Asparagine Metabolism in Tumors Is Linked to Poor Survival in Females with Colorectal Cancer: A Cohort Study
Abstract
:1. Introduction
2. Results
2.1. Population Characteristics
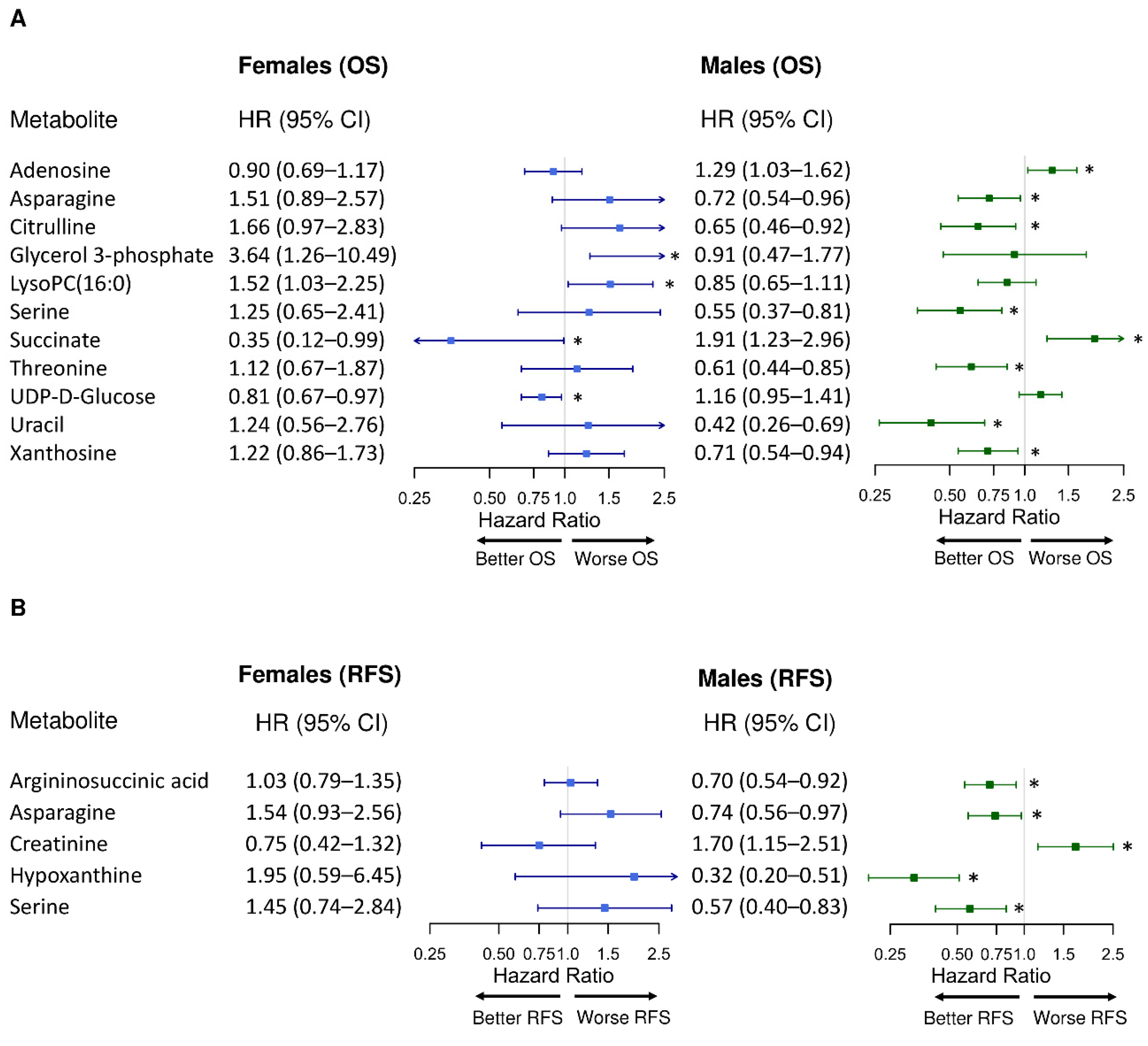
2.2. Sex-Specific Differences in the Associations between Individual Metabolite Abundances and CRC Prognosis
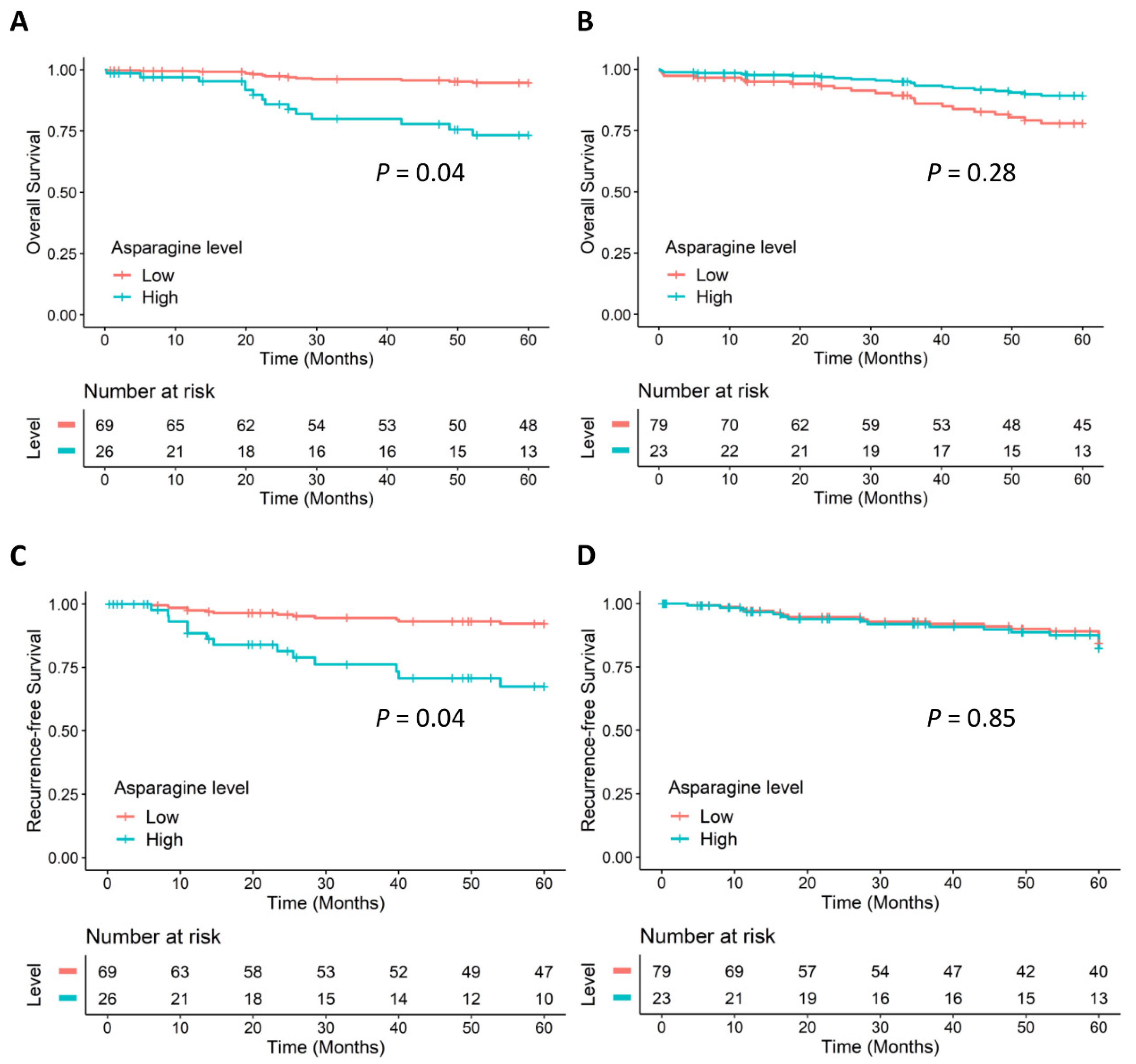
2.3. Sex-Specific Differences in CRC Prognosis Associated with Asparagine Synthesis
2.4. Sex-Specific Differences in CRC Prognosis Associated with Glycolysis and the Pentose Phosphate Pathway
2.5. Sex-Specific Differences in CRC Prognosis Associated with Lysophospholipid Synthesis
2.6. Sex-Specific Differences in CRC Prognosis Associated with Methionine Metabolism
2.7. Sex-Specific Differences in CRC Prognosis Associated with Polyamine Synthesis
3. Discussion
4. Materials and Methods
4.1. Sample Collection and Metabolite Measurements
4.2. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix B
| Metabolite Name | Females | Males | Int. Sex p Value b | ||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | p Value a | HR | 95% CI | p Value a | ||
| Acetyl-lysine | 1.06 | 0.76–1.49 | 0.735 | 0.57 | 0.42–0.78 | <0.001 | 0.025 |
| Adenosine | 1.01 | 0.62–1.63 | 0.976 | 1.42 | 1.04–1.94 | 0.029 | 0.254 |
| ADMA | 1.58 | 0.90–2.78 | 0.109 | 0.51 | 0.33–0.80 | 0.003 | 0.002 |
| Alanine | 1.43 | 0.89–2.30 | 0.144 | 0.65 | 0.46–0.91 | 0.012 | 0.007 |
| Arginine | 1.70 | 0.72–4.03 | 0.227 | 0.53 | 0.28–0.99 | 0.045 | 0.049 |
| Argininosuccinic acid | 1.08 | 0.71–1.64 | 0.713 | 0.58 | 0.40–0.83 | 0.003 | 0.026 |
| Citrulline | 1.76 | 0.97–3.18 | 0.063 | 0.44 | 0.25–0.77 | 0.004 | 0.001 |
| CMP | 1.27 | 0.55–2.90 | 0.578 | 0.57 | 0.36–0.90 | 0.017 | 0.076 |
