Defining Fatty Acid Changes Linked to Rumen Development, Weaning and Growth in Holstein-Friesian Heifers
Abstract
:1. Introduction
2. Experimental Design
Animal Samples
- Calves were born in May and kept indoors in small group accommodations (n = 4) on straw bedding. The calves had free access to hay and drinking water, in addition to restricted access to a commercially available milk replacer and calf concentrate.
- Following weaning at ~6 weeks of age, the calves received a conventional diet based on grass silage and limited concentrates with free access to water.
- From the beginning of July, the calves were housed in 2 groups of 10 but remained indoors on a ration of grass silage and limited concentrates with free access to water.
- During the subsequent summer period (May until October), the heifers were kept on pasture with free access to grass and limited concentrates.
- The heifers returned indoors from October until the end of the study.
3. Procedure
3.1. Untargeted Metabolite Fingerprinting by Flow Infusion Electrospray Ionization High-Resolution Mass Spectrometry (FIE-HRMS)
3.2. Statistical Analysis
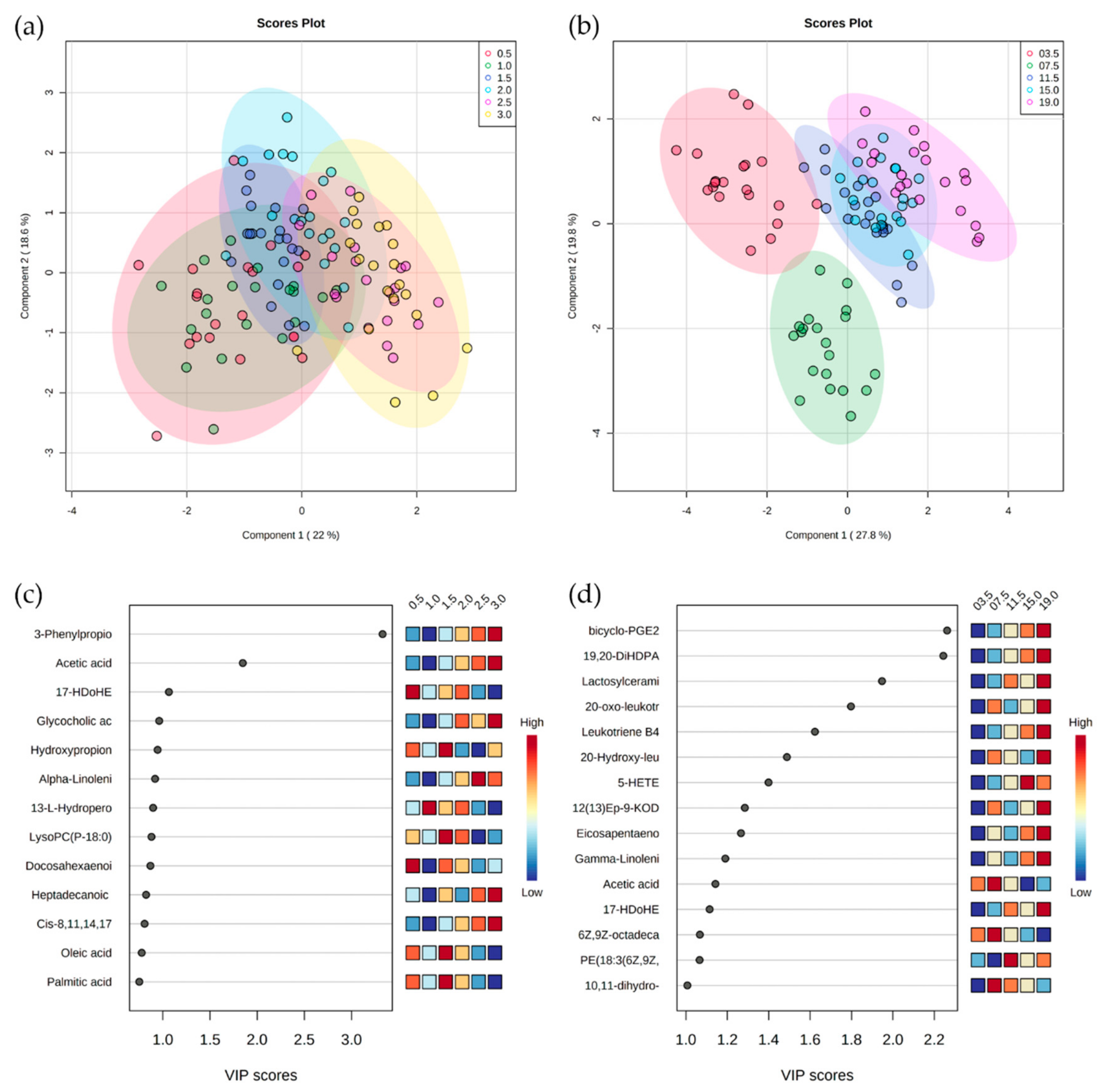
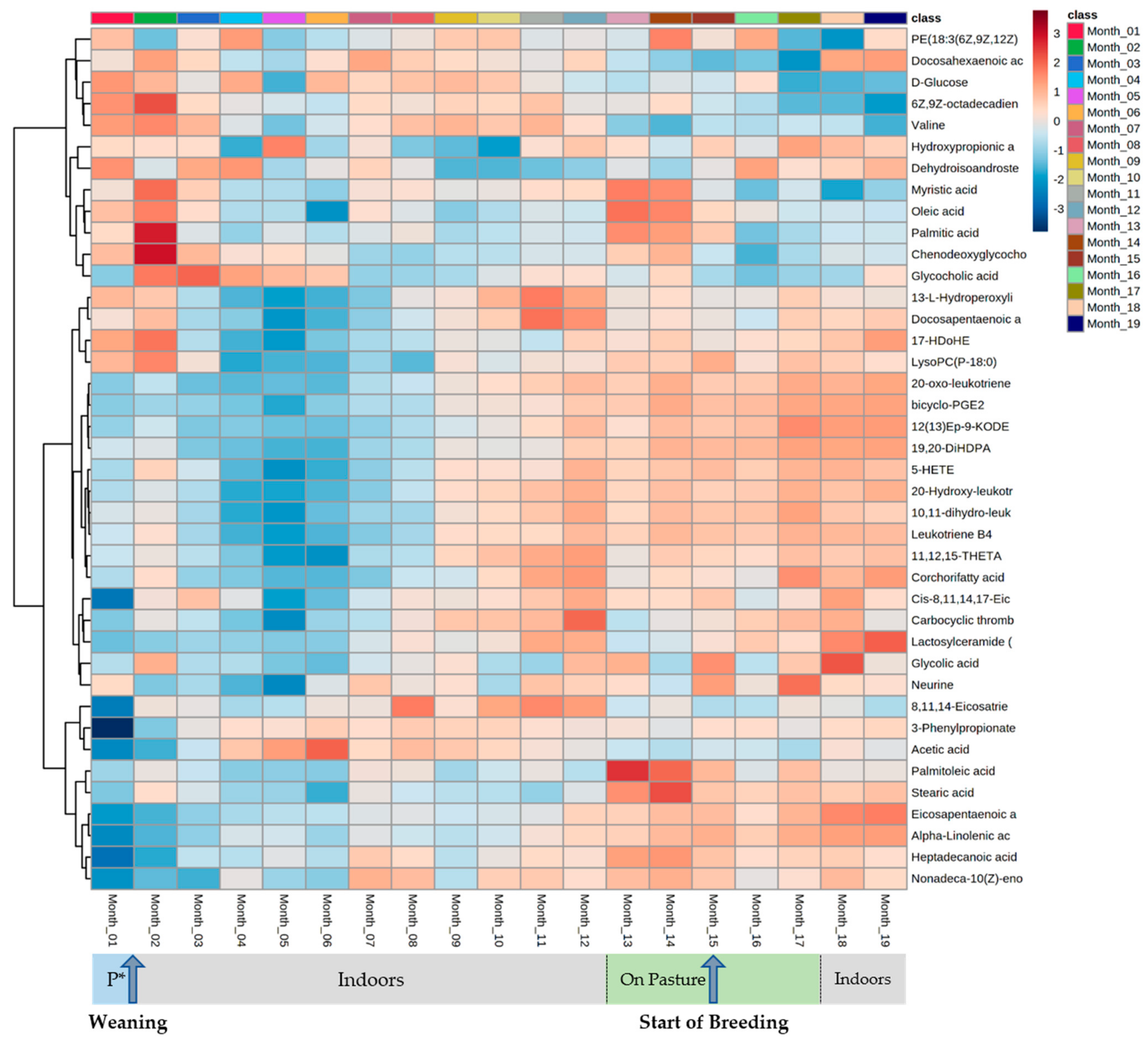
4. Results
5. Discussion
5.1. Serum Metabolomes Show the Influence of VFAs in Rumen Maturation
5.2. Serum Oxylipids Indicate Inflammatory and Innate Immune Responses
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wise, G.H.; Anderson, G.W.; Miller, P.G. Factors affecting the passage of liquids into the rumen of the dairy calf. II. Elevation of the head as milk is consumed. J. Dairy Sci. 1942, 25, 529–536. [Google Scholar] [CrossRef]
- Moran, J. Tropical Dairy Farming: Feeding Management for Small Holder Dairy Farmers in the Humid Tropics; Csiro Publishing: Victoria, Australia, 2005. [Google Scholar]
- Coffey, M.P.; Hickey, J.; Brotherstone, S. Genetic aspects of growth of Holstein-Friesian dairy cows from birth to maturity. J. Dairy Sci. 2006, 89, 322–329. [Google Scholar] [CrossRef] [Green Version]
- Handcock, R.C.; Lopez-Villalobos, N.; McNaughton, L.R.; Back, P.J.; Edwards, G.R.; Hickson, R.E. Live weight and growth of Holstein-Friesian, Jersey, and crossbred dairy heifers in New Zealand. N. Z. J. Agric. Res. 2018, 62, 173–183. [Google Scholar] [CrossRef]
