Identification of New Natural Sources of Flavour and Aroma Metabolites from Solid-State Fermentation of Agro-Industrial By-Products
Abstract
1. Introduction
2. Results
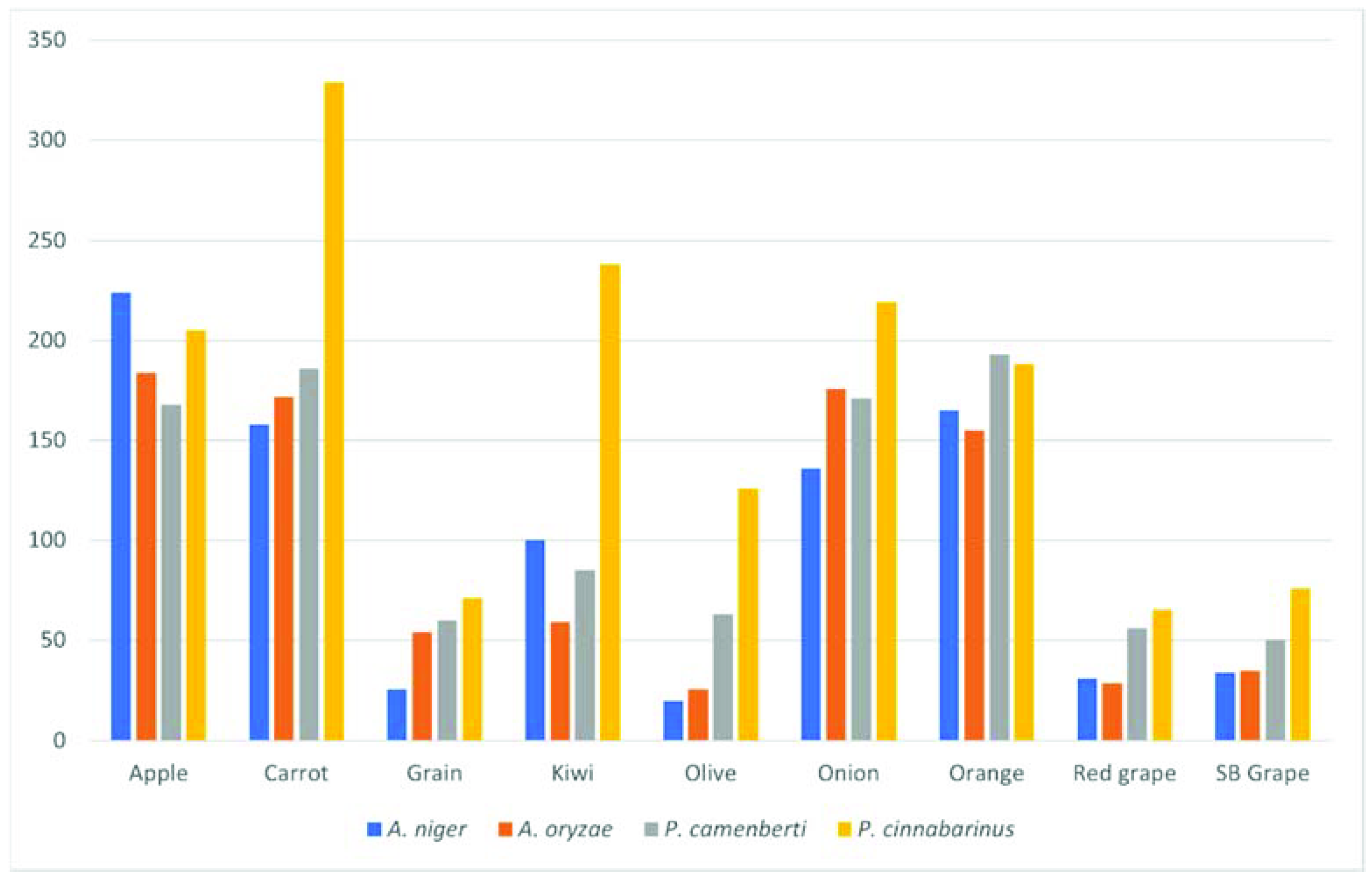
2.1. Solid-State Fermentation Performance
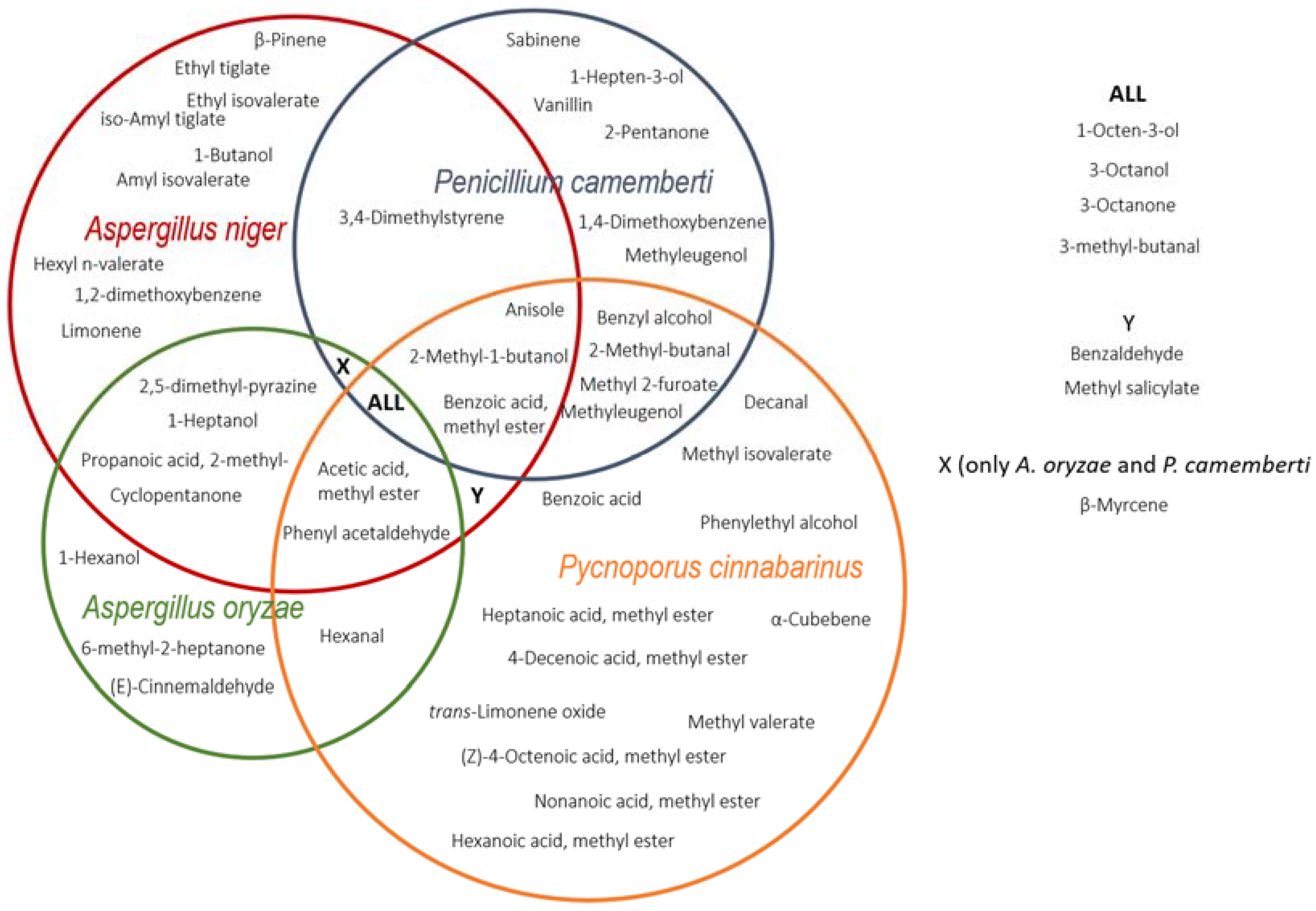
2.2. Profile of Volatile Compounds
3. Discussion
4. Materials and Methods
4.1. Microorganisms
4.2. Solid-State Fermentations
4.3. Headspace Solid-Phase Microextraction (HS-SPME) Coupled to Gas Chromatography–Mass Spectrometry (GC–MS)
4.4. SPME-GCMS Data Processing
4.5. Identification Confirmation and Quantitation of Volatile Compounds
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Compound | Relative Slope of Regression Line a (n ≥ 4) | Intercept of Regression Line a (n ≥ 4) | Coefficient (r2) | Linear Range (μM) |
|---|---|---|---|---|
| Methyl benzoate | 13.819 | 0.0021 | >0.99 | 0.5–7.0 |
| Phenylacetaldehyde | 30.800 | 0.0931 | >0.99 | 10.0–70.0 |
| 1-octen-3-ol | 30.906 | 0.0744 | >0.99 | 10.0–70.0 |
| Phenethyl alcohol | 33.867 | 0.1537 | >0.99 | 10.0–70.0 |
References
- Berger, R.G. Flavours and Fragrances: Chemistry, Bioprocessing and Sustainability I; Springer-Verlag: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Button, J.E.; Dutton, R.J. Cheese microbes. Curr. Biol. 2012, 22, 587–589. [Google Scholar] [CrossRef] [PubMed]
- Goddard, M.R. Microbiology: Mixing wine, chocolate, and coffee. Curr. Biol. Dispatch 2016, 26, R275–R277. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hugenholtz, J. Traditional biotechnology for new foods and beverages. Curr. Opin. Biotechnol. 2013, 24, 155–159. [Google Scholar] [CrossRef]
- Christen, P.; Meza, J.C.; Revah, S. Fruity aroma production in solid state fermentation by Ceratocystis fimbriata: Influence of the substrate type and the presence of precursors. Mycol. Res. 1997, 101, 911–919. [Google Scholar] [CrossRef]
- Christen, P.; Bramorski, A.; Revah, S.; Soccol, C.R. Characterization of volatile compounds produced by Rhizopus strains grown on agro-industrial solid wastes. Bioresour. Technol. 2000, 71, 211–215. [Google Scholar] [CrossRef]
- Soares, M.; Christen, P.; Pandey, A.; Soccol, C.R. Fruity flavour production by Ceratocystis fimbriata grown on coffee husk in solid-state fermentation. Proc. Biochem. 2000, 35, 857–861. [Google Scholar] [CrossRef]
