Solid-State Fermented Okara with Aspergillus spp. Improves Lipid Metabolism and High-Fat Diet Induced Obesity
Abstract
:1. Introduction
2. Results
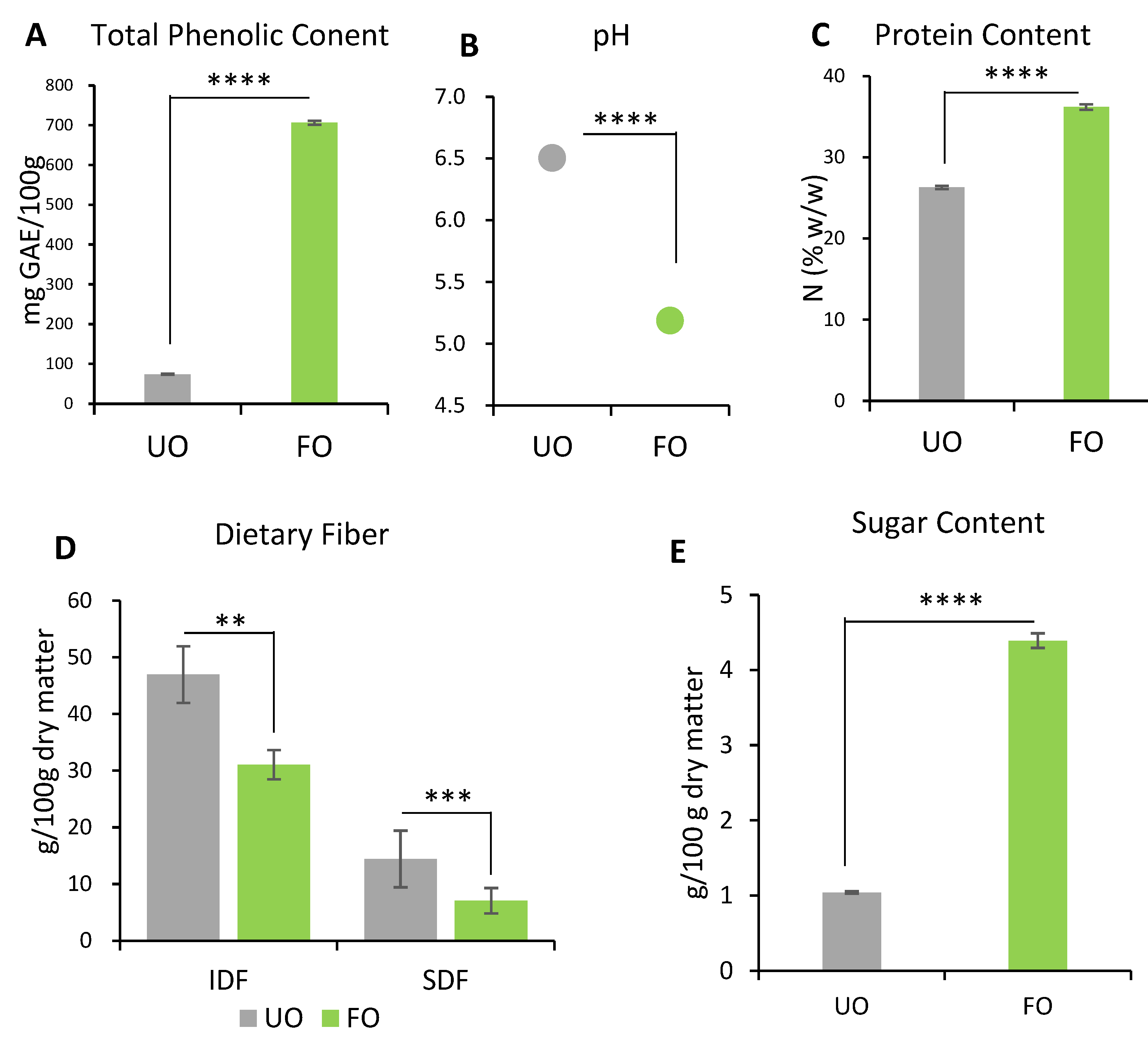
2.1. Composition of Okara Fermented with Aspergillus spp.
2.1.1. TPC and pH
2.1.2. Protein Content
2.1.3. Dietary Fiber
2.1.4. Sugars
2.1.5. Amino Acid
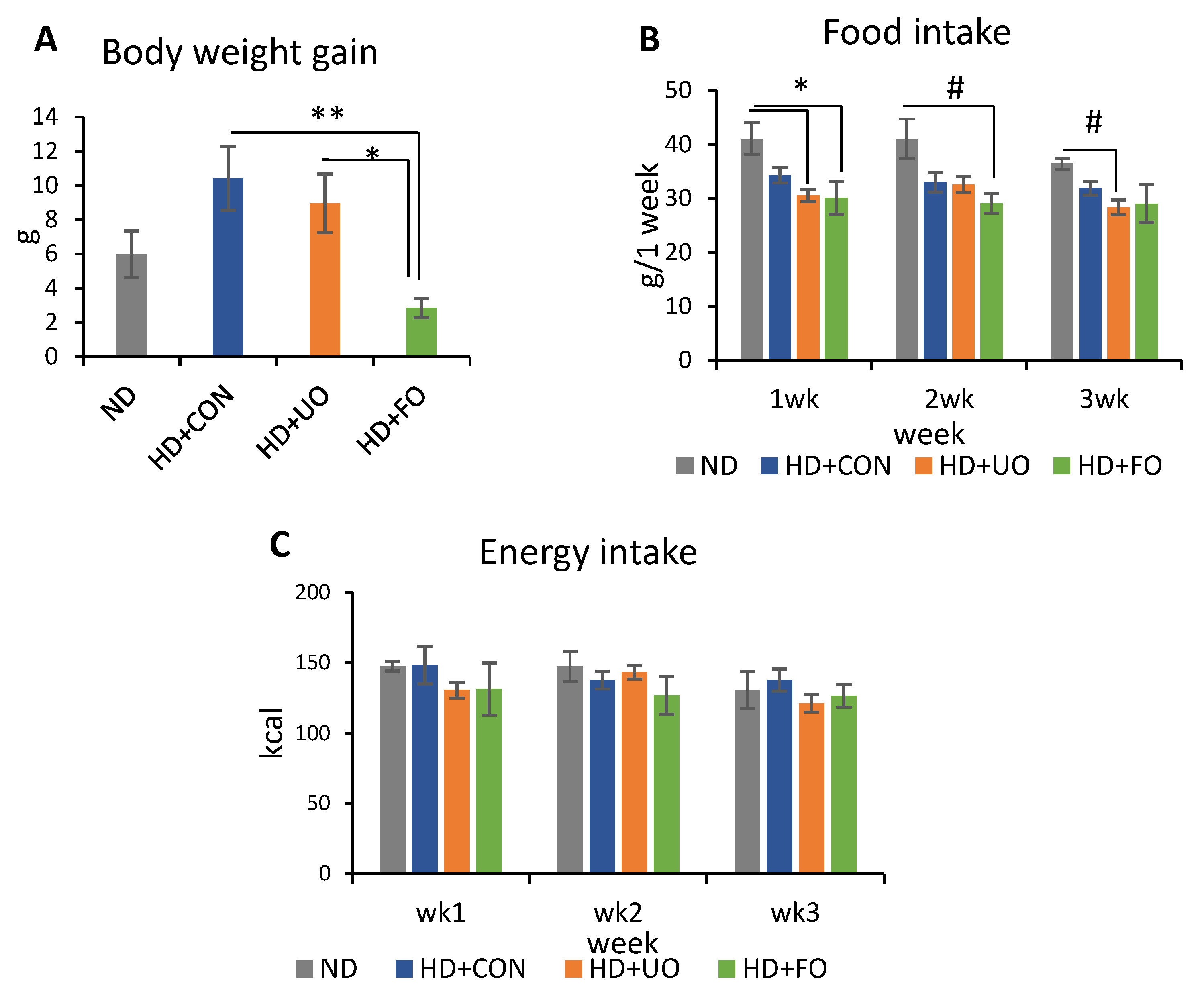
2.2. Animal Experiment
2.2.1. Body Profile (Body Weight Gain, Food Intake, and Energy Intake)
2.2.2. Tissue Weight (Liver Wight and Adipose Weight)
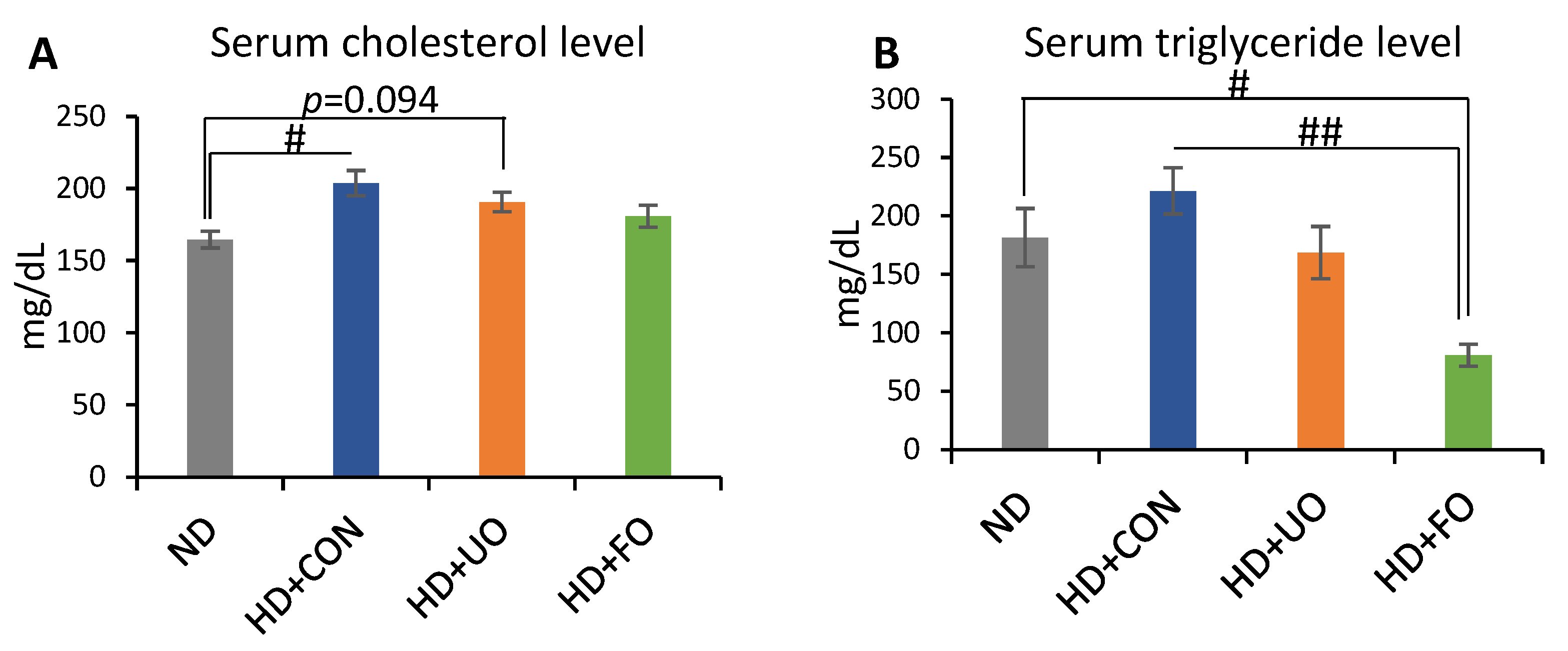
2.2.3. Lipid Profile in the Serum (Cholesterol, Triglyceride)
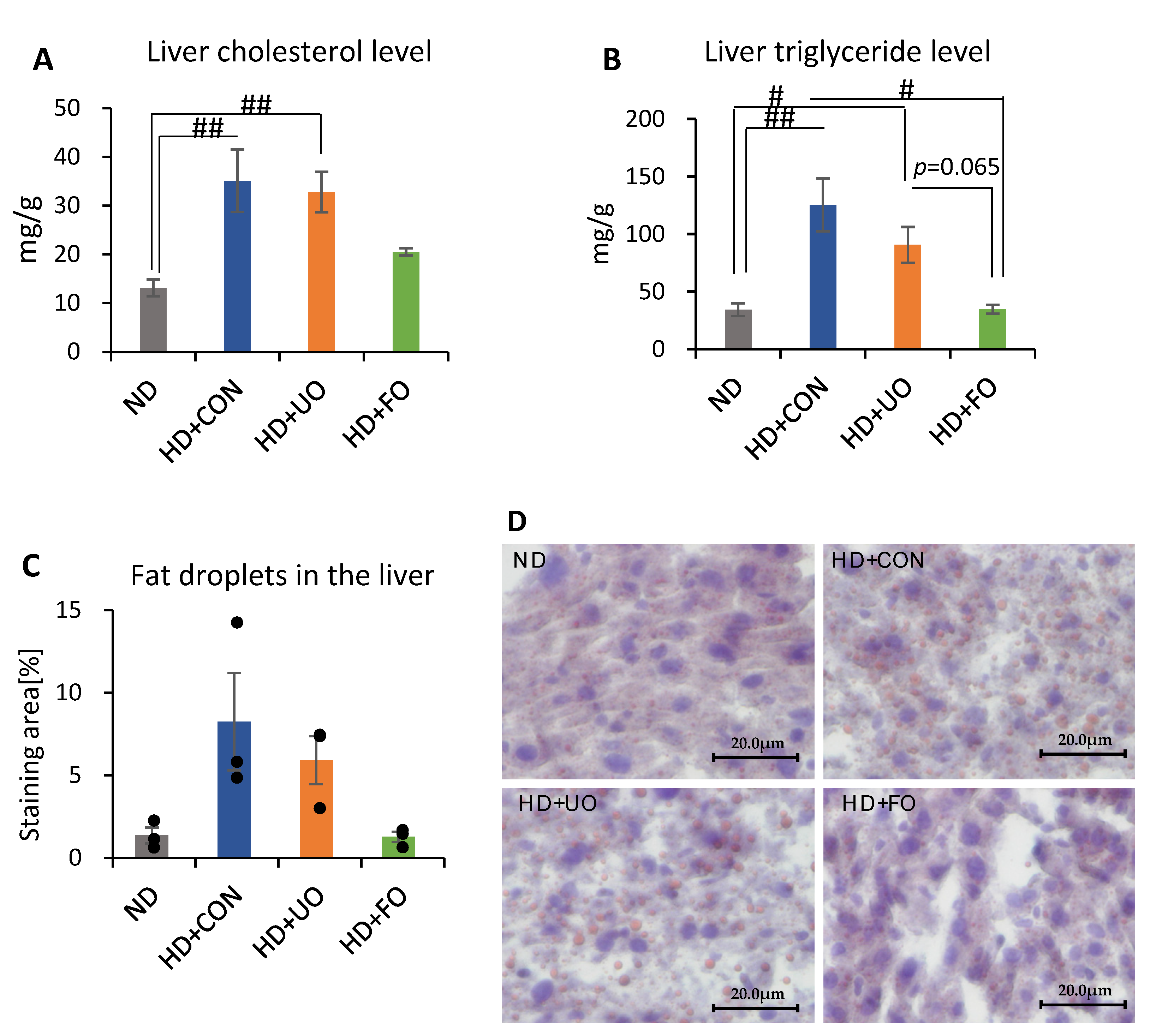
2.2.4. Liver Lipid Profile (Cholesterol, Triglyceride, and Histological Analysis)
2.2.5. mRNA Expression Levels of Genes in Liver
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Fermentation and Characterization
4.2.1. Solid-State Fermentation
4.2.2. Measurement of pH
4.2.3. Determination of TPC
4.2.4. Determination of Protein Content
4.2.5. Extraction of Amino Acids and Sugars
4.2.6. High Performance Liquid Chromatography (HPLC) Analysis of Amino Acids