- Troccon, J.L. Effects of winter feeding during the rearing period on performance and longevity in dairy cattle. Livest. Prod. Sci. 1993, 36, 157–176. [Google Scholar] [CrossRef]
- Boulton, A.C.; Rushton, J.; Wathes, D.C. An empirical analysis of the cost of rearing dairy heifers from birth to first calving and the time taken to repay these costs. Animal 2017, 11, 1372–1380. [Google Scholar] [CrossRef] [Green Version]
- Diao, Q.; Zhang, R.; Fu, T. Review of strategies to promote rumen development in calves. Animals 2019, 9, 490. [Google Scholar] [CrossRef] [Green Version]
- Curtis, G.; McGregor Argo, C.; Jones, D.; Grove-White, D. The impact of early life nutrition and housing on growth and reproduction in dairy cattle. PLoS ONE 2018, 13, e0191687. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Wang, Q.; Lin, X.; Jin, X.; Liu, L.; Wang, C.; Chen, Q.; Liu, J.; Liu, H. The Use of “Omics” in Lactation Research in Dairy Cows. Int. J. Mol. Sci. 2017, 18, 983. [Google Scholar] [CrossRef] [Green Version]
- Paradis, F.; Yue, S.; Grant, J.R.; Stothard, P.; Basarab, J.A.; Fitzsimmons, C. Transcriptomic analysis by RNA sequencing reveals that hepatic interferon-induced genes may be associated with feed efficiency in beef heifers. J. Anim. Sci. 2015, 93, 3331–3341. [Google Scholar] [CrossRef]
- Foroutan, A.; Fitzsimmons, C.; Mandal, R.; Piri-Moghadam, H.; Zheng, J.; Guo, A.C.; Li, C.; Guan, L.L.; Wishart, D.S. The Bovine Metabolome. Metabolites 2020, 10, 233. [Google Scholar] [CrossRef]
- Xue, M.-Y.; Sun, H.-Z.; Wu, X.-H.; Liu, J.-X.; Guan, L.L. Multi-omics reveals that the rumen microbiome and its metabolome together with the host metabolome contribute to individualized dairy cow performance. Microbiome 2020, 8, 64. [Google Scholar] [CrossRef] [PubMed]
- Stergiadis, S.; Cabeza-Luna, I.; Mora-Ortiz, M.; Stewart, R.D.; Dewhurst, R.J.; Humphries, D.J.; Watson, M.; Roehe, R.; Auffret, M.D. Unravelling the role of rumen microbial communities, genes, and activities, on milk fatty acid profile using a combination of omics approaches. Front. Microbiol. 2021, 11, 590441. [Google Scholar] [CrossRef] [PubMed]