- Zheng, L.; Zheng, P.; Sun, Z.; Bai, Y.; Wang, J.; Guo, X. Production of vanillin from waste residue of rice bran oil by Aspergillus niger and Pycnoporus cinnabarinus. Bioresour. Technol. 2007, 98, 1115–1119. [Google Scholar] [CrossRef]
- Rossi, S.C.; Vandenberghe, L.P.S.; Pereira, B.M.P.; Gago, F.D.; Rizzolo, J.A.; Pandey, A.; Soccol, C.R.; Medeiros, A.B.P. Improving fruity aroma production by fungi in SSF using citric pulp. Food Res. Int. 2009, 42, 484–486. [Google Scholar] [CrossRef]
- Hanem, H.; Fadel, M.; Gomaa Mahmoud, M.; Mohamed, M.; Asker, S.; Lotfy, S.N. Characterization and evaluation of coconut aroma produced by Trichoderma viride EMCC-107 in solid state fermentation on sugarcane bagasse. EJBT 2015, 18, 5–9. [Google Scholar] [CrossRef]
- Madrera, R.R.; Bedriñana, R.P.; Valles, B.S. Production and characterization of aroma compounds from apple pomace by solid-state fermentation with selected yeasts. LWT-Food Sci. Technol. 2015, 64, 1342–1353. [Google Scholar] [CrossRef]
- Mantzouridou, F.T.; Paraskevopoulou, A.; Lalou, S. Yeast flavour production by solid state fermentation of orange peel waste. Biochem. Eng. J. 2015, 101, 1–8. [Google Scholar] [CrossRef]
- Castilho, L.R.; Mitchell, D.A.; Freire, D.M.G. Production of polyhydroxyalkanoates (PHAs) from waste materials and by-products by submerged and solid-state fermentation. Biores Technol. 2009, 100, 5996–6009. [Google Scholar] [CrossRef] [PubMed]
- Soccol, C.R.; Ferreira Da Costa, E.S.; Letti, A.J.; Karp, S.G.; Woiciechowski, A.L.; Porto De Souza Vandenberghe, L. Recent developments and innovations in solid state fermentation. Biotechnol. Res. Innov. 2017, 1, 52–71. [Google Scholar] [CrossRef]
- Etschmann, M.; Bluemke, W.; Sell, D.; Schrader, J. Biotechnological production of 2-phenylethanol. Appl. Microbiol. Biotechnol. 2002, 59, 1–8. [Google Scholar] [CrossRef] [PubMed]
- George, B.A. Fernaroli’s Handbook of Flavor Ingredients, 5th ed.; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Baydar, H. Oil-bearing rose (Rosa damascena Mill.) Cultivation and rose oil industry in Turkey. Ind. Crops Prod. 2006, 14, 13–17. [Google Scholar]
- Murahashi, S. The odor of matsutake (Armillaria matsutake Ito er Imai) II. Sci. Pap. Inst. Phys. Chem Res. 1938, 34, 155–172. [Google Scholar]
- Maga, J.A. Mushroom flavor. J. Am. Chem. Soc. 1981, 29, 1–4. [Google Scholar] [CrossRef]
- Kaminski, E.; Stawicki, S.; Wasowicz, E. Volatile flavor compounds produced by molds of Aspergillus, Penicillium, and Fungi imperfecti. Appl. Microbiol. 1974, 27, 1001–1004. [Google Scholar] [CrossRef]