| Glycerol 3-phosphate | 4.20 | 1.24–14.25 | 0.021 | 1.03 | 0.39–2.74 | 0.948 | 0.057 |
| GMP | 1.13 | 0.62–2.07 | 0.686 | 0.59 | 0.39–0.89 | 0.013 | 0.055 |
| Histidine | 1.23 | 0.72–2.08 | 0.451 | 0.73 | 0.53–1.00 | 0.049 | 0.080 |
| Lysine | 1.54 | 0.63–3.74 | 0.341 | 0.54 | 0.30–0.98 | 0.044 | 0.030 |
| LysoPC(16:0) | 2.03 | 1.18–3.49 | 0.010 | 0.77 | 0.52–1.14 | 0.188 | 0.003 |
| LysoPC(16:1) | 1.13 | 0.69–1.86 | 0.632 | 0.67 | 0.45–0.98 | 0.038 | 0.088 |
| Methionine | 2.38 | 0.81–7.00 | 0.114 | 0.35 | 0.17–0.75 | 0.007 | 0.009 |
| Ornithine | 1.63 | 0.59–4.50 | 0.347 | 0.41 | 0.19–0.91 | 0.028 | 0.032 |
| Phenylalanine | 1.68 | 0.55–5.08 | 0.359 | 0.26 | 0.09–0.71 | 0.008 | 0.027 |
| Serine | 2.88 | 0.82–10.15 | 0.100 | 0.34 | 0.16–0.71 | 0.004 | 0.005 |
| Spermine | 2.13 | 1.29–3.52 | 0.003 | 1.16 | 0.82–1.63 | 0.407 | 0.076 |
| Taurine | 0.63 | 0.19–2.11 | 0.451 | 0.34 | 0.14–0.79 | 0.013 | 0.475 |
| Threonine | 1.38 | 0.62–3.08 | 0.432 | 0.39 | 0.20–0.75 | 0.005 | 0.012 |
| Tyrosine | 0.81 | 0.40–1.68 | 0.578 | 0.60 | 0.37–0.99 | 0.044 | 0.325 |
| Uracil | 2.87 | 0.70–11.76 | 0.143 | 0.30 | 0.16–0.58 | <0.001 | 0.005 |
| Xanthosine | 1.21 | 0.85–1.73 | 0.288 | 0.56 | 0.37–0.84 | 0.006 | 0.016 |
| Metabolite Name | Females | Males | Int. Sex p Value b | ||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | p Value a | HR | 95% CI | p Value a | ||
| Arginine | 3.05 | 1.33–7.02 | 0.009 | 1.88 | 0.64–5.52 | 0.252 | 0.218 |
| Asparagine | 2.60 | 1.06–6.37 | 0.036 | 1.02 | 0.56–1.85 | 0.943 | 0.058 |
| Glutathione disulfide | 0.67 | 0.47–0.94 | 0.022 | 0.84 | 0.62–1.15 | 0.274 | 0.264 |
| Hypoxanthine | 6.02 | 0.55–65.93 | 0.142 | 0.37 | 0.17–0.80 | 0.011 | 0.023 |
