Optimising Fluvoxamine Maternal/Fetal Exposure during Gestation: A Pharmacokinetic Virtual Clinical Trials Study
Abstract
1. Introduction
2. Materials and Methods
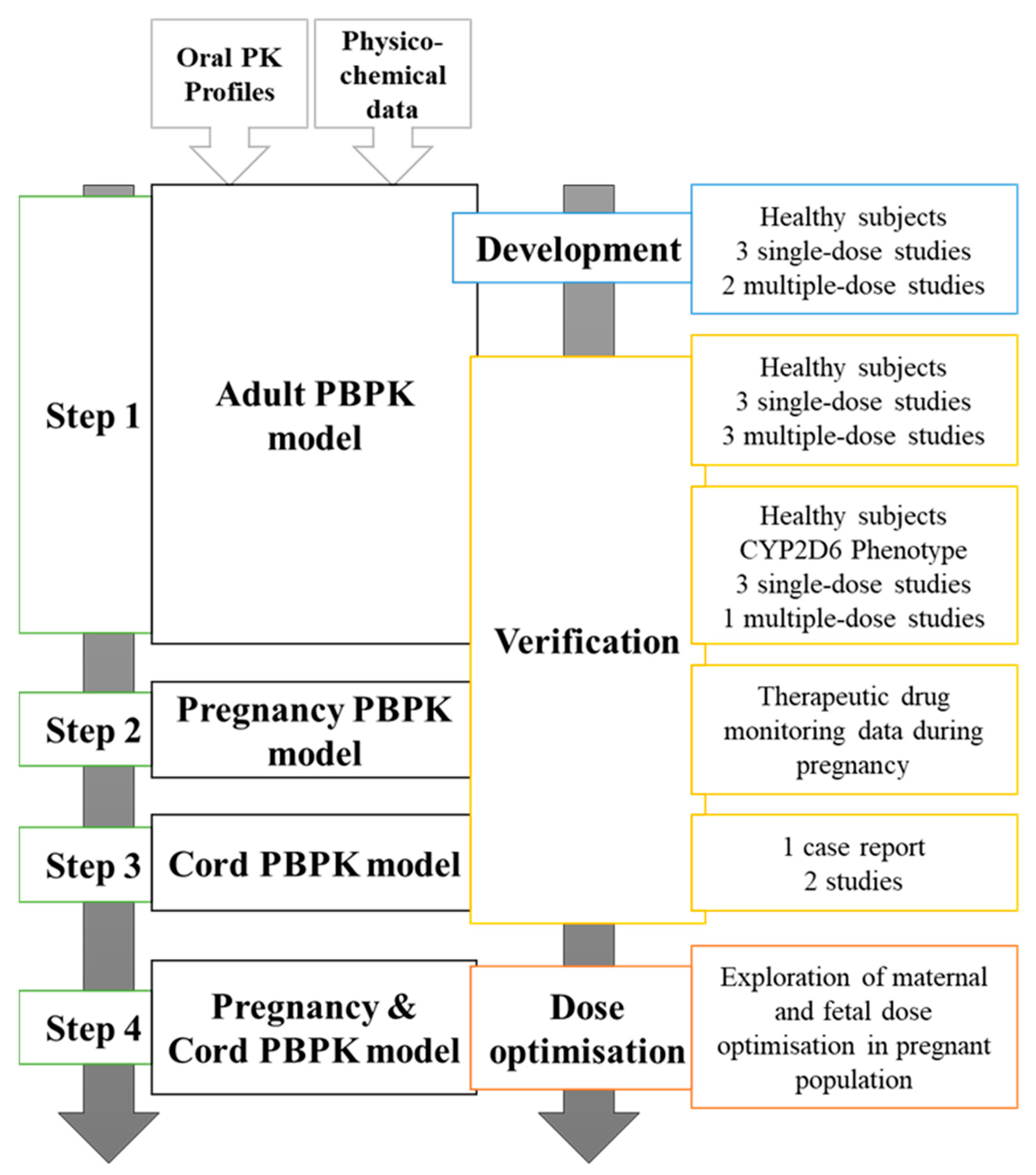
2.1. Step 1: Development and Verification of Fluvoxamine Model in a Healthy Population
2.2. Step 2: Validation of Fluvoxamine PBPK Model in Pregnancy
2.3. Step 3: Validation of Fluvoxamine Fetoplacental PBPK Model
(0.135 × GW) − (0.023 × GW2) + (0.0015 × GW3) − (0.00002 × GW4)
2.4. Step 3: Influence of CYP2D6 Phenotype and Dose Adjustment during Gestation
2.5. Prediction Performance
2.6. Data and Statistical Analysis
3. Results
3.1. Step 1: Development and Validation of Fluvoxamine Model in a Healthy Population
3.2. Step 2: Verification of Fluvoxamine Model in Pregnancy and the Impact of Pregnancy on Fluvoxamine Level
3.3. Step 3: Validation of Fluvoxamine Fetoplacental PBPK Model
3.4. Step 4: Impact of CYP2D6 Phenotype and Dose Adjustment during Gestation
4. Discussion
4.1. Step 1: Validation of Fluvoxamine Model in Healthy Subjects
4.1.1. PBPK Model Parameters
4.1.2. Validation in Healthy Subjects and CYP2D6 Phenotype Populations
4.2. Step 2: Verification of Fluvoxamine Pregnancy Model and the Impact of Pregnancy on Fluvoxamine Concentration
4.2.1. Verification of Fluvoxamine Pregnancy Model
4.2.2. The Impact of Pregnancy on Fluvoxamine Concentration
4.3. Step 3: Validation of Fluvoxamine Fetoplacental PBPK Model
4.4. Step 4: Impact of CYP2D6 Phenotype and Dose Adjustment during Gestation
4.4.1. Impact of CYP2D6 Phenotype in the Pregnant Population
4.4.2. Maternal Plasma Concentration Changes throughout Pregnancy
4.4.3. Umbilical Cord Concentration Changes throughout Pregnancy
4.4.4. Fluvoxamine Dosing Adjustment during Pregnancy
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gaynes, B.N.; Gavin, N.; Meltzer-Brody, S.; Lohr, K.N.; Swinson, T.; Gartlehner, G.; Brody, S.; Miller, W.C. Perinatal depression: Prevalence, screening accuracy, and screening outcomes. Evid. Rep. Technol. Assess (Summ) 2005, 1–8. [Google Scholar] [CrossRef]
- Okagbue, H.I.; Adamu, P.I.; Bishop, S.A.; Oguntunde, P.E.; Opanuga, A.A.; Akhmetshin, E.M. Systematic review of prevalence of antepartum depression during the trimesters of pregnancy. Open Access Maced. J. Med. Sci. 2019, 7, 1555–1560. [Google Scholar] [CrossRef]
- Bennett, H.A.; Einarson, A.; Taddio, A.; Koren, G.; Einarson, T.R. Prevalence of depression during pregnancy: Systematic review. Obstet. Gynecol. 2004, 103, 698–709. [Google Scholar] [CrossRef]
- Wichman, C.L.; Stern, T.A. Diagnosing and treating depression during pregnancy. Prim. Care Companion CNS Disord. 2015, 17. [Google Scholar] [CrossRef]
- Hiemke, C.; Bergemann, N.; Clement, H.W.; Conca, A.; Deckert, J.; Domschke, K.; Eckermann, G.; Egberts, K.; Gerlach, M.; Greiner, C.; et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: Update 2017. Pharmacopsychiatry 2018, 51, e1. [Google Scholar] [CrossRef]
- Andrade, S.E.; Raebel, M.A.; Brown, J.; Lane, K.; Livingston, J.; Boudreau, D.; Rolnick, S.J.; Roblin, D.; Smith, D.H.; Willy, M.E.; et al. Use of antidepressant medications during pregnancy: A multisite study. Am. J. Obstet. Gynecol. 2008, 198. [Google Scholar] [CrossRef]
- Molenaar, N.M.; Bais, B.; Lambregtse-van den Berg, M.P.; Mulder, C.L.; Howell, E.A.; Fox, N.S.; Rommel, A.S.; Bergink, V.; Kamperman, A.M. The international prevalence of antidepressant use before, during, and after pregnancy: A systematic review and meta-analysis of timing, type of prescriptions and geographical variability. J. Affect. Disord. 2020, 264, 82–89. [Google Scholar] [CrossRef]
- Irons, J. Fluvoxamine in the treatment of anxiety disorders. Neuropsychiatr. Dis. Treat. 2005, 1, 289–299. [Google Scholar]
- Stein, D.J.; Westenberg, H.G.M.; Yang, H.C.; Li, D.; Barbato, L.M. Fluvoxamine CR in the long-term treatment of social anxiety disorder: The 12- to 24-week extension phase of a multicentre, randomized, placebo-controlled trial. Int. J. Neuropsychoph. 2003, 6, 317–323. [Google Scholar] [CrossRef][Green Version]
- Escalona, R.; Canive, J.M.; Calais, L.A.; Davidson, J.R. Fluvoxamine treatment in veterans with combat-related post-traumatic stress disorder. Depress. Anxiety 2002, 15, 29–33. [Google Scholar] [CrossRef]
- Milano, W.; Siano, C.; Putrella, C.; Capasso, A. Treatment of bulimia nervosa with fluvoxamine: A randomized controlled trial. Adv. Ther. 2005, 22, 278–283. [Google Scholar] [CrossRef]
- The United States Food and Drug Administration. Drug Approval Package: Luvox (Fluvoxamine Maleate) 25mg, 50mg, and 100mg Tablets. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2007/021519_luvox_toc.cfm (accessed on 2 November 2021).
