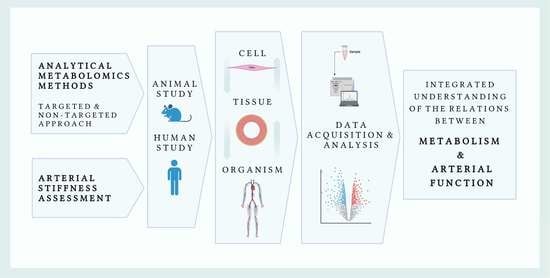
Metabolomics of Arterial Stiffness
Abstract
1. Introduction
2. Arterial Stiffness and Its Prognostic Value
3. Brief Overview of Metabolomics
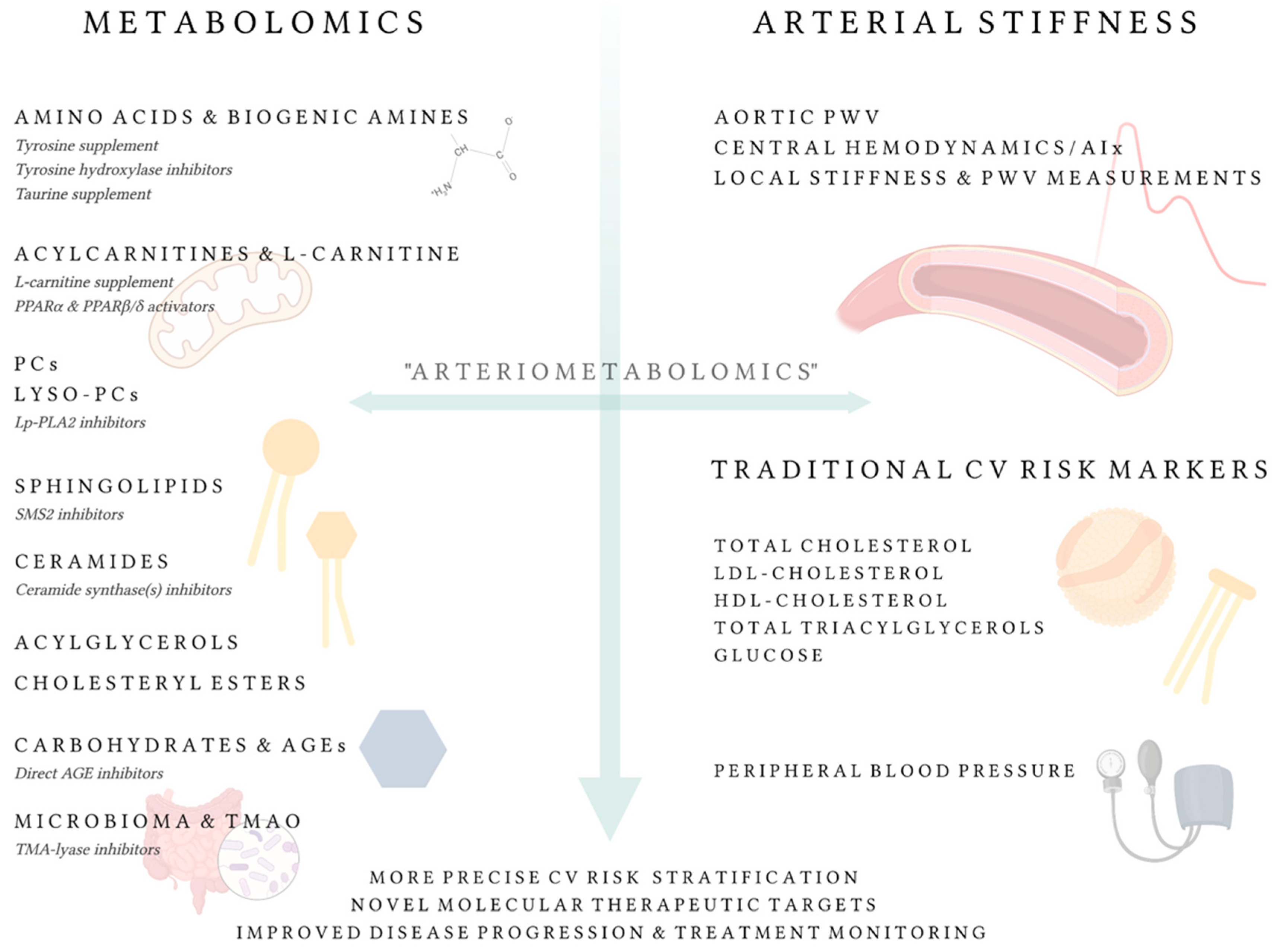
4. ‘Arteriometabolomics’ Approach
4.1. Linkage between Low-Molecular Weight Metabolites and Vascular Research: Current State
4.1.1. L-Carnitine, Trimethylamine N-Oxide, and Acylcarnitines
4.1.2. Phospholipids
4.1.3. Sphingolipids and Ceramides
4.1.4. Acylglycerols and Cholesteryl Esters
4.1.5. Amino Acids and Biogenic Amines
4.1.6. Carbohydrates and Advanced Glycation End-Products
4.1.7. Other Metabolites
4.2. Future Outlook
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nidorf, S.M.; Fiolet, A.T.L.; Mosterd, A.; Eikelboom, J.W.; Schut, A.; Opstal, T.S.J.; The, S.H.K.; Xu, X.-F.; Ireland, M.A.; Lenderink, T.; et al. Colchicine in patients with chronic coronary disease. N. Engl. J. Med. 2020, 383, 1838–1847. [Google Scholar] [CrossRef] [PubMed]
- Tardif, J.-C.; Kouz, S.; Waters, D.D.; Bertrand, O.F.; Diaz, R.; Maggioni, A.P.; Pinto, F.J.; Ibrahim, R.; Gamra, H.; Kiwan, G.S.; et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N. Engl. J. Med. 2019, 381, 2497–2505. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Broch, K.; Anstensrud, A.K.; Woxholt, S.; Sharma, K.; Tøllefsen, I.M.; Bendz, B.; Aakhus, S.; Ueland, T.; Amundsen, B.H.; Damås, J.K.; et al. Randomized trial of interleukin-6 receptor inhibition in patients with acute ST-segment elevation myocardial infarction. J. Am. Coll. Cardiol. 2021, 77, 1845–1855. [Google Scholar] [CrossRef] [PubMed]
- Olsen, M.B.; Gregersen, I.; Sandanger, Ø.; Yang, K.; Sokolova, M.; Halvorsen, B.E.; Gullestad, L.; Broch, K.; Aukrust, P.; Louwe, M.C. Targeting the inflammasome in cardiovascular disease. JACC Basic Transl. Sci. 2022, 7, 84–98. [Google Scholar] [CrossRef]
- Laurent, S.; Chatellier, G.; Azizi, M.; Calvet, D.; Choukroun, G.; Danchin, N.; Delsart, P.; Girerd, X.; Gosse, P.; Khettab, H.; et al. SPARTE study: Normalization of arterial stiffness and cardiovascular events in patients with hypertension at medium to very high risk. Hypertension 2021, 78, 983–995. [Google Scholar] [CrossRef]
- Patti, G.J.; Yanes, O.; Siuzdak, G. Innovation: Metabolomics: The apogee of the omics trilogy. Nat. Rev. Mol. Cell Biol. 2012, 13, 263–269. [Google Scholar] [CrossRef]
- Griffin, J.L.; Atherton, H.J.; Shockcor, J.P.; Atzori, L. Metabolomics as a tool for cardiac research. Nat. Rev. Cardiol. 2011, 8, 630–643. [Google Scholar] [CrossRef]
- McGarrah, R.W.; Crown, S.B.; Zhang, G.-F.; Shah, S.H.; Newgard, C.B. Cardiovascular metabolomics. Circ. Res. 2018, 122, 1238–1258. [Google Scholar] [CrossRef]
- Åstrand, H.; Stålhand, J.; Karlsson, J.; Karlsson, M.; Sonesson, B.; Länne, T. In vivo estimation of the contribution of elastin and collagen to the mechanical properties in the human abdominal aorta: Effect of age and sex. J. Appl. Physiol. 2011, 110, 176–187. [Google Scholar] [CrossRef]
- Van Bussel, B.C.; Schouten, F.; Henry, R.M.; Schalkwijk, C.G.; De Boer, M.R.; Ferreira, I.; Smulders, Y.M.; Twisk, J.W.; Stehouwer, C.D. Endothelial dysfunction and low-grade inflammation are associated with greater arterial stiffness over a 6-year period. Hypertension 2011, 58, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Mäki-Petäjä, K.M.; Elkhawad, M.; Cheriyan, J.; Joshi, F.R.; Östör, A.J.K.; Hall, F.C.; Rudd, J.H.F.; Wilkinson, I.B. Anti-tumor necrosis factor-α therapy reduces aortic inflammation and stiffness in patients with rheumatoid arthritis. Circulation 2012, 126, 2473–2480. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.H.; Vendrov, A.E.; Tchivilev, I.; Niu, X.L.; Molnar, K.C.; Rojas, M.; Carter, J.D.; Tong, H.; Stouffer, G.A.; Madamanchi, N.R.; et al. Mitochondrial oxidative stress in aortic stiffening with age the role of smooth muscle cell function. Arter.-Scler. Thromb. Vasc. Biol. 2012, 32, 745–755. [Google Scholar] [CrossRef] [PubMed]
- Lacolley, P.; Regnault, V.; Segers, P.; Laurent, S. Vascular smooth muscle cells and arterial stiffening: Relevance in development, aging, and disease. Physiol. Rev. 2017, 97, 1555–1617. [Google Scholar] [CrossRef]
- Sell, D.R.; Monnier, V.M. Molecular basis of arterial stiffening: Role of glycation—A mini-review. Gerontology 2012, 58, 227–237. [Google Scholar] [CrossRef]
- Haydar, A.A.; Covic, A.; Colhoun, H.; Rubens, M.; Goldsmith, D.J. Coronary artery calcification and aortic pulse wave velocity in chronic kidney disease patients. Kidney Int. 2004, 65, 1790–1794. [Google Scholar] [CrossRef]
- Ghiadoni, L.; Penno, G.; Giannarelli, C.; Plantinga, Y.; Bernardini, M.; Pucci, L.; Miccoli, R.; Taddei, S.; Salvetti, A.; Del Prato, S. Metabolic syndrome and vascular alterations in normotensive subjects at risk of diabetes mellitus. Hypertension 2008, 51, 440–445. [Google Scholar] [CrossRef]
- Murray, R.; Kitaba, N.; Antoun, E.; Titcombe, P.; Barton, S.; Cooper, C.; Inskip, H.M.; Burdge, G.C.; Mahon, P.A.; Deanfield, J.; et al. Influence of maternal lifestyle and diet on perinatal DNA methylation signatures associated with childhood arterial stiffness at 8 to 9 years. Hypertension 2021, 78, 787–800. [Google Scholar] [CrossRef]
- Lacolley, P.; Regnault, V.; Laurent, S. Mechanisms of arterial stiffening: From mechanotransduction to epigenetics. Arter.-Scler. Thromb. Vasc. Biol. 2020, 40, 1055–1062. [Google Scholar] [CrossRef]
- Wu, S.; Jin, C.; Li, S.; Zheng, X.; Zhang, X.; Cui, L.; Gao, X. Aging, arterial stiffness, and blood pressure association in Chinese adults. Hypertension 2019, 73, 893–899. [Google Scholar] [CrossRef]
- Sun, Z. Aging, arterial stiffness, and hypertension. Hypertension 2015, 65, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Safar, M.E.; Asmar, R.; Benetos, A.; Blacher, J.; Boutouyrie, P.; Lacolley, P.; Laurent, S.; London, G.; Pannier, B.; Protogerou, A.; et al. Interaction between hypertension and arterial stiffness an expert reappraisal. Hypertension 2018, 72, 796–805. [Google Scholar] [CrossRef] [PubMed]
- Prenner, S.B.; Chirinos, J.A. Arterial stiffness in diabetes mellitus. Atherosclerosis 2015, 238, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Townsend, R.R. Arterial stiffness in CKD: A review. Am. J. Kidney Dis. 2019, 73, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Hsu, B.-G.; Tsai, J.-P. Arterial stiffness: A brief review. Tzu-Chi Med. J. 2021, 33, 115–121. [Google Scholar] [CrossRef]
- Vlachopoulos, C.; Terentes-Printzios, D.; Laurent, S.; Nilsson, P.M.; Protogerou, A.D.; Aznaouridis, K.; Xaplanteris, P.; Koutagiar, I.; Tomiyama, H.; Yamashina, A.; et al. Association of estimated pulse wave velocity with survival: A secondary analysis of SPRINT. JAMA Netw. Open 2019, 2, e1912831. [Google Scholar] [CrossRef]
- Hametner, B.; Wassertheurer, S.; Mayer, C.C.; Danninger, K.; Binder, R.K.; Weber, T. Aortic pulse wave velocity predicts cardiovascular events and mortality in patients undergoing coronary angiography: A comparison of invasive measurements and noninvasive estimates. Hypertension 2021, 77, 571–581. [Google Scholar] [CrossRef]
- Vlachopoulos, C.; Aznaouridis, K.; Stefanadis, C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: A systematic review and meta-analysis. J. Am. Coll. Cardiol. 2010, 55, 1318–1327. [Google Scholar] [CrossRef]
- Laurent, S.; Cockcroft, J.; Van Bortel, L.; Boutouyrie, P.; Giannattasio, C.; Hayoz, D.; Pannier, B.; Vlachopoulos, C.; Wilkinson, I.; Struijker-Boudier, H. Expert consensus document on arterial stiffness: Methodological issues and clinical applications. Eur. Heart J. 2006, 27, 2588–2605. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Rosei, E.A.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef]