4.2.7. HPLC Analysis of Sugars
4.2.8. Determination of Dietary Fibre
4.3. Animal Experiments and Profiling
4.3.1. Animals and Experimental Diets
4.3.2. Liver Fat Extraction
4.3.3. Measurement of Lipid Profile in the Serum and Liver
4.3.4. Histology Examination
4.3.5. Real-Time Reverse Transcription PCR (RT-PCR)
4.3.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
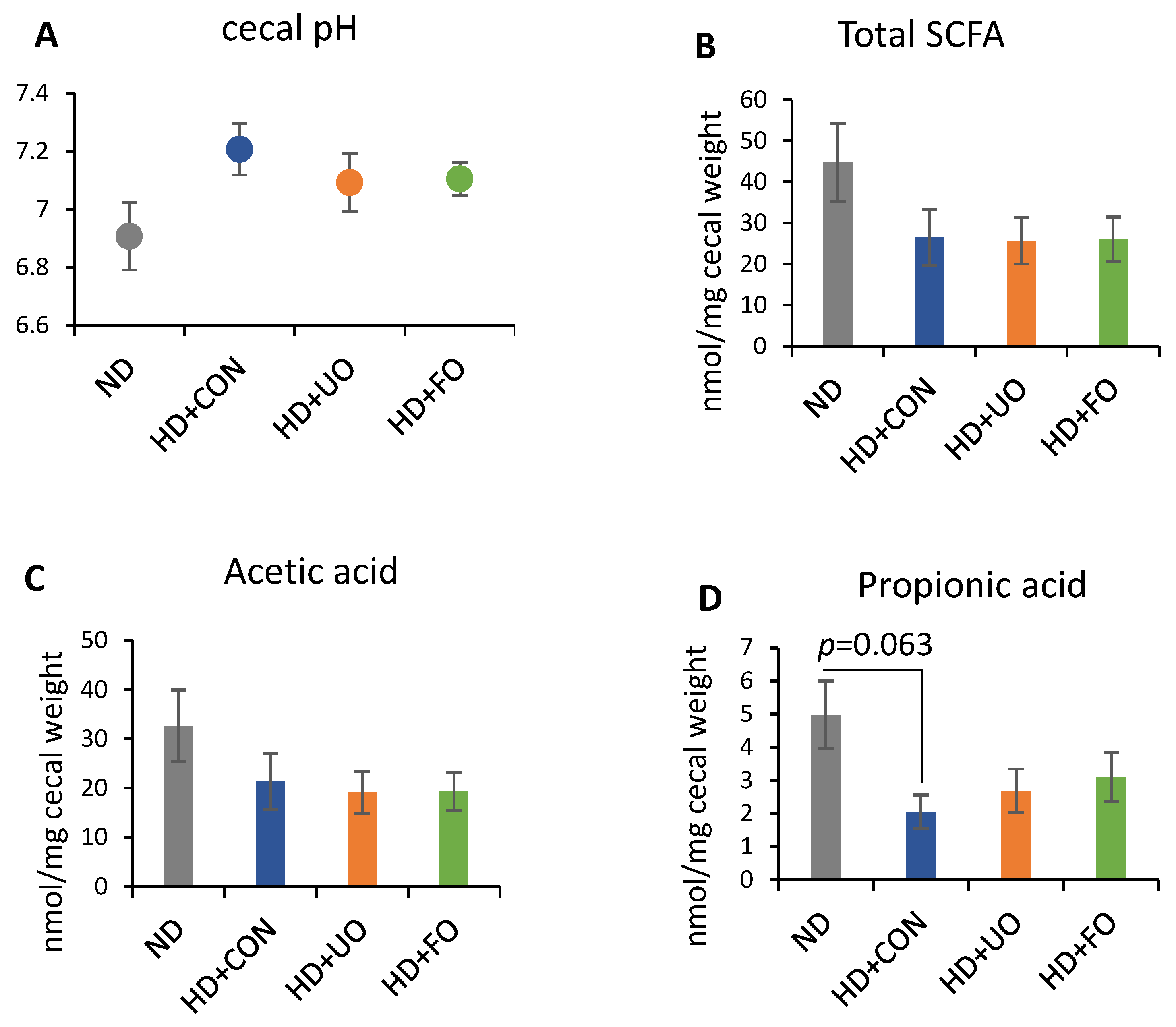
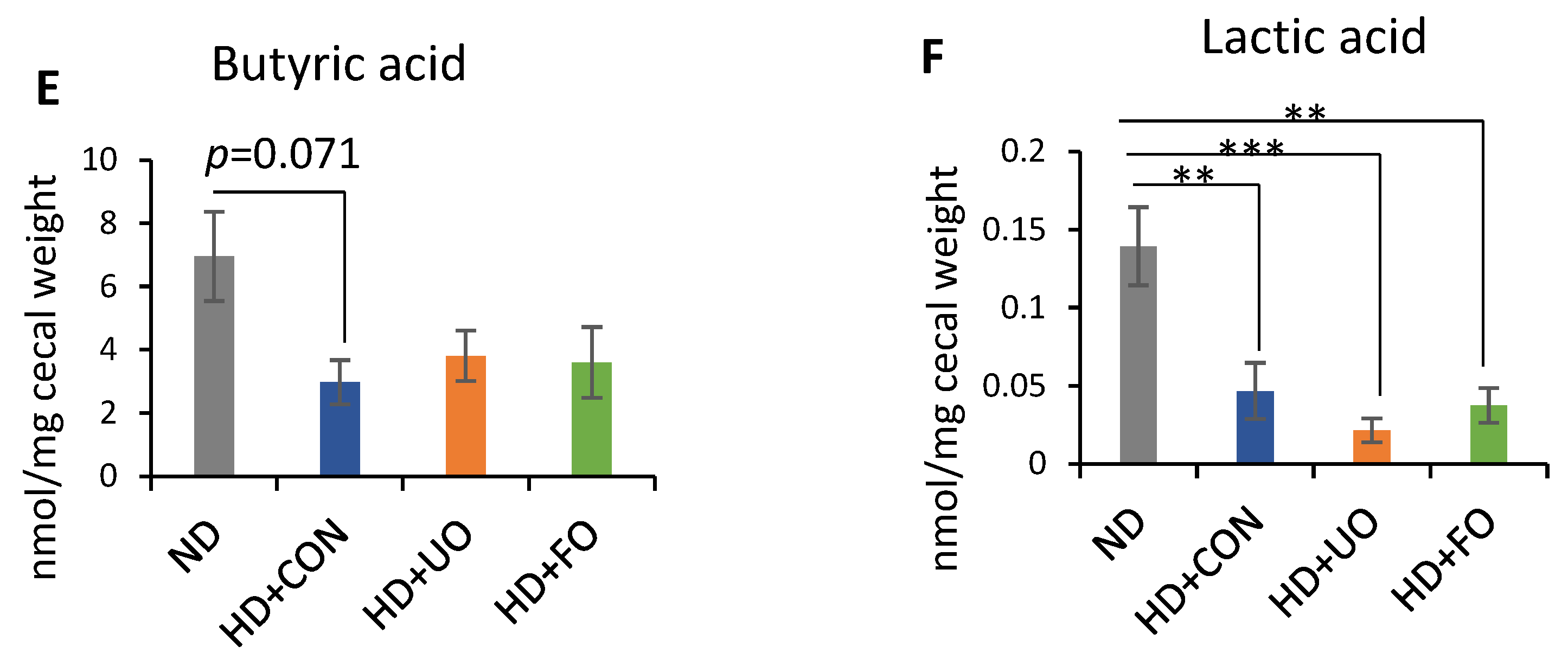
Appendix A
References
- Privatti, R.T.; Rodrigues, C.E.d.C. An Overview of the Composition, Applications, and Recovery Techniques of the Components of Okara Aimed at the Biovalorization of This Soybean Processing Residue. Food Rev. Int. 2021, 1–24. [Google Scholar] [CrossRef]
- Vong, W.C.; Liu, S.-Q. Biovalorisation of okara (soybean residue) for food and nutrition. Trends Food Sci. Technol. 2016, 52, 139–147. [Google Scholar] [CrossRef]
- Shabbir, M.A.; Khan, M.R.; Saeed, M.; Pasha, I.; Khalil, A.A.; Siraj, N. Punicic acid: A striking health substance to combat metabolic syndromes in humans. Lipids Health Dis. 2017, 16, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; Qiao, M.; Lu, F. Composition, nutrition, and utilization of okara (soybean residue). Food Rev. Int. 2012, 28, 231–252. [Google Scholar] [CrossRef]
