Diabetes-Induced Changes in Macrophage Biology Might Lead to Reduced Risk for Abdominal Aortic Aneurysm Development
Abstract
:1. Introduction
2. Results
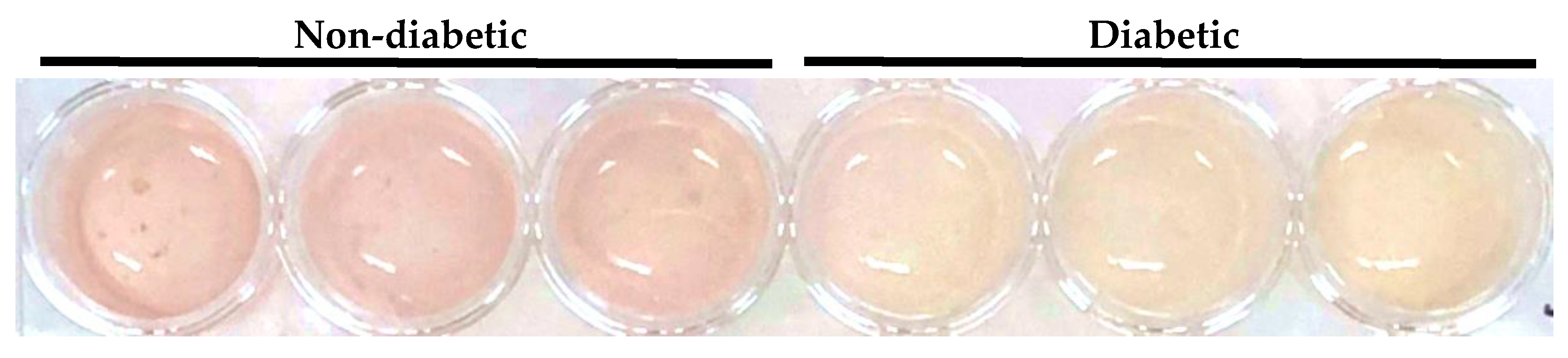
2.1. Acidification of Primary Human Macrophage Culture Media by Treatment with Serum from Diabetic AAA Patients
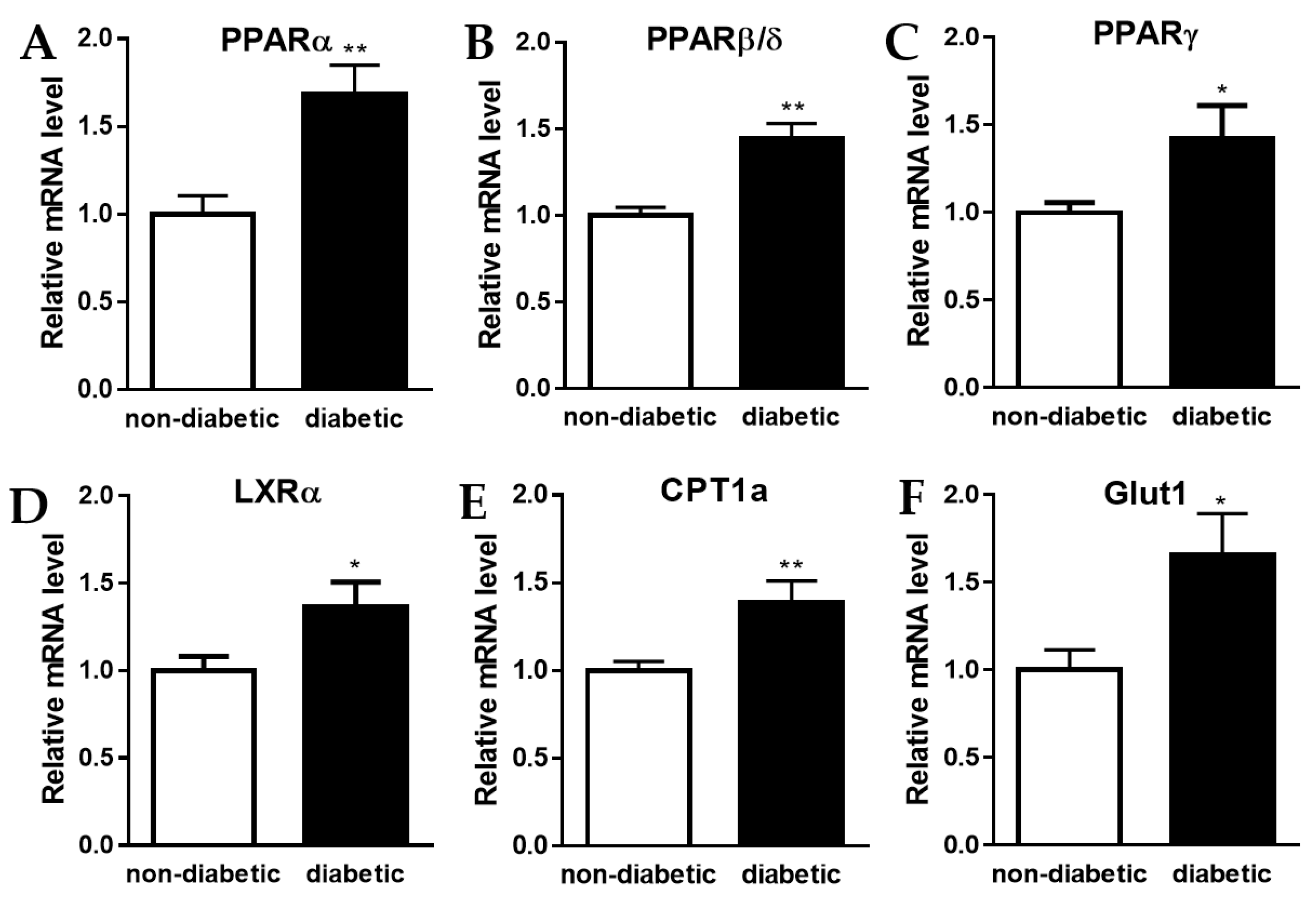
2.2. Increase in Expression of Genes Involved in Oxidative Phosphorylation and Glycolysis
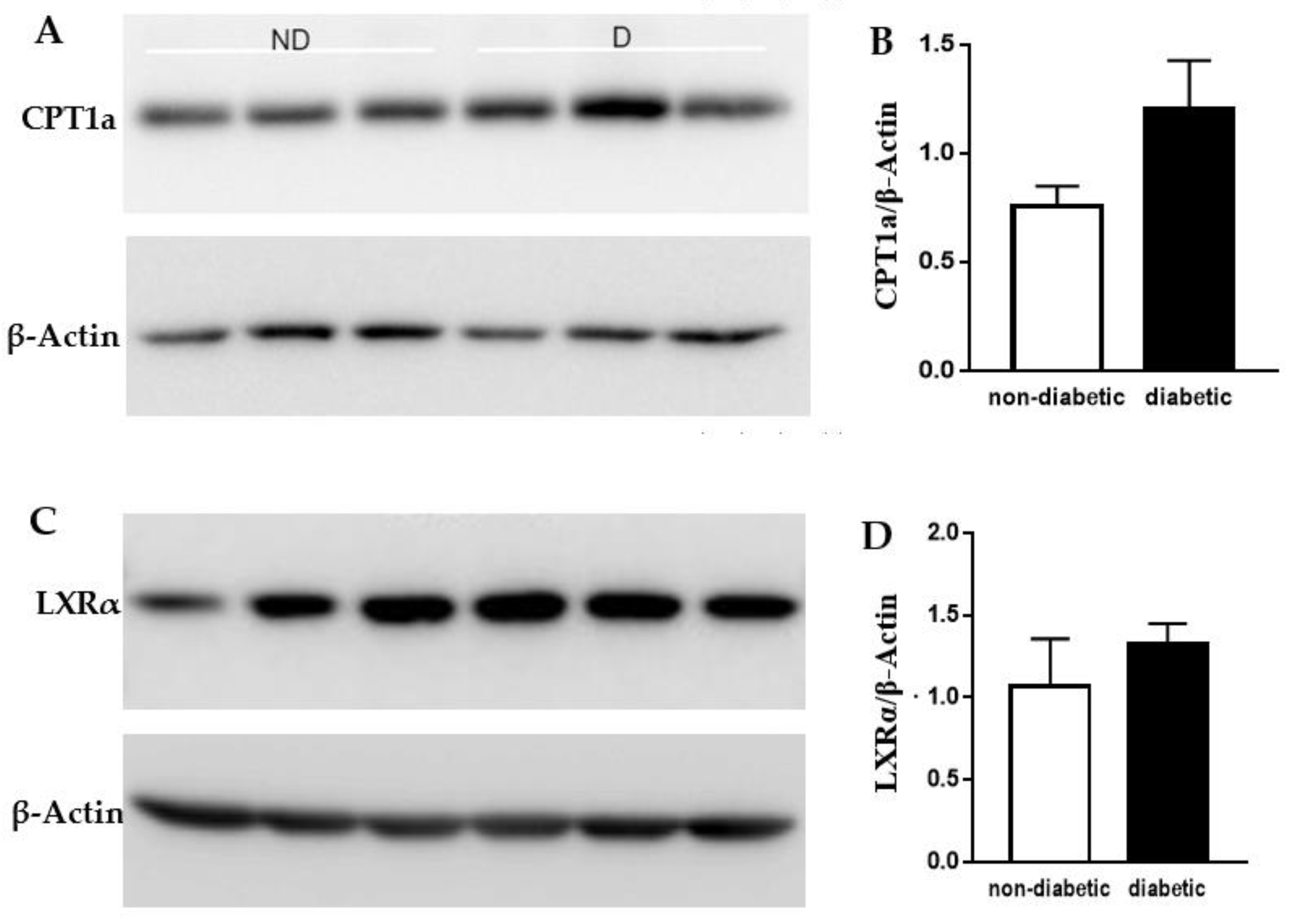
2.3. Protein Levels of CPT1a and LXRα
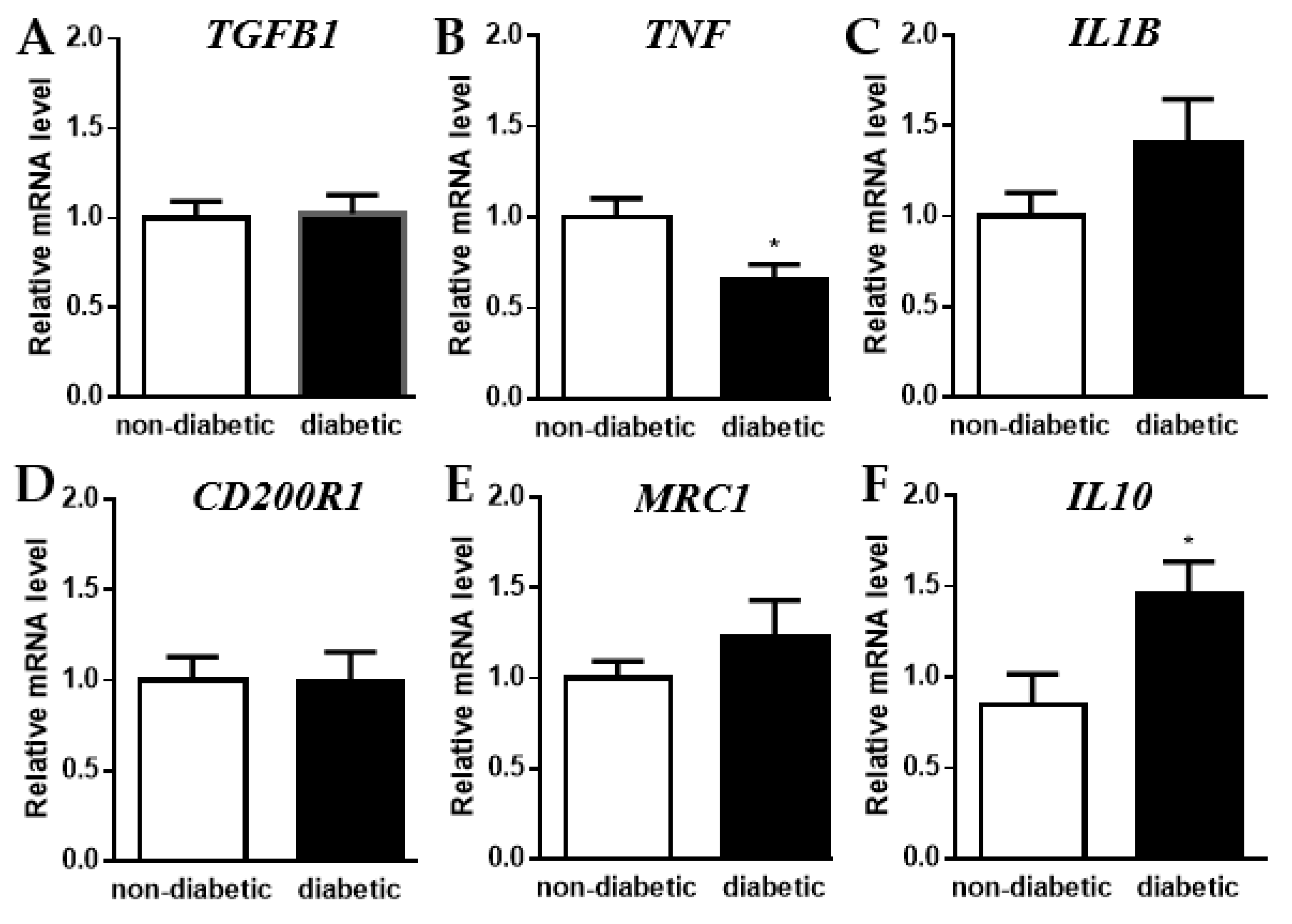
2.4. Shift towards an Anti-Inflammatory State
3. Discussion
4. Materials and Methods
4.1. AAA Patient Serum Samples
4.2. Primary Human Macrophage Cultures
4.3. RNA Extraction and Quantitative Real-Time PCR
4.4. Protein Extraction and Western Blot Analysis
4.5. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Golledge, J.; Muller, J.; Daugherty, A.; Norman, P. Abdominal aortic aneurysm: Pathogenesis and implications for management. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2605–2613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boytard, L.; Spear, R.; Chinetti-Gbaguidi, G.; Acosta-Martin, A.E.; Vanhoutte, J.; Lamblin, N.; Staels, B.; Amouyel, P.; Haulon, S.; Pinet, F. Role of proinflammatory CD68(+) mannose receptor(-) macrophages in peroxiredoxin-1 expression and in abdominal aortic aneurysms in humans. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 431–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golledge, J.; Norman, P.E. Atherosclerosis and abdominal aortic aneurysm: Cause, response, or common risk factors? Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1075–1077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raffort, J.; Lareyre, F.; Clement, M.; Hassen-Khodja, R.; Chinetti, G.; Mallat, Z. Diabetes and aortic aneurysm: Current state of the art. Cardiovasc. Res. 2018, 114, 1702–1713. [Google Scholar] [CrossRef] [PubMed]
