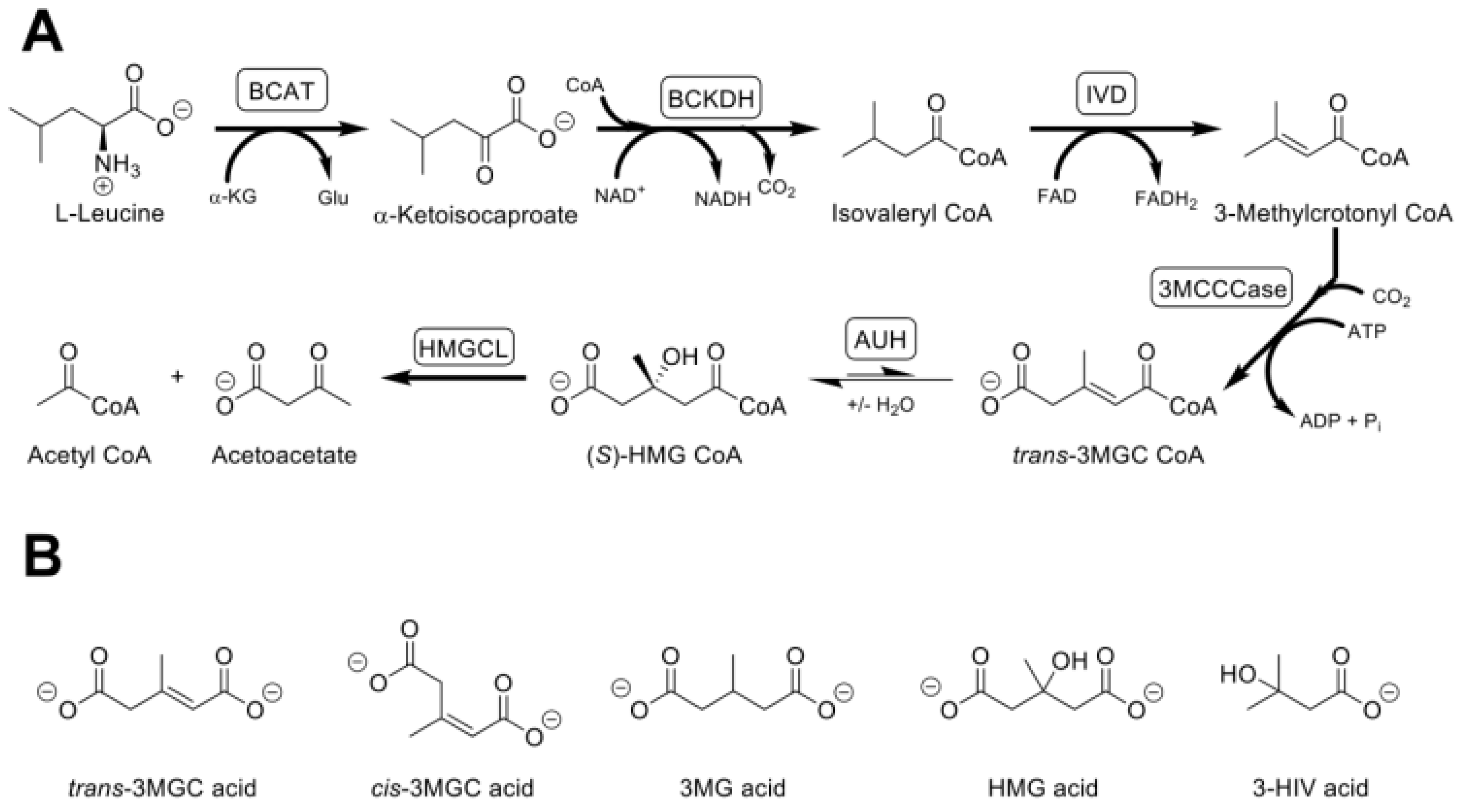
2. Primary and Secondary 3MGC Aciduria
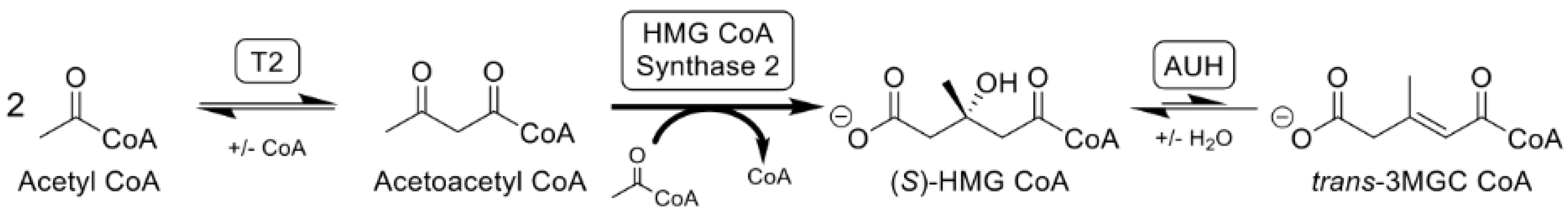
3. Mevalonate Shunt Hypothesis and Secondary 3MGC Aciduria
4. The Acetyl CoA Diversion Pathway
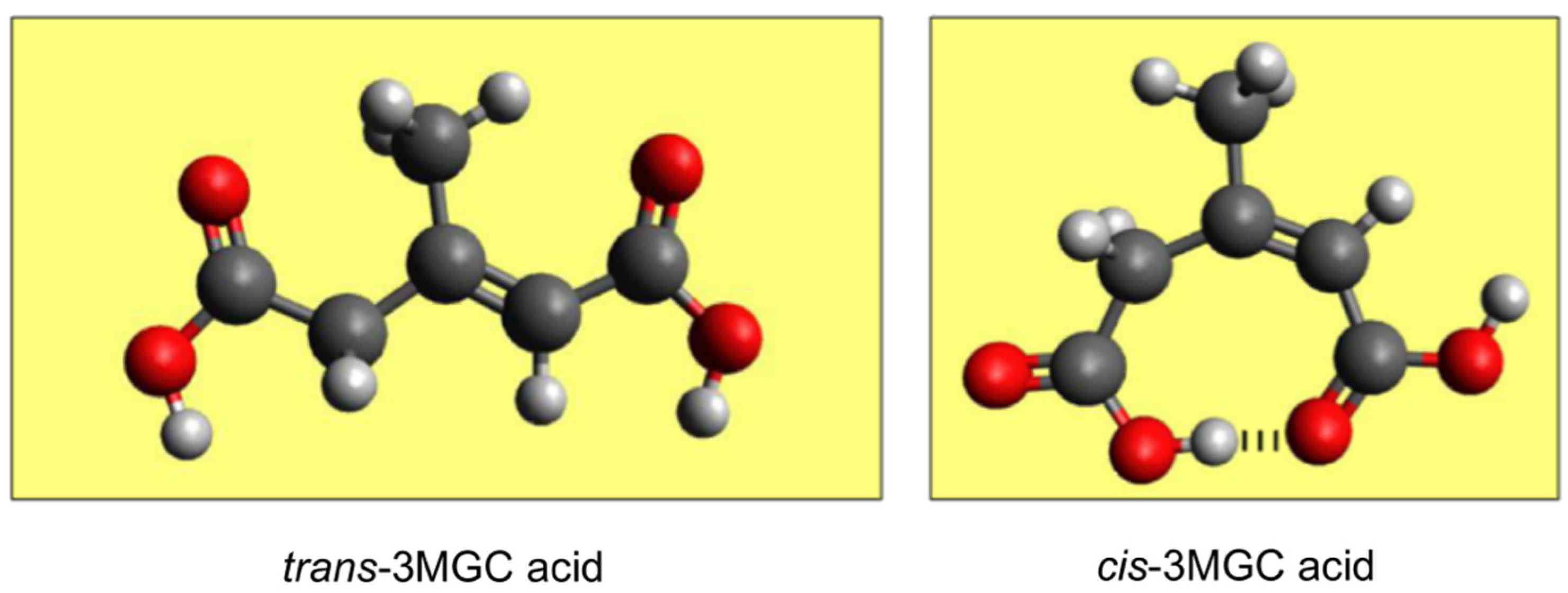
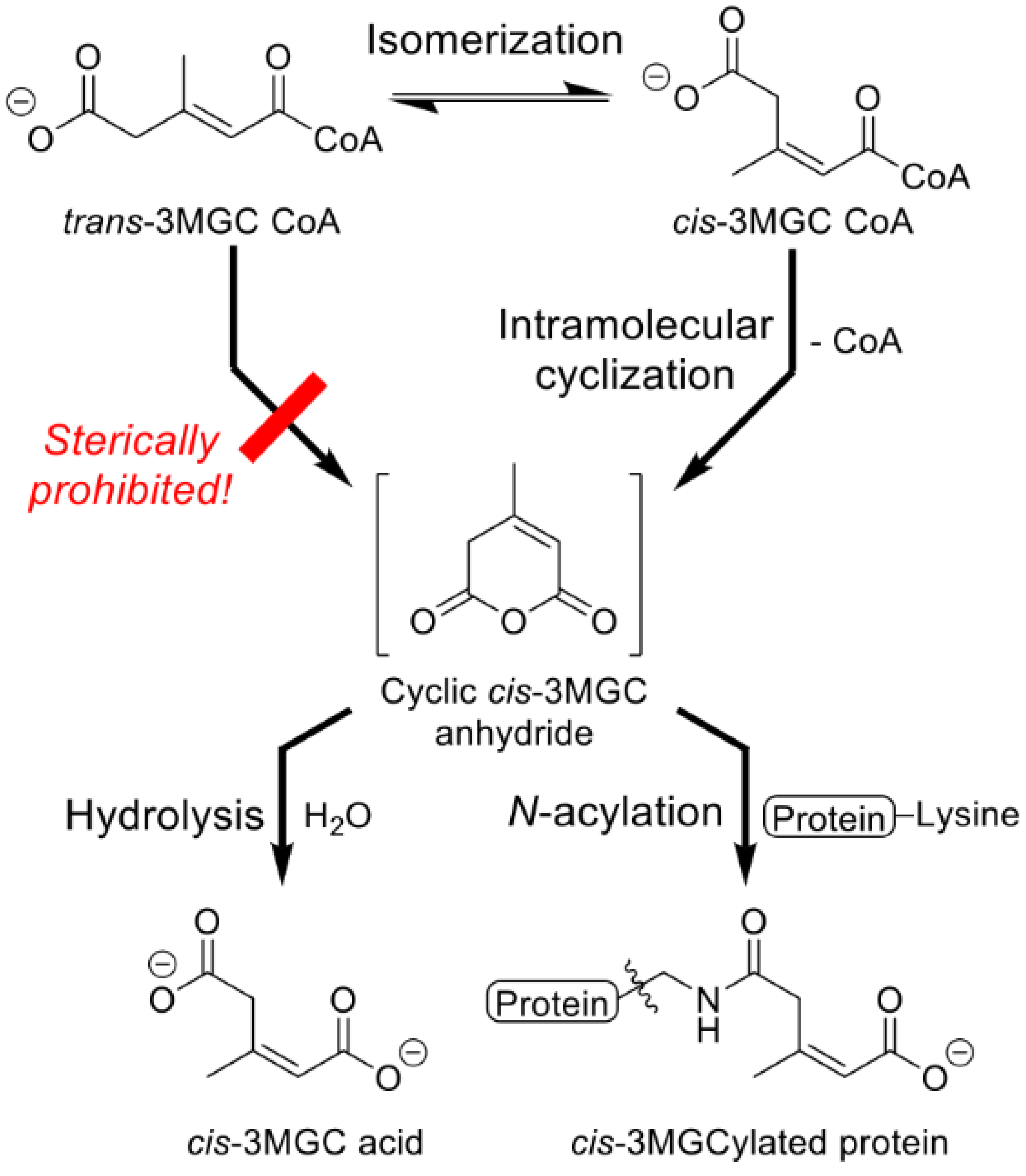
5. Isomerization of trans-3MGC CoA
6. A Non-Enzymatic Reaction Sequence Converts trans-3MGC CoA to cis-3MGC Acid
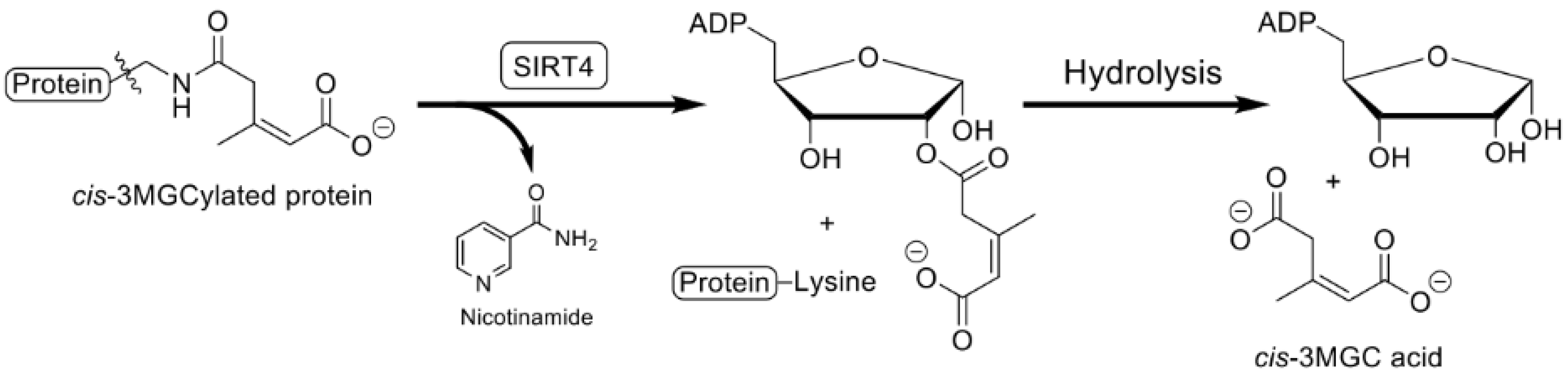
7. Sirtuin 4-Mediated Deacylation
8. A Model IEM Associated with Secondary 3MGC Aciduria
9. Other IEMs Associated with 3MGC Aciduria
10. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Phipps, W.S.; Jones, P.M.; Patel, K. Amino and organic acid analysis: Essential tools in the diagnosis of inborn errors of metabolism. Adv. Clin. Chem. 2019, 92, 59–103. [Google Scholar] [PubMed]
- Villani, G.R.; Gallo, G.; Scolamiero, E.; Salvatore, F.; Ruoppolo, M. Classical organic acidurias: Diagnosis and pathogenesis. Clin. Exp. Med. 2017, 17, 305–323. [Google Scholar] [CrossRef] [PubMed]
- Pié, J.; López-Viñas, E.; Puisac, B.; Menao, S.; Pié, A.; Casale, C.; Ramos, F.J.; Hegardt, F.; Gómez-Puertas, P.; Casals, N. Molecular genetics of HMG-CoA lyase deficiency. Mol. Genet. Metab. 2007, 92, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.E.; Klacking, E.; Ryan, R.O. Inborn errors of metabolism associated with 3-methylglutaconic aciduria. Clin. Chim. Acta 2021, 522, 96–104. [Google Scholar] [CrossRef] [PubMed]
- IJlst, L.; Loupatty, F.J.; Ruiter, J.P.; Duran, M.; Lehnert, W.; Wanders, R.J. 3-Methylglutaconic aciduria type I is caused by mutations in AUH. Am. J. Hum. Genet. 2002, 71, 1463–1466. [Google Scholar] [CrossRef]