- Leal, L.N.; Doelman, J.; Keppler, B.R.; Steele, M.A.; Martin-Tereso, J. Preweaning nutrient supply alters serum metabolomics profiles related to protein and energy metabolism and hepatic function in Holstein heifer calves. J. Dairy Sci. 2021, 104, 7711–7724. [Google Scholar] [CrossRef] [PubMed]
- Hare, K.S.; Leal, L.N.; Romao, J.M.; Hooiveld, G.J.; Soberon, F.; Berends, H.; Van Amburgh, M.E.; Martin-Tereso, J.; Steele, M.A. Preweaning nutrient supply alters mammary gland transcriptome expression relating to morphology, lipid accumulation, DNA synthesis, and RNA expression in Holstein heifer calves. J. Dairy Sci. 2018, 102, 2618–2630. [Google Scholar] [CrossRef]
- Beckmann, M.; Parker, D.; Enot, D.P.; Duval, E.; Draper, J. High-throughput, nontargeted metabolite fingerprinting using nominal mass flow injection electrospray mass spectrometry. Nat. Protoc. 2008, 3, 486–504. [Google Scholar] [CrossRef]
- Chong, J.; Wishart, D.; Xia, J. Using MetaboAnalyst 4.0 for comprehensive and integrative metabolomics data analysis. Curr. Protoc. Bioinform. 2019, 68, e86. [Google Scholar] [CrossRef]
- Mohd Nor, N.; Steeneveld, W.; van Werven, T.; Mourits, M.C.M.; Hogeveen, H. First-calving age and first-lactation milk production on Dutch dairy farms. J. Dairy Sci. 2013, 96, 981–992. [Google Scholar] [CrossRef] [Green Version]
- Phillips, K.M.; Read, C.C.; Kriese-Anderson, L.A.; Rodning, S.P.; Brandebourg, T.D.; Biase, F.H.; Marks, M.L.; Elmore, J.B.; Stanford, M.K.; Dyce, P.W. Plasma metabolomic profiles differ at the time of artificial insemination based on pregnancy outcome, in Bos taurus beef heifers. Sci. Rep. 2018, 8, 13196. [Google Scholar] [CrossRef] [Green Version]
- Gomez, E.; Canela, N.; Herrero, P.; Cereto, A.; Gimeno, I.; Carrocera, S.; Martin-Gonzalez, D.; Murillo, A.; Munoz, M. Metabolites secreted by bovine embryos in vitro predict pregnancies that the recipient plasma metabolome cannot, and vice versa. Metabolites 2021, 11, 162. [Google Scholar] [CrossRef]
- Nagaraja, T.G. Chapter 2—Microbiology of the Rumen. In Rumenology; Millen, D.D., Arrigoni, M.D.B., Pacheco, R.D.L., Eds.; Springer: New York, NY, USA, 2016; pp. 39–61. [Google Scholar]