- The United States Food and Drug Administration. Pregnancy and Lactation Labeling (Drugs) Final Rule. Available online: https://www.fda.gov/drugs/labeling-information-drug-products/pregnancy-and-lactation-labeling-drugs-final-rule (accessed on 16 November 2021).
- The United States Food and Drug Administration. Fluvoxamine Maleate Full Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/021519s018lbl.pdf (accessed on 16 November 2021).
- Byatt, N.; Deligiannidis, K.M.; Freeman, M.P. Antidepressant use in pregnancy: A critical review focused on risks and controversies. Acta Psychiatr. Scand. 2013, 127, 94–114. [Google Scholar] [CrossRef]
- Berard, A.; Zhao, J.P.; Sheehy, O. Antidepressant use during pregnancy and the risk of major congenital malformations in a cohort of depressed pregnant women: An updated analysis of the Quebec Pregnancy Cohort. Bmj Open 2017, 7, e013372. [Google Scholar] [CrossRef]
- Zakiyah, N.; Ter Heijne, L.F.; Bos, J.H.; Hak, E.; Postma, M.J.; Schuiling-Veninga, C.C.M. Antidepressant use during pregnancy and the risk of developing gestational hypertension: A retrospective cohort study. BMC Pregnancy Childbirth 2018, 18, 187. [Google Scholar] [CrossRef]
- Westin, A.A.; Brekke, M.; Molden, E.; Skogvoll, E.; Spigset, O. Selective serotonin reuptake inhibitors and venlafaxine in pregnancy: Changes in drug disposition. PLoS ONE 2017, 12, e0181082. [Google Scholar] [CrossRef]
- Spigset, O.; Axelsson, S.; Norstrom, A.; Hagg, S.; Dahlqvist, R. The major fluvoxamine metabolite in urine is formed by CYP2D6. Eur. J. Clin. Pharmacol. 2001, 57, 653–658. [Google Scholar] [CrossRef]
- Almurjan, A.; Macfarlane, H.; Badhan, R.K.S. Precision dosing-based optimisation of paroxetine during pregnancy for poor and ultrarapid CYP2D6 metabolisers: A virtual clinical trial pharmacokinetics study. J. Pharm. Pharmacol. 2020, 72, 1049–1060. [Google Scholar] [CrossRef]
- Zhuang, X.; Lu, C. PBPK modeling and simulation in drug research and development. Acta Pharm. Sin. B 2016, 6, 430–440. [Google Scholar] [CrossRef]
- Abduljalil, K.; Badhan, R.K.S. Drug dosing during pregnancy-opportunities for physiologically based pharmacokinetic models. J. Pharmacokinet. Pharmacodyn. 2020, 47, 319–340. [Google Scholar] [CrossRef]
- Almurjan, A.; Macfarlane, H.; Badhan, R.K.S. The application of precision dosing in the use of sertraline throughout pregnancy for poor and ultrarapid metabolizer CYP 2C19 subjects: A virtual clinical trial pharmacokinetics study. Biopharm. Drug Dispos. 2021, 42, 252–262. [Google Scholar] [CrossRef]
- Badhan, R.K.S.; Gittins, R. Precision dosing of methadone during pregnancy: A pharmacokinetics virtual clinical trials study. J. Subst. Abuse Treat. 2021, 130, 108521. [Google Scholar] [CrossRef]
- Olafuyi, O.; Badhan, R.K.S. Dose optimization of chloroquine by pharmacokinetic modeling during pregnancy for the treatment of zika virus infection. J. Pharm. Sci. 2019, 108, 661–673. [Google Scholar] [CrossRef]
- Badhan, R.K.S.; Macfarlane, H. Quetiapine dose optimisation during gestation: A pharmacokinetic modelling study. J. Pharm. Pharmacol. 2020, 72, 670–681. [Google Scholar] [CrossRef]
- Qasqas, S.A.; McPherson, C.; Frishman, W.H.; Elkayam, U. Cardiovascular pharmacotherapeutic considerations during pregnancy and lactation. Cardiol. Rev. 2004, 12, 240–261. [Google Scholar] [CrossRef]
- Murphy, M.M.; Scott, J.M.; McPartlin, J.M.; Fernandez-Ballart, J.D. The pregnancy-related decrease in fasting plasma homocysteine is not explained by folic acid supplementation, hemodilution, or a decrease in albumin in a longitudinal study. Am. J. Clin. Nutr. 2002, 76, 614–619. [Google Scholar] [CrossRef]
- Cheung, C.K.; Lao, T.; Swaminathan, R. Urinary excretion of some proteins and enzymes during normal pregnancy. Clin. Chem. 1989, 35, 1978–1980. [Google Scholar] [CrossRef]
- Feghali, M.; Venkataramanan, R.; Caritis, S. Pharmacokinetics of drugs in pregnancy. Semin. Perinatol. 2015, 39, 512–519. [Google Scholar] [CrossRef]
- Davison, J.M.; Dunlop, W. Renal hemodynamics and tubular function normal human pregnancy. Kidney Int. 1980, 18, 152–161. [Google Scholar] [CrossRef]
- Rodgers, T.; Leahy, D.; Rowland, M. Physiologically based pharmacokinetic modeling 1: Predicting the tissue distribution of moderate-to-strong bases. J. Pharm. Sci. 2005, 94, 1259–1276. [Google Scholar] [CrossRef]
- Rodgers, T.; Rowland, M. Physiologically based pharmacokinetic modelling 2: Predicting the tissue distribution of acids, very weak bases, neutrals and zwitterions. J. Pharm. Sci. 2006, 95, 1238–1257. [Google Scholar] [CrossRef]
- Mylan. Faverin 50 mg Film-Coated Tablets, Summary of Product Characteristics (SPC). Available online: https://www.medicines.org.uk/emc/product/1169/smpc#gref (accessed on 12 October 2021).
- Vezmar, S.; Miljkovic, B.; Vucicevic, K.; Timotijevic, I.; Prostran, M.; Todorovic, Z.; Pokrajac, M. Pharmacokinetics and efficacy of fluvoxamine and amitriptyline in depression. J. Pharmacol. Sci. 2009, 110, 98–104. [Google Scholar] [CrossRef]
- Ezuruike, U.; Zhang, M.; Pansari, A.; De Sousa Mendes, M.; Pan, X.; Neuhoff, S.; Gardner, I. Guide to development of compound files for PBPK modeling in the Simcyp population-based simulator. CPT Pharmacomet. Syst. Pharmacol. 2022, 11, 805–821. [Google Scholar] [CrossRef]
- Turner, D.; Musther, H.; Jamei, M.; Rostami-Hodjegan, A. A Mechanistic Model for the Prediction of Equilibrium Blood-to-Plasma Concentration Ratio (B/P) in Human Blood: Basic and Neutral Drugs. In Proceedings of the AAPS Annual Meeting and Exposition, Washington, DC, USA, 23–27 October 2011. [Google Scholar]
- Winiwarter, S.; Bonham, N.M.; Ax, F.; Hallberg, A.; Lennernas, H.; Karlen, A. Correlation of human jejunal permeability (in vivo) of drugs with experimentally and theoretically derived parameters. A multivariate data analysis approach. J. Med. Chem. 1998, 41, 4939–4949. [Google Scholar] [CrossRef]
- De Vries, M.H.; Van Harten, J.; Van Bemmel, P.; Raghoebar, M. Pharmacokinetics of fluvoxamine maleate after increasing single oral doses in healthy subjects. Biopharm. Drug Dispos. 1993, 14, 291–296. [Google Scholar] [CrossRef]
- Van Harten, J.; Van Bemmel, P.; Dobrinska, M.R.; Ferguson, R.K.; Raghoebar, M. Bioavailability of fluvoxamine given with and without food. Biopharm. Drug Dispos. 1991, 12, 571–576. [Google Scholar] [CrossRef]
- Bahrami, G.; Mohammadi, B. Rapid and sensitive bioanalytical method for measurement of fluvoxamine in human serum using 4-chloro-7-nitrobenzofurazan as pre-column derivatization agent: Application to a human pharmacokinetic study. J. Chromatogr. B 2007, 857, 322–326. [Google Scholar] [CrossRef]
- de Vries, M.H.; Raghoebar, M.; Mathlener, I.S.; van Harten, J. Single and multiple oral dose fluvoxamine kinetics in young and elderly subjects. Ther. Drug Monit. 1992, 14, 493–498. [Google Scholar] [CrossRef]
- Fleishaker, J.C.; Hulst, L.K. A pharmacokinetic and pharmacodynamic evaluation of the combined administration of alprazolam and fluvoxamine. Eur. J. Clin. Pharmacol. 1994, 46, 35–39. [Google Scholar] [CrossRef]
- Orlando, R.; De Martin, S.; Andrighetto, L.; Floreani, M.; Palatini, P. Fluvoxamine pharmacokinetics in healthy elderly subjects and elderly patients with chronic heart failure. Br. J. Clin. Pharmacol. 2010, 69, 279–286. [Google Scholar] [CrossRef]
- Debree, H.; Vanderschoot, J.B.; Post, L.C. Fluvoxamine maleate - Disposition in man. Eur. J. Drug Metab. Pharmacokinet. 1983, 8, 175–179. [Google Scholar] [CrossRef]
- The United States Food and Drug Administration. Clinical Pharmacology and Biopharmaceutics Review for Luvox Extended-Release; US FDA: Silver Spring, MD, USA, 2008.