- Nichols, W.W. Clinical measurement of arterial stiffness obtained from noninvasive pressure waveforms. Am. J. Hypertens. 2005, 18, 3S–10S. [Google Scholar] [CrossRef] [PubMed]
- Nichols, W.W.; Edwards, D.G. Arterial elastance and wave reflection augmentation of systolic blood pressure: Deleterious effects and implications for therapy. J. Cardiovasc. Pharmacol. Ther. 2001, 6, 5–21. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, G.F. Aortic stiffness, pressure and flow pulsatility, and target organ damage. J. Appl. Physiol. 2018, 125, 1871–1880. [Google Scholar] [CrossRef]
- Vlachopoulos, C.; Aznaouridis, K.; O’Rourke, M.F.; Safar, M.E.; Baou, K.; Stefanadis, C. Prediction of cardiovascular events and all-cause mortality with central haemodynamics: A systematic review and meta-analysis. Eur. Heart J. 2010, 31, 1865–1871. [Google Scholar] [CrossRef]
- Townsend, R.R.; Wilkinson, I.B.; Schiffrin, E.L.; Avolio, A.P.; Chirinos, J.A.; Cockcroft, J.R.; Heffernan, K.S.; Lakatta, E.G.; McEniery, C.M.; Mitchell, G.F.; et al. Recommendations for improving and standardizing vascular research on arterial stiffness: A scientific statement from the American Heart Association. Hypertension 2015, 66, 698–722. [Google Scholar] [CrossRef] [PubMed]
- Segers, P.; Rietzschel, E.R.; Chirinos, J.A. How to measure arterial stiffness in humans. Arter. Thromb. Vasc. Biol. 2020, 40, 1034–1043. [Google Scholar] [CrossRef]
- Mahmud, A.; Feely, J. Review: Arterial stiffness and the renin-angiotensin-aldosterone system. J. Renin-Angiotensin-Aldosterone Syst. 2004, 5, 102–108. [Google Scholar] [CrossRef]
- London, G.M.; Pannier, B.; Guerin, A.P.; Marchais, S.J.; Safar, M.E.; Cuche, J.L. Cardiac hypertrophy, aortic compliance, peripheral resistance, and wave reflection in end-stage renal disease: Comparative effects of ACE inhibition and calcium channel blockade. Circulation 1994, 90, 2786–2796. [Google Scholar] [CrossRef]
- Ichihara, A.; Hayashi, M.; Koura, Y.; Tada, Y.; Kaneshiro, Y.; Saruta, T. Long-term effects of statins on arterial pressure and stiffness of hypertensives. J. Hum. Hypertens. 2005, 19, 103–109. [Google Scholar] [CrossRef][Green Version]
- Striepe, K.; Jumar, A.; Ott, C.; Karg, M.V.; Schneider, M.P.; Kannenkeril, D.; Schmieder, R.E. Effects of the selective sodium-glucose cotransporter 2 inhibitor empagliflozin on vascular function and central hemodynamics in patients with type 2 diabetes mellitus. Circulation 2017, 136, 1167–1169. [Google Scholar] [CrossRef]
- Cherney, D.Z.I.; Perkins, B.A.; Soleymanlou, N.; Har, R.; Fagan, N.; Johansen, O.E.; Woerle, H.J.; von Eynatten, M.; Broedl, U.C. The effect of empagliflozin on arterial stiffness and heart rate variability in subjects with uncomplicated type 1 diabetes mellitus. Cardiovasc. Diabetol. 2014, 13, 28. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.N.; Buchanich, J.M.; Youk, A.; Brooks, M.M.; Barinas-Mitchell, E.; Conroy, M.B.; Sutton-Tyrrell, K. Reductions in arterial stiffness with weight loss in overweight and obese young adults: Potential mechanisms. Atherosclerosis 2012, 223, 485–490. [Google Scholar] [CrossRef]
- D’Elia, L.; Galletti, F.; La Fata, E.; Sabino, P.; Strazzullo, P. Effect of dietary sodium restriction on arterial stiffness: Systematic review and meta-analysis of the randomized controlled trials. J. Hypertens. 2018, 36, 734–743. [Google Scholar] [CrossRef] [PubMed]
- Jatoi, N.A.; Jerrard-Dunne, P.; Feely, J.; Mahmud, A. Impact of smoking and smoking cessation on arterial stiffness and aortic wave reflection in hypertension. Hypertension 2007, 49, 981–985. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S. Metabolomics for investigating physiological and pathophysiological processes. Physiol. Rev. 2019, 99, 1819–1875. [Google Scholar] [CrossRef] [PubMed]
- Aderemi, A.V.; Ayeleso, A.O.; Oyedapo, O.O.; Mukwevho, E. Metabolomics: A scoping review of its role as a tool for disease biomarker discovery in selected non-communicable diseases. Metabolites 2021, 11, 418. [Google Scholar] [CrossRef]
- Reyon, D.; Khayter, C.; Regan, M.R.; Joung, J.K.; Sander, J.D. Engineering designer transcription activator-like effector nucleases (TALENs) by REAL or REAL-fast assembly. Curr. Protoc. Mol. Biol. 2012, 100, 12–15. [Google Scholar] [CrossRef]
- Ribbenstedt, A.; Ziarrusta, H.; Benskin, J.P. Development, characterization and comparisons of targeted and non-targeted metabolomics methods. PLoS ONE 2018, 13, e0207082. [Google Scholar] [CrossRef]
- Schrimpe-Rutledge, A.C.; Codreanu, S.G.; Sherrod, S.D.; McLean, J.A. Untargeted metabolomics strategies—Challenges and emerging directions. J. Am. Soc. Mass Spectrom. 2016, 27, 1897–1905. [Google Scholar] [CrossRef]
- Emwas, A.H.M. The strengths and weaknesses of NMR spectroscopy and mass spectrometry with particular focus on metabolomics research. Methods Mol. Biol. 2015, 1277, 161–193. [Google Scholar]
- Emwas, A.H.M.; Roy, R.; McKay, R.T.; Tenori, L.; Saccenti, E.; Nagana Gowda, G.A.; Raftery, D.; AlAhmari, F.; Jaremko, L.; Jaremko, M.; et al. NMR spectroscopy for metabolomics research. Metabolites 2019, 9, 123. [Google Scholar] [CrossRef] [PubMed]
- Alseekh, S.; Aharoni, A.; Brotman, Y.; Contrepois, K.; D’Auria, J.; Ewald, J.; Ewald, J.C.; Fraser, P.D.; Giavalisco, P.; Hall, R.D.; et al. Mass spectrometry-based metabolomics: A guide for annotation, quantification and best reporting practices. Nat. Methods 2021, 18, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, S.; Sen, P.; Dickens, A.M.; Hyötyläinen, T.; Orešič, M. An overview of metabolomics data analysis: Current tools and future perspectives. Compr. Anal. Chem. 2018, 82, 387–413. [Google Scholar]
- Xia, J.; Wishart, D.S.; Valencia, A. MetPA: A web-based metabolomics tool for pathway analysis and visualization. Bioinformatics 2011, 26, 2342–2344. [Google Scholar] [CrossRef] [PubMed]
- Karnovsky, A.; Li, S. Pathway analysis for targeted and untargeted metabolomics. Methods Mol. Biol. 2020, 2104, 387–400. [Google Scholar] [CrossRef]
- McGranaghan, P.; Kirwan, J.A.; Garcia-Rivera, M.A.; Pieske, B.; Edelmann, F.; Blaschke, F.; Appunni, S.; Saxena, A.; Rubens, M.; Veledar, E.; et al. Lipid metabolite biomarkers in cardiovascular disease: Discovery and biomechanism translation from human studies. Metabolites 2021, 11, 621. [Google Scholar] [CrossRef]
- Flanagan, J.L.; Simmons, P.A.; Vehige, J.; Willcox, M.D.; Garrett, Q. Role of carnitine in disease. Nutr. Metab. 2010, 7, 30. [Google Scholar] [CrossRef]
- Marcovina, S.M.; Sirtori, C.; Peracino, A.; Gheorghiade, M.; Borum, P.; Remuzzi, G.; Ardehali, H. Translating the basic knowledge of mitochondrial functions to metabolic therapy: Role of L-carnitine. Transl. Res. 2013, 161, 73–84. [Google Scholar] [CrossRef]
- Pons, R.; De Vivo, D.C. Primary and secondary carnitine deficiency syndromes. J. Child Neurol. 1995, 10, S8–S24. [Google Scholar] [CrossRef]
- Mohammadi, M.; Hajhossein Talasaz, A.; Alidoosti, M. Preventive effect of L-carnitine and its derivatives on endothelial dysfunction and platelet aggregation. Clin. Nutr. ESPEN 2016, 15, 1–10. [Google Scholar] [CrossRef]