- Saleem, F.; Ametaj, B.N.; Bouatra, S.; Mandal, R.; Zebeli, Q.; Dunn, S.M.; Wishart, D.S. A metabolomics approach to uncover the effects of grain diets on rumen health in dairy cows. J. Dairy Sci. 2012, 95, 6606–6623. [Google Scholar] [CrossRef] [Green Version]
- Pagella, J.H. Urinary Benzylated Compounds as Potential Markers of Forage Intake and Metabolism of Their Precursors in Ruminants. Ph.D. Thesis, Aberdeen University, Aberdeen, UK, 1998. [Google Scholar]
- Ametaj, B.N.; Zebeli, Q.; Saleem, F.; Psychogios, N.; Lewis, M.J.; Dunn, S.M.; Xia, J.; Wishart, D.S. Metabolomics reveals unhealthy alterations in rumen metabolism with increased proportion of cereal grain in the die of dairy cows. Metabolomics 2010, 6, 583–594. [Google Scholar] [CrossRef]
- Hamada, T.; Maeda, S.; Kameoka, K. Factors influencing growth of rumen, liver, and other organs in kids weaned from milk replacers to solid foods. J. Dairy Sci. 1976, 59, 1110–1118. [Google Scholar] [CrossRef]
- Davis, C.L.; Drackley, J.K. The Development, Nutrition, and Management of the Young Calf; Iowa State University Press: Iowa City, IA, USA, 1998. [Google Scholar]
- Lin, X.Y.; Wang, Y.; Wang, J.; Hou, Q.L.; Hu, Z.Y.; Shi, K.R.; Yan, Z.G.; Wang, Z.H. Effect of initial time of forage supply on growth and rumen development in preweaning calves. Anim. Prod. Sci. 2018, 58, 2224–2232. [Google Scholar] [CrossRef]
- Khan, M.A.; Lee, H.J.; Lee, W.S.; Kim, H.S.; Ki, K.S.; Hur, T.Y.; Suh, G.H.; Kang, S.J.; Choi, Y.J. Structural growth, rumen development, and metabolic and immune responses of Holstein male calves fed milk through step-down and conventional methods. J. Dairy Sci. 2007, 90, 3376–3387. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Guo, Y.; Wen, Z.; Jiang, X.; Ma, X.; Han, X. Weaning stress perturbs gut microbiome and its metabolic profile in piglets. Sci. Rep. 2018, 8, 18068. [Google Scholar] [CrossRef] [Green Version]
- Southey, B.R.; Bolt, C.R.; Rymut, H.E.; Keever, M.R.; Ulanov, A.V.; Li, Z.; Rund, L.A.; Johnson, R.W.; Rodriguez-Zas, S.L. Impact of weaning and maternal immune activation on the metabolism of pigs. Front. Mol. Biosci. 2021, 8, 660764. [Google Scholar] [CrossRef]