- Spigset, O.; Granberg, K.; Hagg, S.; Soderstrom, E.; Dahlqvist, R. Non-linear fluvoxamine disposition. Br. J. Clin. Pharmacol. 1998, 45, 257–263. [Google Scholar] [CrossRef][Green Version]
- Carrillo, J.A.; Dahl, M.L.; Svensson, J.O.; Alm, C.; Rodriguez, I.; Bertilsson, L. Disposition of fluvoxamine in humans is determined by the polymorphic CYP2D6 and also by the CYP1A2 activity. Clin. Pharmacol. Ther. 1996, 60, 183–190. [Google Scholar] [CrossRef]
- Spigset, O.; Granberg, K.; Hagg, S.; Norstrom, A.; Dahlqvist, R. Relationship between fluvoxamine pharmacokinetics and CYP2D6/CYP2C19 phenotype polymorphisms. Eur. J. Clin. Pharmacol. 1997, 52, 129–133. [Google Scholar] [CrossRef]
- Hartter, S.; Grozinger, M.; Weigmann, H.; Roschke, J.; Hiemke, C. Increased bioavailability of oral melatonin after fluvoxamine coadministration. Clin. Pharmacol. Ther. 2000, 67, 1–6. [Google Scholar] [CrossRef]
- Christensen, M.; Tybring, G.; Mihara, K.; Yasui-Furokori, N.; Carrillo, J.A.; Ramos, S.I.; Andersson, K.; Dahl, M.L.; Bertilsson, L. Low daily 10-mg and 20-mg doses of fluvoxamine inhibit the metabolism of both caffeine (cytochrome P4501A2) and omeprazole (cytochrome P4502C19). Clin. Pharmacol. Ther. 2002, 71, 141–152. [Google Scholar] [CrossRef]
- Gaohua, L.; Abduljalil, K.; Jamei, M.; Johnson, T.N.; Rostami-Hodjegan, A. A pregnancy physiologically based pharmacokinetic (p-PBPK) model for disposition of drugs metabolized by CYP1A2, CYP2D6 and CYP3A4. Br. J. Clin. Pharmacol. 2012, 74, 873–885. [Google Scholar] [CrossRef]
- Abduljalil, K. Predicting Drug Pharmacokinetics in Pregnancy, Fetal, and Lactation Using PBPK; 2020. [Google Scholar]
- Ryu, R.J.; Eyal, S.; Easterling, T.R.; Caritis, S.N.; Venkataraman, R.; Hankins, G.; Rytting, E.; Thummel, K.; Kelly, E.J.; Risler, L.; et al. Pharmacokinetics of metoprolol during pregnancy and lactation. J. Clin. Pharmacol. 2016, 56, 581–589. [Google Scholar] [CrossRef]
- Achour, B.; Russell, M.R.; Barber, J.; Rostami-Hodjegan, A. Simultaneous quantification of the abundance of several cytochrome P450 and uridine 5′-diphospho-glucuronosyltransferase enzymes in human liver microsomes using multiplexed targeted proteomics. Drug Metab. Dispos. 2014, 42, 500–510. [Google Scholar] [CrossRef]
- Hostetter, A.; Ritchie, J.C.; Stowe, Z.N. Amniotic fluid and umbilical cord blood concentrations of antidepressants in three women. Biol. Psychiatry 2000, 48, 1032–1034. [Google Scholar] [CrossRef]
- Sit, D.; Perel, J.M.; Wisniewski, S.R.; Helsel, J.C.; Luther, J.F.; Wisner, K.L. Mother-infant antidepressant concentrations, maternal depression, and perinatal events. J. Clin. Psychiatry 2011, 72, 994–1001. [Google Scholar] [CrossRef]
- Rampono, J.; Simmer, K.; Ilett, K.F.; Hackett, L.P.; Doherty, D.A.; Elliot, R.; Kok, C.H.; Coenen, A.; Forman, T. Placental transfer of SSRI and SNRI antidepressants and effects on the neonate. Pharmacopsychiatry 2009, 42, 95–100. [Google Scholar] [CrossRef]
- Edginton, A.N.; Schmitt, W.; Willmann, S. Development and evaluation of a generic physiologically based pharmacokinetic model for children. Clin. Pharmacokinet. 2006, 45, 1013–1034. [Google Scholar] [CrossRef]
- Ginsberg, G.; Hattis, D.; Russ, A.; Sonawane, B. Physiologically based pharmacokinetic (PBPK) modeling of caffeine and theophylline in neonates and adults: Implications for assessing children’s risks from environmental agents. J. Toxicol. Environ. Health A 2004, 67, 297–329. [Google Scholar] [CrossRef]
- Parrott, N.; Davies, B.; Hoffmann, G.; Koerner, A.; Lave, T.; Prinssen, E.; Theogaraj, E.; Singer, T. Development of a physiologically based model for oseltamivir and simulation of pharmacokinetics in neonates and infants. Clin. Pharmacokinet. 2011, 50, 613–623. [Google Scholar] [CrossRef]
- he United States Food and Drug Administration. Summary minutes of the Advisory Committee for Pharmaceutical Science and Clinical Pharmacology; 2012. [Google Scholar]
- The United States Food and Drug Administration. FDA Drug Safety Communication: Selective Serotonin Reuptake Inhibitor (SSRI) Antidepressant Use during Pregnancy and Reports of a Rare Heart and Lung Condition in Newborn Babies. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-selective-serotonin-reuptake-inhibitor-ssri-antidepressant-use-during (accessed on 16 November 2021).
- Einarson, A.; Choi, J.; Einarson, T.R.; Koren, G. Incidence of major malformations in infants following antidepressant exposure in pregnancy: Results of a large prospective cohort study. Can. J. Psychiatry 2009, 54, 242–246. [Google Scholar] [CrossRef]
- Furu, K.; Kieler, H.; Haglund, B.; Engeland, A.; Selmer, R.; Stephansson, O.; Valdimarsdottir, U.A.; Zoega, H.; Artama, M.; Gissler, M.; et al. Selective serotonin reuptake inhibitors and venlafaxine in early pregnancy and risk of birth defects: Population based cohort study and sibling design. BMJ 2015, 350, h1798. [Google Scholar] [CrossRef]
- McElhatton, P.R.; Garbis, H.M.; Elefant, E.; Vial, T.; Bellemin, B.; Mastroiacovo, P.; Arnon, J.; Rodriguez-Pinilla, E.; Schaefer, C.; Pexieder, T.; et al. The outcome of pregnancy in 689 women exposed to therapeutic doses of antidepressants. A collaborative study of the European Network of Teratology Information Services (ENTIS). Reprod. Toxicol. 1996, 10, 285–294. [Google Scholar] [CrossRef]
- Kulin, N.A.; Pastuszak, A.; Sage, S.R.; Schick-Boschetto, B.; Spivey, G.; Feldkamp, M.; Ormond, K.; Matsui, D.; Stein-Schechman, A.K.; Cook, L.; et al. Pregnancy outcome following maternal use of the new selective serotonin reuptake inhibitors - A prospective controlled multicenter study. JAMA-J. Am. Med. Assoc. 1998, 279, 609–610. [Google Scholar] [CrossRef]
- Sivojelezova, A. Fluvoxamine (Luvox (TM)) use in pregnancy. Clin. Pharmacol. Ther. 2004, 75, 25. [Google Scholar] [CrossRef]
- Malm, H.; Artama, M.; Gissler, M.; Ritvanen, A. Selective serotonin reuptake inhibitors and risk for major congenital anomalies reply. Obstet. Gynecol. 2012, 119, 183. [Google Scholar] [CrossRef]
- Abduljalil, K.; Pansari, A.; Jamei, M. Prediction of maternal pharmacokinetics using physiologically based pharmacokinetic models: Assessing the impact of the longitudinal changes in the activity of CYP1A2, CYP2D6 and CYP3A4 enzymes during pregnancy. J. Pharmacokinet. Pharmacodyn. 2020, 47, 361–383. [Google Scholar] [CrossRef]
- European Medicines Agency. Guideline on the Investigation of Bioequivalence. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-investigation-bioequivalence-rev1_en.pdf (accessed on 26 November 2011).