- Lai, Y.-H.; Lee, M.-C.; Ho, G.-J.; Liu, C.-H.; Hsu, B.-G. Association of low serum L-carnitine levels with peripheral arterial stiffness in patients who undergo kidney transplantation. Nutrients 2019, 11, 2000. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.J.; Hsu, B.G.; Lai, Y.H.; Wang, C.H.; Lin, Y.L.; Kuo, C.H.; Tsai, J.P. Association of low serum L-carnitine levels with aortic stiffness in patients with non-dialysis chronic kidney disease. Nutrients 2020, 12, 2918. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Jiang, W.; Chen, G.; Zhu, W.; Ding, W.; Ge, Z.; Tan, Y.; Ma, T.; Cui, G. L-carnitine treatment of insulin resistance: A systematic review and meta-analysis. Adv. Clin. Exp. Med. 2017, 26, 333–338. [Google Scholar] [CrossRef] [PubMed]
- National Library of Medicine (U.S.). Causal Mechanisms in Adolescent Arterial Stiffness. ClinicalTrials.gov Identifier: NCT04128969. October 2019. Available online: https://clinicaltrials.gov/ct2/show/NCT04128969 (accessed on 6 March 2022).
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Koeth, R.A.; Levison, B.S.; Culley, M.K.; Buffa, J.A.; Wang, Z.; Gregory, J.C.; Org, E.; Wu, Y.; Li, L.; Smith, J.D.; et al. γ-butyrobetaine is a proatherogenic intermediate in gut microbial metabolism of L-carnitine to TMAO. Cell Metab. 2014, 20, 799–812. [Google Scholar] [CrossRef]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; DuGar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.-M.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef]
- Schiattarella, G.G.; Sannino, A.; Toscano, E.; Giugliano, G.; Gargiulo, G.; Franzone, A.; Trimarco, B.; Esposito, G.; Perrino, C. Gut microbe-generated metabolite trimethylamine-N-oxide as cardiovascular risk biomarker: A systematic review and dose-response meta-analysis. Eur. Heart J. 2017, 38, 2948–2956. [Google Scholar] [CrossRef]
- Lee, Y.; Nemet, I.; Wang, Z.; Lai, H.T.M.; de Oliveira Otto, M.C.; Lemaitre, R.N.; Fretts, A.M.; Sotoodehnia, N.; Budoff, M.; DiDonato, J.A.; et al. Longitudinal plasma measures of trimethylamine N-oxide and risk of atherosclerotic cardiovascular disease events in community-based older adults. J. Am. Heart Assoc. 2021, 10, e020646. [Google Scholar] [CrossRef]
- Canyelles, M.; Tondo, M.; Cedó, L.; Farràs, M.; Escolà-Gil, J.C.; Blanco-Vaca, F. Trimethylamine N-oxide: A link among diet, gut microbiota, gene regulation of liver and intestine cholesterol homeostasis and HDL function. Int. J. Mol. Sci. 2018, 19, 3228. [Google Scholar] [CrossRef]
- Chen, M.L.; Zhu, X.H.; Ran, L.; Lang, H.D.; Yi, L.; Mi, M.T. Trimethylamine-N-oxide induces vascular inflammation by activating the NLRP3 inflammasome through the SIRT3-SOD2-MtROS signaling pathway. J. Am. Heart Assoc. 2017, 6, e006347. [Google Scholar] [CrossRef]
- Brunt, V.E.; Casso, A.G.; Gioscia-Ryan, R.A.; Sapinsley, Z.J.; Ziemba, B.P.; Clayton, Z.S.; Bazzoni, A.E.; VanDongen, N.S.; Richey, J.J.; Hutton, D.A.; et al. Gut microbiome-derived metabolite trimethylamine N-oxide induces aortic stiffening and increases systolic blood pressure with aging in mice and humans. Hypertension 2021, 78, 499–511. [Google Scholar] [CrossRef]
- Pierce, G.L.; Roy, S.J.; Gimblet, C.J. The gut-arterial stiffness axis: Is TMAO a novel target to prevent age-related aortic stiffening? Hypertension 2021, 78, 512–515. [Google Scholar] [CrossRef]
- Brunt, V.E.; Gioscia-Ryan, R.A.; Casso, A.G.; VanDongen, N.S.; Ziemba, B.P.; Sapinsley, Z.J.; Richey, J.J.; Zigler, M.C.; Neilson, A.P.; Davy, K.P.; et al. Trimethylamine-N-oxide promotes age-related vascular oxidative stress and endothelial dysfunction in mice and healthy humans. Hypertension 2020, 76, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Papandreou, C.; Moré, M.; Bellamine, A. Trimethylamine N-oxide in relation to cardiometabolic health—Cause or effect? Nutrients 2020, 12, 1330. [Google Scholar] [CrossRef] [PubMed]
- Lindskog Jonsson, A.; Caesar, R.; Akrami, R.; Reinhardt, C.; Fak Hallenius, F.; Boren, J.; Backhed, F. Impact of gut microbiota and diet on the development of atherosclerosis in ApoE−/− mice. Arter. Thromb. Vasc. Biol. 2018, 38, 2318–2326. [Google Scholar] [CrossRef] [PubMed]
- Collins, H.L.; Drazul-Schrader, D.; Sulpizio, A.C.; Koster, P.D.; Williamson, Y.; Adelman, S.J.; Owen, K.; Sanli, T.; Bellamine, A. L-carnitine intake and high trimethylamine N-oxide plasma levels correlate with low aortic lesions in ApoE−/− trnsgenic mice expressing CETP. Atherosclerosis 2016, 244, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Aldana-Hernández, P.; Leonard, K.A.; Zhao, Y.Y.; Curtis, J.M.; Field, C.J.; Jacobs, R.L. Dietary choline or trimethylamine N-oxide supplementation does not influence atherosclerosis development in Ldlr−/− and ApoE−/− male mice. J. Nutr. 2020, 150, 249–255. [Google Scholar] [CrossRef]
- Fennema, D.; Phillips, I.R.; Shephard, E.A. Trimethylamine and trimethylamine N-oxide, a flavin-containing monooxygenase 3 (FMO3)-mediated host-microbiome metabolic axis implicated in health and disease. Drug Metab. Dispos. 2016, 44, 1839–1850. [Google Scholar] [CrossRef]
- Wang, S.-C.; Lai, Y.-H.; Liu, C.-H.; Wang, C.-H.; Hsu, B.-G.; Tsai, J.-P. Association between serum indoxyl sulfate levels with carotid-femoral pulse wave velocity in patients with chronic kidney disease. Ren. Fail. 2021, 43, 796–802. [Google Scholar] [CrossRef]
- Barreto, F.C.; Barreto, D.V.; Liabeuf, S.; Meert, N.; Glorieux, G.; Temmar, M.; Choukroun, G.; Vanholder, R.; Massy, Z.A.; European Uremic Toxin Work Group (EUTox). Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin. J. Am. Soc. Nephrol. 2009, 4, 1551–1558. [Google Scholar] [CrossRef]
- Lai, Y.-H.; Wang, C.-H.; Kuo, C.-H.; Lin, Y.-L.; Tsai, J.-P.; Hsu, B.-G. Serum P-cresyl sulfate is a predictor of central arterial stiffness in patients on maintenance hemodialysis. Toxins 2019, 12, 10. [Google Scholar] [CrossRef] [PubMed]
- Menni, C.; Mangino, M.; Cecelja, M.; Psatha, M.; Brosnan, M.J.; Trimmer, J.; Mohney, R.P.; Chowienczyk, P.; Padmanabhan, S.; Spector, T.D.; et al. Metabolomic study of carotid–femoral pulse-wave velocity in women. J. Hypertens. 2015, 33, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Törmälä, R.; Appt, S.; Clarkson, T.B.; Groop, P.-H.; Rönnback, M.; Ylikorkala, O.; Mikkola, T.S. Equol production capability is associated with favorable vascular function in postmenopausal women using tibolone; no effect with soy supplementation. Atherosclerosis 2008, 198, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Kashtanova, D.; Tkacheva, O.; Popenko, A.; Egshatyan, L.; Tyakht, A.; Alexeev, D.; Kotovskaya, Y.; Plokhova, E.; Boytsov, S. Gut microbiota and vascular biomarkers in patients without clinical cardiovascular diseases. Artery Res. 2017, 18, 41–48. [Google Scholar] [CrossRef]
- Menni, C.; Lin, C.; Cecelja, M.; Mangino, M.; Matey-Hernandez, M.L.; Keehn, L.; Mohney, R.P.; Steves, C.; Spector, T.D.; Kuo, C.-F.; et al. Gut microbial diversity is associated with lower arterial stiffness in women. Eur. Heart J. 2018, 39, 2390–2397. [Google Scholar] [CrossRef] [PubMed]
- Dinakis, E.; Nakai, M.; Gill, P.A.; Yiallourou, S.; Sata, Y.; Muir, J.; Carrington, M.; Head, G.A.; Kaye, D.M.; Marques, F.Z. The gut microbiota and their metabolites in human arterial stiffness. Heart Lung Circ. 2021, 30, 1716–1725. [Google Scholar] [CrossRef]
- McCoin, C.S.; Knotts, T.A.; Ono-Moore, K.D.; Oort, P.J.; Adams, S.H. Long-chain acylcarnitines activate cell stress and myokine release in C2C12 myotubes: Calcium-dependent and -independent effects. Am. J. Physiol. Metab. 2015, 308, E990–E1000. [Google Scholar] [CrossRef]
- Rutkowsky, J.M.; Knotts, T.A.; Ono-Moore, K.D.; McCoin, C.S.; Huang, S.; Schneider, D.; Singh, S.; Adams, S.H.; Hwang, D.H. Acylcarnitines activate proinflammatory signaling pathways. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E1378–E1387. [Google Scholar] [CrossRef]