- Takagi, H.; Umemoto, T.; Group, A. Negative association of diabetes with rupture of abdominal aortic aneurysm. Diabetes Vasc. Dis. Res. 2016, 13, 341–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, A.; Cooper, J.A.; Fabricius, M.; Humphries, S.E.; Ashton, H.A.; Hafez, H. An analysis of drug modulation of abdominal aortic aneurysm growth through 25 years of surveillance. J. Vasc. Surg. 2010, 52, 55–61 e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le, M.T.; Jamrozik, K.; Davis, T.M.; Norman, P.E. Negative association between infra-renal aortic diameter and glycaemia: The Health in Men Study. Eur. J. Vasc. Endovasc. Surg. 2007, 33, 599–604. [Google Scholar] [CrossRef]
- Kristensen, K.L.; Dahl, M.; Rasmussen, L.M.; Lindholt, J.S. Glycated Hemoglobin Is Associated With the Growth Rate of Abdominal Aortic Aneurysms: A Substudy from the VIVA (Viborg Vascular) Randomized Screening Trial. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 730–736. [Google Scholar] [CrossRef] [Green Version]
- Lareyre, F.; Moratal, C.; Zereg, E.; Carboni, J.; Panaia-Ferrari, P.; Bayer, P.; Jean-Baptiste, E.; Hassen-Khodja, R.; Chinetti, G.; Raffort, J. Association of abdominal aortic aneurysm diameter with insulin resistance index. Biochem. Med. 2018, 28, 030702. [Google Scholar] [CrossRef]
- Lareyre, F.; Moratal, C.; Chikande, J.; Jean-Baptiste, E.; Hassen-Khodja, R.; Neels, J.; Chinetti, G.; Raffort, J. Investigation of Plasma Inflammatory Profile in Diabetic Patients with Abdominal Aortic Aneurysm: A Pilot Study. Vasc. Endovascular. Surg. 2018, 52, 597–601. [Google Scholar] [CrossRef]