- Jones, D.E.; Perez, L.; Ryan, R.O. 3-Methylglutaric acid in energy metabolism. Clin. Chim. Acta 2020, 502, 233–239. [Google Scholar] [CrossRef]
- Wortmann, S.B.; Kluijtmans, L.A.; Sequeira, S.; Wevers, R.A.; Morava, E. Leucine loading test is only discriminative for 3-methylglutaconic aciduria due to AUH defect. JIMD Rep. 2014, 16, 1–6. [Google Scholar] [CrossRef]
- Kelley, R.I.; Cheatham, J.P.; Clark, B.J.; Nigro, M.A.; Powell, B.R.; Sherwood, G.W.; Sladky, J.T.; Swisher, W.P. X-linked dilated cardiomyopathy with neutropenia, growth retardation, and 3-methylglutaconic aciduria. J. Pediatr. 1991, 119, 738–747. [Google Scholar] [CrossRef]
- Wortmann, S.B.; Kluijtmans, L.A.; Engelke, U.F.H.; Wevers, R.A.; Morava, E. The 3-methylglutaconic acidurias: What’s new? J. Inherit. Metab. Dis. 2012, 35, 13–22. [Google Scholar] [CrossRef]
- Kelley, R.I.; Kratz, L. 3-methylglutaconic acidemia in Smith-Lemli-Opitz syndrome. Pediatr. Res. 1995, 37, 671–674. [Google Scholar] [CrossRef][Green Version]
- Edmond, J.; Popják, G. Transfer of carbon atoms from mevalonate to n-fatty acids. J. Biol. Chem. 1974, 249, 66–71. [Google Scholar] [CrossRef]
- Su, B.; Ryan, R.O. Metabolic biology of 3-methylglutaconic acid-uria: A new perspective. J. Inherit. Metab. Dis. 2014, 37, 359–368. [Google Scholar] [CrossRef]
- Gabriel, J.L.; Plaut, G.W. Inhibition of bovine heart NAD-specific isocitrate dehydrogenase by reduced pyridine nucleotides: Modulation of inhibition by ADP, NAD+, Ca2+, citrate, and isocitrate. Biochemistry 1984, 23, 2773–2778. [Google Scholar] [CrossRef]