- Noe, D.A. Criteria for reporting noncompartmental estimates of half-life and area under the curve extrapolated to infinity. Pharm. Stat. 2020, 19, 101–112. [Google Scholar] [CrossRef]
- Britz, H.; Hanke, N.; Volz, A.K.; Spigset, O.; Schwab, M.; Eissing, T.; Wendl, T.; Frechen, S.; Lehr, T. Physiologically-Based Pharmacokinetic Models for CYP1A2 Drug-Drug Interaction Prediction: A Modeling Network of Fluvoxamine, Theophylline, Caffeine, Rifampicin, and Midazolam. CPT Pharmacomet. Syst. Pharmacol. 2019, 8, 296–307. [Google Scholar] [CrossRef]
- Abduljalil, K.; Cain, T.; Humphries, H.; Rostami-Hodjegan, A. Deciding on success criteria for predictability of pharmacokinetic parameters from in vitro studies: An analysis based on in vivo observations. Drug Metab. Dispos. 2014, 42, 1478–1484. [Google Scholar] [CrossRef]
- Miura, M.; Ohkubo, T. Identification of human cytochrome P450 enzymes involved in the major metabolic pathway of fluvoxamine. Xenobiotica 2007, 37, 169–179. [Google Scholar] [CrossRef]
- Tracy, T.S.; Venkataramanan, R.; Glover, D.D.; Caritis, S.N.; National Institute for Child Health; Human Development Network of Maternal-Fetal-Medicine Units. Temporal changes in drug metabolism (CYP1A2, CYP2D6 and CYP3A Activity) during pregnancy. Am. J. Obstet. Gynecol. 2005, 192, 633–639. [Google Scholar] [CrossRef]
- Wadelius, M.; Darj, E.; Frenne, G.; Rane, A. Induction of CYP2D6 in pregnancy. Clin. Pharmacol. Ther. 1997, 62, 400–407. [Google Scholar] [CrossRef]
- Spigset, O.; Hagg, S.; Soderstrom, E.; Dahlqvist, R. Lack of correlation between fluvoxamine clearance and CYP1A2 activity as measured by systemic caffeine clearance. Eur. J. Clin. Pharmacol. 1999, 54, 943–946. [Google Scholar] [CrossRef]
- Yu, T.; Campbell, S.C.; Stockmann, C.; Tak, C.; Schoen, K.; Clark, E.A.; Varner, M.W.; Spigarelli, M.G.; Sherwin, C.M. Pregnancy-induced changes in the pharmacokinetics of caffeine and its metabolites. J. Clin. Pharmacol. 2016, 56, 590–596. [Google Scholar] [CrossRef]
- Dawes, M.; Chowienczyk, P.J. Drugs in pregnancy. Pharmacokinetics in pregnancy. Best Pract. Res. Clin. Obstet. Gynaecol. 2001, 15, 819–826. [Google Scholar] [CrossRef][Green Version]
- van Harten, J.; Duchier, J.; Devissaguet, J.P.; van Bemmel, P.; de Vries, M.H.; Raghoebar, M. Pharmacokinetics of fluvoxamine maleate in patients with liver cirrhosis after single-dose oral administration. Clin. Pharmacokinet. 1993, 24, 177–182. [Google Scholar] [CrossRef]
- Orlando, R.; Padrini, R.; Perazzi, M.; De Martin, S.; Piccoli, P.; Palatini, P. Liver dysfunction markedly decreases the inhibition of cytochrome P450 1A2-mediated theophylline metabolism by fluvoxamine. Clin. Pharmacol. Ther. 2006, 79, 489–499. [Google Scholar] [CrossRef]
- van Harten, J. Overview of the pharmacokinetics of fluvoxamine. Clin. Pharmacokinet. 1995, 29 (Suppl. 1), 1–9. [Google Scholar] [CrossRef]
- DeVane, C.L.; Gill, H.S. Clinical pharmacokinetics of fluvoxamine: Applications to dosage regimen design. J. Clin. Psychiatry 1997, 58 (Suppl. 5), 7–14. [Google Scholar]
- Matsuoka, S.; Hori, S.; Satoh, H.; Nagamatsu, T.; Fujii, T.; Sawada, Y. Quantitative prediction of fetal plasma concentration of fluvoxamine during dosage-tapering to the mother. Placenta 2017, 58, 74–81. [Google Scholar] [CrossRef]
- Hicks, J.K.; Bishop, J.R.; Sangkuhl, K.; Muller, D.J.; Ji, Y.; Leckband, S.G.; Leeder, J.S.; Graham, R.L.; Chiulli, D.L.; A, L.L.; et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2D6 and CYP2C19 Genotypes and Dosing of Selective Serotonin Reuptake Inhibitors. Clin. Pharmacol. Ther. 2015, 98, 127–134. [Google Scholar] [CrossRef]
- Brouwer, J.; Nijenhuis, M.; Soree, B.; Guchelaar, H.J.; Swen, J.J.; van Schaik, R.H.N.; Weide, J.V.; Rongen, G.; Buunk, A.M.; de Boer-Veger, N.J.; et al. Dutch Pharmacogenetics Working Group (DPWG) guideline for the gene-drug interaction between CYP2C19 and CYP2D6 and SSRIs. Eur. J. Hum. Genet. 2022, 30, 1114–1120. [Google Scholar] [CrossRef]
- Tasnif, Y.; Morado, J.; Hebert, M.F. Pregnancy-related pharmacokinetic changes. Clin. Pharmacol. Ther. 2016, 100, 53–62. [Google Scholar] [CrossRef]
- Hodge, L.S.; Tracy, T.S. Alterations in drug disposition during pregnancy: Implications for drug therapy. Expert Opin. Drug Metab. Toxicol. 2007, 3, 557–571. [Google Scholar] [CrossRef]
- Anderson, G.D. Pregnancy-induced changes in pharmacokinetics: A mechanistic-based approach. Clin. Pharmacokinet. 2005, 44, 989–1008. [Google Scholar] [CrossRef]
- Badaoui, S.; Hopkins, A.M.; Rodrigues, A.D.; Miners, J.O.; Sorich, M.J.; Rowland, A. Application of Model Informed Precision Dosing to Address the Impact of Pregnancy Stage and CYP2D6 Phenotype on Foetal Morphine Exposure. AAPS J. 2021, 23, 15. [Google Scholar] [CrossRef]
- Giaginis, C.; Theocharis, S.; Tsantili-Kakoulidou, A. Current toxicological aspects on drug and chemical transport and metabolism across the human placental barrier. Expert Opin. Drug Metab. Toxicol. 2012, 8, 1263–1275. [Google Scholar] [CrossRef]
- Ewing, G.; Tatarchuk, Y.; Appleby, D.; Schwartz, N.; Kim, D. Placental transfer of antidepressant medications: Implications for postnatal adaptation syndrome. Clin. Pharmacokinet. 2015, 54, 359–370. [Google Scholar] [CrossRef]
- Evseenko, D.; Paxton, J.W.; Keelan, J.A. Active transport across the human placenta: Impact on drug efficacy and toxicity. Expert Opin. Drug Metab. Toxicol. 2006, 2, 51–69. [Google Scholar] [CrossRef]
- Wishart, D.S.; Feunang, Y.D.; Guo, A.C.; Lo, E.J.; Marcu, A.; Grant, J.R.; Sajed, T.; Johnson, D.; Li, C.; Sayeeda, Z.; et al. DrugBank 5.0: A major update to the DrugBank database for 2018. Nucleic Acids Res. 2018, 46, D1074–D1082. [Google Scholar] [CrossRef]
- Browne, V.A.; Julian, C.G.; Toledo-Jaldin, L.; Cioffi-Ragan, D.; Vargas, E.; Moore, L.G. Uterine artery blood flow, fetal hypoxia and fetal growth. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20140068. [Google Scholar] [CrossRef]
- Schoretsanitis, G.; Westin, A.A.; Stingl, J.C.; Deligiannidis, K.M.; Paulzen, M.; Spigset, O. Antidepressant transfer into amniotic fluid, umbilical cord blood breast milk: A systematic review combined analysis. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 107, 110228. [Google Scholar] [CrossRef]
- Mulder, H.; Wilmink, F.W.; Beumer, T.L.; Tamminga, W.J.; Jedema, J.N.; Egberts, A.C. The association between cytochrome P450 2D6 genotype and prescription patterns of antipsychotic and antidepressant drugs in hospitalized psychiatric patients: A retrospective follow-up study. J. Clin. Psychopharmacol. 2005, 25, 188–191. [Google Scholar] [CrossRef]
- Berard, A.; Gaedigk, A.; Sheehy, O.; Chambers, C.; Roth, M.; Bozzo, P.; Johnson, D.; Kao, K.; Lavigne, S.; Wolfe, L.; et al. Association between CYP2D6 Genotypes and the Risk of Antidepressant Discontinuation, Dosage Modification and the Occurrence of Maternal Depression during Pregnancy. Front. Pharmacol. 2017, 8, 402. [Google Scholar] [CrossRef]
- Rau, T.; Wohlleben, G.; Wuttke, H.; Thuerauf, N.; Lunkenheimer, J.; Lanczik, M.; Eschenhagen, T. CYP2D6 genotype: Impact on adverse effects and nonresponse during treatment with antidepressants-a pilot study. Clin. Pharmacol. Ther. 2004, 75, 386–393. [Google Scholar] [CrossRef]
- Misri, S.; Eng, A.B.; Abizadeh, J.; Blackwell, E.; Spidel, A.; Oberlander, T.F. Factors impacting decisions to decline or adhere to antidepressant medication in perinatal women with mood and anxiety disorders. Depress. Anxiety 2013, 30, 1129–1136. [Google Scholar] [CrossRef]
- COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. Available online: https://www.covid19treatmentguidelines.nih.gov/ (accessed on 26 October 2021).
