Beneficial Effects of the Consumption of Hot-Water Extracts of Thinned Immature Mangos (Mangifera indica “Irwin”) on the Hypertriglyceridemia of Apolipoprotein E-Deficient Mice
Abstract
1. Introduction
2. Results
2.1. Growth Parameters and Organ Masses
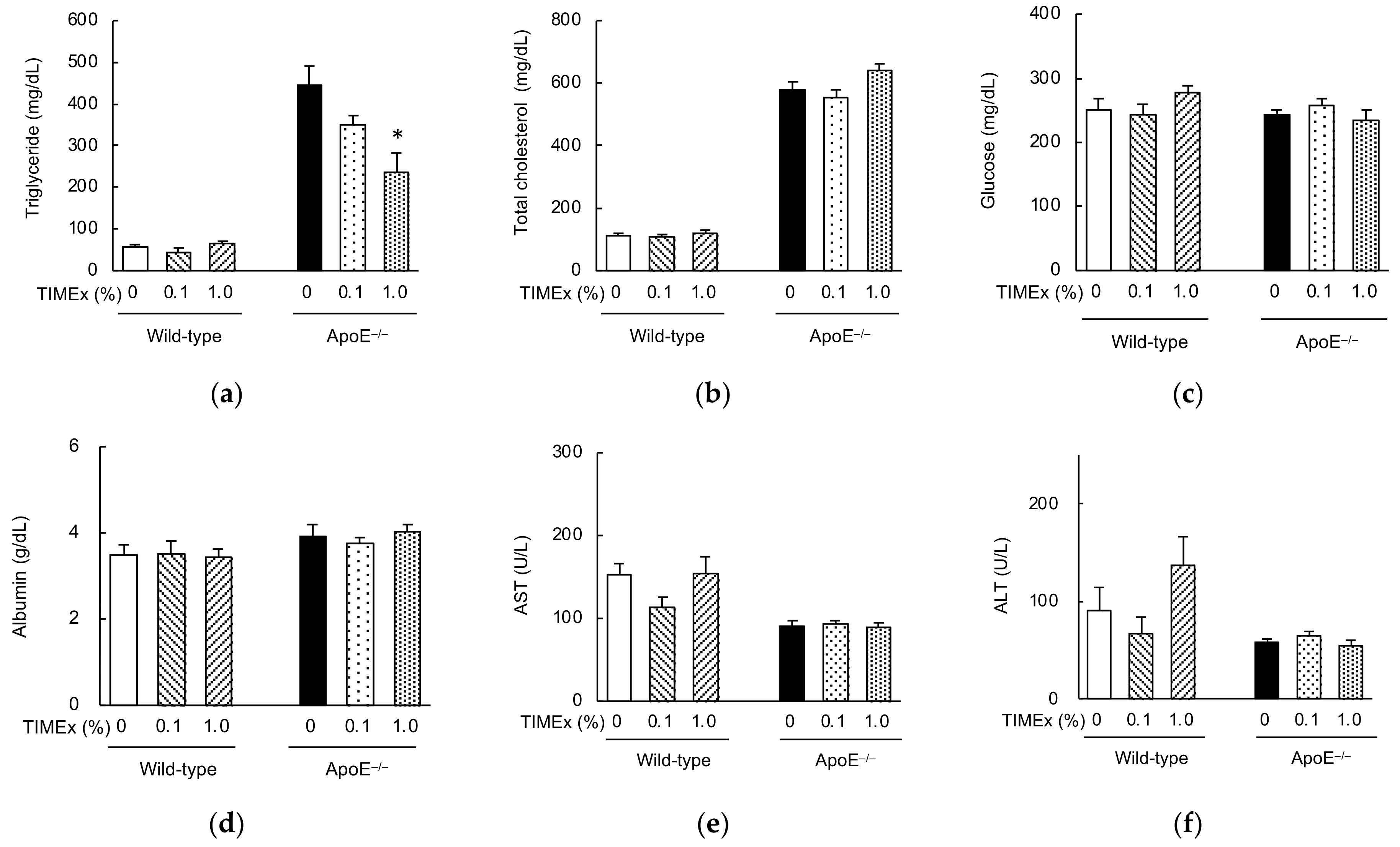
2.2. Serum Lipid Concentrations
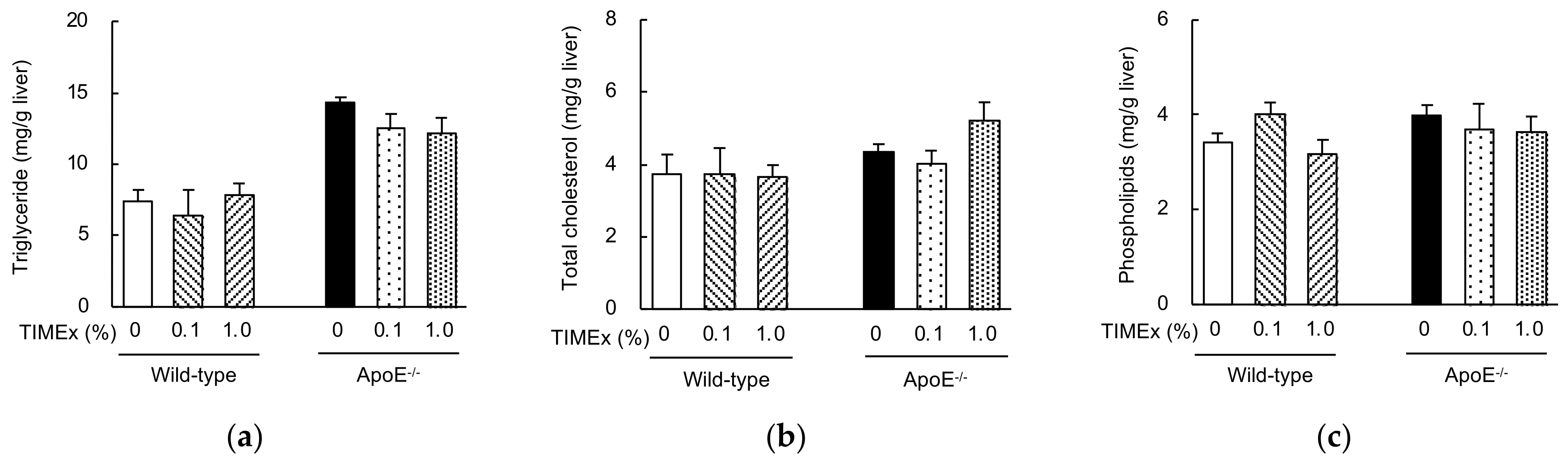
2.3. Hepatic Lipid Concentrations
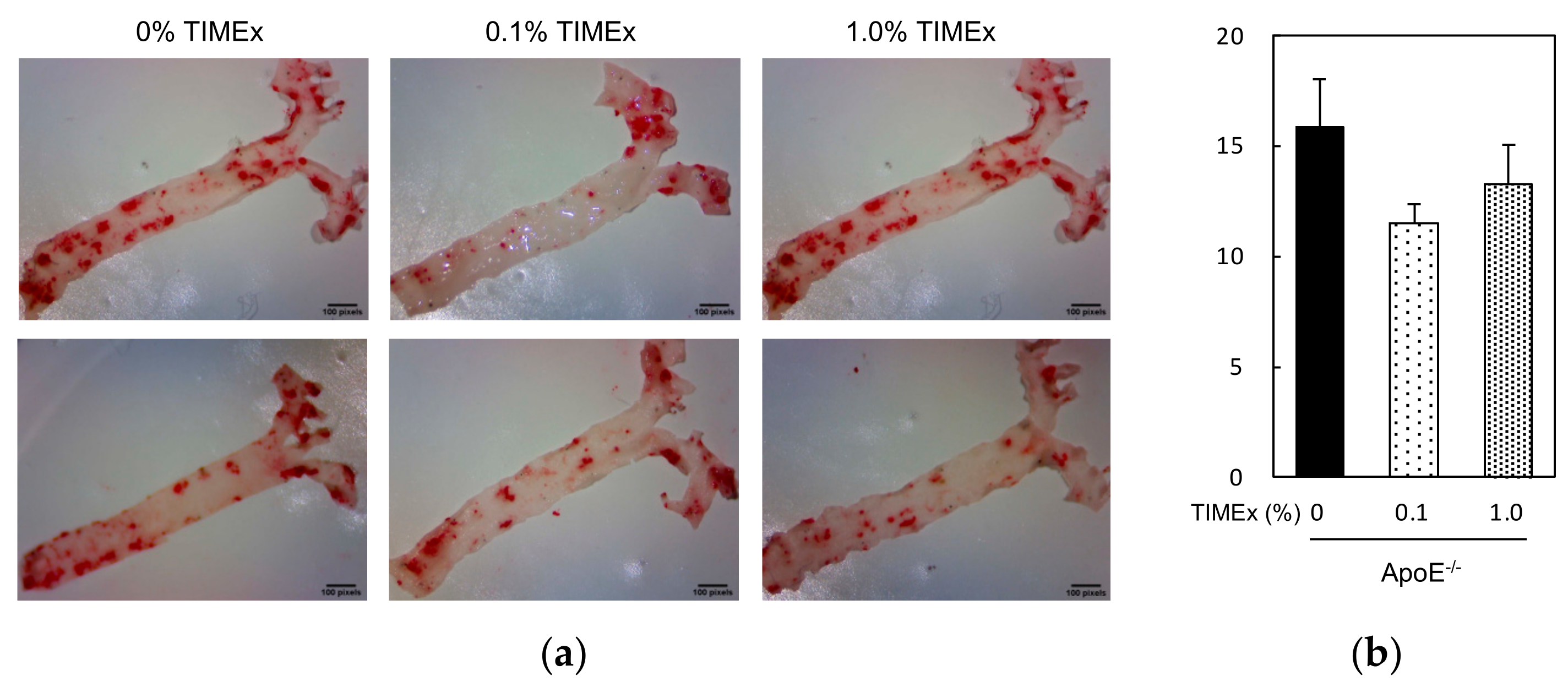
2.4. Atherosclerotic Plaque Lesions
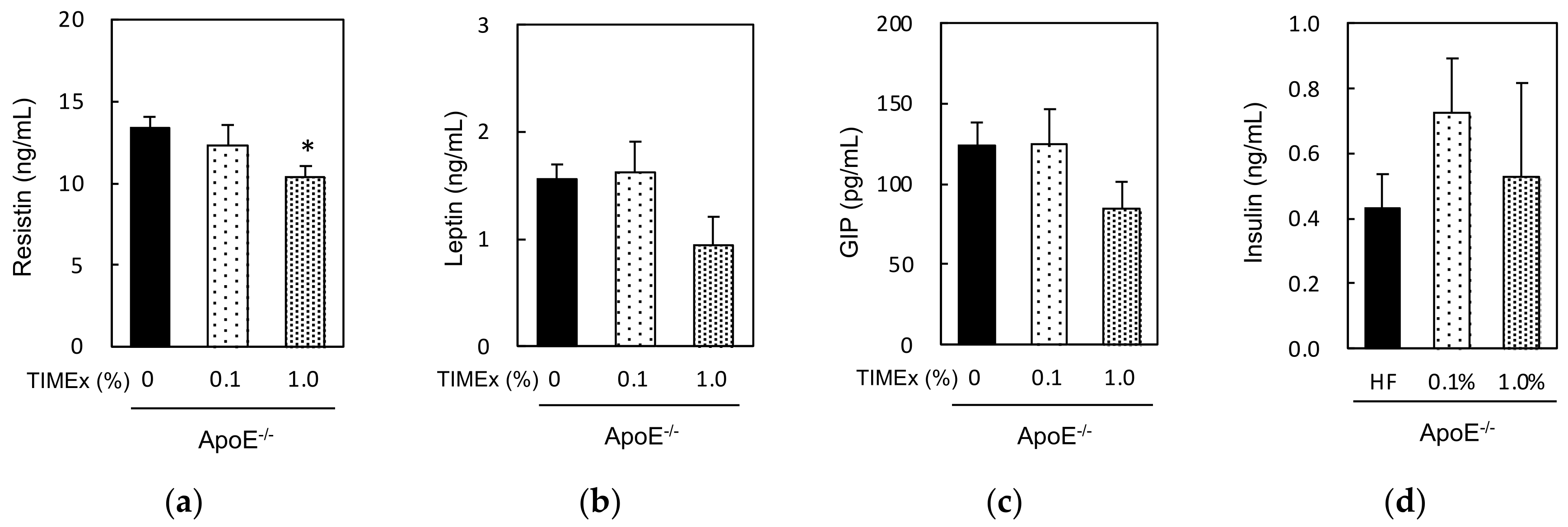
2.5. Serum Hormone Concentrations
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Experimental Materials and Preparations
4.2.1. Immature Mango Fruits
4.2.2. Hot-Water Extraction
4.3. Animal Experiments
4.3.1. Institutional Approval of the Study Protocol
4.3.2. Animal Housing, Diet, and Experiments
4.3.3. Quantification of Atherosclerotic Lesions
4.3.4. Blood Chemistry and Adipokines
4.3.5. Hepatic Lipid Analysis
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, L.; Parhofer, K.G. Diabetic dyslipidemia. Metabolism 2014, 63, 1469–1479. [Google Scholar] [CrossRef] [PubMed]