- Chinopoulos, C. Which way does the citric acid cycle turn during hypoxia? The critical role of α-ketoglutarate dehydrogenase complex. J. Neurosci. Res. 2013, 91, 1030–1043. [Google Scholar] [CrossRef]
- Apitz-Castro, R.; Rehn, K.; Lynen, F. Beta methylcrotonyl-CoA-carboxylase. Crystallization and some physical properties. Eur. J. Biochem. 1970, 16, 71–79. [Google Scholar] [CrossRef]
- Mack, M.; Schniegler-Mattox, U.; Peters, V.; Hoffmann, G.F.; Liesert, M.; Buckel, W.; Zschocke, J. Biochemical characterization of human 3-methylglutaconyl-CoA hydratase and its role in leucine metabolism. FEBS J. 2006, 273, 2012–2022. [Google Scholar] [CrossRef]
- Young, R.; Jones, D.E.; Diacovich, L.; Witkowski, A.; Ryan, R.O. trans-3-methylglutaconyl CoA isomerization-dependent protein acylation. Biochem. Biophys. Res. Commun. 2021, 534, 261–265. [Google Scholar] [CrossRef]
- Iles, R.A.; Jago, J.R.; Williams, S.R.; Chalmers, R.A. 3-Hydroxy-3-methylglutaryl-CoA lyase deficiency studied using 2-dimensional proton nuclear magnetic resonance spectroscopy. FEBS Lett. 1986, 203, 49–53. [Google Scholar] [CrossRef]
- Engelke, U.F.; Kremer, B.; Kluijtmans, L.A.; van der Graaf, M.; Morava, E.; Loupatty, F.J.; Wanders, R.J.; Moskau, D.; Loss, S.; van den Bergh, E.; et al. NMR spectroscopic studies on the late onset form of 3-methylglutaconic aciduria type I and other defects in leucine metabolism. NMR Biomed. 2006, 19, 271–278. [Google Scholar] [CrossRef]
- Kelley, R.I. Quantification of 3-methylglutaconic acid in urine, plasma, and amniotic fluid by isotope-dilution gas chromatography/mass spectrometry. Clin. Chim. Acta 1993, 220, 157–164. [Google Scholar] [CrossRef]
