Effects of pH and Osmotic Changes on the Metabolic Expressions of Bacillus subtilis Strain 168 in Metabolite Pathways including Leucine Metabolism
Abstract
1. Introduction
2. Results
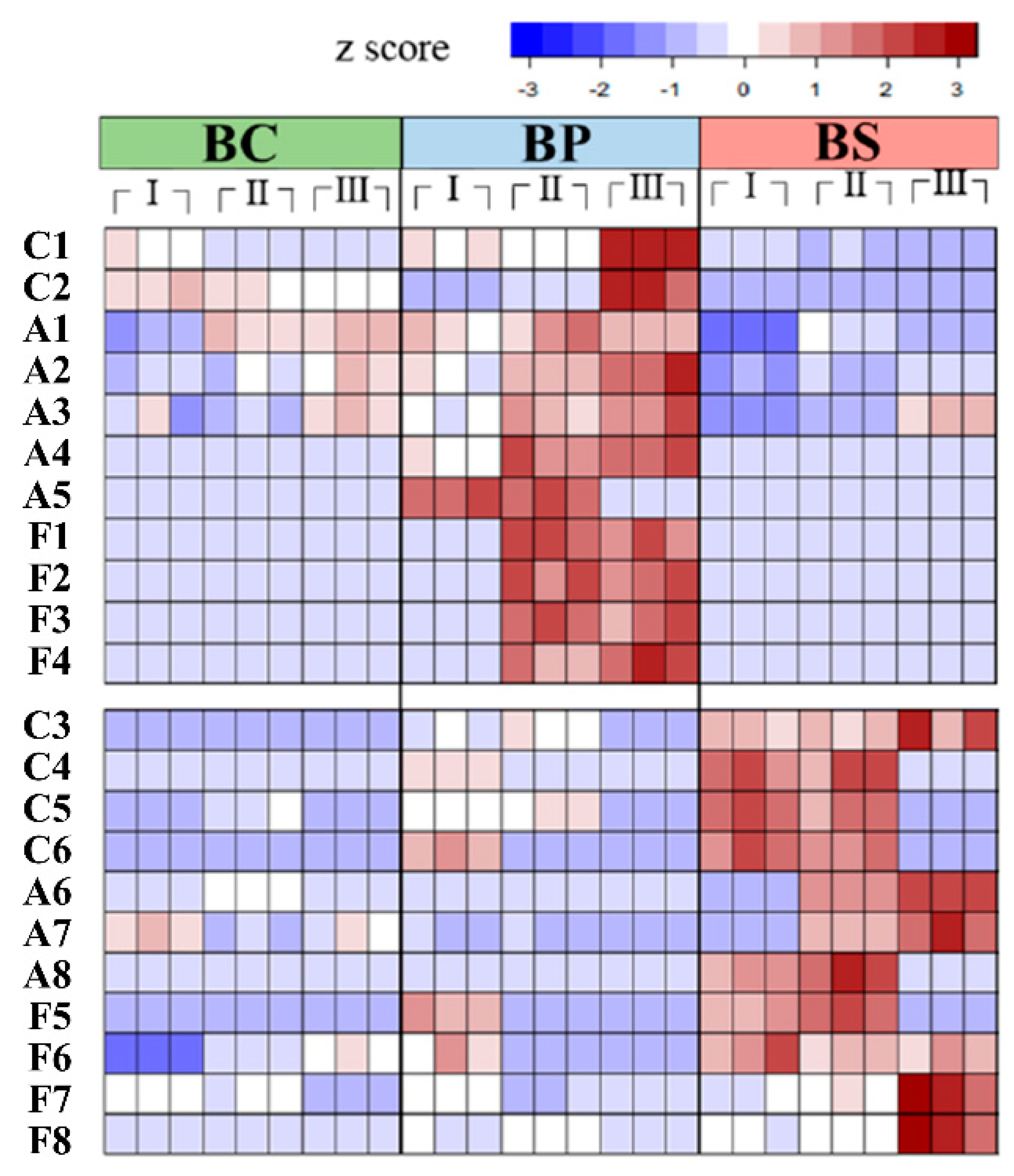
2.1. Analysis of Primary and Secondary Volatile Metabolite Profiles in B. subtilis
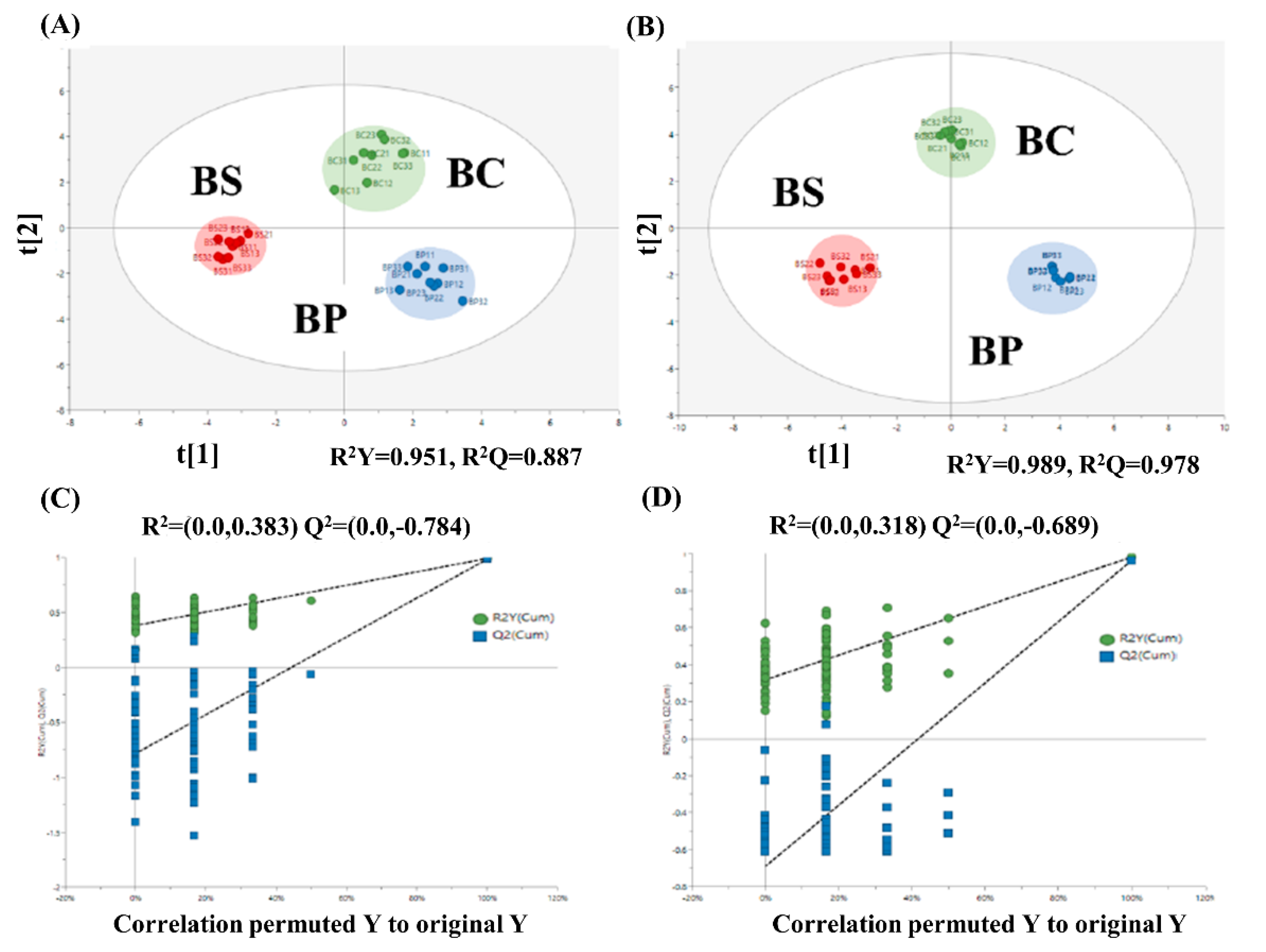
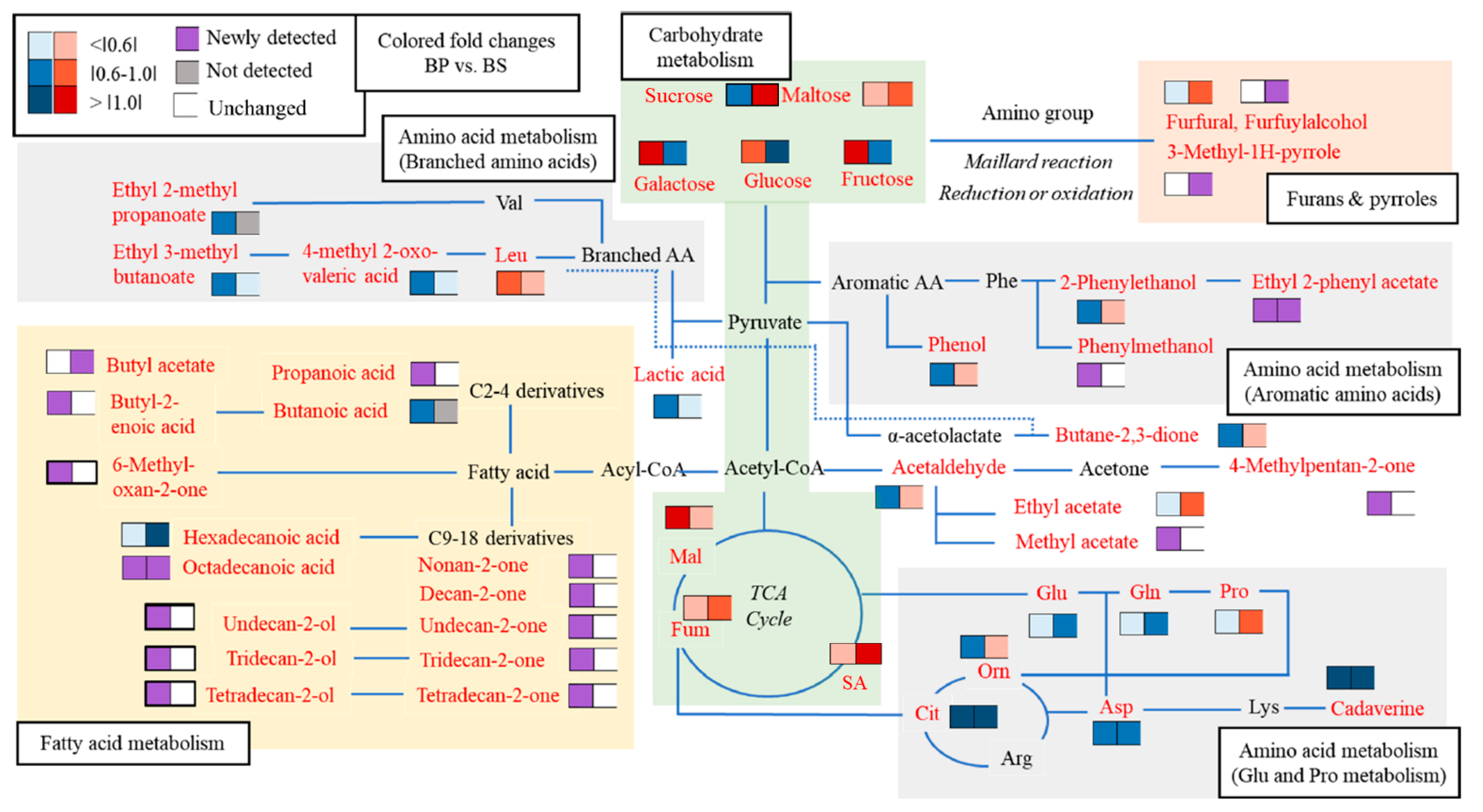
2.2. The Changes of Metabolic Pathways in Bacillus subtilis
2.3. Leucine Metabolism
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Strain and Cultivation of Bacillus subtilis
4.3. Extraction Non-Volatile Primary Metabolites and GC–TOF/MS Analysis
4.4. Extraction of Volatile Secondary Metabolites and GC–MS Analysis
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Buchholz, A.; Hurlebaus, J.; Wandrey, C.; Takors, R. Metabolomics: Quantification of intracellular metabolite dynamics. Biomol. Eng. 2002, 19, 5–15. [Google Scholar] [CrossRef]
- Park, M.K.; Kim, Y.-S. Mass spectrometry based metabolomics approach on the elucidation of volatile metabolites formation in fermented foods: A mini review. Food Sci. Biotechnol. 2021, 30, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Stein, T. Bacillus subtilis antibiotics: Structures, syntheses and specific functions. Mol. Microbiol. 2005, 56, 845–857. [Google Scholar] [CrossRef]
- Beaumont, M. Flavouring composition prepared by fermentation with Bacillus spp. Int. J. Food Microbiol. 2002, 75, 189–196. [Google Scholar] [CrossRef]
- Lee, E.J.; Kim, J.K. Characteristics of taste components of chongkukjang fermented with Bacillus subtilis. Food Sci. Biotechnol. 2004, 13, 572–575. [Google Scholar]
- Liu, Y.; Song, H.; Luo, H. Correlation between the key aroma compounds and gDNA copies of Bacillus during fermentation and maturation of natto. Food Res. Int. 2018, 112, 175–183. [Google Scholar] [CrossRef]