- Aguer, C.; McCoin, C.S.; Knotts, T.A.; Thrush, A.B.; Ono-Moore, K.; McPherson, R.; Dent, R.; Hwang, D.H.; Adams, S.H.; Harper, M.E. Acylcarnitines: Potential implications for skeletal muscle insulin resistance. FASEB J. 2015, 29, 336–345. [Google Scholar] [CrossRef]
- Ha, C.Y.; Kim, J.Y.; Paik, J.K.; Kim, O.Y.; Paik, Y.H.; Lee, E.J.; Lee, J.H. The association of specific metabolites of lipid metabolism with markers of oxidative stress, inflammation and arterial stiffness in men with newly diagnosed type 2 diabetes. Clin. Endocrinol. 2012, 76, 674–682. [Google Scholar] [CrossRef]
- Paapstel, K.; Kals, J.; Eha, J.; Tootsi, K.; Ottas, A.; Piir, A.; Zilmer, M. Metabolomic profiles of lipid metabolism, arterial stiffness and hemodynamics in male coronary artery disease patients. IJC Metab. Endocr. 2016, 11, 13–18. [Google Scholar] [CrossRef]
- Tootsi, K.; Kals, J.; Zilmer, M.; Paapstel, K.; Ottas, A.; Märtson, A. Medium- and long-chain acylcarnitines are associated with osteoarthritis severity and arterial stiffness in end-stage osteoarthritis patients: A case-control study. Int. J. Rheum. Dis. 2018, 21, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.S.; Gao, F.; Liu, J.; Fridianto, K.T.; Ching, J.; Tan, R.S.; Wong, J.-I.; Chua, S.J.; Leng, S.; Zhong, L.; et al. Metabolomic profile of arterial stiffness in aged adults. Diabetes Vasc. Dis. Res. 2018, 15, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Toral, M.; Romero, M.; Jiménez, R.; Mahmoud, A.M.; Barroso, E.; Gómez-Guzmán, M.; Sánchez, M.; Cogolludo, Á.; García-Redondo, A.B.; Briones, A.M.; et al. Carnitine palmitoyltransferase-1 up-regulation by PPAR-β/δ prevents lipid-induced endothelial dysfunction. Clin. Sci. 2015, 129, 823–837. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.-C.; den Tseng, C.; Lu, S.-C.; Liang, J.-T.; Wu, M.-S.; Tsai, M.-S.; Hsu, K.-L. Effects of acetyl-L-carnitine and oxfenicine on aorta stiffness in diabetic rats. Eur. J. Clin. Investig. 2010, 40, 1002–1010. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Zhang, T.; Wang, C.; Huang, Z.; Luo, X.; Deng, Y. A review on phospholipids and their main applications in drug delivery systems. Asian J. Pharm. Sci. 2015, 10, 81–98. [Google Scholar] [CrossRef]
- Kim, O.; Lim, H.; Lee, M.; Kim, J.; Lee, J. Association of fatty acid composition in serum phospholipids with metabolic syndrome and arterial stiffness. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 366–374. [Google Scholar] [CrossRef]
- Anderson, S.G.; Sanders, T.A.B.; Cruickshank, J.K. Plasma fatty acid composition as a predictor of arterial stiffness and mortality. Hypertension 2009, 53, 839–845. [Google Scholar] [CrossRef]
- Lee, M.-H.; Kwon, N.; Yoon, S.R.; Kim, O.Y. Serum phospholipid docosahexaenoic acid is inversely associated with arterial stiffness in metabolically healthy men. Clin. Nutr. Res. 2016, 5, 190–203. [Google Scholar] [CrossRef]
- Nishizawa, H.; Hamazaki, K.; Hamazaki, T.; Fujioka, S.; Sawazaki, S. The relationship between tissue RBC n-3 fatty acids and pulse wave velocity. In Vivo 2006, 20, 307–310. [Google Scholar]
- Hall, W.L.; Sanders, K.A.; Sanders, T.A.B.; Chowienczyk, P.J. A high-fat meal enriched with eicosapentaenoic acid reduces postprandial arterial stiffness measured by digital volume pulse analysis in healthy men. J. Nutr. 2008, 138, 287–291. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Van Der Veen, J.N.; Kennelly, J.P.; Wan, S.; Vance, J.E.; Vance, D.E.; Jacobs, R.L. The critical role of phosphatidylcholine and phosphatidylethanolamine metabolism in health and disease. Biochim. Biophys. Acta (BBA) Biomembr. 2017, 1859, 1558–1572. [Google Scholar] [CrossRef] [PubMed]
- Subbaiah, P.V.; Albers, J.J.; Chen, C.H.; Bagdade, J.D. Low density lipoprotein-activated lysolecithin acylation by human plasma lecithin-cholesterol acyltransferase. Identity of lysolecithin acyltransferase and lecithin-cholesterol acyltransferase. J. Biol. Chem. 1980, 255, 9275–9280. [Google Scholar] [CrossRef]
- Sekas, G.; Patton, G.M.; Lincoln, E.C.; Robins, S.J. Origin of plasma lysophosphatidylcholine: Evidence for direct hepatic secretion in the rat. J. Lab. Clin. Med. 1985, 105, 190–194. [Google Scholar]
- Matsumoto, T.; Kobayashi, T.; Kamata, K. Mechanisms underlying lysophosphatidylcholine-induced potentiation of vascular contractions in the Otsuka Long-Evans Tokushima Fatty (OLETF) rat aorta. Br. J. Pharmacol. 2006, 149, 931–941. [Google Scholar] [CrossRef]
- Hara, S.; Shike, T.; Takasu, N.; Mizui, T. Lysophosphatidylcholine promotes cholesterol efflux from mouse macrophage foam cells. Arter. Thromb. Vasc. Biol. 1997, 17, 1258–1266. [Google Scholar] [CrossRef]
- Paapstel, K.; Kals, J.; Eha, J.; Tootsi, K.; Ottas, A.; Piir, A.; Jakobson, M.; Lieberg, J.; Zilmer, M. Inverse relations of serum phosphatidylcholines and lysophosphatidylcholines with vascular damage and heart rate in patients with atherosclerosis. Nutr. Metab. Cardiovasc. Dis. 2017, 28, 44–52. [Google Scholar] [CrossRef]
- Kurotani, K.; Karunapema, P.; Jayaratne, K.; Sato, M.; Hayashi, T.; Kajio, H.; Fukuda, S.; Hara, H.; Okazaki, O.; Jayatilleke, A.U.; et al. Circulating odd-chain saturated fatty acids were associated with arteriosclerosis among patients with diabetes, dyslipidemia, or hypertension in Sri Lanka but not Japan. Nutr. Res. 2018, 50, 82–93. [Google Scholar] [CrossRef]
- Petersen, K.S.; Keogh, J.B.; Lister, N.; Weir, J.M.; Meikle, P.J.; Clifton, P.M. Association between dairy intake, lipids and vascular structure and function in diabetes. World J. Diabetes 2017, 8, 202–212. [Google Scholar] [CrossRef]
- Polonis, K.; Wawrzyniak, R.; Daghir-Wojtkowiak, E.; Szyndler, A.; Chrostowska, M.; Melander, O.; Hoffmann, M.; Kordalewska, M.; Raczak-Gutknecht, J.; Bartosińska, E.; et al. Metabolomic signature of Early Vascular Aging (EVA) in hypertension. Front. Mol. Biosci. 2020, 7, 12. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, O.Y.; Paik, J.K.; Kwon, D.Y.; Kim, H.J.; Lee, J.H. Association of age-related changes in circulating intermediary lipid metabolites, inflammatory and oxidative stress markers, and arterial stiffness in middle-aged men. Age 2013, 35, 1507–1519. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Jung, S.; Kim, S.Y.; Lee, S.-H.; Lee, J.H. Prehypertension-associated elevation in circulating lysophosphatidlycholines, Lp-PLA2 activity, and oxidative stress. PLoS ONE 2014, 9, e96735. [Google Scholar] [CrossRef] [PubMed]
- Kugiyama, K.; Sugiyama, S.; Ogata, N.; Oka, H.; Doi, H.; Ota, Y.; Yasue, H. Burst production of superoxide anion in human endothelial cells by lysophosphatidylcholine. Atherosclerosis 1999, 143, 201–204. [Google Scholar] [CrossRef]
- Murohara, T.; Scalia, R.; Lefer, A.M. Lysophosphatidylcholine promotes P-selectin expression in platelets and endothelial cells. Possible involvement of protein kinase C activation and its inhibition by nitric oxide donors. Circ. Res. 1996, 78, 780–789. [Google Scholar] [CrossRef]
- Zhang, R.; Bai, N.; So, J.; Laher, I.; MacLeod, K.M.; Rodrigues, B. The ischemic metabolite lysophosphatidylcholine increases rat coronary arterial tone by endothelium-dependent mechanisms. J. Mol. Cell. Cardiol. 2009, 47, 112–120. [Google Scholar] [CrossRef]
- Rao, S.P.; Riederer, M.; Lechleitner, M.; Hermansson, M.; Desoye, G.; Hallström, S.; Graier, W.F.; Frank, S. Acyl chain-dependent effect of lysophosphatidylcholine on endothelium-dependent vasorelaxation. PLoS ONE 2013, 8, e65155. [Google Scholar] [CrossRef]