- Jones, D.E.; Ricker, J.D.; Geary, L.M.; Kosma, D.K.; Ryan, R.O. Isomerization of 3-methylglutaconic acid. JIMD Rep. 2020, 58, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.E.; Romenskaia, I.; Kosma, D.K.; Ryan, R.O. Role of non-enzymatic chemical reactions in 3-methylglutaconic aciduria. FEBS J. 2022. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Wagner, G.R.; Bhatt, D.P.; O’Connell, T.M.; Thompson, J.W.; Dubois, L.G.; Backos, D.S.; Yang, H.; Mitchell, G.A.; Ilkayeva, O.R.; Stevens, R.D.; et al. A class of reactive acyl-CoA species reveals the non-enzymatic origins of protein acylation. Cell Metab. 2017, 25, 823–837.e8. [Google Scholar] [CrossRef]
- Anderson, K.A.; Huynh, F.K.; Fisher-Wellman, K.; Stuart, J.D.; Peterson, B.S.; Douros, J.D.; Wagner, G.R.; Thompson, J.W.; Madsen, A.S.; Green, M.F.; et al. SIRT4 is a lysine deacylase that controls leucine metabolism and insulin secretion. Cell Metab. 2017, 25, 838–855. [Google Scholar] [CrossRef] [PubMed]
- Bedalov, A.; Chowdhury, S.; Simon, J.A. Biology, chemistry, and pharmacology of sirtuins. Methods Enzymol. 2016, 574, 183–211. [Google Scholar] [PubMed]
- Betsinger, C.N.; Cristea, I.M. Mitochondrial function, metabolic regulation, and human disease viewed through the prism of sirtuin 4 (SIRT4) functions. J. Proteome Res. 2019, 18, 1929–1938. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.L.; Bowron, A.; Gonzalez, I.L.; Groves, S.J.; Newbury-Ecob, R.; Clayton, N.; Martin, R.P.; Tsai-Goodman, B.; Garratt, V.; Ashworth, M.; et al. Barth syndrome. Orphanet J. Rare Dis. 2013, 8, 23. [Google Scholar] [CrossRef]