- Borjesson, T.; Stollman, U.; Schnurer, J. Volatile metabolites produced by six fungal species compared with other indicators of fungal growth on cereal grains. Appl. Environ. Microbiol. 1992, 58, 2599–2605. [Google Scholar] [CrossRef]
- Zawirska-Wojtasiak, R. Optical purity of (R)-(−)-1-octen-3-ol in the aroma of various species of edible mushrooms. Food Chem. 2004, 86, 113–118. [Google Scholar] [CrossRef]
- Husson, F.; Krumov, K.N.; Cases, E.; Cayot, P.; Bisakowski, B.; Kermasha, S.; Belin, J.-M. Influence of medium composition and structure on the biosynthesis of the natural flavour 1-octen-3-ol by Penicillium camemberti. Proc. Biochem. 2005, 40, 1395–1400. [Google Scholar] [CrossRef]
- De Carvalho, D.S.; Dionísio, A.P.; Dos Santos, R.; Boguzs, S.; Godoy, H.T.; Pastore, G.M. Production of 1-octen-3-ol by Neurospora species isolated from beiju in different culture medium. Ital. Oral Surg. 2011, 1, 1694–1699. [Google Scholar] [CrossRef]
- Van Leuven, I.; Van Caelenberg, T.; Dirinck, P. Aroma characterisation of Gouda-type cheeses. Int. Dairy J. 2008, 18, 790–800. [Google Scholar] [CrossRef]
- San Juan, F.; Cacho, J.; Ferreira, V.; Escudero, A. Differences in chemical composition of aroma among red wines of different price category. In Flavour Science; Ferreira, V., Lopez, R., Eds.; Elsevier Inc: Berlin, Germany, 2013; pp. 117–122. [Google Scholar] [CrossRef]
- Crafack, M.; Keul, H.; Eskildsen, C.E.; Petersen, M.A.; Saerens, S.; Blennow, A.; Skovmand-Larsen, M.; Swiegers, J.H.; Petersen, G.B.; Heimdal, H.; et al. Impact of starter cultures and fermentation techniques on the volatile aroma and sensory profile of chocolate. Food Res. Int. 2014, 63, 306–316. [Google Scholar] [CrossRef]
- Sunarharum, W.B.; Williams, D.J.; Smyth, H.E. Complexity of coffee flavor: A compositional and sensory perspective. FRIN 2014, 62, 315–325. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, L.; Ali, N.S.; Cano-Lamadrid, M.; Noguera-Artiaga, L.; Lipan, L.; Carbonell-Barrachina, Á.A.; Sendra, E. Flavors and aromas. In Postharvest Physiology and Biochemistry of Fruits and Vegetables; Yahia, E.M., Ed.; Woodhead Publishing: Cambridge, UK, 2018; pp. 385–404. [Google Scholar] [CrossRef]
- Agosin, E.; Odier, E. Solid-state fermentation, lignin degradation and resulting digestibility of wheat straw fermented by selected white-rot fungi. Appl. Microbiol. Biotechnol. 1985, 21, 397–403. [Google Scholar] [CrossRef]