- Croset, M.; Brossard, N.; Polette, A.; Lagarde, M. Characterization of plasma unsaturated lysophosphatidylcholines in human and rat. Biochem. J. 2000, 345, 61–67. [Google Scholar] [CrossRef]
- Stegemann, C.; Pechlaner, R.; Willeit, P.; Langley, S.R.; Mangino, M.; Mayr, U.; Menni, C.; Moayyeri, A.; Santer, P.; Rungger, G.; et al. Lipidomics profiling and risk of cardiovascular disease in the prospective population-based Bruneck study. Circulation 2014, 129, 1821–1831. [Google Scholar] [CrossRef]
- Meikle, P.J.; Wong, G.; Tsorotes, D.; Barlow, C.K.; Weir, J.M.; Christopher, M.J.; MacIntosh, G.L.; Goudey, B.; Stern, L.; Kowalczyk, A.; et al. Plasma lipidomic analysis of stable and unstable coronary artery disease. Arter. Thromb. Vasc. Biol. 2011, 31, 2723–2732. [Google Scholar] [CrossRef]
- Duivenvoorden, R.; Holleboom, A.G.; van den Bogaard, B.; Nederveen, A.J.; de Groot, E.; Hutten, B.A.; Schimmel, A.W.; Hovingh, G.K.; Kastelein, J.J.P.; Kuivenhoven, J.A.; et al. Cholesterol acyltransferase gene mutations have accelerated atherogenesis as assessed by carotid 3.0-T magnetic resonance imaging: Carriers of lecithin. J. Am. Coll. Cardiol. 2011, 58, 2481–2487. [Google Scholar] [CrossRef]
- Rasmiena, A.A.; Ng, T.W.; Meikle, P.J. Metabolomics and ischaemic heart disease. Clin. Sci. 2013, 124, 289–306. [Google Scholar] [CrossRef] [PubMed]
- Hannun, Y.A.; Obeid, L.M. Sphingolipids and their metabolism in physiology and disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Turpin-Nolan, S.M.; Brüning, J.C. The role of ceramides in metabolic disorders: When size and localization matters. Nat. Rev. Endocrinol. 2020, 16, 224–233. [Google Scholar] [CrossRef]
- Maceyka, M.; Spiegel, S. Sphingolipid metabolites in inflammatory disease. Nature 2014, 510, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Salvia, R.; Halbac-Cotoara-zamfir, R.; Cividino, S.; Gutterman, D.D.; Quaranta, G. Manipulation of the sphingolipid rheostat influences the mediator of flow-induced dilation in the human microvasculature. J. Am. Heart Assoc. 2019, 8, e013153. [Google Scholar]
- Li, Y.; Zhang, W.; Li, J.; Sun, Y.; Yang, Q.; Wang, S.; Luo, X.; Wang, W.; Wang, K.; Bai, W.; et al. The imbalance in the aortic ceramide/sphingosine-1-phosphate rheostat in ovariectomized rats and the preventive effect of estrogen. Lipids Health Dis. 2020, 19, 95. [Google Scholar] [CrossRef] [PubMed]
- Cogolludo, A.; Villamor, E.; Perez-Vizcaino, F.; Moreno, L. Ceramide and regulation of vascular tone. Int. J. Mol. Sci. 2019, 20, 411. [Google Scholar] [CrossRef]
- Bhat, O.M.; Yuan, X.; Cain, C.; Salloum, F.N.; Li, P. Medial calcification in the arterial wall of smooth muscle cell-specific Smpd1 transgenic mice: A ceramide-mediated vasculopathy. J. Cell. Mol. Med. 2020, 24, 539–553. [Google Scholar] [CrossRef]
- Chun, L.; Junlin, Z.; Aimin, W.; Niansheng, L.; Benmei, C.; Minxiang, L. Inhibition of ceramide synthesis reverses endothelial dysfunction and atherosclerosis in streptozotocin-induced diabetic rats. Diabetes Res. Clin. Pract. 2011, 93, 77–85. [Google Scholar] [CrossRef]
- Holland, W.L.; Brozinick, J.T.; Wang, L.-P.; Hawkins, E.D.; Sargent, K.M.; Liu, Y.; Narra, K.; Hoehn, K.L.; Knotts, T.A.; Siesky, A.; et al. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab. 2007, 5, 167–179. [Google Scholar] [CrossRef]
- Skácel, J.; Slusher, B.S.; Tsukamoto, T. Small molecule inhibitors targeting biosynthesis of ceramide, the central hub of the sphingolipid network. J. Med. Chem. 2021, 64, 279–297. [Google Scholar] [CrossRef]
- Chatterjee, S.; Bedja, D.; Mishra, S.; Amuzie, C.; Avolio, A.; Kass, D.A.; Berkowitz, D.; Renehan, M. Inhibition of glycosphingolipid synthesis ameliorates atherosclerosis and arterial stiffness in Apolipoprotein E −/− mice and rabbits fed a high-fat and -cholesterol diet. Circulation 2014, 129, 2403–2413. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Jung, S.; Lee, S.H.; Lee, J.H. Association between arterial stiffness and serum L-octanoylcarnitine and lacto-sylceramide in overweight middle-aged subjects: 3-year follow-up study. PLoS ONE 2015, 10, e0119519. [Google Scholar]
- Jung, S.; Kim, M.; Lee, Y.J.; Lee, S.H.; Lee, J.H. Associations between metabolomic-identified changes of biomarkers and arterial stiffness in subjects progressing to impaired fasting glucose. Clin. Endocrinol. 2015, 83, 196–204. [Google Scholar] [CrossRef]
- Seth, S.K.; Newman, H.A.I. Sphingomyelin and other phospholipid metabolism in the rabbit atheromatous and normal aorta. Circ. Res. 1975, 36, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Edsfeldt, A.; Dunér, P.; Ståhlman, M.; Mollet, I.G.; Asciutto, G.; Grufman, H.; Nitulescu, M.; Persson, A.F.; Fisher, R.M.; Melander, O.; et al. Sphingolipids contribute to human atherosclerotic plaque inflammation. Arter. Thromb. Vasc. Biol. 2016, 36, 1132–1140. [Google Scholar] [CrossRef]
- Jiang, X.-C.; Paultre, F.; Pearson, T.A.; Reed, R.G.; Francis, C.K.; Lin, M.; Berglund, L.; Tall, A.R. Plasma sphingomyelin level as a risk factor for coronary artery disease. Arter. Thromb. Vasc. Biol. 2000, 20, 2614–2618. [Google Scholar] [CrossRef]
- Li, C.; He, J.; Li, S.; Chen, W.; Bazzano, L.; Sun, X.; Shen, L.; Liang, L.; Shen, Y.; Gu, X.; et al. Novel metabolites are associated with augmentation index and pulse wave velocity: Findings from the Bogalusa heart study. Am. J. Hypertens. 2019, 32, 547–556. [Google Scholar] [CrossRef]
- Nelson, J.C.; Jiang, X.-C.; Tabas, I.; Tall, A.; Shea, S. Plasma sphingomyelin and subclinical atherosclerosis: Findings from the multi-ethnic study of atherosclerosis. Am. J. Epidemiol. 2006, 163, 903–912. [Google Scholar] [CrossRef]
- Yu, Z.; Peng, Q.; Huang, Y. Potential therapeutic targets for atherosclerosis in sphingolipid metabolism. Clin. Sci. 2019, 133, 763–776. [Google Scholar] [CrossRef]
- Sigruener, A.; Kleber, M.E.; Heimerl, S.; Liebisch, G.; Schmitz, G.; Maerz, W. Glycerophospholipid and sphingolipid species and mortality: The Ludwigshafen Risk and Cardiovascular Health (LURIC) study. PLoS ONE 2014, 9, e85724. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.L.E.; Stone, S.J.; Koliwad, S.; Harris, C.; Farese, R.V., Jr. Thematic review series: Glycerolipids DGAT enzymes and triacylglycerol biosynthesis. J. Lipid Res. 2008, 49, 2283–2301. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.; Cooney, G.J.; Kraegen, E.W.; Bruce, C.R. Fatty acid metabolism, energy expenditure and insulin resistance in muscle. J. Endocrinol. 2014, 220, T61–T79. [Google Scholar] [CrossRef] [PubMed]
- Erion, D.M.; Shulman, G.I. Diacylglycerol-mediated insulin resistance. Nat. Med. 2010, 16, 400–402. [Google Scholar] [CrossRef]
- Coen, P.M.; Goodpaster, B.H. Role of intramyocelluar lipids in human health. Trends Endocrinol. Metab. 2012, 23, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, H.; Meikle, P.J.; Mamtani, M.; Weir, J.M.; Barlow, C.K.; Jowett, J.B.; Bellis, C.; Dyer, T.D.; Johnson, M.P.; Rainwater, D.L.; et al. Plasma lipidomic profile signature of hypertension in Mexican American families: Specific role of diacylglycerols. Hypertension 2013, 62, 621–626. [Google Scholar] [CrossRef]
- Pramfalk, C.; Eriksson, M.; Parini, P. Cholesteryl esters and ACAT. Eur. J. Lipid Sci. Technol. 2012, 114, 624–633. [Google Scholar] [CrossRef]
- Kunnen, S.; van Eck, M. Lecithin: Cholesterol acyltransferase: Old friend or foe in atherosclerosis? J. Lipid Res. 2012, 53, 1783–1799. [Google Scholar] [CrossRef]