- Mills, D.S.; Marchant-Forde, J.N. The Encyclopedia of Applied Animal Behaviour and Welfare; CABI: Oxfordshire, UK, 2010. [Google Scholar]
- Carroll, J.A.; Arthington, J.D.; Chase, C.C. Early weaning alters the acute phase reaction to an endotoxin challenge in beef calves. J. Anim. Sci. J. 2009, 87, 4167–4172. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.-H.; Yang, J.-Y.; Upadhaya, S.D.; Lee, H.-J.; Yun, C.-H.; Ha, J.K. The stress of weaning influences serum levels of acute-phase proteins, iron-binding proteins, inflammatory cytokines, cortisol, and leukocyte subsets in Holstein calves. J. Vet. Sci. 2011, 12, 151–157. [Google Scholar] [CrossRef] [Green Version]
- Sordillo, L.M. Symposium review: Oxylipids and the regulation of bovine mammary inflammatory responses. J. Dairy Sci. 2018, 101, 5629–5641. [Google Scholar] [CrossRef] [Green Version]
- Calder, P.C. Polyunsaturated fatty acids and inflammatory processes: New twists in an old tale. Biochimie 2009, 91, 791–795. [Google Scholar] [CrossRef]
- Hedi, H.; Norbet, G. 5-Lipoxygenase pathway, dendritic cells and adaptive immunity. J. Biomed. Biotechnol. 2004, 2004, 638598. [Google Scholar] [CrossRef] [Green Version]
- Rola-Pleszczynski, M.; Stankova, J. Leukotriene B4 enhances interleukin-6 (IL-6) production and IL6 messenger RNA accumulation in human monocytes in vitro: Transcriptional and posttranscriptional mechanisms. Blood 1992, 80, 1004–1011. [Google Scholar] [CrossRef] [Green Version]
- Dubois, C.M.; Bissonnette, E.; Rola-Pleszczynski, M. Asbestos fibers and silica particles stimulate rat alveolar macrophages to release tumor necrosis factor: Autoregulatory role of leukotriene B4. Am. Rev. Respir. Dis. 1989, 139, 1257–1264. [Google Scholar] [CrossRef] [PubMed]
- Cavallini, D.; Mammi, L.M.E.; Buonaiuto, G.; Palmonari, A.; Valle, E.; Formigoni, A. Immune-metabolic-inflammatory markers in Holstein cows exposed to a nutritional and environmental stressing challenge. J. Anim. Physiol. Anim. Nutr. 2021, 105, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Kent-Dennis, C.; Pasternak, A.; Plaizier, J.C.; Penner, G.B. Potential for a localized immune response by the ruminal epithelium in nonpregnant heifers following a short-term subacute ruminal acidosis challenge. J. Dairy Sci. 2019, 102, 7556–7569. [Google Scholar] [CrossRef]