- Miller, P.C.; Ren, M.; Schlame, M.; Toth, M.J.; Phoon, C.K.L. A Bayesian analysis to determine the prevalence of Barth Syndrome in the pediatric population. J. Pediatr. 2020, 217, 139–144. [Google Scholar] [CrossRef]
- Bione, S.; D’Adamo, P.; Maestrini, E.; Gedeon, A.K.; Bolhuis, P.A.; Toniolo, D. A novel X-linked gene, G4.5. is responsible for Barth syndrome. Nat. Genet. 1996, 12, 385–389. [Google Scholar] [CrossRef]
- Ikon, N.; Ryan, R.O. Cardiolipin and mitochondrial cristae organization. Biochim. Biophys. Acta 2017, 1859, 1156–1163. [Google Scholar] [CrossRef]
- Schlame, M.; Towbin, J.A.; Heerdt, P.M.; Jehle, R.; DiMauro, S.; Blanck, T.J. Deficiency of tetralinoleoyl-cardiolipin in Barth syndrome. Ann. Neurol. 2002, 51, 634–637. [Google Scholar] [CrossRef]
- Valianpour, F.; Mitsakos, V.; Schlemmer, D.; Towbin, J.A.; Taylor, J.M.; Ekert, P.G.; Thorburn, D.R.; Munnich, A.; Wanders, R.J.; Barth, P.G.; et al. Monolysocardiolipins accumulate in Barth syndrome but do not lead to enhanced apoptosis. J. Lipid Res. 2005, 46, 1182–1195. [Google Scholar] [CrossRef]
- Van Werkhoven, M.A.; Thorburn, D.R.; Gedeon, A.K.; Pitt, J.J. Monolysocardiolipin in cultured fibroblasts is a sensitive and specific marker for Barth Syndrome. J. Lipid Res. 2006, 47, 2346–2351. [Google Scholar] [CrossRef]
- Barth, P.G.; Scholte, H.; Berden, J.A.; Van der Klei-Van Moorsel, J.M.; Luyt-Houwen, I.E.; Van‘t Veer-Korthof, E.T.; Van der Harten, J.J.; Sobotka-Plojhar, M.A. An X-linked mitochondrial disease affecting cardiac muscle, skeletal muscle and neutrophil leucocytes. J. Neurol. Sci. 1983, 62, 327–355. [Google Scholar] [CrossRef]