- Rahouti, M.; Seigle-Murandi, F.; Steiman, R.; Eriksson, K.E. Metabolism of ferulic acid by Paecilomyces variotii and Pestalotia palmarum. Appl. Environ. Microbiol. 1989, 55, 2391–2398. [Google Scholar] [CrossRef]
- Tillett, R.; Walker, J.R.L. Metabolism of ferulic acid by a Penicillium sp. Arch. Microbiol. 1990, 154, 206–208. [Google Scholar] [CrossRef]
- Lesage-Meessen, L.; Delattre, M.; Haon, M.; Thibault, J.F.; Ceccaldi, B.C.; Brunerie, P.; Asther, M. A two-step bioconversion process for vanillin production from ferulic acid combining Aspergillus niger and Pycnoporus cinnabarinus. J. Biotechnol. 1996, 50, 107–113. [Google Scholar] [CrossRef]
- Martín García, A.I.; Moumen, A.; Yáñez Ruiz, D.R.; Molina Alcaide, E. Chemical composition and nutrients availability for goats and sheep of two-stage olive cake and olive leaves. Anim. Feed Sci. Technol. 2003, 107, 61–74. [Google Scholar] [CrossRef]
- Alu’datt, M.H.; Alli, I.; Ereifej, K.; Alhamad, M.; Al-Tawaha, A.R.; Rababah, T. Optimisation, characterisation and quantification of phenolic compounds in olive cake. Food Chem. 2010, 123, 117–122. [Google Scholar] [CrossRef]
- Bello, M.O.; Olabanji, I.O.; Abdul-Hammed, M.; Okunade, T.D. Characterization of domestic onion wastes and bulb (Allium cepa L.): Fatty acids and metal contents. Int. Food Res. J. 2013, 20, 2153–2158. [Google Scholar]
- Lawall, C.H.; Forman, L. The analysis of vanilla extract. J. Am. Pharma Assoc. 1914, 3, 25–28. [Google Scholar] [CrossRef]
- Walton, N.J.; Mayer, M.J.; Narbad, A. Molecules of interest vanillin. Phytochem 2003, 63, 505–515. [Google Scholar] [CrossRef]
| Compound | Substrate | Microorganism | Fold-Change a | Descriptor b |
|---|---|---|---|---|
| 1-Butanol | Apple pomace | A. niger | 3 | Oily-banana |
| 2-methyl-1-butanol | Onion pulp | A. niger | 2 | Black truffle |
| Onion pulp | P. camemberti | 191 | ||
| Onion pulp | P. cinnabarinus | 6 | ||
| Orange pomace | P. camemberti | 28 | ||
| 1-Heptanol | Brewer’s spent grain | A. oryzae | 60 | Pleasant, cosmetic |
| Brewer’s spent grain | A. niger | 10 | ||
| 1-Hepten-3-ol | Onion pulp | P. camemberti | 165 | Oily, green, metallic |
| 1-Hexanol | Brewer’s spent grain | A. oryzae | 7 | Fresh cut grass |
| 1-Octen-3-ol | Brewer’s spent grain | P. camemberti | 24 | Mushroom alcohol |