- Womersley, K.; Ripullone, K.; Agius, M. What are the risks associated with different Selective Serotonin Re-uptake Inhibitors (SSRIs) to treat depression and anxiety in pregnancy? An evaluation of current evidence. Psychiatr. Danub. 2017, 29, 629–644. [Google Scholar]
- The Medicines Healthcare products Regulatory Agency United Kingdom. Guidance: Selective Serotonin Reuptake Inhibitors (SSRIs) and Serotonin and Noradrenaline Reuptake Inhibitors (SNRIs): Use and Safety. Available online: https://www.gov.uk/government/publications/ssris-and-snris-use-and-safety/selective-serotonin-reuptake-inhibitors-ssris-and-serotonin-and-noradrenaline-reuptake-inhibitors-snris-use-and-safety#safety-concerns-with-ssrisnri-use-in-pregnancy (accessed on 17 November 2021).











| Parameters | Fluvoxamine | Notes |
|---|---|---|
| Compound type | Monoprotic Base | |
| Molecular weight (g/mol) | 318.3 | |
| Log P | 3 | |
| pKa 1 | 8.7 | |
| fu | 0.14 | |
| B/P | 0.826 | Predicted in Simcyp® based on Log P, plasma pH, haematocrit, and fu [36,37] |
| Vss (L/kg) | 35.48 | Full PBPK model with Kp scalar of 13 |
| Kp | 13 | Estimated using Simcyp® parameter estimation function |
| ka (h−1) | 0.15 | Optimised through sensitivity analysis [14] |
| fa | 0.8 | Optimised through sensitivity analysis [14] |
| Lag time (h) | 0 | |
| Absorption Model | First Order | |
| Distribution Model | Full PBPK | |
| CLPDM and CLPDF | 0.253 | Predicted from HBD and PSA information using [38] |
| Study | Study Design | Number of Subjects | Age 1 (Years) | Dosing Regimen |
|---|---|---|---|---|
| Studies used for Model Development | ||||
| De Vries et al. [39] | Crossover with 7 days washout between each dose | 12 healthy males | 22–41 | 25 mg/50 mg/100 mg single-dose under fasted conditions |
| Van Harten et al. [40] | Crossover with 7 days washout between each period | 8 healthy males and 4 healthy females | 18–30 | 50 mg single-dose under fed and fasted conditions |
| Bahrami and Mohammadi [41] | Crossover bioequivalence study with 3 weeks washout period | 24 healthy males | 27.2 ± 3.1 | 100 mg single-dose |
| de Vries et al. [42] | Multiple-dose | 3 healthy males and 3 healthy females | 25–31 | 50 mg on day 1, followed by 50 mg twice daily from day 4 to day 31 |
| Fleishaker and Hulst [43] | Multiple-dose | 10 healthy males and 10 healthy females | 20–44 | 50 mg daily for 3 days, followed by 100 mg daily for 7 days |
| Studies used for Model Validation | ||||
| Orlando et al. [44] | Single-dose | 10 healthy males | 35 ± 7 | 50 mg single-dose under fasted condition |
| Debree at al. [45] | Single-dose | 9 healthy males and 1 healthy female | 20–25 | 100 mg single-dose under fasted condition |
| USFDA [46] | Single-dose Study code: S1141107 | 15 healthy males and 13 healthy females | 20.3–44.7 | 100 mg single-dose under fasted condition |
| Spigset et al. [47] | Multiple-dose | 10 healthy males | 28.9 ± 5.2 | 12.5 mg twice daily for 1st week, followed by 25 mg twice daily for 2nd week, 50 mg twice daily for 3rd week, and 100 mg twice daily for 4th week |
| USFDA [46] | Multiple-dose Study code: 1098001 | 12 healthy males with EM CYP2D6 | 19–43 | 100 mg daily for 10 days under fasting conditions |
| USFDA [46] | Multiple-dose Study code: 1098002 | 12 healthy males with EM CYP2D6 | 21–44 | 100 mg daily for 10 days under fasting conditions |
| Studies used for validation with CYP2D6 EM and PM population | ||||
| Carrillo et al. [48] | Single-dose | EM: 3 healthy males and 2 healthy females; PM: 2 healthy males and 1 healthy female | EM: 26–40 PM: 31–49 | 50 mg single-dose under fasting conditions |
| Spigset et al. [49] | Single-dose | EM: 7 healthy males and 3 healthy females; PM: 5 healthy males | EM: 28.7 ± 8.1 PM: 24.0 ± 1.6 | 50 mg single-dose under fasting conditions |
| Hartter et al. [50] | Single-dose | EM: 4 healthy males; PM: 1 healthy male | 34–55 | 50 mg single-dose |
| Christensen et al. [51] | Single-dose and Multiple-dose | EM: 7 healthy subjects; PM: 5 healthy subjects | 22–45 | Period 1: EM—50 mg single-dose PM—25 mg single-dose Period 2: EM—25 mg twice daily for 7 days PM—25 mg daily for 7 days Period 3: EM—10 mg twice daily for 7 days PM—10 mg daily for 7 days |
| References | Dosing | PK Parameters | Observed | Predicted | Predicted/ Observed |
|---|---|---|---|---|---|
| Model Development | |||||
| Geometric Mean (Range) | |||||
| De Vries et al. [39] | Single dose 25 mg | Cmax (ng/mL) | 8.80 (4.70–13.00) | 7.77 (3.19–20.49) | 0.88 |
| AUCinf (ng/mL·h) | 209.00 (117.00–425.00) | 230.67 (97.76–571.74) | 1.10 | ||
| Tmax (h) 1 | 5.00 (1.00–8.00) | 5.66 (3.40–13.35) | 1.13 | ||
| Single dose 50 mg | Cmax (ng/mL) | 17.00 (8.40–28.00) | 16.00 (6.39–40.97) | 0.94 | |
| AUCinf (ng/mL·h) | 448.00 (166.00–1115.00) | 719.24 (254.16–3113.83) | 1.61 | ||
| Tmax (h) 1 | 4.80 (2.00–8.00) | 5.67 (3.40–13.40) | 1.03 | ||
| Single dose 100 mg | Cmax (ng/mL) | 36.00 (21.00–60.00) | 32.01 (12.78–81.95) | 0.89 | |
| AUCinf (ng/mL·h) | 927.00 (325.00–2146.00) | 1693.24 (585.86–10825.67) | 1.83 | ||
| Tmax (h) 1 | 4.50 (3.00–6.00) | 5.68 (3.40–13.35) | 1.04 | ||
| Van Harten et al. [40] | Single dose 50 mg—Fast | Cmax (ng/mL) | 15.40 (7.50–27.00) | 16.00 (6.39–40.97) | 1.04 |
| AUC0–32 h (ng/mL·h) | 237.00 (102.00–571.00) | 324.19 (139.89–710.72) | 1.37 | ||
| Tmax (h) 1 | 6.00 (3.00–12.00) | 5.67 (3.40–13.35) | 0.95 | ||
| Single dose 50 mg—Fed | Cmax (ng/mL) | 15.50 (10.00–32.00) | 16.00 (6.39–40.97) | 1.03 | |
| AUC0–32 h (ng/mL·h) | 223.00 (65.00–587.00) | 324.19 (139.89–710.72) | 1.45 | ||
| Tmax (h) 1 | 7.00 (2.00–12.00) | 5.67 (3.40–13.35) | 0.81 | ||
| de Vries et al. [42] | Single dose 50 mg—Day 1 | Cmax (ng/mL) | 30.00 (13.10) | 17.82 (9.63) | 0.59 |
| AUCinf (ng/mL·h) | 652.00 (319.00) | 882.08 (717.30) | 1.35 | ||
| Tmax (h)1 | 6.00 (4.00–8.00) | 5.63 (3.25–14.50) | 0.94 | ||
| Multiple doses of 50 mg twice daily from Day 4 to Day 31 | Cmax (ng/mL) | 93.00 (96.16) | 81.55 (60.34) | 0.88 | |
| AUC0–12 h (ng/mL·h) | 873.00 (782.44) | 920.77 (707.31) | 1.05 | ||
| Tmax (h) 1 | 5.00 (1.00–10.00) | 3.48 (2.65–4.20) | 0.70 | ||