| LysoPC(16:0) | 1.94 | 1.11–3.37 | 0.020 | 0.69 | 0.39–1.22 | 0.202 | 0.025 |
| LysoPE(18:2) | 1.75 | 0.79–3.89 | 0.167 | 0.40 | 0.17–0.94 | 0.035 | 0.059 |
| Methionine | 3.40 | 1.10–10.53 | 0.034 | 1.12 | 0.43–2.89 | 0.814 | 0.095 |
| N1-acetylspermine | 0.95 | 0.66–1.36 | 0.781 | 3.20 | 1.32–7.76 | 0.010 | 0.016 |
| Succinate | 0.87 | 0.25–3.11 | 0.834 | 0.07 | 0.01–0.92 | 0.043 | 0.143 |
| Variable a | OS | RFS | ||||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p Value | Int. Sex p Value b | HR | 95% CI | p Value | Int. Sex p Value b | |
| Asparagine | 1.28 | 0.74–2.22 | 0.377 | 0.035 | 2.50 | 1.27–4.91 | 0.008 | 0.010 |
| Aspartate | 1.24 | 0.80–1.93 | 0.345 | 0.967 | 1.59 | 0.96–2.63 | 0.071 | 0.447 |
| Glutamate | 0.68 | 0.26–1.80 | 0.438 | 0.692 | 0.43 | 0.13–1.41 | 0.165 | 0.078 |
| Glutamine | 0.65 | 0.24–1.75 | 0.393 | 0.932 | 0.21 | 0.07–0.63 | 0.005 | 0.716 |
| AMP | 0.74 | 0.47–1.17 | 0.198 | 0.444 | 0.86 | 0.50–1.48 | 0.585 | 0.319 |
| Sex = Male (ref: female) | 2.02 | 0.94–4.32 | 0.070 | - | 0.97 | 0.40–2.34 | 0.947 | - |
| Anatomic location = RCC (ref: LCC) | 0.81 | 0.37–1.78 | 0.598 | - | 0.68 | 0.28–1.70 | 0.415 | - |
| Clinical stage = late (ref: early) | 4.46 | 1.55–12.78 | 0.005 | - | 1.32 | 0.37–4.62 | 0.669 | - |
| Chemotherapy = yes (ref: no) | 1.05 | 0.36–3.10 | 0.924 | 2.58 | 0.67–9.96 | 0.166 | ||
| Age | 1.10 | 1.04–1.16 | <0.001 | - | 0.98 | 0.92–1.05 | 0.604 | - |
| KRAS = mutant (ref: wild type) | 1.09 | 0.50–2.37 | 0.832 | - | 1.48 | 0.60–3.69 | 0.398 | - |
| Variable a | OS | RFS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Females | Males | Females | Males | |||||||||
| HR | 95% CI | p Value | HR | 95% CI | p Value | HR | 95% CI | p Value | HR | 95% CI | p Value | |