- Xu, Y.; Sutachan, J.J.; Plesken, H.; Kelley, R.I.; Schlame, M. Characterization of lymphoblast mitochondria from patients with Barth syndrome. Lab. Investig. 2005, 85, 823–830. [Google Scholar] [CrossRef]
- Acehan, D.; Xu, Y.; Stokes, D.L.; Schlame, M. Comparison of lymphoblast mitochondria from normal subjects and patients with Barth syndrome using electron microscopic tomography. Lab. Investig. 2007, 87, 40–48. [Google Scholar] [CrossRef]
- Ikon, N.; Ryan, R.O. Barth syndrome: Connecting cardiolipin to cardiomyopathy. Lipids 2017, 52, 99–108. [Google Scholar] [CrossRef]
- Wortmann, S.B.; Duran, M.; Anikster, Y.; Barth, P.G.; Sperl, W.; Zschocke, J.; Morava, E.; Wevers, R.A. Inborn errors of metabolism with 3-methylglutaconic aciduria as discriminative feature: Proper classification and nomenclature. J. Inherit. Metab. Dis. 2013, 36, 923–928. [Google Scholar] [CrossRef]
- Mayr, J.A.; Havlícková, V.; Zimmermann, F.; Magler, I.; Kaplanová, V.; Jesina, P.; Pecinová, A.; Nusková, H.; Koch, J.; Sperl, W.; et al. Mitochondrial ATP synthase deficiency due to a mutation in the ATP5E gene for the F1 epsilon subunit. Hum. Mol. Genet. 2010, 19, 3430–3439. [Google Scholar] [CrossRef]
- Tort, F.; García-Silva, M.T.; Ferrer-Cortès, X.; Navarro-Sastre, A.; Garcia-Villoria, J.; Coll, M.J.; Vidal, E.; Jiménez-Almazán, J.; Dopazo, J.; Briones, P.; et al. Exome sequencing identifies a new mutation in SERAC1 in a patient with 3-methylglutaconic aciduria. Mol. Genet. Metab. 2013, 110, 73–77. [Google Scholar] [CrossRef]