- Hahne, H.; Mader, U.; Otto, A.; Bonn, F.; Steil, L.; Bremer, E.; Hecker, M.; Becher, D. A comprehensive proteomics and transcriptomics analysis of Bacillus subtilis salt stress adaptation. J. Bacteriol. 2010, 192, 870–882. [Google Scholar] [CrossRef]
- Olajuyigbe, F.M.; Ajele, J.O. Production dynamics of extracellular protease from Bacillus species. Afr. J. Biotechnol. 2005, 4, 776–779. [Google Scholar]
- Kohlstedt, M.; Sappa, P.K.; Mayer, H.; Maab, S.; Zaprasis, A.; Hoffman, T.; Becker, J.; Steil, L.; Hecker, M.; van Dijl, J.N.; et al. Adaptation of Bacillus subtilis carbon core metabolism to simultaneous nutrient limitation and osmotic challenge: A multi-omics perspective. Environ. Microbiol. 2014, 16, 1898–1917. [Google Scholar] [CrossRef]
- Sharma, K.M.; Kumar, R.; Panwar, S.; Kumar, A. Microbial alkaline proteases: Optimization of production parameters and their properties. J. Genet. Eng. Biotechnol. 2017, 15, 115–126. [Google Scholar] [CrossRef]
- Fleming-Jones, M.E.; Smith, R.E. Volatile organic compounds in foods: A five year study. J. Agric. Food Chem. 2003, 51, 8120–8127. [Google Scholar] [CrossRef] [PubMed]
- Layman, D.K. The role of leucine in weight loss diets and glucose homeostasis. J. Nutr. 2003, 133, 261S–267S. [Google Scholar] [CrossRef] [PubMed]
- Patti, M.E.; Brambilla, E.; Luzi, L.; Landaker, E.J.; Kahn, C.R. Bidirectional modulation of insulin action by amino acids. J. Clin. Invest. 1998, 101, 1519–1529. [Google Scholar] [CrossRef] [PubMed]
- Borris, R.; Danchin, A.; Harwood, C.R.; Médigue, C.; Rocha, E.P.C.; Sekowska, A.; Vallenet, D. Bacillus subtilis, the model gram-positive bacterium: 20 years of annotation refinement. Microb. Biotechnol. 2017, 11, 3–17. [Google Scholar] [CrossRef]