- Lareyre, F.; Clement, M.; Raffort, J.; Pohlod, S.; Patel, M.; Esposito, B.; Master, L.; Finigan, A.; Vandestienne, M.; Stergiopulos, N.; et al. TGFbeta (Transforming Growth Factor-beta) Blockade Induces a Human-Like Disease in a Nondissecting Mouse Model of Abdominal Aortic Aneurysm. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 2171–2181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raffort, J.; Lareyre, F.; Clement, M.; Hassen-Khodja, R.; Chinetti, G.; Mallat, Z. Monocytes and macrophages in abdominal aortic aneurysm. Nat. Rev. Cardiol. 2017, 14, 457–471. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S. Foundations of Immunometabolism and Implications for Metabolic Health and Disease. Immunity 2017, 47, 406–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dale, M.A.; Ruhlman, M.K.; Baxter, B.T. Inflammatory cell phenotypes in AAAs: Their role and potential as targets for therapy. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1746–1755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mookerjee, S.A.; Goncalves, R.L.S.; Gerencser, A.A.; Nicholls, D.G.; Brand, M.D. The contributions of respiration and glycolysis to extracellular acid production. Biochim. Biophys. Acta 2015, 1847, 171–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montaigne, D.; Butruille, L.; Staels, B. PPAR control of metabolism and cardiovascular functions. Nat. Rev. Cardiol. 2021, 18, 809–823. [Google Scholar] [CrossRef] [PubMed]