| Arithmetic Mean (SD) | |||||
| Fleishaker and Hulst [43] | Single dose 50 mg—Day 1 | Cmax (ng/mL) | 21.50 (4.89) | 16.77 (6.70) | 0.78 |
| AUC0–24 h (ng/mL·h) | 328.00 (84.60) | 283.65 (107.45) | 0.86 | ||
| Tmax (h) | 5.70 (1.49) | 5.67 (1.45) | 0.99 | ||
| Multiple doses of 50 mg daily for 3 days followed by 100 mg daily for the 7 days | Cmax (ng/mL) | 99.30 (35.00) | 80.32 (36.45) | 0.81 | |
| AUC0–24 h (ng/mL·h) | 1762.00 (737.00) | 1614.20 (786.04) | 0.92 | ||
| Tmax (h) | 7.95 (4.91) | 4.75 (0.78) | 0.60 | ||
| Bahrami and Mohammadi [41] | Single dose 100 mg—Test | Cmax (ng/mL) | 46.20 (29.00) | 34.65 (13.94) | 0.75 |
| AUC0–48 h (ng/mL·h) | 866.20 (480.00) | 872.88 (351.91) | 1.01 | ||
| AUCinf (ng/mL·h) | 1308.00 (781.00) | 1641.58 (902.86) | 1.26 | ||
| Tmax (h) | 5.30 (2.00) | 5.68 (1.45) | 1.07 | ||
| Single dose 100 mg—Reference | Cmax (ng/mL) | 48.50 (28.00) | 34.65 (13.94) | 0.71 | |
| AUC0–48 h (ng/mL·h) | 802.20 (360.00) | 872.88 (351.91) | 1.09 | ||
| AUCinf (ng/mL·h) | 1224.90 (430.00) | 1641.58 (902.86) | 1.34 | ||
| Tmax (h) | 5.60 (2.10) | 5.68 (1.45) | 1.01 | ||
| Model Validation | |||||
| Arithmetic Mean (SD) | |||||
| Orlando et al. [44] | Single dose 50 mg | Cmax (ng/mL) | 15.00 (3.00) | 17.32 (6.97) | 1.15 |
| AUCinf (ng/mL·h) | 304.00 (84.00) | 820.98 (452.53) | 2.70 | ||
| Tmax (h) 2 | 5.00 (4.00–8.00) | 5.47 (3.40–13.40) | 1.08 | ||
| Geometric Mean (SD) | |||||
| Debree et al. [45] | Single dose 100 mg | Cmax (ng/mL) | 49.30 (17.00) | 32.01 (13.94) | 0.65 |
| AUC0–24 h (ng/mL·h) | 523.90 (122.90) | 545.44 (228.11) | 1.04 | ||
| AUCinf (ng/mL·h) | 817.00 (194.30) | 958.18 (472.25) | 1.17 | ||
| Tmax (h) 1 | 5.00 (2.00–8.00) | 5.68 (3.40–13.40) | 1.14 | ||
| Arithmetic Mean (SD) | |||||
| USFDA [46] | Single dose 100 mg | Cmax (ng/mL) | 41.88 (18.99) | 34.65 (13.94) | 0.83 |
| AUCinf (ng/mL·h) | 959.33 (520.71) | 2071.01 (1435.11) | 2.16 | ||
| Tmax (h) 1 | 6.00 (4.00–16.00) | 5.68 (3.40–13.35) | 0.95 | ||
| Spigset et al. [47] | Week 1—12.5 mg twice daily for 7 days | Cmax (nmol/L) | 25.10 (9.40) | 57.96 (29.62) | 2.31 |
| AUC12 h (nmol.hr/L) | 236.00 (95.00) | 652.00 (339.68) | 2.76 | ||
| Week 1—25 mg twice daily for 7 days | Cmax (nmol/L) | 76.30 (22.10) | 107.53 (60.63) | 1.41 | |
| AUC12 h (nmol.hr/L) | 745.00 (258.00) | 1457.38 (795.37) | 1.96 | ||
| Week 1—50 mg twice daily for 7 days | Cmax (nmol/L) | 244.00 (97.90) | 261.50 (141.94) | 1.07 | |
| AUC12 h (nmol/L·hr) | 2391.00 (949.00) | 2960.86 (1643.50) | 1.24 | ||
| Week 1—100 mg twice daily for 7 days | Cmax (nmol/L) | 738.00 (314.00) | 439.88 (254.36) | 0.60 | |
| AUC12 h (nmol·hr/L) | 7545.00 (3239.00) | 5943.80 (3317.30) | 0.79 | ||
| USFDA [46] | Multiple doses of 100 mg daily for 10 days (Prot. C) | Cmax (ng/mL) | 107.00 (73.52) | 79.41 (31.39) | 0.74 |
| AUC0–24 h (ng/mL·h) | 1738.55 (1392.42) | 1587.74 (669.76) | 0.91 | ||
| Multiple doses of 100 mg daily for 10 days (Prot. D) | Cmax (ng/mL) | 129.59 (62.86) | 85.97 (42.84) | 0.66 | |
| AUC0–24 h (ng/mL·h) | 2109.30 (1085.63) | 1677.97 (905.71) | 0.80 | ||
| Model validation for EM and PM CYP2D6 Phenotype population | |||||
| Carrillo et al. [48] | Single dose 50 mg -EM CYP2D6 | Cmax (nmol/L) | 85.90 (42.50) | 61.03 (28.98) | 0.71 |
| AUC0–32 h (nmol/L·h) 3 | 1097.90 (180.35) | 1220.62 (55.36) | 1.11 | ||
| AUCinf (nmol/L·h) | 1352.00 (733.00) | 2065.41 (1033.85) | 1.53 | ||
| Tmax (h) | 4.4 (2.1) | 5.57 (1.52) | 1.27 | ||
| Single dose 50 mg -PM CYP2D6 | Cmax (nmol/L) | 178.10 (27.50) | 78.81 (33.53) | 0.44 | |
| AUC0–72 h (nmol/L·h) 3 | 4648.59 (237.46) | 2889.62 (118.60) | 0.62 | ||
| AUCinf (nmol/L·h) | 5290.00 (332.00) | 6287.38 (2990.77) | 1.19 | ||
| Tmax (h) | 4.60 (2.30) | 7.01 (1.82) | 1.52 | ||
| Spigset et al. [49] | Single dose 50 mg -EM CYP2D6 | Cmax (nmol/L) | 44.50 (12.30) | 61.03 (28.98) | 1.37 |
| AUC0–48 h (nmol/L·h) 3 | 870.00 (110.00) | 1530.00 (70.00) | 1.76 | ||
| AUCinf (nmol/L·h) | 1000.00 (410.00) | 2610.00 (1280.00) | 2.61 | ||
| Tmax (h) | 7.80 (2.40) | 5.57 (1.52) | 0.71 | ||
| Single dose 50 mg -PM CYP2D6 | Cmax (nmol/L) | 50.40 (17.80) | 78.81 (33.53) | 1.56 | |
| AUC0–48 h (nmol/L·h) 3 | 1090.00 (160.00) | 2280.00 (90.00) | 2.09 | ||
| AUCinf (nmol/L·h) | 1310.00 (670.00) | 4950.00 (2220.00) | 3.78 | ||
| Tmax (h) | 6.60 (2.10) | 7.01 (1.82) | 1.06 | ||
| Hartter et al. [50] | Single dose 50 mg -EM CYP2D6 | Cmax (ng/mL) | 13.00 (3.7) | 19.43 (9.22) | 1.49 |
| AUC0–28 h (mcg/L·h) | 185.50 (33.60) | 360.58 (163.21) | 1.94 | ||
| Single dose 50 mg -PM CYP2D6 4 | Cmax (ng/mL) | 48.00 | 25.09 (10.67) | 0.52 | |
| AUC0–28 h (mcg/L·h) | 612.00 | 512.06 (206.59) | 0.84 | ||
| Chtistensen et al. [51] | Single dose 50 mg -EM CYP2D6 | Cmax (nmol/L) | 58.14 (37.90) | 61.03 (28.98) | 1.05 |
| Single dose 25 mg -PM CYP2D6 | Cmax (nmol/L) | 23.20 (2.28) | 39.41 (16.76) | 1.70 | |
| Pharmacokinetic Metric | Gestational Week (GW) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 (Baseline) | 5 | 10 | 15 | 20 | 25 | 30 | 35 | 40 (Full Term) | |
| Steady-state Cmin (ng/mL) | 75.23 (52.73) | 76.34 (56.97) | 71.37 (55.5) | 65.72 (53.73) | 59.84 (51.76) | 54.04 (49.62) | 48.52 (47.31) | 43.32 (44.8) | 38.4 (42.05) |
| % change from baseline | 0 | 1.48 | −5.13 | −12.64 | −20.46 | −28.17 | −35.50 | −42.42 | −48.96 |
| p-value 1 | 0.9998 | 0.9966 | 0.6707 | 0.1718 | 0.0215 | 0.0016 | <0.0001 | <0.0001 | |
| % Cmin < 60 ng/mL 2 (%) | 46 | 48 | 54 | 58 | 66 | 68 | 74 | 80 | 85 |
| Steady-state Cmax (ng/mL) | 112.5 (64.17) | 113.1 (67.3) | 106.1 (65.56) | 97.87 (63.45) | 89.09 (61.06) | 80.29 (58.43) | 71.85 (55.61) | 64.03 (52.63) | 56.96 (49.53) |
| % change from baseline | 0 | 0.53 | −5.69 | −13.00 | −20.81 | −28.63 | −36.13 | −43.08 | −49.37 |
| p-value 1 | >0.9999 | 0.9739 | 0.3875 | 0.0385 | 0.0012 | <0.0001 | <0.0001 | <0.0001 | |
| % Cmax < 60 ng/mL 2 (%) | 17 | 20 | 23 | 29 | 38 | 45 | 56 | 60 | 67 |
| Gestational Week (GW) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Daily Dose | Phenotype | Pharmacokinetic Metric | 0 (Baseline) | 5 | 10 | 15 | 20↓ | 25 | 30 | 35 | 40 (Full Term) |