- Lee, D.P.S.; Gan, A.X.; Kim, J.E. Incorporation of biovalorised okara in biscuits: Improvements of nutritional, antioxidant, physical, and sensory properties. LWT 2020, 134, 109902. [Google Scholar] [CrossRef]
- Ostermann-Porcel, M.V.; Quiroga-Panelo, N.; Rinaldoni, A.N.; Campderrós, M.E. Incorporation of okara into gluten-free cookies with high quality and nutritional value. J. Food Qual. 2017, 2017, 4071585. [Google Scholar] [CrossRef] [Green Version]
- Pandey, A. Solid-state fermentation. Biochem. Eng. J. 2003, 13, 81–84. [Google Scholar] [CrossRef]
- Krishna, C. Solid-State Fermentation Systems—An Overview. Crit. Rev. Biotechnol. 2005, 25, 1–30. [Google Scholar] [CrossRef]
- Park, H.-S.; Jun, S.-C.; Han, K.-H.; Hong, S.-B.; Yu, J.-H. Diversity, Application, and Synthetic Biology of Industrially Important Aspergillus Fungi. Adv. Appl. Microbiol. 2017, 100, 161–202. [Google Scholar] [CrossRef]
- Vong, W.C.; Hua, X.Y.; Liu, S.-Q. Solid-state fermentation with Rhizopus oligosporus and Yarrowia lipolytica improved nutritional and flavour properties of okara. LWT 2018, 90, 316–322. [Google Scholar] [CrossRef]
- Santos, V.A.Q.; Nascimento, C.G.; Schmidt, C.A.; Mantovani, D.; Dekker, R.F.; da Cunha, M.A.A. Solid-state fermentation of soybean okara: Isoflavones biotransformation, antioxidant activity and enhancement of nutritional quality. Lwt 2018, 92, 509–515. [Google Scholar] [CrossRef]
- Gupta, S.; Lee, J.J.L.; Chen, W.N. Analysis of Improved Nutritional Composition of Potential Functional Food (Okara) after Probiotic Solid-State Fermentation. J. Agric. Food Chem. 2018, 66, 5373–5381. [Google Scholar] [CrossRef] [PubMed]
- Sitanggang, A.B.; Sinaga, W.S.L.; Wie, F.; Fernando, F.; Krusong, W. Enhanced antioxidant activity of okara through solid state fermentation of GRAS Fungi. Food Sci. Technol. 2020, 40, 178–186. [Google Scholar] [CrossRef] [Green Version]
- Villanueva, M.; Yokoyama, W.; Hong, Y.; Barttley, G.; Rupérez, P. Effect of high-fat diets supplemented with okara soybean by-product on lipid profiles of plasma, liver and faeces in Syrian hamsters. Food Chem. 2011, 124, 72–79. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.-S.; Yu, O.-K.; Byun, M.-S.; Cha, Y.-S. Okara, a soybean by-product, prevents high fat diet-induced obesity and improves serum lipid profiles in C57BL/6J mice. Food Sci. Biotechnol. 2016, 25, 607–613. [Google Scholar] [CrossRef]
- Chan, L.Y.; Takahashi, M.; Lim, P.J.; Aoyama, S.; Makino, S.; Ferdinandus, F.; Ng, S.Y.C.; Arai, S.; Fujita, H.; Tan, H.C. Eurotium cristatum fermented okara as a potential food ingredient to combat diabetes. Sci. Rep. 2019, 9, 17536. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.Y.; Daily, J.W., III; Kim, H.J.; Park, S. Antidiabetic effects of fermented soybean products on type 2 diabetes. Nutr. Res. 2010, 30, 1–13. [Google Scholar] [CrossRef]
- Roobab, U.; Batool, Z.; Manzoor, M.F.; Shabbir, M.A.; Khan, M.R.; Aadil, R.M. Sources, formulations, advanced delivery and health benefits of probiotics. Curr. Opin. Food Sci. 2020, 32, 17–28. [Google Scholar] [CrossRef]
- WHO. Obesity and Overweight. Available online: https://www.who.int/en/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 7 July 2021).