- Fras, Z.; Jug, B.; Penson, P.E.; Rizzo, M. Challenges and opportunities on lipid metabolism disorders diagnosis and therapy: Novel insights and future perspective. Metabolites 2021, 11, 611. [Google Scholar] [CrossRef] [PubMed]
- Banach, M.; Penson, P.E.; Vrablik, M.; Bunc, M.; Dyrbus, K.; Fedacko, J.; Gaita, D.; Gierlotka, M.; Jarai, Z.; Magda, S.L.; et al. Optimal use of lipid-lowering therapy after acute coronary syndromes: A position paper endorsed by the International Lipid Expert Panel (ILEP). Pharmacol Res. 2021, 166, 105499. [Google Scholar] [CrossRef] [PubMed]
- Fernandes Silva, L.; Vangipurapu, J.; Laakso, M. The “Common Soil Hypothesis” revisited-risk factors for type 2 diabetes and cardiovascular disease. Metabolites 2021, 11, 691. [Google Scholar] [CrossRef] [PubMed]
- Nuthikattu, S.; Milenkovic, D.; Rutledge, J.; Villablanca, A. The western diet regulates hippocampal microvascular gene expression: An integrated genomic analyses in female mice. Sci. Rep. 2019, 9, 19058. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Major Tropical Fruits–Market Review. Available online: https://www.fao.org/3/ca5692en/ca5692en.pdf (accessed on 15 January 2022).