- Peng, S.; Guo, W.; Morrisett, J.D.; Johnstone, M.T.; Hamilton, J.A. Quantification of cholesteryl esters in human and rabbit atherosclerotic plaques by magic-angle spinning 13C-NMR. Arter. Thromb. Vasc. Biol. 2000, 20, 2682–2688. [Google Scholar] [CrossRef]
- Miller, C.D.; Thomas, M.J.; Hiestand, B.; Samuel, M.P.; Wilson, M.D.; Sawyer, J.; Rudel, L.L. Cholesteryl esters associated with Acyl-CoA: Cholesterol acyltransferase predict coronary artery disease in patients with symptoms of acute coronary syndrome. Acad. Emerg. Med. 2012, 19, 673–682. [Google Scholar] [CrossRef]
- van Popele, N.M.; Grobbee, D.E.; Bots, M.L.; Asmar, R.; Topouchian, J.; Reneman, R.S.; Hoeks, A.P.G.; van der Kuip, D.A.M.; Hofman, A.; Witteman, J.C.M. Association between arterial stiffness and atherosclerosis: The Rotterdam study. Stroke 2001, 32, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Wu, G. Amino acids: Metabolism, functions, and nutrition. Amino Acids 2009, 37, 41. [Google Scholar] [CrossRef] [PubMed]
- Zagura, M.; Kals, J.; Kilk, K.; Serg, M.; Kampus, P.; Eha, J.; Soomets, U.; Zilmer, M. Metabolomic signature of arterial stiffness in male patients with peripheral arterial disease. Hypertens. Res. 2015, 38, 840–846. [Google Scholar] [CrossRef]
- Kauko, A.; Palmu, J.; Jousilahti, P.; Havulinna, A.; Salomaa, V.; Niiranen, T. Associations between circulating metabolites and arterial stiffness. J. Hum. Hypertens. 2021, 35, 809–811. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhang, K.; Zhu, Z.; Cui, M.; An, Y.; Wang, Y.; Suo, C.; Fan, M.; Jin, L.; Tian, W.; et al. Associations between serum metabolites and subclinical atherosclerosis in a Chinese population: The Taizhou imaging study. Aging 2020, 12, 15302–15313. [Google Scholar] [CrossRef] [PubMed]
- Zhenyukh, O.; González-Amor, M.; Rodrigues-Diez, R.R.; Esteban, V.; Ruiz-Ortega, M.; Salaices, M.; Mas, S.; Briones, A.M.; Egido, J. Branched-chain amino acids promote endothelial dysfunction through increased reactive oxygen species generation and inflammation. J. Cell. Mol. Med. 2018, 22, 4948–4962. [Google Scholar] [CrossRef]
- Lee, C.C.; Watkins, S.M.; Lorenzo, C.; Wagenknecht, L.E.; Il’Yasova, D.; Chen, Y.-D.I.; Haffner, S.M.; Hanley, A.J. Branched-chain amino acids and insulin metabolism: The Insulin Resistance Atherosclerosis Study (IRAS). Diabetes Care 2016, 39, 582–588. [Google Scholar] [CrossRef]
- Neishabouri, S.H.; Hutson, S.M.; Davoodi, J. Chronic activation of mTOR complex 1 by branched chain amino acids and organ hypertrophy. Amino Acids 2015, 47, 1167–1182. [Google Scholar] [CrossRef]
- Tobias, D.K.; Lawler, P.R.; Harada, P.H.; Demler, O.V.; Ridker, P.M.; Manson, J.E.; Cheng, S.; Mora, S. Circulating branched-chain amino acids and incident cardiovascular disease in a prospective cohort of US women. Circ. Genom. Precis. Med. 2018, 11, e002157. [Google Scholar] [CrossRef]
- Hellmuth, C.; Kirchberg, F.F.; Lass, N.; Harder, U.; Peissner, W.; Koletzko, B.; Reinehr, T. Tyrosine is associated with insulin resistance in longitudinal metabolomic profiling of obese children. J. Diabetes Res. 2016, 2016, 2108909. [Google Scholar] [CrossRef]
- Monirujjaman, M.; Ferdouse, A. Metabolic and physiological roles of branched-chain amino acids. Adv. Mol. Biol. 2014, 2014, 364976. [Google Scholar] [CrossRef]
- Cañes, L.; Alonso, J.; Ballester-Servera, C.; Varona, S.; Escudero, J.R.; Andrés, V.; Rodríguez, C.; Martínez-González, J. Targeting tyrosine hydroxylase for abdominal aortic aneurysm: Impact on inflammation, oxidative stress, and vascular remodeling. Hypertension 2021, 78, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.-J.; Hsu, B.-G.; Wang, J.-H.; Lai, Y.-H.; Dongoran, R.A.; Liu, C.-H. Serum indoxyl sulfate as a potential biomarker of aortic arterial stiffness in coronary artery disease. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 2320–2327. [Google Scholar] [CrossRef] [PubMed]
- Katakami, N.; Omori, K.; Taya, N.; Arakawa, S.; Takahara, M.; Matsuoka, T.-A.; Tsugawa, H.; Furuno, M.; Bamba, T.; Fukusaki, E.; et al. Plasma metabolites associated with arterial stiffness in patients with type 2 diabetes. Cardiovasc. Diabetol. 2020, 19, 75. [Google Scholar] [CrossRef] [PubMed]
- Zapolski, T.; Kamińska, A.; Kocki, T.; Wysokiński, A.; Urbanska, E.M. Aortic stiffness—Is kynurenic acid a novel marker? Cross-sectional study in patients with persistent atrial fibrillation. PLoS ONE 2020, 15, e0236413. [Google Scholar] [CrossRef]
- Erasmus, D.; Mels, C.M.C.; Louw, R.; Lindeque, J.Z.; Kruger, R. Urinary metabolites and their link with premature arterial stiffness in black boys: The ASOS study. Pulse 2018, 6, 144–153. [Google Scholar] [CrossRef]
- Chang, Y.C.; Wang, C.H.; Lai, Y.H.; Lin, Y.L.; Kuo, C.H.; Hsu, B.G.; Tsai, J.P. Low serum 3-methyl histidine level is associated with aortic stiffness in maintenance hemodialysis patients. Ther. Apher. Dial. 2021. [Google Scholar] [CrossRef]
- Kimble, R.; Murray, L.; Keane, K.M.; Haggerty, K.; Howatson, G.; Lodge, J.K. The influence of tart cherries (Prunus cerasus) on vascular function and the urinary metabolome: A randomised placebo-controlled pilot study. J. Nutr. Sci. 2021, 10, e73. [Google Scholar] [CrossRef]
- Cziráki, A.; Lenkey, Z.; Sulyok, E.; Szokodi, I.; Koller, A. L-arginine-nitric oxide-asymmetric dimethylarginine pathway and the coronary circulation: Translation of basic science results to clinical practice. Front. Pharmacol. 2020, 11, 569914. [Google Scholar] [CrossRef]
- Schulman, S.P.; Becker, L.C.; Kass, D.A.; Champion, H.C.; Terrin, M.L.; Forman, S.; Ernst, K.V.; Kelemen, M.D.; Townsend, S.N.; Capriotti, A.; et al. L-arginine therapy in acute myocardial infarction: The Vascular Interaction with Age in Myocardial Infarction (VINTAGE MI) randomized clinical trial. JAMA 2006, 295, 58–64. [Google Scholar] [CrossRef]
- Kakimoto, Y.; Akazawa, S. Isolation and identification of N-G, N-G- and N-G,N’-G-dimethyl-arginine, N-epsilon-mono-, di-, and trimethyllysine, and glucosylgalactosyl- and galactosyl-delta-hydroxylysine from human urine. J. Biol. Chem. 1970, 245, 5751–5758. [Google Scholar] [CrossRef]
- Ogawa, T.; Kimoto, M.; Sasaoka, K. Purification and properties of a new enzyme, N, N-dimethylarginine dimethylaminohydrolase, from rat kidney. J. Biol. Chem. 1989, 264, 10205–10209. [Google Scholar] [CrossRef]
- Kals, J.; Kampus, P.; Kals, M.; Teesalu, R.; Zilmer, K.; Pulges, A.; Zilmer, M. Arterial elasticity is associated with endothelial vasodilatory function and asymmetric dimethylarginine level in healthy subjects. Scand. J. Clin. Lab. Investig. 2007, 67, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-N.; Lu, P.-C.; Lo, M.-H.; Lin, I.-C.; Tain, Y.-L. The association between nitric oxide pathway, blood pressure abnormalities, and cardiovascular risk profile in pediatric chronic kidney disease. Int. J. Mol. Sci. 2019, 20, 5301. [Google Scholar] [CrossRef]
- Chien, S.-J.; Lin, I.-C.; Hsu, C.-N.; Lo, M.-H.; Tain, Y.-L. Homocysteine and arginine-to-asymmetric dimethylarginine ratio associated with blood pressure abnormalities in children with early chronic kidney disease. Circ. J. 2015, 79, 2031–2037. [Google Scholar] [CrossRef]
- Masaki, N.; Hakuno, D.; Toya, T.; Shiraishi, Y.; Kujiraoka, T.; Namba, T.; Yada, H.; Kimura, K.; Miyazaki, K.; Adachi, T. Association between brachial-ankle pulse wave velocity and the ratio of L-arginine to asymmetric dimethylarginine in patients undergoing coronary angiography. J. Cardiol. 2015, 65, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Klima, Ł.; Kawecka-Jaszcz, K.; Stolarz-Skrzypek, K.; Menne, J.; Fijorek, K.; Olszanecka, A.; Wojciechowska, W.; Bilo, G.; Czarnecka, D. Structure and function of large arteries in hypertension in relation to oxidative stress markers. Kardiol. Pol. 2013, 71, 917–923. [Google Scholar] [CrossRef]