| Asparagine | 6.79 | 1.43–32.16 | 0.016 | 0.77 | 0.42–1.42 | 0.407 | 15.23 | 2.23–104.27 | 0.006 | 1.37 | 0.66–2.82 | 0.397 |
| Aspartate | 1.01 | 0.35–2.92 | 0.984 | 1.33 | 0.76–2.32 | 0.319 | 1.38 | 0.42–4.49 | 0.594 | 1.55 | 0.81–2.97 | 0.190 |
| Glutamate | 0.30 | 0.04–1.99 | 0.211 | 1.21 | 0.34–4.31 | 0.771 | 0.32 | 0.03–3.50 | 0.348 | 0.42 | 0.08–2.12 | 0.291 |
| Glutamine | 0.12 | 0.01–0.93 | 0.044 | 0.96 | 0.24–3.74 | 0.949 | 0.02 | 0.002–0.21 | 0.001 | 0.39 | 0.07–2.11 | 0.276 |
| AMP | 0.99 | 0.35–2.77 | 0.981 | 0.62 | 0.34–1.13 | 0.117 | 1.07 | 0.33–3.45 | 0.915 | 0.76 | 0.32–1.88 | 0.554 |
| KRAS = mutant (ref: wild type) | 1.34 | 0.32–5.67 | 0.69 | 0.54 | 0.17–1.68 | 0.286 | 1.69 | 0.36–7.94 | 0.505 | 0.77 | 0.16–3.76 | 0.748 |
| Anatomic location = RCC (ref: LCC) | 1.50 | 0.43–5.31 | 0.527 | 0.83 | 0.26–2.71 | 0.759 | 0.82 | 0.21–3.25 | 0.777 | 0.76 | 0.17–3.47 | 0.721 |
| Clinical stage = late (ref: early) | 9.66 | 1.34–69.57 | 0.024 | 8.09 | 1.42–46.22 | 0.019 | 1.31 | 0.19–9.03 | 0.782 | 0.61 | 0.07–4.78 | 0.636 |
| Chemotherapy = Yes (ref: no) | 1.80 | 0.37–8.74 | 0.468 | 0.46 | 0.07–2.85 | 0.402 | 3.55 | 0.47–26.49 | 0.217 | 3.33 | 0.37–29.78 | 0.283 |
| Age | 1.09 | 0.97–1.23 | 0.154 | 1.14 | 1.05–1.231 | 0.001 | 1.05 | 0.94–1.17 | 0.407 | 0.95 | 0.87–1.05 | 0.331 |
References
- Colorectal Cancer Statistics. Available online: https://www.cdc.gov/cancer/colorectal/statistics/index.htm (accessed on 13 October 2021).
- Amin, M.B.; Edge, S.B. AJCC Cancer Staging Manual; Springer: Berlin, Germany, 2017. [Google Scholar]
- TNM Classification of Malignant Tumours. Available online: https://www.uicc.org/resources/tnm (accessed on 13 October 2021).