- Joseph, S.B.; Castrillo, A.; Laffitte, B.A.; Mangelsdorf, D.J.; Tontonoz, P. Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nat. Med. 2003, 9, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Kolliniati, O.; Ieronymaki, E.; Vergadi, E.; Tsatsanis, C. Metabolic Regulation of Macrophage Activation. J. Innate Immun. 2021, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, R.; Gu, H.; Zhang, E.; Qu, J.; Cao, W.; Huang, X.; Yan, H.; He, J.; Cai, Z. Metabolic reprogramming in macrophage responses. Biomark Res. 2021, 9, 1. [Google Scholar] [CrossRef]
- Roszer, T. Understanding the Mysterious M2 Macrophage through Activation Markers and Effector Mechanisms. Mediat. Inflamm. 2015, 2015, 816460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.X.; Zhang, S.X.; Wu, H.J.; Rong, X.L.; Guo, J. M2b macrophage polarization and its roles in diseases. J. Leukoc. Biol. 2019, 106, 345–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halvorsen, B.; Santilli, F.; Scholz, H.; Sahraoui, A.; Gulseth, H.L.; Wium, C.; Lattanzio, S.; Formoso, G.; Di Fulvio, P.; Otterdal, K.; et al. LIGHT/TNFSF14 is increased in patients with type 2 diabetes mellitus and promotes islet cell dysfunction and endothelial cell inflammation in vitro. Diabetologia 2016, 59, 2134–2144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lefevre, L.; Gales, A.; Olagnier, D.; Bernad, J.; Perez, L.; Burcelin, R.; Valentin, A.; Auwerx, J.; Pipy, B.; Coste, A. PPARgamma ligands switched high fat diet-induced macrophage M2b polarization toward M2a thereby improving intestinal Candida elimination. PLoS ONE 2010, 5, e12828. [Google Scholar] [CrossRef] [PubMed]
- Zeyda, M.; Farmer, D.; Todoric, J.; Aszmann, O.; Speiser, M.; Gyori, G.; Zlabinger, G.J.; Stulnig, T.M. Human adipose tissue macrophages are of an anti-inflammatory phenotype but capable of excessive pro-inflammatory mediator production. Int. J. Obes. 2007, 31, 