| 50 mg | UM CYP2D6 | Steady-state Cmin (ng/mL) | 20.34 (11.28) | 20.19 (13.18) | 18.19 (12.16) | 16.04 (10.99) | 15.90 (11.52) | 13.43 (9.68) | 11.40 (8.40) | 9.61 (7.21) | 8.05 (6.15) |
| % change from baseline | −0.72 | −10.55 | −21.13 | −21.82 | −33.94 | −43.93 | −52.75 | −60.40 | |||
| p-value 1 | 0.9999 | 0.5661 | 0.0229 | 0.0171 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |||
| % Cmin < 60 ng/mL 2 | 100 | 98 | 98 | 99 | 100 | 100 | 100 | 100 | 100 | ||
| Steady-state Cmax (ng/mL) | 35.82 (16.28) | 35.09 (17.59) | 31.98 (16.38) | 28.49 (14.99) | 28.57 (16.75) | 24.98 (14.64) | 21.80 (13.08) | 18.86 (11.57) | 16.20 (10.16) | ||
| % change from baseline | −2.04 | −10.72 | −20.47 | −20.24 | −30.27 | −39.15 | −47.34 | −54.77 | |||
| p-value 1 | 0.9995 | 0.3221 | 0.0037 | 0.0043 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |||
| % Cmax > 230 ng/mL2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| EM CYP2D6 | Steady-state Cmin (ng/mL) | 30.14 (19.33) | 28.07 (16.06) | 25.80 (15.07) | 23.27 (13.90) | 22.36 (14.29) | 19.69 (12.66) | 17.00 (11.05) | 14.54 (9.56) | 12.36 (8.20) | |
| % change from baseline | −6.87 | −14.40 | −22.80 | −25.82 | −34.67 | −43.61 | −51.75 | −59.01 | |||
| p-value 1 | 0.8536 | 0.1452 | 0.0033 | 0.0006 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |||
| % Cmin < 60 ng/mL 2 | 93 | 95 | 98 | 98 | 97 | 98 | 99 | 100 | 100 | ||
| Steady-state Cmax (ng/mL) | 46.99 (23.79) | 44.73 (20.46) | 41.44 (19.29) | 37.65 (17.91) | 36.64 (18.90) | 32.91 (17.07) | 29.11 (15.27) | 25.53 (13.55) | 22.20 (11.94) | ||
| % change from baseline | −4.81 | −11.80 | −19.89 | −22.02 | −29.96 | −38.04 | −45.68 | −52.76 | |||
| p-value 1 | 0.9358 | 0.1602 | 0.0019 | 0.0004 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |||
| % Cmax > 230 ng/mL 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| PM CYP2D6 | Steady-state Cmin (ng/mL) | 68.51 (36.54) | 67.54 (32.65) | 66.21 (31.92) | 64.58 (30.97) | 60.80 (29.70) | 57.69 (26.25) | 55.29 (25.09) | 52.68 (23.83) | 49.88 (22.49) | |
| % change from baseline | −1.43 | −3.37 | −5.75 | −11.26 | −15.80 | −19.30 | −23.10 | −27.19 | |||
| p-value 1 | 0.9997 | 0.9944 | 0.9102 | 0.302 | 0.0564 | 0.0102 | 0.0011 | <0.0001 | |||
| % Cmin < 60 ng/mL 2 | 44 | 45 | 48 | 50 | 58 | 60 | 65 | 65 | 69 | ||
| Steady-state Cmax (ng/mL) | 90.43 (40.94) | 89.40 (37.15) | 87.17 (36.23) | 84.38 (35.04) | 80.17 (34.19) | 76.21 (30.35) | 73.03 (29.04) | 69.54 (27.62) | 65.77 (26.08) | ||
| % change from baseline | −1.14 | −3.60 | −6.68 | −11.34 | −15.72 | −19.24 | −23.10 | −27.27 | |||
| p-value 1 | 0.9997 | 0.9848 | 0.7071 | 0.164 | 0.0184 | 0.0018 | <0.0001 | <0.0001 | |||
| % Cmax > 230 ng/mL2 | 2 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | ||
| 100 mg | UM CYP2D6 | Steady-state Cmin (ng/mL) | 40.70 (22.58) | 40.41 (26.40) | 36.41 (24.34) | 32.11 (22.01) | 31.82 (23.08) | 26.89 (19.39) | 22.82 (16.82) | 19.23 (14.45) | 16.11 (12.31) |
| % change from baseline | −0.72 | −10.55 | −21.13 | −21.82 | −33.94 | −43.93 | −52.75 | −60.41 | |||
| p-value 1 | 0.9999 | 0.5666 | 0.023 | 0.0172 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |||
| % Cmin < 60 ng/mL 2 | 78 | 78 | 84 | 92 | 90 | 94 | 94 | 97 | 99 | ||
| Steady-state Cmax (ng/mL) | 71.70 (32.59) | 70.23 (35.22) | 64.01 (32.80) | 57.02 (30.01) | 57.18 (33.54) | 49.99 (29.32) | 43.63 (26.19) | 37.75 (23.18) | 32.42 (20.34) | ||
| % change from baseline | −2.04 | −10.72 | −20.46 | −20.24 | −30.27 | −39.15 | −47.34 | −54.77 | |||
| p-value 1 | 0.9995 | 0.3225 | 0.0038 | 0.0043 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |||
| % Cmax > 230 ng/mL 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| EM CYP2D6 | Steady-state Cmin (ng/mL) | 60.33 (38.70) | 56.19 (32.17) | 51.64 (30.17) | 46.57 (27.84) | 44.76 (28.65) | 39.42 (25.37) | 34.03 (22.14) | 29.11 (19.15) | 24.73 (16.43) | |
| % change from baseline | −6.87 | −14.40 | −22.81 | −25.82 | −34.66 | −43.60 | −51.75 | −59.01 | |||
| p-value 1 | 0.8538 | 0.1455 | 0.0033 | 0.0006 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |||
| % Cmin < 60 ng/mL 2 | 60 | 62 | 72 | 78 | 78 | 83 | 89 | 96 | 97 | ||
| Steady-state Cmax (ng/mL) | 94.04 (47.64) | 89.52 (40.96) | 82.95 (38.62) | 75.34 (35.85) | 73.35 (37.87) | 65.87 (34.20) | 58.27 (30.60) | 51.09 (27.15) | 44.43 (23.91) | ||
| % change from baseline | −4.81 | −11.80 | −19.89 | −22.01 | −29.96 | −38.04 | −45.67 | −52.75 | |||
| p-value 1 | 0.9359 | 0.1607 | 0.0019 | 0.0004 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |||
| % Cmax > 230 ng/mL 2 | 2 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | ||
| PM CYP2D6 | Steady-state Cmin (ng/mL) | 137.03 (73.09) | 135.07 (65.31) | 132.41 (63.84) | 129.15 (61.95) | 121.60 (59.40) | 115.38 (52.50) | 110.58 (50.17) | 105.37 (47.66) | 99.77 (44.98) | |
| % change from baseline | −1.43 | −3.37 | −5.75 | −11.26 | −15.80 | −19.30 | −23.10 | −27.19 | |||
| p-value 1 | 0.9997 | 0.9944 | 0.9103 | 0.302 | 0.0565 | 0.0102 | 0.0011 | <0.0001 | |||
| % Cmin < 60 ng/mL 2 | 7 | 7 | 7 | 7 | 11 | 11 | 13 | 17 | 19 | ||
| Steady-state Cmax (ng/mL) | 180.86 (81.88) | 178.80 (74.3) | 174.34 (72.45) | 168.77 (70.08) | 160.34 (68.38) | 152.43 (60.71) | 146.05 (58.08) | 139.08 (55.23) | 131.54 (52.17) | ||
| % change from baseline | −1.14 | −3.60 | −6.69 | −11.34 | −15.72 | −19.24 | −23.10 | −27.27 | |||
| p-value 1 | 0.9997 | 0.9848 | 0.707 | 0.1641 | 0.0184 | 0.0018 | <0.0001 | <0.0001 | |||
| % Cmax > 230 ng/mL 2 | 22 | 18 | 15 | 13 | 16 | 7 | 4 | 4 | 4 | ||
| 300 mg | UM CYP2D6 | Steady-state Cmin (ng/mL) | 146.24 (76.37) | 144.43 (87.69) | 130.69 (81.21) | 115.76 (73.81) | 114.73 (77.53) | 97.84 (65.77) | 83.65 (57.51) | 70.98 (49.80) | 59.88 (42.77) |
| % change from baseline | −1.24 | −10.63 | −20.84 | −21.55 | −33.09 | −42.80 | −51.46 | −59.06 | |||
| p-value 1 | 0.9997 | 0.4863 | 0.0144 | 0.0103 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |||
| % Cmin < 60 ng/mL 2 | 9 | 13 | 15 | 21 | 28 | 32 | 38 | 51 | 65 | ||
| Steady-state Cmax (ng/mL) | 184.13 (87.29) | 180.98 (97.07) | 164.56 (90.20) | 146.38 (82.30) | 146.20 (89.44) | 126.68 (77.14) | 109.74 (68.24) | 94.32 (59.82) | 80.52 (52.01) | ||