- Nakagawa-Senda, H.; Hori, M.; Matsuda, T.; Ito, H. Prognostic Impact of Tumor Location in Colon Cancer: The Monitoring of Cancer Incidence in Japan (MCIJ) Project. BMC Cancer 2019, 19, 431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, C.; Jiang, F.; Lin, H.; Li, S. Clinical Characteristics and Prognosis of Different Primary Tumor Location in Colorectal Cancer: A Population-Based Cohort Study. Clin. Transl. Oncol. 2019, 21, 1524–1531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doleman, B.; Mills, K.T.; Lim, S.; Zelhart, M.D.; Gagliardi, G. Body Mass Index and Colorectal Cancer Prognosis: A Systematic Review and Meta-Analysis. Tech. Coloproctol. 2016, 20, 517–535. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Wu, X.; Wu, B.; Pei, D.; Zhang, L.; Wei, L. The Relationship between Diabetes and Colorectal Cancer Prognosis: A Meta-Analysis Based on the Cohort Studies. PLoS ONE 2017, 12, e0176068. [Google Scholar] [CrossRef] [Green Version]
- Xu, G.; Zhang, M.; Zhu, H.; Xu, J. A 15-Gene Signature for Prediction of Colon Cancer Recurrence and Prognosis Based on SVM. Gene 2017, 604, 33–40. [Google Scholar] [CrossRef]
- Pietrzyk, Ł.; Korolczuk, A.; Matysek, M.; Arciszewski, M.B.; Torres, K. Clinical Value of Detecting Tumor Endothelial Marker 8 (ANTXR1) as a Biomarker in the Diagnosis and Prognosis of Colorectal Cancer. Cancer Manag. Res. 2021, 13, 3113–3122. [Google Scholar] [CrossRef]
- Peng, Q.; Zhang, X.; Min, M.; Zou, L.; Shen, P.; Zhu, Y. The Clinical Role of Microrna-21 as a Promising Biomarker in the Diagnosis and Prognosis of Colorectal Cancer: A Systematic Review and Meta-Analysis. Oncotarget 2017, 8, 44893–44909. [Google Scholar] [CrossRef] [Green Version]
- He, T.; Zhang, M.; Zheng, R.; Zheng, S.; Linghu, E.; Herman, J.G.; Guo, M. Methylation of SLFN11 Is a Marker of Poor Prognosis and Cisplatin Resistance in Colorectal Cancer. Epigenomics 2017, 9, 849–862. [Google Scholar] [CrossRef] [Green Version]
- Hsu, C.-H.; Hsiao, C.-W.; Sun, C.-A.; Wu, W.-C.; Yang, T.; Hu, J.-M.; Liao, Y.-C.; Huang, C.-H.; Chen, C.-Y.; Lin, F.-H.; et al. Multiple Gene Promoter Methylation and Clinical Stage in Adjacent Normal Tissues: Effect on Prognosis of Colorectal Cancer in Taiwan. Sci. Rep. 2020, 10, 145. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, D.R.; Patel, R.; Kirsch, D.G.; Lewis, C.A.; Vander Heiden, M.G.; Locasale, J.W. Metabolomics in Cancer Research and Emerging Applications in Clinical Oncology. CA Cancer J. Clin. 2021, 71, 333–358. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, Y.; Zhao, W.; Deng, K.; Wang, Z.; Yang, C.; Ma, L.; Openkova, M.S.; Hou, Y.; Li, K. Metabolomics for Biomarker Discovery in the Diagnosis, Prognosis, Survival and Recurrence of Colorectal Cancer: A Systematic Review. Oncotarget 2017, 8, 35460–35472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ose, J.; Gigic, B.; Brezina, S.; Lin, T.; Baierl, A.; Geijsen, A.J.; van Roekel, E.; Robinot, N.; Gicquiau, A.; Achaintre, D.; et al. Targeted plasma metabolic profiles and risk of recurrence in Stage ii and III Colorectal Cancer Patients: Results from an International Cohort Consortium. Metabolites 2021, 11, 129. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.D.; Yang, D.; Sunakawa, Y.; Zhang, W.; Ning, Y.; Matsusaka, S.; Okazaki, S.; Miyamoto, Y.; Suenaga, M.; Schirripa, M.; et al. Impact of Sex, Age, and Ethnicity/Race on the Survival of Patients with Rectal Cancer in the United States from 1988 to 2012. Oncotarget 2016, 7, 53668–53678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abancens, M.; Bustos, V.; Harvey, H.; McBryan, J.; Harvey, B.J. Sexual Dimorphism in Colon Cancer. Front. Oncol. 2020, 10, 607909. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, F.; Tomasello, G.; Borgonovo, K.; Ghidini, M.; Turati, L.; Dallera, P.; Passalacqua, R.; Sgroi, G.; Barni, S. Prognostic Survival Associated with Left-Sided vs Right-Sided Colon Cancer. JAMA Oncol. 2017, 3, 211. [Google Scholar] [CrossRef]
- Hansen, I.O.; Jess, P. Possible better long-term survival in left versus right-sided colon cancer—A systematic review. Dan. Med. J. 2012, 59, A4444. [Google Scholar]
- Majek, O.; Gondos, A.; Jansen, L.; Emrich, K.; Holleczek, B.; Katalinic, A.; Nennecke, A.; Eberle, A.; Brenner, H. Sex Differences in Colorectal Cancer Survival: Population-Based Analysis of 164,996 Colorectal Cancer Patients in Germany. PLoS ONE 2013, 8, e68077. [Google Scholar] [CrossRef] [Green Version]
- Cai, Y.; Rattray, N.J.; Zhang, Q.; Mironova, V.; Santos-Neto, A.; Hsu, K.-S.; Rattray, Z.; Cross, J.R.; Zhang, Y.; Paty, P.B.; et al. Sex Differences in Colon Cancer Metabolism Reveal a Novel Subphenotype. Sci. Rep. 2020, 10, 4905. [Google Scholar] [CrossRef] [Green Version]
- Richards, N.G.; Kilberg, M.S. Asparagine Synthetase Chemotherapy. Annu. Rev. Biochem. 2006, 75, 629–654. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Fan, J.; Venneti, S.; Cross, J.R.; Takagi, T.; Bhinder, B.; Djaballah, H.; Kanai, M.; Cheng, E.H.; Judkins, A.R.; et al. Asparagine Plays a Critical Role in Regulating Cellular Adaptation to Glutamine Depletion. Mol. Cell 2014, 56, 205–218. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.H.; Chung, Y.; Cheng, C.-T.; Ouyang, C.; Fu, Y.; Kuo, C.-Y.; Chi, K.K.; Sadeghi, M.; Chu, P.; Kung, H.-J.; et al. Autophagic Reliance Promotes Metabolic Reprogramming in Oncogenic Kras-Driven Tumorigenesis. Autophagy 2018, 14, 1481–1498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krall, A.S.; Xu, S.; Graeber, T.G.; Braas, D.; Christofk, H.R. Asparagine Promotes Cancer Cell Proliferation through Use as an Amino Acid Exchange Factor. Nat. Commun. 2016, 7, 11457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knott, S.R.; Wagenblast, E.; Khan, S.; Kim, S.Y.; Soto, M.; Wagner, M.; Turgeon, M.-O.; Fish, L.; Erard, N.; Gable, A.L.; et al. Asparagine bioavailability governs metastasis in a model of breast cancer. Nature 2018, 554, 378–381. [Google Scholar] [CrossRef]
- Du, F.; Chen, J.; Liu, H.; Cai, Y.; Cao, T.; Han, W.; Yi, X.; Qian, M.; Tian, D.; Nie, Y.; et al. Sox12 promotes colorectal cancer cell proliferation and metastasis by regulating asparagine synthesis. Cell Death Dis. 2019, 10, 239. [Google Scholar] [CrossRef] [PubMed]