| Brewer’s spent grain | P. cinnabarinus | 19 | ||
| Kiwifruit peels | A. oryzae | 31 | ||
| Kiwifruit peels | A. niger | 29 | ||
| Kiwifruit peels | P. cinnabarinus | 68 | ||
| Kiwifruit peels | P. camemberti | 33 | ||
| Onion pulp | A. niger | 87 | ||
| Onion pulp | A. oryzae | 38 | ||
| Onion pulp | P. camemberti | 3118 | ||
| Onion pulp | P. cinnabarinus | 1555 | ||
| 6-methyl-2-heptanone | Brewer’s spent grain | A. oryzae | 54 | Camphorous |
| 2-Pentanone | Apple pomace | P. camemberti | 8 | Fruity |
| 3-Octanol | Carrot pomace | A. oryzae | 2 | Mushroom, herbal, citrus |
| Carrot pomace | P. cinnabarinus | 163 | ||
| Brewer’s spent grain | A. niger | 109 | ||
| Brewer’s spent grain | P. cinnabarinus | 261 | ||
| Brewer’s spent grain | P. camemberti | 100 | ||
| Kiwifruit peels | P. cinnabarinus | 84 | ||
| Kiwifruit peels | P. camemberti | 5 | ||
| Onion pulp | A. niger | 423 | ||
| Onion pulp | A. oryzae | 705 | ||
| Onion pulp | P. camemberti | 43 | ||
| Onion pulp | P. cinnabarinus | 182 | ||
| Red grape marc | A. niger | 48 | ||
| Red grape marc | A. oryzae | 215 | ||
| White grape marc | A. niger | 50 | ||
| White grape marc | A. oryzae | 102 | ||
| 3-Octanone | Brewer’s spent grain | A. niger | 26 | Herbal, lavender, nectarine |
| Brewer’s spent grain | P. camemberti | 221 | ||
| Brewer’s spent grain | P. cinnabarinus | 20 | ||
| Kiwifruit peels | A. oryzae | 9 | ||
| Kiwifruit peels | A. niger | 4 | ||
| Kiwifruit peels | P. cinnabarinus | 14 | ||
| Kiwifruit peels | P. camemberti | 5 | ||
| Onion pulp | A. niger | 224 | ||
| Onion pulp | A. oryzae | 193 | ||
| Onion pulp | P. camemberti | 220 | ||
| Onion pulp | P. cinnabarinus | 85 | ||
| Red grape marc | A. oryzae | 58 | ||
| Red grape marc | A. niger | 30 | ||
| White grape marc | A. niger | 20 | ||
| White grape marc | A. oryzae | 25 | ||
| Methyl dec-4-enoate | Apple pomace | P. cinnabarinus | 112 | Tropical, fishy |
| Carrot pomace | P. cinnabarinus | 354 | ||
| Methyl oct-4-enoate | Carrot pomace | P. cinnabarinus | 207 | Fresh pineapple |
| Methyl acetate | Apple pomace | A. niger | 8 | Ethereal, sweet, fruity |
| Apple pomace | A. oryzae | 5 | ||
| Carrot pomace | P. cinnabarinus | 261 | ||
| Carrot pomace | P. cinnabarinus | 195 | ||
| Orange pomace | A. niger | 65 | ||
| Orange pomace | A. oryzae | 14 | ||
| α.-Cubebene | Apple pomace | P. cinnabarinus | 61 | Herbal |
| Amyl isovalerate | Apple pomace | A. niger | 71 | Fruity |