- Tachtsis, B.; Camera, D.; Lacham-Kaplan, O.L. Potential roles of n-3 PUFAs during skeletal muscle growth and regeneration. Nutrients 2018, 10, 309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDonnell, R.P.; Doherty, J.V.; Earley, B.; Clarke, A.M.; Kenny, D.A. Effect of supplementation with n-3 polyunsaturated fatty acids and/or β-glucans on performance, feeding behaviour and immune status of Holstein Friesian bull calves during the pre- and post-weaning periods. J. Anim. Sci. Biotechnol. 2019, 10, 7. [Google Scholar] [CrossRef] [Green Version]
- Garcia, M.; Shin, J.H.; Schlaefli, A.; Greco, L.F.; Maunsell, F.P.; Thatcher, W.W.; Santos, J.E.P.; Staples, C.R. Increasing intake of essential fatty acids from milk replacer benefits performance, immune responses, and health of preweaned Holstein calves. J. Dairy Sci. 2015, 98, 458–477. [Google Scholar] [CrossRef] [Green Version]



| Class | Subclass | Metabolites | Mode | Age (Months) | |||
|---|---|---|---|---|---|---|---|
| 0.5–3.0 | 0.5–19.0 | ||||||
| F-Value | p-Value | F-Value | p-Value | ||||
| Carboxylic acids and derivatives | Amino acids, peptides and analogues | Valine | Pos | - | - | 1.31 × 101 | 2.06 × 10−48 |
| Carboxylic acids | Acetic acid | Neg | 1.69 × 101 | 2.77 × 10−11 | 6.48 × 101 | 1.72 × 10−165 | |
| Fatty acyls | Eicosanoids | 20-Hydroxy-leukotriene B4 | Neg | 2.79 × 100 | 2.55 × 10−2 | 4.21 × 101 | 9.68 × 10−127 |
| 5-Hete | Neg | 1.05 × 101 | 1.23 × 10−7 | 3.19 × 101 | 2.97 × 10−104 | ||
| Leukotriene B4 | Neg | 5.24 × 100 | 3.95 × 10−4 | 2.91 × 101 | 2.02 × 10−97 | ||
| 10,11-dihydro-leukotriene B4 | Neg | - | - | 2.00 × 101 | 5.99 × 10−72 | ||
| 11,12,15-THETA | Neg | - | - | 1.68 × 101 | 1.74 × 10−61 | ||
| 20-oxo-leukotriene B4 | Neg | - | - | 4.21 × 101 | 8.13 × 10−127 | ||
| bicyclo-PGE2 | Neg | - | - | 4.09 × 101 | 1.93 × 10−124 | ||
| Carbocyclic thromboxane A2 | Neg | - | - | 7.86 × 100 | 5.11 × 10−28 | ||
| Fatty acids and conjugates | 11,14,17-Eicosatrienoic acid | Neg | - | - | 2.47 × 100 | 2.65 × 10−5 | |