1420–1428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bourlier, V.; Zakaroff-Girard, A.; Miranville, A.; De Barros, S.; Maumus, M.; Sengenes, C.; Galitzky, J.; Lafontan, M.; Karpe, F.; Frayn, K.N.; et al. Remodeling phenotype of human subcutaneous adipose tissue macrophages. Circulation 2008, 117, 806–815. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhou, Y.Z.; Wu, Y.; Wu, Q.Y.; Liao, X.B.; Fu, X.M.; Zhou, X.M. Diverse roles of macrophage polarization in aortic aneurysm: Destruction and repair. J. Transl. Med. 2018, 16, 354. [Google Scholar] [CrossRef] [Green Version]
- Fujimura, N.; Xiong, J.; Kettler, E.B.; Xuan, H.; Glover, K.J.; Mell, M.W.; Xu, B.; Dalman, R.L. Metformin treatment status and abdominal aortic aneurysm disease progression. J. Vasc. Surg. 2016, 64, 46–54.e8. [Google Scholar] [CrossRef] [Green Version]
- Jing, Y.; Wu, F.; Li, D.; Yang, L.; Li, Q.; Li, R. Metformin improves obesity-associated inflammation by altering macrophages polarization. Mol. Cell Endocrinol. 2018, 461, 256–264. [Google Scholar] [CrossRef]
- Schmidt, A.M. 22016 ATVB Plenary Lecture: Receptor for Advanced Glycation Endproducts and Implications for the Pathogenesis and Treatment of Cardiometabolic Disorders: Spotlight on the Macrophage. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 613–621. [Google Scholar] [CrossRef] [Green Version]
- Colin, S.; Fanchon, M.; Belloy, L.; Bochem, A.E.; Copin, C.; Derudas, B.; Stroes, E.S.; Hovingh, G.K.; Kuivenhoven, J.A.; Dallinga-Thie, G.M.; et al. HDL does not influence the polarization of human monocytes toward an alternative phenotype. Int. J. Cardiol. 2014, 172, 179–184. [Google Scholar] [CrossRef]
| Characteristic | Non-Diabetics (n = 6) | Diabetics (n = 6) |
|---|---|---|
| Age (years) | 70.2 ± 2.4 | 69.0 ± 2.8 |