| Anisole | Brewer’s spent grain | P. camemberti | 8 | Aniseed |
| Brewer’s spent grain | P. cinnabarinus | 786 | ||
| Orange pomace | A. niger | 21 | ||
| Orange pomace | P. cinnabarinus | 351 | ||
| Benzaldehyde | Brewer’s spent grain | P. cinnabarinus | 2 | Cherry, almond |
| Orange pomace | A. niger | 2 | ||
| 1,2-dimethoxybenzene | Orange pomace | A. niger | 92 | Insect attractant |
| 1,4-Dimethoxybenzene | Onion pulp | P. camemberti | 164 | Intense sweet, floral |
| 3,4-Dimethylstyrene | Orange pomace | A. niger | 150 | Green, floral, smoky |
| Orange pomace | P. camemberti | 245 | ||
| Benzoic acid | Carrot pomace | P. cinnabarinus | 272 | Balsamic |
| Kiwifruit peels | P. cinnabarinus | 29 | ||
| Onion pulp | P. cinnabarinus | 138 | ||
| Methyl benzoate | Orange pomace | P. camemberti | 16 | Feijoa, ylang ylang, wintergreen |
| Orange pomace | P. cinnabarinus | 33 | ||
| Orange pomace | A. niger | 27 | ||
| Benzyl alcohol | Brewer’s spent grain | P. camemberti | 31 | Precursor and solvent |
| Brewer’s spent grain | P. cinnabarinus | 17 | ||
| β-Pinene | Kiwifruit peels | A. niger | 2 | Herbal, pine |
| β-Myrcene | Apple pomace | A. oryzae | 2 | Clove-like |
| Kiwifruit peels | P. camemberti | 2 | ||
| Sabinene | Brewer’s spent grain | P. camemberti | 8 | Spicy, black pepper |
| 2-methylbutanal | Brewer’s spent grain | P. camemberti | 15 | Musty, chocolate |
| Brewer’s spent grain | P. cinnabarinus | 13 | ||
| 3-methylbutanal | Carrot pomace | P. cinnabarinus | 61 | Peach, malty, fatty, chocolate, peach |
| Kiwifruit peels | P. cinnabarinus | 33 | ||
| Kiwifruit peels | P. camemberti | 4 | ||
| Onion pulp | A. oryzae | 15 | ||
| Onion pulp | A. niger | 8 | ||
| Brewer’s spent grain | A. niger | 11 | ||
| Brewer’s spent grain | A. oryzae | 10 | ||
| Onion pulp | A. oryzae | 307 | ||
| Onion pulp | A. niger | 53 | ||
| Ethyl isovalerate | Apple pomace | A. niger | 210 | Fruity |
| (E)-Cinnemaldehyde | Red grape marc | A. oryzae | 40 | Cinnamon |
| Cyclopentanone | Kiwifruit peels | A. oryzae | 37 | Minty |
| Kiwifruit peels | A. niger | 17 | ||
| Decanal | Olive cake | P. cinnabarinus | 5 | Citrus |
| (D)-Limonene | Carrot pomace | A. niger | 6 | Citrus |
| Ethyl tiglate | Red grape marc | A. niger | 129 | Tutti frutti, green olive |
| Methyl heptanoate | Carrot pomace | P. cinnabarinus | 106 | Fruity, green, waxy |
| Hexanal | Brewer’s spent grain | A. oryzae | 3 | Fresh cut grass |