- Global Burden of Disease Collaborative Network. Global Burden of Disease Study 2015 (GBD 2015) Obesity and Overweight Prevalence 1980–2015; Institute for Health Metrics and Evaluation: Seattle, DA, USA, 2017. [Google Scholar]
- Chooi, Y.C.; Ding, C.; Magkos, F. The epidemiology of obesity. Metabolism 2019, 92, 6–10. [Google Scholar] [CrossRef] [Green Version]
- Singh, G.M.; Danaei, G.; Farzadfar, F.; Stevens, G.A.; Woodward, M.; Wormser, D.; Kaptoge, S.; Whitlock, G.; Qiao, Q.; Lewington, S.; et al. The Age-Specific Quantitative Effects of Metabolic Risk Factors on Cardiovascular Diseases and Diabetes: A Pooled Analysis. PLoS ONE 2013, 8, e65174. [Google Scholar] [CrossRef]
- Lauby-Secretan, B.; Scoccianti, C.; Loomis, D.; Grosse, Y.; Bianchini, F.; Straif, K. Body fatness and cancer—Viewpoint of the IARC Working Group. N. Engl. J. Med. 2016, 375, 794–798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimura, A.; Esaki, H.; Mori, K.; Kumagai, M.; Nakamura, Y. Evaluation of an Okara Beni Koji Based on Antioxidant Properties, and an Application to Cookie Making. Nippon Shokuhin Kagaku Kogaku Kaishi 2014, 61, 409–417. [Google Scholar] [CrossRef] [Green Version]
- Shin, H.-Y.; Kim, S.-M.; Lee, J.H.; Lim, S.-T. Solid-state fermentation of black rice bran with Aspergillus awamori and Aspergillus oryzae: Effects on phenolic acid composition and antioxidant activity of bran extracts. Food Chem. 2019, 272, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Mojsov, K. Aspergillus enzymes for food industries. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2016; pp. 215–222. [Google Scholar]
- Wyman, C.; Decker, S.; Himmel, M.; Brady, J.; Skopec, C.; Viikari, L. Hydrolysis of Cellulose and Hemicellulose. In Polysaccharides; Taylor & Francis Group: Abingdon, UK, 2004; pp. 995–1034. [Google Scholar] [CrossRef]
- Lee, D.E.; Lee, S.; Jang, E.S.; Shin, H.W.; Moon, B.S.; Lee, C.H. Metabolomic profiles of Aspergillus oryzae and Bacillus amyloliquefaciens during rice koji fermentation. Molecules 2016, 21, 773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hyeon, H.; Min, C.W.; Moon, K.; Cha, J.; Gupta, R.; Park, S.U.; Kim, S.T.; Kim, J.K. Metabolic profiling-based evaluation of the fermentative behavior of Aspergillus oryzae and Bacillus subtilis for soybean residues treated at different temperatures. Foods 2020, 9, 117. [Google Scholar] [CrossRef] [Green Version]
- Olukomaiya, O.O.; Adiamo, O.Q.; Fernando, W.C.; Mereddy, R.; Li, X.; Sultanbawa, Y. Effect of solid-state fermentation on proximate composition, anti-nutritional factor, microbiological and functional properties of lupin flour. Food Chem. 2020, 315, 126238. [Google Scholar] [CrossRef]
- Pownall, T.L.; Udenigwe, C.C.; Aluko, R.E. Amino Acid Composition and Antioxidant Properties of Pea Seed (Pisum sativum L.) Enzymatic Protein Hydrolysate Fractions. J. Agric. Food Chem. 2010, 58, 4712–4718. [Google Scholar] [CrossRef] [PubMed]
- Pasquier, B.; Armand, M.; Castelain, C.; Guillon, F.; Borel, P.; Lafont, H.; Lairon, D. Emulsification and lipolysis of triacylglycerols are altered by viscous soluble dietary fibres in acidic gastric medium in vitro. Biochem. J. 1996, 314, 269–275. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.S.; Lee, Y.; Kim, J.; Sohn, E.; Kim, C.S.; Lee, Y.M.; Jo, K.; Shin, S.; Song, Y.; Kim, J.H.; et al. Inhibitory Activities of Cudrania tricuspidata Leaves on Pancreatic Lipase In Vitro and Lipolysis In Vivo. Evid.-Based Complement. Altern. Med. 2012, 2012, 878365. [Google Scholar] [CrossRef] [Green Version]
- Shen, Y.; Song, S.J.; Keum, N.; Park, T. Olive Leaf Extract Attenuates Obesity in High-Fat Diet-Fed Mice by Modulating the Expression of Molecules Involved in Adipogenesis and Thermogenesis. Evid.-Based Complement. Altern. Med. 2014, 2014, 971890. [Google Scholar] [CrossRef] [Green Version]