| 12(13)Ep-9-KODE | Neg | 1.11 × 101 | 5.15 × 10−8 | 4.67 × 101 | 1.19 × 10−135 | ||
| 17-HDoHE | Neg | 8.09 × 100 | 4.06 × 10−6 | 1.27 × 101 | 4.62 × 10−47 | ||
| 19,20-DiHDPA | Neg | 5.15 × 100 | 4.54 × 10−4 | 2.73 × 101 | 1.01 × 10−92 | ||
| 9,10,13-TriHOME | Neg | 4.45 × 100 | 1.49 × 10−3 | - | - | ||
| Cis-8,11,14,17-Eicosatetraenoic acid | Neg | 5.01 × 100 | 5.77 × 10−4 | 3.89 × 100 | 4.15 × 10−11 | ||
| Docosahexaenoic acid | Neg | 8.02 × 100 | 4.49 × 10−6 | 3.79 × 100 | 1.12 × 10−10 | ||
| Docosapentaenoic acid | Neg | - | - | 1.30 × 101 | 3.97 × 10−48 | ||
| Eicosapentaenoic acid | Neg | - | - | 2.32 × 101 | 2.78 × 10−81 | ||
| Eicosenoic acid | Neg | 7.42 × 100 | 1.13 × 10−5 | - | - | ||
| Heptadecanoic acid | Neg | 4.17 × 100 | 2.41 × 10−3 | 9.65 × 100 | 2.68 × 10−35 | ||
| Myristic acid | Neg | 6.94 × 100 | 2.41 × 10−5 | 9.50 × 100 | 1.01 × 10−34 | ||
| Nonadeca-10(Z)-enoic acid | Neg | - | - | 2.06 × 100 | 7.96 × 10−4 | ||
| Oleic acid | Neg | 6.16 × 100 | 8.58 × 10−5 | 6.22 × 100 | 4.23 × 10−21 | ||
| Palmitic acid | Neg | 2.38 × 101 | 1.49 × 10−14 | 1.25 × 101 | 2.06 × 10−46 | ||
| Palmitoleic acid | Neg | - | - | 3.74 × 100 | 1.86 × 10−10 | ||
| Stearic acid | Neg | - | - | 1.72 × 100 | 9.85 × 10−3 | ||
| Fatty amides | Oleamide | Neg | 7.38 × 100 | 1.20 × 10−5 | - | - | |
| Lineolic acids and derivatives | 13-L-Hydroperoxylinoleic acid | Neg | 7.75 × 100 | 6.83 × 10−6 | 2.33 × 101 | 1.05 × 10−81 | |
| 6Z,9Z-octadecadienoic acid | Neg | 5.23 × 100 | 4.00 × 10−4 | 5.61 × 100 | 1.81 × 10−18 | ||
| Alpha-linolenic acid | Neg | 3.60 × 100 | 6.36 × 10−3 | 4.11 × 101 | 9.48 × 10−125 | ||
| Corchorifatty acid F | Neg | 9.28 × 100 | 6.84 × 10−7 | 2.40 × 101 | 1.22 × 10−83 | ||
| Glycerophospholipids | Glycerophosphocholines | LysoPC(P-18:0) | Neg | 1.11 × 101 | 4.83 × 10−8 | 2.96 × 101 | 9.10 × 10−99 |
| Glycerophosphoethanolamines | PE(22:6) 1 | Neg | 1.31 × 101 | 3.14 × 10−9 | - | - | |
| Glycerophosphoethanolamines | PE(18:3) 2 | Neg | - | - | 9.65 × 100 | 2.53 × 10−35 | |
| Hydroxy acids and derivatives | Alpha hydroxy acids and derivatives | Glycolic acid | Neg | 5.96 × 100 | 1.18 × 10−4 | 4.84 × 100 | 3.64 × 10−15 |