- Food and Agriculture Orgnization of the United Nations. Post-Harvest Management of Mango for Quality and Safety Assurance. Available online: https://www.fao.org/3/I8239EN/i8239en.pdf (accessed on 15 January 2022).
- Maldonado-Celis, M.E.; Yahia, E.M.; Bedoya, R.; Landazuri, P.; Loango, N.; Aguillon, J.; Restrepo, B.; Guerrero Ospina, J.C. Chemical composition of mango (Mangifera indica L.) fruit: Nutritional and phytochemical compounds. Front. Plant. Sci. 2019, 10, 1073. [Google Scholar] [CrossRef]
- Smith, J.C.; Biasi, W.V.; Holstege, D.; Mitcham, E.J. Effect of passive drying on ascorbic acid, alpha-tocopherol, and beta-carotene in tomato and mango. J. Food Sci. 2018, 83, 1412–1421. [Google Scholar] [CrossRef]
- Qi, J.; Li, K.; Shi, Y.; Li, Y.; Dong, L.; Liu, L.; Li, M.; Ren, H.; Liu, X.; Fang, C.; et al. Cross-species comparison of metabolomics to decipher the metabolic diversity in ten fruits. Metabolites 2021, 11, 164. [Google Scholar] [CrossRef]
- Lebaka, V.R.; Wee, Y.J.; Ye, W.; Korivi, M. Nutritional composition and bioactive compounds in three different parts of mango fruit. Int. J. Environ. Res. Public Health 2021, 18, 741. [Google Scholar] [CrossRef]
- Burge, G.K.; Spence, C.B.; Marshall, R.R. Kiwifruit: Effects of thinning on fruit size, vegetative growth, and return bloom. N. Z. J. Exp. Agric. 1987, 151, 317–324. [Google Scholar] [CrossRef]
- Elfving, D.C.; Schechter, I. Fruit count, fruit weight, and yield relationships in ‘Delicious’ apple trees on nine rootstock. HortScience 1993, 28, 793–795. [Google Scholar] [CrossRef]
- Yeshitela, T.; Robbertse, P.J.; Fivas, J. Effects of fruit thinning on ‘Sensation’ mango (Mangifera indica) trees with respect to fruit quantity, quality and tree phenology. Expl. Agric. 2004, 40, 433–444. [Google Scholar] [CrossRef][Green Version]
- National Mango Board. Mango Handling and Ripening Protocol. Available online: https://www.mango.org/wp-content/uploads/2017/10/Mango_Handling_and_Ripening_Protocol_Eng.pdf (accessed on 15 January 2022).