- Toda, K.; Kawada, K.; Iwamoto, M.; Inamoto, S.; Sasazuki, T.; Shirasawa, S.; Hasegawa, S.; Sakai, Y. Metabolic alterations caused by KRAS mutations in colorectal cancer contribute to cell adaptation to glutamine depletion by upregulation of asparagine synthetase. Neoplasia 2016, 18, 654–665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gwinn, D.M.; Lee, A.G.; Briones-Martin-del-Campo, M.; Conn, C.S.; Simpson, D.R.; Scott, A.I.; Le, A.; Cowan, T.M.; Ruggero, D.; Sweet-Cordero, E.A. Oncogenic kras regulates amino acid homeostasis and asparagine biosynthesis via ATF4 and alters sensitivity to L-asparaginase. Cancer Cell 2018, 33, 91–107. [Google Scholar] [CrossRef] [Green Version]
- Wong, C.C.; Qian, Y.; Li, X.; Xu, J.; Kang, W.; Tong, J.H.; To, K.-F.; Jin, Y.; Li, W.; Chen, H.; et al. SLC25A22 promotes proliferation and survival of colorectal cancer cells with KRAS mutations and xenograft tumor progression in mice via intracellular synthesis of aspartate. Gastroenterology 2016, 151, 945–960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Chung, A.C.K.; Li, S.; Wu, L.; Xu, J.; Yu, J.; Wong, C.; Cai, Z. LC-MS-based metabolomics revealed slc25a22 as an essential regulator of aspartate-derived amino acids and polyamines in KRAS-mutant colorectal cancer. Oncotarget 2017, 8, 101333–101344. [Google Scholar] [CrossRef] [Green Version]
- Hinze, L.; Labrosse, R.; Degar, J.; Han, T.; Schatoff, E.M.; Schreek, S.; Karim, S.; McGuckin, C.; Sacher, J.R.; Wagner, F.; et al. Exploiting the therapeutic interaction of Wnt pathway activation and asparaginase for colorectal cancer therapy. Cancer Discov. 2020, 10, 1690–1705. [Google Scholar] [CrossRef]
- Chiu, M.; Taurino, G.; Bianchi, M.G.; Kilberg, M.S.; Bussolati, O. Asparagine synthetase in Cancer: Beyond acute lymphoblastic leukemia. Front. Oncol. 2020, 9, 1480. [Google Scholar] [CrossRef]
- Montrose, D.C.; Saha, S.; Foronda, M.; McNally, E.M.; Chen, J.; Zhou, X.K.; Ha, T.; Krumsiek, J.; Buyukozkan, M.; Verma, A.; et al. Exogenous and endogenous sources of serine contribute to colon cancer metabolism, growth, and resistance to 5-Fluorouracil. Cancer Res. 2021, 81, 2275–2288. [Google Scholar] [CrossRef] [PubMed]
- Grant, G.A. D-3-phosphoglycerate dehydrogenase. Front. Mol. Biosci. 2018, 5, 110. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Kim, J.G.; Seo, A.N.; Park, S.Y.; Kim, H.J.; Park, J.S.; Choi, G.S.; Jeong, J.Y.; Jun, D.Y.; Yoon, G.S.; et al. Clinical implication of serine metabolism-associated enzymes in colon cancer. Oncology 2015, 89, 351–359. [Google Scholar] [CrossRef]
- Bednarz-Misa, I.; Fleszar, M.G.; Zawadzki, M.; Kapturkiewicz, B.; Kubiak, A.; Neubauer, K.; Witkiewicz, W.; Krzystek-Korpacka, M. L-arginine/no pathway metabolites in colorectal cancer: Relevance as disease biomarkers and predictors of adverse clinical outcomes following surgery. J. Clin. Med. 2020, 9, 1782. [Google Scholar] [CrossRef] [PubMed]
- Curis, E.; Nicolis, I.; Moinard, C.; Osowska, S.; Zerrouk, N.; Bénazeth, S.; Cynober, L. Almost all about citrulline in mammals. Amino Acids 2005, 29, 177–205. [Google Scholar] [CrossRef] [PubMed]
- Pegg, A.E. Regulation of ornithine decarboxylase. J. Biol. Chem. 2006, 281, 14529–14532. [Google Scholar] [CrossRef] [Green Version]
- Laukaitis, C.M.; Gerner, E.W. DFMO: Targeted risk reduction therapy for colorectal neoplasia. Best Pract. Res. Clin. Gastroenterol. 2011, 25, 495–506. [Google Scholar] [CrossRef] [Green Version]
- S0820, Adenoma and Second Primary Prevention Trial. Available online: https://clinicaltrials.gov/ct2/show/NCT01349881 (accessed on 13 October 2021).