- Lin, I.-C.; Hsu, C.-N.; Lo, M.-H.; Chien, S.-J.; Tain, Y.-L. Low urinary citrulline/arginine ratio associated with blood pressure abnormalities and arterial stiffness in childhood chronic kidney disease. J. Am. Soc. Hypertens. 2015, 10, 115–123. [Google Scholar] [CrossRef]
- Tayama, J.; Munakata, M.; Yoshinaga, K.; Toyota, T. Higher plasma homocysteine concentration is associated with more advanced systematic arterial stiffness and greater blood pressure response to stress in hypertensive patients. Hypertens. Res. 2006, 29, 403–409. [Google Scholar] [CrossRef][Green Version]
- van Dijk, S.C.; Smulders, Y.M.; Enneman, A.W.; Swart, K.M.A.; van Wijngaarden, J.P.; Ham, A.C.; van Schoor, N.M.; Dhonukshe-Rutten, R.A.M.; de Groot, L.C.P.G.M.; Lips, P.; et al. Homocysteine level is associated with aortic stiffness in elderly: Cross-sectional results from the B-PROOF study. J. Hypertens. 2013, 31, 952–959. [Google Scholar] [CrossRef]
- Nakhai-Pour, H.R.; Grobbee, D.E.; Bots, M.L.; Muller, M.; van der Schouw, Y.T. Circulating homocysteine and large arterial stiffness and thickness in a population-based sample of middle-aged and elderly men. J. Hum. Hypertens. 2007, 21, 942–948. [Google Scholar] [CrossRef] [PubMed]
- Welch, G.N.; Loscalzo, J. Homocysteine and atherothrombosis. N. Engl. J. Med. 1998, 338, 1042–1050. [Google Scholar] [CrossRef] [PubMed]
- Tawakol, A.; Omland, T.; Gerhard, M.; Wu, J.T.; Creager, M.A. Hyperhomocyst (e) inemia is associated with impaired endo-thelium-dependent vasodilation in humans. Circulation 1997, 95, 1119–1121. [Google Scholar] [CrossRef] [PubMed]
- Charpio, P.; Bescond, A.; Augier, T.; Chareyre, C.; Fraterno, M.; Rolland, P.-H.; Garçon, D. Hyperhomocysteinemia induces elastolysis in minipig arteries: Structural consequences, arterial site specificity and effect of captopril-hydrochlorothiazide. Matrix Biol. 1998, 17, 559–574. [Google Scholar] [CrossRef]
- Wilkinson, I.B.; Megson, I.L.; MaCallum, H.; Rooijmans, D.F.; Johnson, S.M.; Boyd, J.L.; Cockcroft, J.R.; Webb, D.J. Acute methionine loading does not alter arterial stiffness in humans. J. Cardiovasc. Pharmacol. 2001, 37, 1–5. [Google Scholar] [CrossRef]
- Doupis, J.; Eleftheriadou, I.; Kokkinos, A.; Perrea, D.; Pavlatos, S.; Gonis, A.; Katsilambros, N.; Tentolouris, N. Acute hyper-homocysteinemia impairs endothelium function in subjects with type 2 diabetes mellitus. Exp. Clin. Endocrinol. Diabetes 2010, 118, 453–458. [Google Scholar] [CrossRef]
- Nestel, P.J.; Chronopoulos, A.; Cehun, M. Arterial stiffness is rapidly induced by raising the plasma homocysteine concentration with methionine. Atherosclerosis 2003, 171, 83–86. [Google Scholar] [CrossRef]
- Durga, J.; Bots, M.L.; Schouten, E.G.; Grobbee, D.E.; Kok, F.J.; Verhoef, P. Effect of 3 y of folic acid supplementation on the progression of carotid intima-media thickness and carotid arterial stiffness in older adults. Am. J. Clin. Nutr. 2011, 93, 941–949. [Google Scholar] [CrossRef]
- van Dijk, R.A.J.M.; Rauwerda, J.A.; Steyn, M.; Twisk, J.W.R.; Stehouwer, C.D.A. Long-term homocysteine-lowering treatment with folic acid plus pyridoxine is associated with decreased blood pressure but not with improved brachial artery endothelium-dependent vasodilation or carotid artery stiffness: A 2-year, randomized, placebo-controlled trial. Arter.-Scler. Thromb. Vasc. Biol. 2001, 21, 2072–2079. [Google Scholar]
- Furuki, K.; Adachi, H.; Enomoto, M.; Otsuka, M.; Fukami, A.; Kumagae, S.-I.; Matsuoka, H.; Nanjo, Y.; Kakuma, T.; Imaizumi, T. Plasma Level of Asymmetric Dimethylarginine (ADMA) as a predictor of carotid intima-media thickness progression: Six-year prospective study using carotid ultrasonography. Hypertens. Res. 2008, 31, 1185–1189. [Google Scholar] [CrossRef]
- Baliou, S.; Adamaki, M.; Ioannou, P.; Pappa, A.; Panayiotidis, M.I.; Spandidos, D.A.; Christodoulou, I.; Kyriakopoulos, A.M.; Zoumpourlis, V. Protective role of taurine against oxidative stress (Review). Mol. Med. Rep. 2021, 24, 605. [Google Scholar] [CrossRef] [PubMed]
- Satoh, H.; Kang, J. Modulation by taurine of human arterial stiffness and wave reflection. Adv. Exp. Med. Biol. 2009, 643, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Ra, S.G.; Choi, Y.; Akazawa, N.; Ohmori, H.; Maeda, S. Taurine supplementation attenuates delayed increase in exercise-induced arterial stiffness. Appl. Physiol. Nutr. Metab. 2016, 41, 618–623. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Wang, B.; Li, Y.; Sun, F.; Li, P.; Xia, W.; Zhou, X.; Li, Q.; Wang, X.; Chen, J.; et al. Taurine supplementation lowers blood pressure and improves vascular function in prehypertension: Randomized, double-blind, placebo-controlled study. Hypertension 2016, 67, 541–549. [Google Scholar] [CrossRef]
- Tuomilehto, J.; Qiao, Q.; Borch-Johnsen, K.; Balkau, B. Glucose tolerance and mortality: Comparison of WHO and American Diabetes Association diagnostic criteria. Lancet 1999, 354, 617–621. [Google Scholar]
- Kobayashi, R.; Sakazaki, M.; Nagai, Y.; Asaki, K.; Hashiguchi, T.; Negoro, H. Effects of different types of carbohydrates on arterial stiffness: A comparison of isomaltulose and sucrose. Nutrients 2021, 13, 4493. [Google Scholar] [CrossRef]
- Kobayashi, R.; Sato, K.; Sakazaki, M.; Nagai, Y.; Iwanuma, S.; Ohashi, N.; Hashiguchi, T. Acute effects of difference in glucose intake on arterial stiffness in healthy subjects. Cardiol. J. 2021, 28, 446–452. [Google Scholar] [CrossRef]
- Chavakis, T.; Bierhaus, A.; Nawroth, P.P. RAGE (Receptor for Advanced Glycation End products): A central player in the inflammatory response. Microbes Infect. 2004, 6, 1219–1225. [Google Scholar] [CrossRef] [PubMed]
- Kemeny, S.F.; Figueroa, D.S.; Andrews, A.M.; Barbee, K.A.; Clyne, A.M. Glycated collagen alters endothelial cell actin alignment and nitric oxide release in response to fluid shear stress. J. Biomech. 2011, 44, 1927–1935. [Google Scholar] [CrossRef]
- Sveen, K.A.; Dahl-Jørgensen, K.; Stensaeth, K.H.; Angel, K.; Seljeflot, I.; Sell, D.R.; Monnier, V.M.; Hanssen, K.F. Glucosepane and oxidative markers in skin collagen correlate with intima media thickness and arterial stiffness in long-term type 1 diabetes. J. Diabetes Complicat. 2015, 29, 407–412. [Google Scholar] [CrossRef]
- Semba, R.D.; Sun, K.; Schwartz, A.V.; Varadhan, R.; Harris, T.B.; Satterfield, S.; Garcia, M.; Ferrucci, L.; Newman, A.B. Serum carboxymethyl-lysine, an advanced glycation end product, is associated with arterial stiffness in older adults. J. Hypertens. 2015, 33, 797–803. [Google Scholar] [CrossRef] [PubMed]
- van Eupen, M.G.A.; Schram, M.T.; van Sloten, T.T.; Scheijen, J.; Sep, S.J.S.; van der Kallen, C.J.; Dagnelie, P.C.; Koster, A.; Schaper, N.; Henry, R.M.A.; et al. Skin autofluorescence and pentosidine are associated with aortic stiffening: The Maastricht study. Hypertension 2016, 68, 956–963. [Google Scholar] [CrossRef] [PubMed]
- van der Bruggen, M.M.; Spronck, B.; Delhaas, T.; Reesink, K.D.; Schalkwijk, C.G. The putative role of methylglyoxal in arterial stiffening: A review. Heart Lung Circ. 2021, 30, 1681–1693. [Google Scholar] [CrossRef] [PubMed]