- Moriyama, T.; Kishimoto, K.; Nagai, K.; Urade, R.; Ogawa, T.; Utsumi, S.; Maruyama, N.; Maebuchi, M. Soybean beta-conglycinin diet suppresses serum triglyceride levels in normal and genetically obese mice by induction of beta-oxidation, downregulation of fatty acid synthase, and inhibition of triglyceride absorption. Biosci. Biotechnol. Biochem. 2004, 68, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Fukui, K.; Kojima, M.; Tachibana, N.; Kohno, M.; Takamatsu, K.; Hirotsuka, M.; Kito, M. Effects of soybean beta-conglycinin on hepatic lipid metabolism and fecal lipid excretion in normal adult rats. Biosci. Biotechnol. Biochem. 2004, 68, 1153–1155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freudenberg, A.; Petzke, K.J.; Klaus, S. Comparison of high-protein diets and leucine supplementation in the prevention of metabolic syndrome and related disorders in mice. J. Nutr. Biochem. 2012, 23, 1524–1530. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J.; Han, S.-F.; Zhang, W.; Xu, J.-Y.; Tong, X.; Yin, X.-B.; Yuan, L.-X.; Qin, L.-Q. Chronic leucine supplementation improves lipid metabolism in C57BL/6J mice fed with a high-fat/cholesterol diet. Food Nutr. Res. 2016, 60, 31304–31310. [Google Scholar] [CrossRef] [Green Version]
- Arakawa, M.; Masaki, T.; Nishimura, J.; Seike, M.; Yoshimatsu, H. The effects of branched-chain amino acid granules on the accumulation of tissue triglycerides and uncoupling proteins in diet-induced obese mice. Endocr. J. 2011, 58, 161–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rani, V.; Deep, G.; Singh, R.K.; Palle, K.; Yadav, U.C.S. Oxidative stress and metabolic disorders: Pathogenesis and therapeutic strategies. Life Sci. 2016, 148, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, K.; Olejnik, A.; Szwajgier, D.; Olkowicz, M. Inhibitory activity of chokeberry, bilberry, raspberry and cranberry polyphenol-rich extract towards adipogenesis and oxidative stress in differentiated 3T3-L1 adipose cells. PLoS ONE 2017, 12, e0188583. [Google Scholar] [CrossRef] [Green Version]
- Seyedan, A.; Alshawsh, M.A.; Alshagga, M.A.; Koosha, S.; Mohamed, Z. Medicinal Plants and Their Inhibitory Activities against Pancreatic Lipase: A Review. Evid.-Based Complement. Altern. Med. 2015, 2015, 973143. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.S.; Zhang, J.; Zhang, L.; Huang, J.; Wang, Y. Mechanisms of body weight reduction and metabolic syndrome alleviation by tea. Mol. Nutr. Food Res. 2016, 60, 160–174. [Google Scholar] [CrossRef]
- Abdul Rahman, H.; Saari, N.; Abas, F.; Ismail, A.; Mumtaz, M.W.; Abdul Hamid, A. Anti-obesity and antioxidant activities of selected medicinal plants and phytochemical profiling of bioactive compounds. Int. J. Food Prop. 2017, 20, 2616–2629. [Google Scholar] [CrossRef]
- Kohno, M.; Hirotsuka, M.; Kito, M.; Matsuzawa, Y. Decreases in serum triacylglycerol and visceral fat mediated by dietary soybean β-conglycinin. J. Atheroscler. Thromb. 2006, 13, 247–255. [Google Scholar] [CrossRef] [Green Version]
- Abboud, K.Y.; Reis, S.K.; Martelli, M.E.; Zordão, O.P.; Tannihão, F.; De Souza, A.Z.Z.; Assalin, H.B.; Guadagnini, D.; Rocha, G.Z.; Saad, M.J.A. Oral glutamine supplementation reduces obesity, pro-inflammatory markers, and improves insulin sensitivity in DIO wistar rats and reduces waist circumference in overweight and obese humans. Nutrients 2019, 11, 536. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Chen, J.; Sun, B.; Wang, Z.; Zhu, J.; Yue, Z.; Zhang, Y.; Shan, A.; Ma, Q.; Wang, J. Leucine, but not isoleucine or valine, affects serum lipid profiles and browning of WAT in mice. Food Funct. 2021, 12, 6712–6724. [Google Scholar] [CrossRef]