| BMI (kg/m2) | 26.7 ± 3.5 | 29.1 ± 3.6 |
| Smoking | 5/6 | 5/6 |
| Hypertension | 3/6 | 5/6 |
| Dyslipidemia | 2/6 | 1/6 |
| AAA diameter (mm) | 50.8 ± 2.1 | 50.7 ± 4.7 |
| Symptomatic | 4/6 | 4/6 |
| Glycemia (mmol/L) | 7.8 ± 1.4 | 7.5 ± 1.9 |
| Diabetes treatment | 0/6 | 6/6 ***† |
| Insulin therapy | 0/6 | 1/6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chinetti, G.; Carboni, J.; Murdaca, J.; Moratal, C.; Sibille, B.; Raffort, J.; Lareyre, F.; Baptiste, E.J.; Hassen-Khodja, R.; Neels, J.G. Diabetes-Induced Changes in Macrophage Biology Might Lead to Reduced Risk for Abdominal Aortic Aneurysm Development. Metabolites 2022, 12, 128. https://doi.org/10.3390/metabo12020128
Chinetti G, Carboni J, Murdaca J, Moratal C, Sibille B, Raffort J, Lareyre F, Baptiste EJ, Hassen-Khodja R, Neels JG. Diabetes-Induced Changes in Macrophage Biology Might Lead to Reduced Risk for Abdominal Aortic Aneurysm Development. Metabolites. 2022; 12(2):128. https://doi.org/10.3390/metabo12020128
Chicago/Turabian StyleChinetti, Giulia, Joseph Carboni, Joseph Murdaca, Claudine Moratal, Brigitte Sibille, Juliette Raffort, Fabien Lareyre, Elixène Jean Baptiste, Réda Hassen-Khodja, and Jaap G. Neels. 2022. "Diabetes-Induced Changes in Macrophage Biology Might Lead to Reduced Risk for Abdominal Aortic Aneurysm Development" Metabolites 12, no. 2: 128. https://doi.org/10.3390/metabo12020128
APA StyleChinetti, G., Carboni, J., Murdaca, J., Moratal, C., Sibille, B., Raffort, J., Lareyre, F., Baptiste, E. J., Hassen-Khodja, R., & Neels, J. G. (2022). Diabetes-Induced Changes in Macrophage Biology Might Lead to Reduced Risk for Abdominal Aortic Aneurysm Development. Metabolites, 12(2), 128. https://doi.org/10.3390/metabo12020128