- Tajiri, H.; Tanaka, W.; Takashima, M.; Matsuyama, H.; Sugita, T.; Hidaka, K.; Sakakibara, H. Subchronic safety evaluation of hot-water extract from thinned immature mangos (Mangifera indica ‘Irwin’): 90-days oral toxicity study in rats. Toxicol. Rep. 2021, 8, 1046–1053. [Google Scholar] [CrossRef] [PubMed]
- Cao, H. Adipocytokines in obesity and metabolic disease. J. Endocrinol. 2014, 220, T47–T59. [Google Scholar] [CrossRef]
- Farkhondeh, T.; Llorens, S.; Pourbagher-Shahri, A.M.; Ashrafizadeh, M.; Talebi, M.; Shakibaei, M.; Samarghandian, S. An overview of the role of adipokines in cardiometabolic diseases. Molecules 2020, 25, 5218. [Google Scholar] [CrossRef]
- Heimburger, S.M.; Bergmann, N.C.; Augustin, R.; Gasbjerg, L.S.; Christensen, M.B.; Knop, F.K. Glucose-dependent insulinotropic polypeptide (GIP) and cardiovascular disease. Peptides 2020, 125, 170174. [Google Scholar] [CrossRef]
- Yamashita, K.; Yatsuya, H.; Muramatsu, T.; Toyoshima, H.; Murohara, T.; Tamakoshi, K. Association of coffee consumption with serum adiponectin, leptin, inflammation and metabolic markers in Japanese workers: A cross-sectional study. Nutr Diabetes 2012, 2, e33. [Google Scholar] [CrossRef]
- Mahley, R.W. Apolipoprotein E: From cardiovascular disease to neurodegenerative disorders. J. Mol. Med. 2016, 94, 739–746. [Google Scholar] [CrossRef]
- Krishnan, M.; Hwang, J.S.; Kim, M.; Kim, Y.J.; Seo, J.H.H.; Jung, J.; Ha, E. Beta-hydroxybutyrate impedes the progression of alzheimer’s disease and atherosclerosis in ApoE-deficient mice. Nutrients 2020, 12, 471. [Google Scholar] [CrossRef]
- Emini Veseli, B.; Perrotta, P.; De Meyer, G.R.A.; Roth, L.; Van der Donckt, C.; Martinet, W.; De Meyer, G.R.Y. Animal models of atherosclerosis. Eur. J. Pharm. 2017, 816, 3–13. [Google Scholar] [CrossRef]
- Han, B.H.; Seo, C.S.; Yoon, J.J.; Kim, H.Y.; Ahn, Y.M.; Eun, S.Y.; Hong, M.H.; Lee, J.G.; Shin, H.K.; Lee, H.S.; et al. The inhibitory effect of ojeoksan on early and advanced atherosclerosis. Nutrients 2018, 10, 1256. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, H.; Tanaka, W.; Miyoshi, N.; Miyazaki, T.; Michimoto, H.; Sakakibara, H. Beneficial effects of the consumption of sun-dried radishes (Raphanus sativus cv. YR-Hyuga-Risou) on dyslipidemia in apolipoprotein E-deficient mice. J. Food Biochem. 2021, 45, e13727. [Google Scholar] [CrossRef]
- Angulo, P.; Lindor, K.D. Non-alcoholic fatty liver disease. J. Gastroenterol. Hepatol. 2002, 17, S186–S190. [Google Scholar] [CrossRef] [PubMed]
- Wadhawan, M.; Anand, A.C. Coffee and liver disease. J. Clin. Exp. Hepatol. 2016, 6, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Keller, U. Nutritional laboratory markers in malnutrition. J. Clin. Med. 2019, 8, 775. [Google Scholar] [CrossRef] [PubMed]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef]
- Yasunaga, E.; Fukuda, S.; Takata, D.; Spreer, W.; Sardsud, V.; Nakano, K. Quality changes in fresh mango fruits (Mangifera indica L. ‘Nam Dok Mai’) under actual distribution temperature profile from Thailand to Japan. Environ. Control. Biol. 2018, 56, 45–49. [Google Scholar] [CrossRef]