- Rehman, T.; Shabbir, M.A.; Inam-Ur-Raheem, M.; Manzoor, M.F.; Ahmad, N.; Liu, Z.W.; Ahmad, M.H.; Siddeeg, A.; Abid, M.; Aadil, R.M. Cysteine and homocysteine as biomarker of various diseases. Food Sci. Nutr. 2020, 8, 4696–4707. [Google Scholar] [CrossRef] [PubMed]
- Høie, L.H.; Morgenstern, E.C.A.; Gruenwald, J.; Graubaum, H.-J.; Busch, R.; Lüder, W.; Zunft, H.-J.F. A double-blind placebo-controlled clinical trial compares the cholesterol-lowering effects of two different soy protein preparations in hypercholesterolemic subjects. Eur. J. Nutr. 2005, 44, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef] [PubMed]


| Average (g/100 g Dry Matter) | ||
|---|---|---|
| Sample/Sugar | UO | FO |
| Fructose | ND | ND |
| Glucose | ND | 4.392 ± 0.098 |
| Sucrose | 1.042 ± 0.016 | ND |
| Maltose | ND | ND |
| Total sugar | 1.042 ± 0.016 | 4.392 ± 0.098 |
| Average (mg/100 g Dry Weight) | |||
|---|---|---|---|
| Amino Acid | Unfermented Okara | Fermented Okara | Fold Change |
| Asp | 3.221 ± 0.051 | 58.366 ± 0.798 | 18 |
| Glu | 10.509 ± 0.103 | 322.036 ± 3.060 | 31 |
| Asn | 0.677 ± 0.014 | 72.094 ± 0.648 | 106 |
| Ser | 0.847 ± 0.019 | 47.399 ± 0.540 | 56 |
| Gln | 0.639 ± 0.004 | 521.395 ± 3.955 | 816 |
| His/Gly | 1.767 ± 0.129 | 44.09 ± 0.405 | 25 |
| Thr | 0.824 ± 0.014 | 39.603 ± 1.000 | 48 |
| Arg | 7.185 ± 0.054 | 45.768 ± 1.197 | 6 |
| Ala | 7.156 ± 0.083 | 141.764 ± 1.410 | 20 |
| Tyr | 2.986 ± 0.051 | 36.48 ± 0.583 | 12 |
| Cys | ND | 39.609 ± 8.261 | * |
| Val/Met | 3.198 ± 0.053 | 23.171 ± 0.220 | 7 |
| Trp | 5.636 ± 0.091 | 10.911 ± 0.206 | 2 |
| Phe | 5.79 ± 0.065 | 15.497 ± 0.400 | 3 |
| Ile | 1.312 ± 0.011 | 8.463 ± 0.077 | 6 |
| Leu | 3.151 ± 0.058 | 14.406 ± 0.228 | 5 |
| Lys | 1.815 ± 0.229 | 23.569 ± 2.540 | 13 |
| Pro | 2.117 ± 0.163 | 16.665 ± 0.682 | 8 |
| Overall amino acids | 58.812 ± 0.376 | 1481.286 ± 10.331 | 25 |
| ND | HD + CON | HD + UO | HD + FO | |
|---|---|---|---|---|
| Normal diet | 100 | 0 | 0 | 0 |
| High-fat diet | 0 | 80 | 80 | 80 |
| Starch | 0 | 4.8 | 0 | 0 |
| Casein | 0 | 11.5 | 0 | 0 |
| Corn oil | 0 | 3.7 | 0 | 0 |
| Unfermented okara | 0 | 0 | 20 | 0 |
| Fermented okara with Aspergillus spp. | 0 | 0 | 0 | 20 |
| Energy (kcal/100 g) | 359 | 432 | 428 | 436 |
| Unfermented Okara (UO) | Fermented Okara with Aspergillus spp. (FO) | |
|---|---|---|
| Carbohydrates | 10.9 | 16 |
| Protein | 26.29 | 36.2 |
| Total Dietary Fibre | 61.37 | 38.13 |
| Fat | 10.9 | 8.88 |
| Total calorie (kcal/100 g) | 246.86 | 288.72 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ichikawa, N.; Ng, L.S.; Makino, S.; Goh, L.L.; Lim, Y.J.; Ferdinandus; Sasaki, H.; Shibata, S.; Lee, C.-L.K. Solid-State Fermented Okara with Aspergillus spp. Improves Lipid Metabolism and High-Fat Diet Induced Obesity. Metabolites 2022, 12, 198. https://doi.org/10.3390/metabo12030198
Ichikawa N, Ng LS, Makino S, Goh LL, Lim YJ, Ferdinandus, Sasaki H, Shibata S, Lee C-LK. Solid-State Fermented Okara with Aspergillus spp. Improves Lipid Metabolism and High-Fat Diet Induced Obesity. Metabolites. 2022; 12(3):198. https://doi.org/10.3390/metabo12030198
Chicago/Turabian StyleIchikawa, Natsumi, Li Shiuan Ng, Saneyuki Makino, Luo Lin Goh, Yun Jia Lim, Ferdinandus, Hiroyuki Sasaki, Shigenobu Shibata, and Chi-Lik Ken Lee. 2022. "Solid-State Fermented Okara with Aspergillus spp. Improves Lipid Metabolism and High-Fat Diet Induced Obesity" Metabolites 12, no. 3: 198. https://doi.org/10.3390/metabo12030198
APA StyleIchikawa, N., Ng, L. S., Makino, S., Goh, L. L., Lim, Y. J., Ferdinandus, Sasaki, H., Shibata, S., & Lee, C.-L. K. (2022). Solid-State Fermented Okara with Aspergillus spp. Improves Lipid Metabolism and High-Fat Diet Induced Obesity. Metabolites, 12(3), 198. https://doi.org/10.3390/metabo12030198