- Forbes, J.M.; Thorpe, S.R.; Thallas-Bonke, V.; Pete, J.; Thomas, M.C.; Deemer, E.R.; Bassal, S.; El-Osta, A.; Long, D.M.; Panag-iotopoulos, S.; et al. Modulation of soluble receptor for advanced glycation end products by angiotensin-converting en-zyme-1 inhibition in diabetic nephropathy. J. Am. Soc. Nephrol. 2005, 16, 2363–2372. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Sato, E.; Fujiwara, N.; Kawagoe, Y.; Takeuchi, M.; Maeda, S.; Yamagishi, S.-I. Atorvastatin reduces proteinuria in non-diabetic chronic kidney disease patients partly via lowering serum levels of Advanced Glycation End products (AGEs). Oxidative Med. Cell. Longev. 2010, 3, 304–307. [Google Scholar] [CrossRef]
- Sourris, K.C.; Watson, A.; Jandeleit-Dahm, K. Inhibitors of Advanced Glycation End product (AGE) formation and accumulation. In Handbook of Experimental Pharmacology: Reactive Oxygen Species; Schmidt, H.H.W., Ghezzi, P., Cuadrado, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2021; Volume 264, pp. 395–423. [Google Scholar]
- el Ridi, R.; Tallima, H. Physiological functions and pathogenic potential of uric acid: A review. J. Adv. Res. 2017, 8, 487–493. [Google Scholar] [CrossRef]
- Saito, Y.; Tanaka, A.; Node, K.; Kobayashi, Y. Uric acid and cardiovascular disease: A clinical review. J. Cardiol. 2021, 78, 51–57. [Google Scholar] [CrossRef]
- Alem, M.M.; Alshehri, A.; Cahusac, P.; Walters, M.R. Effect of xanthine oxidase inhibition on arterial stiffness in patients with chronic heart failure. Clin. Med. Insights Cardiol. 2018, 12, 1179546818779584. [Google Scholar] [CrossRef]
- Canepa, M.; Viazzi, F.; Strait, J.B.; Ameri, P.; Pontremoli, R.; Brunelli, C.; Studenski, S.; Ferrucci, L.; Lakatta, E.G.; Alghatrif, M. Longitudinal association between serum uric acid and arterial stiffness: Results from the Baltimore longitudinal study of aging. Hypertension 2017, 69, 228–235. [Google Scholar] [CrossRef]
- Chen, X.; Li, Y.; Sheng, C.-S.; Huang, Q.-F.; Zheng, Y.; Wang, J.-G. Association of serum uric acid with aortic stiffness and pressure in a Chinese workplace setting. Am. J. Hypertens. 2010, 23, 387–392. [Google Scholar] [CrossRef]
- Liu, X.; Wu, J.; Wu, H.; Yi, C.; Huang, F.; Yu, X.; Yang, X. Association of serum uric acid with arterial stiffness in peritoneal dialysis patients. Kidney Blood Press. Res. 2018, 43, 1451–1458. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.G.; Rosticci, M.; Fogacci, F.; Grandi, E.; D’Addato, S.; Borghi, C.; Brisighella Heart Study Group. High serum uric acid is associated to poorly controlled blood pressure and higher arterial stiffness in hypertensive subjects. Eur. J. Intern. Med. 2017, 37, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.G.; Salvi, P.; D’Addato, S.; Rosticci, M.; Borghi, C. Association between serum uric acid, hypertension, vascular stiffness and subclinical atherosclerosis: Data from the Brisighella heart study. J. Hypertens. 2014, 32, 57–64. [Google Scholar] [CrossRef]
- Albu, A.; Para, I.; Porojan, M. Uric acid and arterial stiffness. Ther. Clin. Risk Manag. 2020, 16, 39–54. [Google Scholar] [CrossRef]
- George, J.; Struthers, A.D. Role of urate, xanthine oxidase and the effects of allopurinol in vascular oxidative stress. Vasc. Health Risk Manag. 2009, 5, 265–272. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Man, A.W.C.; Li, H.; Xia, N. Resveratrol and the Interaction between gut microbiota and arterial remodelling. Nutrients 2020, 12, 119. [Google Scholar] [CrossRef] [PubMed]
- Lilamand, M.; Kelaiditi, E.; Guyonnet, S.; Antonelli Incalzi, R.; Raynaud-Simon, A.; Vellas, B.; Cesari, M. Flavonoids and arterial stiffness: Promising perspectives. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 698–704. [Google Scholar] [CrossRef]
- de Bruyne, T.; Steenput, B.; Roth, L.; de Meyer, G.R.Y.; dos Santos, C.N.; Valentová, K.; Dambrova, M.; Hermans, N. Dietary polyphenols targeting arterial stiffness: Interplay of contributing mechanisms and gut microbiome-related metabolism. Nutrients 2019, 11, 578. [Google Scholar] [CrossRef] [PubMed]
- Ponte, B.; Pruijm, M.; Ackermann, D.; Ehret, G.; Ansermot, N.; Staessen, J.A.; Vogt, B.; Pechère-Bertschi, A.; Burnier, M.; Martin, P.Y.; et al. Associations of urinary caffeine and caffeine metabolites with arterial stiffness in a large population-based study. Mayo Clin. Proc. 2018, 93, 586–596. [Google Scholar] [CrossRef]
- Karatzi, K.; Papaioannou, T.G.; Psaltopoulou, T.; Tousoulis, D. Caffeine effects on arterial stiffness: To Drink or not to drink? Mayo Clin. Proc. 2018, 93, 1149–1150. [Google Scholar] [CrossRef] [PubMed]
- Haam, J.-H.; Kim, Y.-S.; Cho, D.-Y.; Chun, H.; Choi, S.-W.; Lee, Y.K.; Lim, S.W.; Koo, H.S.; Kim, M.J. Elevated levels of urine isocitrate, hydroxymethylglutarate, and formiminoglutamate are associated with arterial stiffness in Korean adults. Sci. Rep. 2021, 11, 10180. [Google Scholar] [CrossRef] [PubMed]
- Larijani, V.N.; Ahmadi, N.; Zeb, I.; Khan, F.; Flores, F.; Budoff, M. Beneficial effects of aged garlic extract and coenzyme Q10 on vascular elasticity and endothelial function: The FAITH randomized clinical trial. Nutrition 2013, 29, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.; Morbini, M.; Rosticci, M.; D’Addato, S.; Grandi, E.; Borghi, C. Middle-term dietary supplementation with red yeast rice plus coenzyme Q10 improves lipid pattern, endothelial reactivity and arterial stiffness in moderately hypercholesterolemic subjects. Ann. Nutr. Metab. 2016, 68, 213–219. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Cho, W.-J.; Kim, J.-K.; Lee, D.-C. Effects of coenzyme Q10 on arterial stiffness, metabolic parameters, and fatigue in obese subjects: A double-blind randomized controlled study. J. Med. Food 2011, 14, 386–390. [Google Scholar] [CrossRef]
- Laurent, S.; Boutouyrie, P.; Cunha, P.G.; Lacolley, P.; Nilsson, P.M. Concept of extremes in vascular aging: From early vascular aging to supernormal vascular aging. Hypertension 2019, 74, 308–318. [Google Scholar] [CrossRef]
- Rhee, E.P.; Clish, C.B.; Ghorbani, A.; Larson, M.G.; Elmariah, S.; McCabe, E.; Yang, Q.; Cheng, S.; Pierce, K.; Deik, A.; et al. A combined epidemiologic and metabolomic approach improves CKD prediction. J. Am. Soc. Nephrol. 2013, 24, 1330–1338. [Google Scholar] [CrossRef]
- Rhee, E.P.; Clish, C.B.; Wenger, J.; Roy, J.; Elmariah, S.; Pierce, K.A.; Bullock, K.; Anderson, A.H.; Gerszten, R.E.; Feldman, H.I. Metabolomics of chronic kidney disease progression: A case-control analysis in the Chronic Renal Insufficiency Cohort study. Am. J. Nephrol. 2016, 43, 366–374. [Google Scholar] [CrossRef]
- Lavi, S.; McConnell, J.P.; Rihal, C.S.; Prasad, A.; Mathew, V.; Lerman, L.O.; Lerman, A. Local production of lipoprotein-associated phospholipase A2 and lysophosphatidylcholine in the coronary circulation: Association with early coronary atherosclerosis and endothelial dysfunction in humans. Circulation 2007, 115, 2715–2721. [Google Scholar] [CrossRef]
- Lavi, S.; Yang, E.H.; Prasad, A.; Mathew, V.; Barsness, G.W.; Rihal, C.S.; Lerman, L.O.; Lerman, A. The interaction between coronary endothelial dysfunction, local oxidative stress, and endogenous nitric oxide in humans. Hypertension 2008, 51, 127–133. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paapstel, K.; Kals, J. Metabolomics of Arterial Stiffness. Metabolites 2022, 12, 370. https://doi.org/10.3390/metabo12050370
Paapstel K, Kals J. Metabolomics of Arterial Stiffness. Metabolites. 2022; 12(5):370. https://doi.org/10.3390/metabo12050370
Chicago/Turabian StylePaapstel, Kaido, and Jaak Kals. 2022. "Metabolomics of Arterial Stiffness" Metabolites 12, no. 5: 370. https://doi.org/10.3390/metabo12050370
APA StylePaapstel, K., & Kals, J. (2022). Metabolomics of Arterial Stiffness. Metabolites, 12(5), 370. https://doi.org/10.3390/metabo12050370