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgozoglu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Primers 2019, 5, 56. [Google Scholar] [CrossRef]
- Nergiz-Unal, R.; Kuijpers, M.J.; de Witt, S.M.; Heeneman, S.; Feijge, M.A.; Garcia Caraballo, S.C.; Biessen, E.A.; Haenen, G.R.; Cosemans, J.M.; Heemskerk, J.W. Atheroprotective effect of dietary walnut intake in ApoE-deficient mice: Involvement of lipids and coagulation factors. Thromb. Res. 2013, 131, 411–417. [Google Scholar] [CrossRef]
- Li, J.; Lin, S.; Vanhoutte, P.M.; Woo, C.W.; Xu, A. Ak.kkermansia muciniphila protects gainst therosclerosis by preventing metabolic endotoxemia-induced inflammation in Apoe−/− mice. Circulation 2016, 133, 2434–2446. [Google Scholar] [CrossRef]
- Ley, R.E. Obesity and the human microbiome. Curr. Opin. Gastroenterol. 2010, 26, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Vijay-Kumar, M.; Aitken, J.D.; Carvalho, F.A.; Cullender, T.C.; Mwangi, S.; Srinivasan, S.; Sitaraman, S.V.; Knight, R.; Ley, R.E.; Gewirtz, A.T. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science 2010, 328, 228–231. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Wu, H.; Yang, X.; Li, Y.; Zhang, Z.; Chen, F.; Zhao, L.; Zhang, C. Lactobacillus mucosae strain promoted by a high-fiber diet in genetic obese child alleviates lipid metabolism and modifies gut microbiota in ApoE(−/−) mice on a western diet. Microorganisms 2020, 8, 1225. [Google Scholar] [CrossRef]
- Zhang, L.; Shi, M.; Ji, J.; Hu, X.; Chen, F. Gut microbiota determines the prevention effects of Luffa cylindrica (L.) Roem supplementation against obesity and associated metabolic disorders induced by high-fat diet. FASEB J. 2019, 33, 10339–10352. [Google Scholar] [CrossRef]
- Zhou, X.L.; Yan, B.B.; Xiao, Y.; Zhou, Y.M.; Liu, T.Y. Tartary buckwheat protein prevented dyslipidemia in high-fat diet-fed mice associated with gut microbiota changes. Food Chem. Toxicol. 2018, 119, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Yue, J.; Hui, N.; Zhi, Y.; Hayat, K.; Yang, X.; Zhang, D.; Chu, S.; Zhou, P. Anti-hyperlipidemia and gut microbiota community regulation effects of selenium-rich cordyceps militaris polysaccharides on the high-fat diet-fed mice model. Foods 2021, 10, 2252. [Google Scholar] [CrossRef]
- Wang, F.; Zhu, H.; Hu, M.; Wang, J.; Xia, H.; Yang, X.; Yang, L.; Sun, G. Perilla oil supplementation improves hypertriglyceridemia and gut dysbiosis in diabetic KKAy mice. Mol. Nutr. Food Res. 2018, 62, e1800299. [Google Scholar] [CrossRef]
- Ojo, B.; El-Rassi, G.D.; Payton, M.E.; Perkins-Veazie, P.; Clarke, S.; Smith, B.J.; Lucas, E.A. Mango supplementation modulates gut microbial dysbiosis and short-chain fatty acid production independent of body weight reduction in C57BL/6 mice fed a high-fat diet. J. Nutr. 2016, 146, 1483–1491. [Google Scholar] [CrossRef]
- Chang, S.C.; Lee, M.S.; Lin, C.J.; Chen, M.L. Dietary fiber content and composition of fruits in Taiwan. Asia Pac. J. Clin. Nutr. 1998, 7, 206–210. [Google Scholar]