| Beta hydroxy acids and derivatives | Hydroxypropionic acid | Neg | 5.64 × 101 | 1.37 × 10−24 | 6.42 × 101 | 1.52 × 10−164 | |
| Organonitrogen compounds | Quaternary ammonium salts | Neurine | Pos | - | - | 5.80 × 100 | 3.70 × 10−19 |
| Organooxygen compounds | Carbohydrates and carbohydrate conjugates | D-Glucose | Neg | 1.18 × 101 | 1.96 × 10−8 | 2.11 × 101 | 4.75 × 10−75 |
| Phenylpropanoic acids | - | 3-Phenylpropionate | Neg | 2.94 × 101 | 7.81 × 10−17 | 4.69 × 101 | 3.73 × 10−136 |
| Sphingolipids | Glycosphingolipids | Lactosylceramide3 | Pos | - | - | 2.17 × 101 | 1.22 × 10−76 |
| Steroids and steroid derivatives | Bile acids, alcohols and derivatives | Chenodeoxyglycocholic acid | Neg | 8.62 × 100 | 1.80 × 10−6 | 1.30 × 101 | 4.11 × 10−48 |
| Glycocholic acid | Neg | 7.27 × 100 | 1.43 × 10−5 | 6.23 × 100 | 3.85 × 10−21 | ||
| Steroid esters | CE(22:6) 4 | Pos | 3.20 × 100 | 1.55 × 10−2 | - | - | |
| Steroidal glycosides | DHEA 3-glucuronide | Pos | 2.85 × 100 | 2.74 × 10−2 | 2.33 × 100 | 9.65 × 10−5 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taylor, E.N.; Han, J.; Fan, C.; Beckmann, M.; Hewinson, G.; Rooke, D.; Koets, A.P.; Mur, L.A.J. Defining Fatty Acid Changes Linked to Rumen Development, Weaning and Growth in Holstein-Friesian Heifers. Metabolites 2022, 12, 374. https://doi.org/10.3390/metabo12050374
Taylor EN, Han J, Fan C, Beckmann M, Hewinson G, Rooke D, Koets AP, Mur LAJ. Defining Fatty Acid Changes Linked to Rumen Development, Weaning and Growth in Holstein-Friesian Heifers. Metabolites. 2022; 12(5):374. https://doi.org/10.3390/metabo12050374
Chicago/Turabian StyleTaylor, Emma N., Jiwan Han, Congying Fan, Manfred Beckmann, Glyn Hewinson, David Rooke, Ad P. Koets, and Luis A. J. Mur. 2022. "Defining Fatty Acid Changes Linked to Rumen Development, Weaning and Growth in Holstein-Friesian Heifers" Metabolites 12, no. 5: 374. https://doi.org/10.3390/metabo12050374
APA StyleTaylor, E. N., Han, J., Fan, C., Beckmann, M., Hewinson, G., Rooke, D., Koets, A. P., & Mur, L. A. J. (2022). Defining Fatty Acid Changes Linked to Rumen Development, Weaning and Growth in Holstein-Friesian Heifers. Metabolites, 12(5), 374. https://doi.org/10.3390/metabo12050374