- Bro, R.; Smilde, A.K. Principal component analysis. Anal. Methods 2014, 6, 2812–2831. [Google Scholar] [CrossRef]
- Triba, M.N.; Le Moyec, L.; Amathieu, R.; Goossens, C.; Bouchemal, N.; Nahon, P. PLS/OPLS models in metabolomics: The impact of permutation of dataset rows on the K-fold cross-validation quality parameters. Mol. Biosyst. 2015, 11, 13–19. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, K.; Zhang, D.; Duan, H.; Liu, Y.; Guo, M. Metabolite accumulation and metabolic network in developing roots of Rehmannia glutinosa reveals its root developmental mechanism and quality. Sci. Rep. 2018, 8, 14127. [Google Scholar] [CrossRef]
- Vandamme, E.J.; Soetaert, W. Bioflavours and fragrances via fermentation and biocatalysis. J. Chem. Technol. Biotechnol. 2002, 77, 1323–1332. [Google Scholar] [CrossRef]
- Brill, J.; Hoffmann, T.; Bleisteiner, M.; Bremer, E. Osmotically controlled synthesis of the compatible solute proline is critical for cellular defense of Bacillus subtilis against high osmolarity. J. Bacteriol. 2011, 193, 5335–5346. [Google Scholar] [CrossRef]
- Huang, J.; Hirji, R.; Adam, L.; Rozwadowski, K.L.; Hammerlindl, J.K.; Keller, W.A.; Selvaraj, F. Genetic engineering of glycinebetaine production toward enhancing stress tolerance in plants: Metabolic limitations. Plant Physiol. 2000, 122, 747–756. [Google Scholar] [CrossRef]
- Garg, A.K.; Kim, J.K.; Owens, T.G.; Ranwala, A.P.; Choi, Y.D.; Kochian, L.V.; Wu, R.J. Trehalose accumulation in rice plants confers high tolerance levels to different abiotic stresses. Proc. Natl. Acad. Sci. USA 2002, 99, 15898–15903. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wei, H.; Cao, J.; Liu, R.; Wang, Y.; Zheng, C. Expression of Bacillus subtilis proBA genes and reduction of feedback inhibition of proline synthesis increases proline production and confers osmotolerance in transgenic arabidopsis. BMB Rep. 2007, 40, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, I.M.; Guido, L.F. Impact of wort amino acids on beer flavour: A review. Fermentation 2018, 4, 23. [Google Scholar] [CrossRef]
- Jo, Y.; Cho, I.H.; Song, C.K.; Shin, H.W.; Kim, Y.-S. Comparison of fermented soybean paste (Doenjang) prepared by different methods based on profiling of volatile compounds. J. Food Sci. 2011, 76, C368–C379. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.; Juwono, N.K.P.; Leong, S.S.J.; Chang, M.W. Production of fatty acid-derived valuable chemicals in synthetic microbes. Front. Bioeng. Biotechnol. 2014, 2, 78. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, A.; McNeil, B.A.; Stuart, D.T. Biosynthesis of fatty alcohols in engineered microbial cell factories: Advances and limitations. Front. Bioeng. Biotechnol. 2020, 8, 610936. [Google Scholar] [CrossRef]
- Qu, G.; Guo, J.; Yang, D.; Sun, Z. Biocatalysis of carboxylic acid reductases: Phylogenesis, catalytic mechanism and potential applications. Green Chem. 2018, 20, 777–792. [Google Scholar] [CrossRef]
- Azudin, N.Y.; Mashitah, M.; Abd Shukor, S.R. Optimization of isoamyl acetate production in a solvent-free system. J. Food Qual. 2013, 36, 441–446. [Google Scholar] [CrossRef]
- Liu, M.; Bienfait, B.; Sacher, O.; Gasteiger, J.; Siezen, R.J.; Nauta, A.; Geurts, J.M.W. Combining chemoinformatics with bioinformatics: In silico prediction of bacterial flavor forming pathways by a chemical systems biology approach “reverse pathway engineering”. PLoS ONE 2014, 9, e84769. [Google Scholar] [CrossRef]