- Li, W.; Zhang, K.; Yang, H. Pectin alleviates high fat (lard) diet-induced nonalcoholic fatty liver disease in mice: Possible role of short-chain fatty acids and gut microbiota regulated by pectin. J. Agric. Food Chem. 2018, 66, 8015–8025. [Google Scholar] [CrossRef]
- Imran, M.; Arshad, M.S.; Butt, M.S.; Kwon, J.H.; Arshad, M.U.; Sultan, M.T. Mangiferin: A natural miracle bioactive compound against lifestyle related disorders. Lipids Health Dis. 2017, 16, 84. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.L.; Li, C.Y.; Zhang, B.; Liu, Y.D.; Lu, B.M.; Shi, Z.; An, N.; Zhao, L.K.; Zhang, J.J.; Bao, J.K.; et al. Mangiferin facilitates islet regeneration and beta-cell proliferation through upregulation of cell cycle and beta-cell regeneration regulators. Int. J. Mol. Sci. 2014, 15, 9016–9035. [Google Scholar] [CrossRef]
- Hegelund, M.H.; Faurholt-Jepsen, D.; Bygbjerg, I.C. Prevention of opportunistic non-communicable diseases. Int. Health 2020, 12, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Ros, E. The role of the Mediterranean diet on weight loss and obesity-related diseases. Rev. Endocr. Metab. Disord. 2020, 21, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, D.; Sharma, S. Abdominal obesity, adipokines and non-communicable diseases. J. Steroid Biochem. Mol. Biol. 2020, 203, 105737. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.D.; Liang, C.H.; Liu, E.H.; Mau, J.L. Antioxidant properties of water extracts from parchinng green tea. J. Food Biochem. 2010, 34, 477–500. [Google Scholar] [CrossRef]
- Hoang, M.H.; Jia, Y.; Mok, B.; Jun, H.J.; Hwang, K.Y.; Lee, S.J. Kaempferol ameliorates symptoms of metabolic syndrome by regulating activities of liver X receptor-beta. J. Nutr. Biochem. 2015, 26, 868–875. [Google Scholar] [CrossRef]
- Matsumoto, M.; Sata, M.; Fukuda, D.; Tanaka, K.; Soma, M.; Hirata, Y.; Nagai, R. Orally administered eicosapentaenoic acid reduces and stabilizes atherosclerotic lesions in ApoE-deficient mice. Atherosclerosis 2008, 197, 524–533. [Google Scholar] [CrossRef]
- Tang, S.L.; Zhao, Z.W.; Liu, S.M.; Wang, G.; Yu, X.H.; Zou, J.; Wang, S.Q.; Dai, X.Y.; Fu, M.G.; Zheng, X.L.; et al. Pregnancy-associated plasma protein-A accelerates atherosclerosis by regulating reverse cholesterol transport and inflammation. Circ. J. 2019, 83, 515–523. [Google Scholar] [CrossRef]
- Yokoyama, D.; Tanaka, W.; Hashizume, Y.; Tandia, M.; Sakono, M.; Shimoi, K.; Sakakibara, H. Daily consumption of monoglucosyl-rutin prevents high-fat diet-induced obesity by suppressing gastric inhibitory polypeptide secretion in mice. Funct. Foods Health. Dis. 2018, 8, 353–371. [Google Scholar] [CrossRef]
| Measurements | Wild-Type (n = 6) | ApoE−/− (n = 9) | ||||
|---|---|---|---|---|---|---|
| 0% TIMEx | 0.1% TIMEx | 1.0% TIMEx | 0% TIMEx | 0.1% TIMEx | 1.0% TIMEx | |
| Body mass (g) | ||||||
| week 0 | 23.9 ± 0.4 | 23.8 ± 0.4 | 23.9 ± 0.6 | 23.5 ± 0.5 | 23.2 ± 0.6 | 23.4 ± 0.3 |
| week 8 | 29.3 ± 0.5 | 28.1 ± 0.7 | 29.0 ± 0.5 | 28.2 ± 2.3 | 28.4 ± 0.9 | 28.1 ± 0.8 |
| Body mass gain (g) | 5.4 ± 0.4 | 4.2 ± 0.5 | 5.1 ± 0.4 | 4.7 ± 0.7 | 5.3 ± 0.6 | 4.7 ± 0.6 |