| Kiwifruit peels | P. cinnabarinus | 4 | ||
| Olive cake | P. cinnabarinus | 10 | ||
| Methyl hexanoate | Apple pomace | P. cinnabarinus | 133.85 | Pineapple, fatty |
| Carrot pomace | P. cinnabarinus | 75.89 | ||
| Orange pomace | P. cinnabarinus | 17.40 | ||
| Hexyl valerate | Apple pomace | A. niger | 1.49 | Green, brandy |
| iso-Amyl tiglate | Apple pomace | A. niger | 21.04 | herbal |
| trans-Limonene oxide | Olive cake | P. cinnabarinus | 36.57 | Minty, citrus |
| Methyl 2-furoate | Apple pomace | P. cinnabarinus | 130.75 | Caramel, musty, fungal |
| Carrot pomace | P. cinnabarinus | 624.14 | ||
| Brewer’s spent grain | P. camemberti | 11.04 | ||
| Brewer’s spent grain | P. cinnabarinus | 687.33 | ||
| Kiwifruit peels | P. cinnabarinus | 588.31 | ||
| Kiwifruit peels | P. camemberti | 7.72 | ||
| Olive cake | P. cinnabarinus | 138.46 | ||
| Onion pulp | P. camemberti | 17.89 | ||
| Onion pulp | P. cinnabarinus | 816.65 | ||
| Orange pomace | P. cinnabarinus | 58.68 | ||
| Methyl isovalerate | Kiwifruit peels | P. cinnabarinus | 1294.67 | Fruity |
| Onion pulp | P. cinnabarinus | 139.85 | ||
| Red grape marc | P. cinnabarinus | 395.00 | ||
| Methyl salicylate | Orange pomace | A. niger | 15.49 | Wintergreen mint, root beer |
| Orange pomace | P. cinnabarinus | 16.38 | ||
| Methyl valerate | Carrot pomace | P. cinnabarinus | 44.37 | Sweet, fruity |
| Methyleugenol | Apple pomace | P. camemberti | 845.19 | Spicy, clove |
| Apple pomace | P. cinnabarinus | 41.88 | ||
| Orange pomace | P. camemberti | 48.28 | ||
| Methyl nonanoate | Carrot pomace | P. cinnabarinus | 90.73 | Pear, tropical, waxy |
| Phenyl acetaldehyde | Carrot pomace | A. niger | 3.15 | Honey, floral |
| Carrot pomace | A. oryzae | 1.52 | ||
| Brewer’s spent grain | A. oryzae | 3.47 | ||
| Brewer’s spent grain | P. cinnabarinus | 77.04 | ||
| Olive cake | P. cinnabarinus | 5.73 | ||
| Phenylethyl alcohol | Brewer’s spent grain | P. cinnabarinus | 77.04 | Rose |
| 2-methylpropanoate | Carrot pomace | A. oryzae | 187.92 | Rancid butter |
| Carrot pomace | A. niger | 62.03 | ||
| 2,5-Dimethylpyrazine | Carrot pomace | A. niger | 3.68 | Nutty, musty |
| Carrot pomace | A. oryzae | 2.95 | ||
| Onion pulp | A. niger | 2.83 | ||
| Vanillin | Olive cake | P. camemberti | 1.14 | Vanilla |
| Apple Pomace | Brewer’s Spent Grain | Carrot Pomace | Kiwifruit Peels | Olive Cake | Onion Pulp | Orange Pomace | Red Grape Marc |