- Zhou, J.; He, L.; Gao, Y.; Han, N.; Zhang, R.; Wu, Q.; Li, J.; Tang, X.; Xu, B.; Ding, J.; et al. Characterization of a novel low-temperature-active, alkaline and sucrose-tolerant invertase. Sci. Rep. 2016, 6, 32081. [Google Scholar] [CrossRef]
- Serrano-Ruiz, J.C.; Luque, R.; Sepúlveda-Escribano, A. Transformations of biomass derived platform molecules: From high added-value chemicals to fuels via aqueous-phase processing. Chem. Soc. Rev. 2011, 40, 5266–5281. [Google Scholar] [CrossRef] [PubMed]
- Sadh, P.K.; Kumar, S.; Chawla, P.; Duhan, J.S. Fermentation: A boon for production of bioactive compounds by processing of food industries wastes (by-products). Molecules 2018, 23, 2560. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, T.; Wensing, A.; Brosius, M.; Steil, L.; Völker, U.; Bremer, E. Osmotic control of opuA expression in Bacillus subtilis and its modulation in response to intracellular glycine betaine and proline pools. J. Bacteriol. 2013, 195, 510–522. [Google Scholar] [CrossRef] [PubMed]
- Umair, S.; Leung, Y.; Bland, R.; Simpson, H. Enzymes of the ornithine–glutamate– proline pathway in the sheep abomasal nematode parasites Haemonchus contortus and Teladorsagia circumcincta. Exp. Parasitol. 2011, 129, 115–119. [Google Scholar] [CrossRef]
- Sun, Y.-L.; Hong, S.-K. Exogenous proline mitigates the detrimental effects of saline and alkaline stresses in Leymus chinensis (Trin.). J. Plant Biotechnol. 2010, 37, 529–538. [Google Scholar] [CrossRef][Green Version]
- Yamauchi, N. The pathway of leucine to mevalonate in halophilic Archaea: Efficient incorporation of leucine into isoprenoidal lipid with the involvement of isovaleryl-CoA dehydrogenase in Halobacterium salinarum. Biosci. Biotechnol. Biochem. 2010, 74, 443–446. [Google Scholar] [CrossRef]