| Food intake (kcal/mouse/day) | 12.1 | 12.0 | 13.7 | 12.9 ± 0.6 | 13.0 ± 0.3 | 14.2 ± 0.5 |
| TIMEx consumption (mg/mouse/day) | - | 2.6 | 27.7 | - | 2.8 ± 0.2 | 28.6 ± 1.0 |
| Water intake (g/mouse/day) | 2.9 | 2.4 | 2.7 | 2.7 ± 0.3 | 2.8 ± 0.3 | 3.3 ± 0.7 |
| Relative organ masses (g/100 g body mass) | ||||||
| liver | 3.75 ± 0.09 | 3.58 ± 0.19 | 4.20 ± 0.08 * | 4.88 ± 0.13 | 4.73 ± 0.10 | 4.31 ± 0.24 |
| kidney | 1.21 ± 0.222 | 1.40 ± 0.04 | 1.31 ± 0.04 | 1.43 ± 0.03 | 1.38 ± 0.08 | 1.41 ± 0.05 |
| spleen | 0.37 ± 0.07 | 0.46 ± 0.02 | 0.43 ± 0.02 | 0.46 ± 0.02 | 0.44 ± 0.01 | 0.41 ± 0.01 |
| visceral fat | 1.14 ± 0.23 | 1.02 ± 0.14 | 1.49 ± 0.16 | 2.08 ± 0.21 | 2.63 ± 0.36 | 2.22 ± 0.29 |
| brown fat | 0.26 ± 0.05 | 0.28 ± 0.01 | 0.35 ± 0.02 | 0.39 ± 0.04 | 0.39 ± 0.03 | 0.30 ± 0.05 |
| Ingredients | AIN-93M | Experimental Diets | ||
|---|---|---|---|---|
| 0% TIMEx | 0.1% TIMEx | 1.0% TIMEx | ||
| β-Cornstarch (g) | 46.6 | 30.6 | 30.5 | 29.6 |
| α-Cornstarch (g) | 15.5 | 15.5 | 15.5 | 15.5 |
| Casein (g) | 14.0 | 14.0 | 14.0 | 14.0 |
| Soybean oil (g) | 4.0 | 4.0 | 4.0 | 4.0 |
| Lard (g) | - | 16.0 | 16.0 | 16.0 |
| Sucrose (g) | 10.0 | 10.0 | 10.0 | 10.0 |
| Cellulose (g) | 5.0 | 5.0 | 5.0 | 5.0 |
| Vitamin mixture (g) | 1.0 | 1.0 | 1.0 | 1.0 |
| Mineral mixture (g) | 3.5 | 3.5 | 3.5 | 3.5 |
| l-Cysteine (g) | 0.18 | 0.18 | 0.18 | 0.18 |
| Choline bitartrate (g) | 0.25 | 0.25 | 0.25 | 0.25 |
| t-Butylhydroquinone (g) | 0.0008 | 0.0008 | 0.0008 | 0.0008 |
| TIMEx (g) | - | - | 0.1 | 1.0 |
| Energy (kcal/g) | 3.80 | 4.60 | 4.60 | 4.56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tajiri, H.; Tanaka, W.; Matsuyama, H.; Sugita, T.; Hidaka, K.; Yokoyama, D.; Sakakibara, H. Beneficial Effects of the Consumption of Hot-Water Extracts of Thinned Immature Mangos (Mangifera indica “Irwin”) on the Hypertriglyceridemia of Apolipoprotein E-Deficient Mice. Metabolites 2022, 12, 116. https://doi.org/10.3390/metabo12020116
Tajiri H, Tanaka W, Matsuyama H, Sugita T, Hidaka K, Yokoyama D, Sakakibara H. Beneficial Effects of the Consumption of Hot-Water Extracts of Thinned Immature Mangos (Mangifera indica “Irwin”) on the Hypertriglyceridemia of Apolipoprotein E-Deficient Mice. Metabolites. 2022; 12(2):116. https://doi.org/10.3390/metabo12020116
Chicago/Turabian StyleTajiri, Hayato, Wataru Tanaka, Hiroki Matsuyama, Takuya Sugita, Kenta Hidaka, Daigo Yokoyama, and Hiroyuki Sakakibara. 2022. "Beneficial Effects of the Consumption of Hot-Water Extracts of Thinned Immature Mangos (Mangifera indica “Irwin”) on the Hypertriglyceridemia of Apolipoprotein E-Deficient Mice" Metabolites 12, no. 2: 116. https://doi.org/10.3390/metabo12020116
APA StyleTajiri, H., Tanaka, W., Matsuyama, H., Sugita, T., Hidaka, K., Yokoyama, D., & Sakakibara, H. (2022). Beneficial Effects of the Consumption of Hot-Water Extracts of Thinned Immature Mangos (Mangifera indica “Irwin”) on the Hypertriglyceridemia of Apolipoprotein E-Deficient Mice. Metabolites, 12(2), 116. https://doi.org/10.3390/metabo12020116