- Wanders, D.; Hobson, K.; Ji, X. Methionine restriction and Cancer Biology. Nutrients 2020, 12, 684. [Google Scholar] [CrossRef] [Green Version]
- Ruoppolo, M.; Scolamiero, E.; Caterino, M.; Mirisola, V.; Franconi, F.; Campesi, I. Female and male human babies have distinct blood metabolomic patterns. Mol. Biosyst. 2015, 11, 2483–2492. [Google Scholar] [CrossRef]
- Bell, J.A.; Santos Ferreira, D.L.; Fraser, A.; Soares, A.L.; Howe, L.D.; Lawlor, D.A.; Carslake, D.; Davey Smith, G.; O’Keeffe, L.M. Sex differences in systemic metabolites at four life stages: Cohort Study with repeated metabolomics. BMC Med. 2021, 19, 58. [Google Scholar] [CrossRef]
- Clocchiatti, A.; Cora, E.; Zhang, Y.; Dotto, G.P. Sexual dimorphism in cancer. Nat. Rev. Cancer 2016, 16, 330–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kovács, T.; Szabó-Meleg, E.; Ábrahám, I.M. Estradiol-induced epigenetically mediated mechanisms and regulation of gene expression. Int. J. Mol. Sci. 2020, 21, 3177. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Unno, T.; Kim, B.-Y.; Park, M.-S. Sex differences in gut microbiota. World J. Men’s Health 2020, 38, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Pei, L.; Xia, H.; Tang, Q.; Bi, F. Role of oncogenic kras in the prognosis, diagnosis and treatment of colorectal cancer. Mol. Cancer 2021, 20, 143. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Garcia, E.; Argiles, G.; Elez, E.; Tabernero, J. BRAF mutant colorectal cancer: Prognosis, treatment, and New Perspectives. Ann. Oncol. 2017, 28, 2648–2657. [Google Scholar] [CrossRef]
- Popat, S.; Hubner, R.; Houlston, R.S. Systematic review of microsatellite instability and colorectal cancer prognosis. J. Clin. Oncol. 2005, 23, 609–618. [Google Scholar] [CrossRef]
| Characteristics | No. of Patients | 5-Year OS | 5-Year RFS | |||||
|---|---|---|---|---|---|---|---|---|
| Deaths, No. | Rate, % a | pb | Cases, No. | Rate, % a | pb | |||
| Age at diagnosis, y | ≤60 | 19 | 2 | 88.9 | 0.048 | 3 | 84.2 | 0.21 |
| 61–69 | 64 | 9 | 83.4 | 14 | 72.4 | |||
| 70–79 | 81 | 15 | 78.7 | 12 | 81.4 | |||
| ≥80 | 33 | 11 | 61.5 | 1 | 96.3 | |||
| Sex, n | Male | 102 | 23 | 74.3 | 0.18 | 17 | 77.5 | 0.48 |
| Female | 95 | 14 | 83.2 | 13 | 84.0 | |||
| Clinical stage, n | I | 47 | 3 | 92.5 | 0.001 | 5 | 88.4 | 0.09 |
| II | 86 | 13 | 82.4 | 11 | 82.0 | |||
| III | 64 | 21 | 63.5 | 14 | 73.8 | |||
| Chemotherapy, n | Yes | 66 | 18 | 68.5 | 0.03 | 15 | 73.1 | 0.03 |
| No | 131 | 19 | 83.7 | 15 | 85.0 | |||
| Anatomic tumor location, n | Left | 99 | 17 | 81.2 | 0.42 | 19 | 77.2 | 0.23 |
| Right | 98 | 20 | 75.6 | 11 | 85.3 | |||
| Prognosis | Sex | Model 1 a | Model 2 b | ||||
|---|---|---|---|---|---|---|---|
| HR (95% CI) c | p | pinteractiond | HR (95% CI) c | p | pinteractiond | ||
| OS | Females | 6.39 (1.78–22.91) | 0.004 | 0.02 | 5.68 (1.06–30.61) | 0.04 | 0.052 |
| Males | 0.57 (0.36–0.91) | 0.02 | 0.46 (0.11–1.84) | 0.27 | |||
| RFS | Females | 4.36 (1.39–3.68) | 0.01 | 0.003 | 4.89 (1.07–22.39) | 0.04 | 0.03 |
| Males | 0.96 (0.61–1.50) | 0.86 | 1.15 (0.27–4.80) | 0.85 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, X.; Cai, Y.; Lu, L.; Huang, H.; Yan, H.; Paty, P.B.; Muca, E.; Ahuja, N.; Zhang, Y.; Johnson, C.H.; et al. Asparagine Metabolism in Tumors Is Linked to Poor Survival in Females with Colorectal Cancer: A Cohort Study. Metabolites 2022, 12, 164. https://doi.org/10.3390/metabo12020164
Shen X, Cai Y, Lu L, Huang H, Yan H, Paty PB, Muca E, Ahuja N, Zhang Y, Johnson CH, et al. Asparagine Metabolism in Tumors Is Linked to Poor Survival in Females with Colorectal Cancer: A Cohort Study. Metabolites. 2022; 12(2):164. https://doi.org/10.3390/metabo12020164
Chicago/Turabian StyleShen, Xinyi, Yuping Cai, Lingeng Lu, Huang Huang, Hong Yan, Philip B. Paty, Engjel Muca, Nita Ahuja, Yawei Zhang, Caroline H. Johnson, and et al. 2022. "Asparagine Metabolism in Tumors Is Linked to Poor Survival in Females with Colorectal Cancer: A Cohort Study" Metabolites 12, no. 2: 164. https://doi.org/10.3390/metabo12020164
APA StyleShen, X., Cai, Y., Lu, L., Huang, H., Yan, H., Paty, P. B., Muca, E., Ahuja, N., Zhang, Y., Johnson, C. H., & Khan, S. A. (2022). Asparagine Metabolism in Tumors Is Linked to Poor Survival in Females with Colorectal Cancer: A Cohort Study. Metabolites, 12(2), 164. https://doi.org/10.3390/metabo12020164