- Torres, S.; Baigorí, M.D.; Swathy, S.; Pandey, A.; Castro, G.R. Enzymatic synthesis of banana flavour (isoamyl acetate) by Bacillus licheniformis S-86 esterase. Food Res. Int. 2009, 42, 454–460. [Google Scholar] [CrossRef]
- Niu, Y.; Zhu, Q.; Xiao, Z. Characterization of perceptual interactions among ester aroma compounds found in Chinese Moutai Baijiu by gas chromatography-olfactometry, odor Intensity, olfactory threshold and odor activity value. Food Res. Int. 2020, 131, 108986. [Google Scholar] [CrossRef]
- Park, M.K.; Seo, J.-A.; Kim, Y.S. Comparative study on metabolic changes of Aspergillus oryzae isolated from fermented foods according to culture conditions. Int. J. Food Microbiol. 2019, 307, 108270. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, M.-K.; Lee, S.; Kim, Y.-S. Effects of pH and Osmotic Changes on the Metabolic Expressions of Bacillus subtilis Strain 168 in Metabolite Pathways including Leucine Metabolism. Metabolites 2022, 12, 112. https://doi.org/10.3390/metabo12020112
Park M-K, Lee S, Kim Y-S. Effects of pH and Osmotic Changes on the Metabolic Expressions of Bacillus subtilis Strain 168 in Metabolite Pathways including Leucine Metabolism. Metabolites. 2022; 12(2):112. https://doi.org/10.3390/metabo12020112
Chicago/Turabian StylePark, Min-Kyung, Soyeon Lee, and Young-Suk Kim. 2022. "Effects of pH and Osmotic Changes on the Metabolic Expressions of Bacillus subtilis Strain 168 in Metabolite Pathways including Leucine Metabolism" Metabolites 12, no. 2: 112. https://doi.org/10.3390/metabo12020112
APA StylePark, M.-K., Lee, S., & Kim, Y.-S. (2022). Effects of pH and Osmotic Changes on the Metabolic Expressions of Bacillus subtilis Strain 168 in Metabolite Pathways including Leucine Metabolism. Metabolites, 12(2), 112. https://doi.org/10.3390/metabo12020112