- Wortmann, S.B.; Espeel, M.; Almeida, L.; Reimer, A.; Bosboom, D.; Roels, F.; de Brouwer, A.P.; Wevers, R.A. Inborn errors of metabolism in the biosynthesis and remodeling of phospholipids. J. Inherit. Metab. Dis. 2015, 38, 99–110. [Google Scholar] [CrossRef]
- Cupo, R.R.; Shorter, J. Skd3 (human ClpB) is a potent mitochondrial protein disaggregase that is inactivated by 3-methylglutaconic aciduria-linked mutations. Elife 2020, 9, e55279. [Google Scholar] [CrossRef]
- Carrozzo, R.; Verrigni, D.; Rasmussen, M.; de Coo, R.; Amartino, H.; Bianchi, M.; Buhas, D.; Mesli, S.; Naess, K.; Born, A.P.; et al. Succinate-CoA ligase deficiency due to mutations in SUCLA2 and SUCLG1: Phenotype and genotype correlations in 71 patients. J. Inherit. Metab. Dis. 2016, 39, 243–252. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jones, D.E.; Jennings, E.A.; Ryan, R.O. Diversion of Acetyl CoA to 3-Methylglutaconic Acid Caused by Discrete Inborn Errors of Metabolism. Metabolites 2022, 12, 377. https://doi.org/10.3390/metabo12050377
Jones DE, Jennings EA, Ryan RO. Diversion of Acetyl CoA to 3-Methylglutaconic Acid Caused by Discrete Inborn Errors of Metabolism. Metabolites. 2022; 12(5):377. https://doi.org/10.3390/metabo12050377
Chicago/Turabian StyleJones, Dylan E., Elizabeth A. Jennings, and Robert O. Ryan. 2022. "Diversion of Acetyl CoA to 3-Methylglutaconic Acid Caused by Discrete Inborn Errors of Metabolism" Metabolites 12, no. 5: 377. https://doi.org/10.3390/metabo12050377
APA StyleJones, D. E., Jennings, E. A., & Ryan, R. O. (2022). Diversion of Acetyl CoA to 3-Methylglutaconic Acid Caused by Discrete Inborn Errors of Metabolism. Metabolites, 12(5), 377. https://doi.org/10.3390/metabo12050377