|---|---|---|---|---|---|---|---|
| Amyl isovalerate | Benzyl alcohol | 2,5-dimethyl-pyrazine | Cyclopentanone | Decanal | 1,4-Dimethoxy benzene | Benzoic acid, methyl ester | Cinnemaldehyde, (E)- |
| 1-Butanol | 1-Heptanol | Heptanoic acid, methyl ester | β-Pinene | trans-Limonene oxide | 1-Hepten-3-ol | 1,2-Dimethoxy benzene | Ethyl tiglate |
| α-Cubebene | 1-Hexanol | Limonene | Vanillin | 3,4-Dimethyl styrene | |||
| Ethyl isovalerate | 2-Methyl-butanal | Methyl valerate | Methyl salicylate | ||||
| Hexyl n-valerate | 6-Methyl-2-heptanone | Nonanoic acid, methyl ester | |||||
| iso-Amyl tiglate | Phenylethyl alcohol | 4-Octenoic acid, methyl ester, (Z)- | |||||
| 2-Pentanone | Sabinene | Propanoic acid, 2-methyl- |
| Compound | Value (USD/kg) a | Annual Consumption (kg) b | Substrate | Microorganism | Yield (g/kg) c |
|---|---|---|---|---|---|
| Methyl benzoate | USD 335 * | 590 | Orange | Aspergillus niger | 0.173 ± 0.0003 |
| Phenylacetaldehyde | USD 450 * | 106 | SBG | Pycnorporus cinnabarinus | 1.493 ± 0.384 |
| 1-Octen-3-ol | USD 4800 * | 250 | Onion | Aspergillus oryzae | 1.297 ± 0.107 |
| Phenylethyl alcohol | USD 500 * | 1240 | SBG | Pycnorporus cinnabarinus | 0.970 ± 0.242 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lindsay, M.A.; Granucci, N.; Greenwood, D.R.; Villas-Boas, S.G. Identification of New Natural Sources of Flavour and Aroma Metabolites from Solid-State Fermentation of Agro-Industrial By-Products. Metabolites 2022, 12, 157. https://doi.org/10.3390/metabo12020157
Lindsay MA, Granucci N, Greenwood DR, Villas-Boas SG. Identification of New Natural Sources of Flavour and Aroma Metabolites from Solid-State Fermentation of Agro-Industrial By-Products. Metabolites. 2022; 12(2):157. https://doi.org/10.3390/metabo12020157
Chicago/Turabian StyleLindsay, Melodie A., Ninna Granucci, David R. Greenwood, and Silas G. Villas-Boas. 2022. "Identification of New Natural Sources of Flavour and Aroma Metabolites from Solid-State Fermentation of Agro-Industrial By-Products" Metabolites 12, no. 2: 157. https://doi.org/10.3390/metabo12020157
APA StyleLindsay, M. A., Granucci, N., Greenwood, D. R., & Villas-Boas, S. G. (2022). Identification of New Natural Sources of Flavour and Aroma Metabolites from Solid-State Fermentation of Agro-Industrial By-Products. Metabolites, 12(2), 157. https://doi.org/10.3390/metabo12020157