| % change from baseline | −1.72 | −10.63 | −20.50 | −20.60 | −31.20 | −40.40 | −48.78 | −56.27 | |||
| p-value 1 | 0.9996 | 0.378 | 0.0062 | 0.0058 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |||
| % Cmax > 230 ng/mL 2 | 28 | 31 | 22 | 13 | 15 | 10 | 6 | 6 | 2 | ||
| EM CYP2D6 | Steady-state Cmin (ng/mL) | 209.09 (124.25) | 196.46 (103.95) | 181.21 (97.8) | 164.03 (90.56) | 157.89 (95.00) | 139.86 (84.78) | 121.61 (74.65) | 104.83 (65.14) | 89.70 (56.41) | |
| % change from baseline | −6.04 | −13.33 | −21.55 | −24.48 | −33.11 | −41.84 | −49.86 | −57.10 | |||
| p-value 1 | 0.8938 | 0.1619 | 0.0034 | 0.0006 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |||
| % Cmin < 60 ng/mL 2 | 5 | 3 | 4 | 5 | 11 | 13 | 20 | 25 | 38 | ||
| Steady-state Cmax (ng/mL) | 249.43 (134.54) | 236.20 (114.15) | 218.50 (107.52) | 198.28 (99.70) | 192.26 (105.20) | 171.75 (94.46) | 150.93 (83.85) | 131.50 (73.82) | 113.68 (64.51) | ||
| % change from baseline | −5.30 | −12.40 | −20.51 | −22.92 | −31.14 | −39.49 | −47.28 | −54.42 | |||
| p-value 1 | 0.917 | 0.1583 | 0.0023 | 0.0005 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |||
| % Cmax > 230 ng/mL 2 | 50 | 47 | 37 | 27 | 25 | 21 | 16 | 9 | 3 | ||
| PM CYP2D6 | Steady-state Cmin (ng/mL) | 446.83 (223.35) | 441.25 (200.60) | 432.13 (196.01) | 420.84 (190.08) | 396.98 (182.97) | 377.01 (161.66) | 361.44 (154.58) | 344.51 (146.93) | 326.3 (138.77) | |
| % change from baseline | −1.25 | −3.29 | −5.82 | −11.16 | −15.63 | −19.11 | −22.90 | −26.97 | |||
| p-value 1 | 0.9997 | 0.9936 | 0.8757 | 0.2526 | 0.0398 | 0.006 | 0.0005 | <0.0001 | |||
| % Cmin < 60 ng/mL 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Steady-state Cmax (ng/mL) | 500.13 (236.64) | 494.14 (213.61) | 482.70 (208.5) | 468.46 (201.93) | 443.69 (195.69) | 421.55 (173.34) | 403.91 (165.78) | 384.70 (157.60) | 363.99 (148.84) | ||
| % change from baseline | −1.20 | −3.49 | −6.33 | −11.28 | −15.71 | −19.24 | −23.08 | −27.22 | |||
| p-value 1 | 0.9997 | 0.9902 | 0.7867 | 0.1985 | 0.0257 | 0.0031 | 0.0002 | <0.0001 | |||
| % Cmax > 230 ng/mL 2 | 94 | 93 | 93 | 93 | 90 | 89 | 87 | 86 | 81 | ||
| Gestational Week (GW) | |||||||
|---|---|---|---|---|---|---|---|
| Daily Dose | Phenotype | Pharmacokinetic Metric | 20 | 25 | 30 | 35 | 40 (Full Term) |
| 50 mg | UM CYP2D6 | Steady-state Cmin (ng/mL) | 8.60 (6.46) | 9.61 (7.34) | 9.84 (7.70) | 9.54 (7.63) | 8.98 (7.31) |
| % change from GW-20 | 11.69 | 14.40 | 10.96 | 4.43 | |||
| p-value * | 0.7359 | 0.5769 | 0.7761 | 0.9888 | |||
| Steady-state Cmax (ng/mL) | 15.36 (9.22) | 17.65 (10.80) | 18.35 (11.55) | 17.88 (11.56) | 16.8 (11.13) | ||
| % change from GW-20 | 14.91 | 19.48 | 16.42 | 9.39 | |||
| p-value * | 0.3882 | 0.1715 | 0.3036 | 0.7637 | |||
| EM CYP2D6 | Steady-state Cmin (ng/mL) | 11.90 (8.25) | 13.76 (9.60) | 14.28 (10.08) | 14.02 (9.99) | 13.34 (9.58) | |
| % change from GW-20 | 15.71 | 20.08 | 17.88 | 12.13 | |||
| p-value * | 0.4456 | 0.2287 | 0.3151 | 0.6341 | |||
| Steady-state Cmax (ng/mL) | 19.37 (10.81) | 22.79 (12.78) | 24 (13.63) | 23.71 (13.64) | 22.58 (13.16) | ||
| % change from GW-20 | 17.67 | 23.92 | 22.42 | 16.58 | |||
| p-value * | 0.1931 | 0.041 | 0.0594 | 0.2244 | |||
| PM CYP2D6 | Steady-state Cmin (ng/mL) | 33.00 (16.85) | 40.91 (19.52) | 47.10 (22.46) | 51.39 (24.44) | 54.31 (25.74) | |
| % change from GW-20 | 23.95 | 42.72 | 55.71 | 64.58 | |||
| p-value * | 0.0424 | <0.0001 | <0.0001 | <0.0001 | |||
| Steady-state Cmax (ng/mL) | 43.22 (19.21) | 53.59 (22.26) | 61.42 (25.56) | 66.47 (27.65) | 69.54 (28.91) | ||
| % change from GW-20 | 23.98 | 42.11 | 53.78 | 60.88 | |||
| p-value * | 0.0139 | <0.0001 | <0.0001 | <0.0001 | |||
| 100 mg | UM CYP2D6 | Steady-state Cmin (ng/mL) | 17.22 (12.94) | 19.23 (14.69) | 19.69 (15.43) | 19.10 (15.28) | 17.98 (14.63) |
| % change from GW-20 | 11.68 | 14.39 | 10.95 | 4.41 | |||
| p-value * | 0.7365 | 0.5781 | 0.777 | 0.9889 | |||
| Steady-state Cmax (ng/mL) | 30.75 (18.46) | 35.33 (21.62) | 36.73 (23.13) | 35.8 (23.15) | 33.63 (22.29) | ||
| % change from GW-20 | 14.90 | 19.47 | 16.42 | 9.38 | |||
| p-value * | 0.3889 | 0.1721 | 0.3042 | 0.7647 | |||
| EM CYP2D6 | Steady-state Cmin (ng/mL) | 23.81 (16.54) | 27.55 (19.23) | 28.59 (20.2) | 28.07 (20.02) | 26.70 (19.20) | |
| % change from GW-20 | 15.71 | 20.07 | 17.86 | 12.11 | |||
| p-value * | 0.4463 | 0.2297 | 0.3164 | 0.6357 | |||
| Steady-state Cmax (ng/mL) | 38.77 (21.65) | 45.63 (25.60) | 48.05 (27.31) | 47.47 (27.33) | 45.20 (26.36) | ||
| % change from GW-20 | 17.67 | 23.92 | 22.42 | 16.58 | |||
| p-value * | 0.1935 | 0.0412 | 0.0598 | 0.2253 | |||
| PM CYP2D6 | Steady-state Cmin (ng/mL) | 66.00 (33.70) | 81.81 (39.04) | 94.20 (44.93) | 102.77 (48.89) | 108.63 (51.48) | |
| % change from GW-20 | 23.95 | 42.72 | 55.71 | 64.58 | |||
| p-value * | 0.0425 | <0.0001 | <0.0001 | <0.0001 | |||
| Steady-state Cmax (ng/mL) | 86.44 (38.42) | 107.18 (44.52) | 122.84 (51.12) | 132.93 (55.31) | 139.06 (57.81) | ||
| % change from GW-20 | 23.98 | 42.11 | 53.78 | 60.87 | |||
| p-value * | 0.0139 | <0.0001 | <0.0001 | <0.0001 | |||
| 300 mg | UM CYP2D6 | Steady-state Cmin (ng/mL) | 62.05 (43.4) | 69.96 (49.76) | 72.27 (52.72) | 70.72 (52.68) | 67.06 (50.82) |
| % change from GW-20 | 12.75 | 16.48 | 13.98 | 8.08 | |||
| p-value * | 0.6345 | 0.4114 | 0.5583 | 0.892 | |||
| Steady-state Cmax (ng/mL) | 78.68 (49.54) | 89.65 (57.41) | 92.71 (61.00) | 90.21 (60.84) | 84.94 (58.56) | ||
| % change from GW-20 | 13.95 | 17.84 | 14.66 | 7.96 | |||
| p-value * | 0.4771 | 0.2612 | 0.4329 | 0.8636 | |||
| EM CYP2D6 | Steady-state Cmin (ng/mL) | 83.97 (54.74) | 97.78 (64.14) | 102.33 (67.94) | 101.33 (67.89) | 97.10 (65.53) | |
| % change from GW-20 | 16.45 | 21.87 | 20.67 | 15.63 | |||
| p-value * | 0.3650 | 0.1401 | 0.1708 | 0.3843 | |||
| Steady-state Cmax (ng/mL) | 101.77 (60.36) | 119.11 (70.95) | 124.76 (75.21) | 123.01 (75.01) | 117.28 (72.28) | ||
| % change from GW-20 | 17.04 | 22.59 | 20.87 | 15.24 | |||
| p-value * | 0.2577 | 0.0774 | 0.112 | 0.3306 | |||
| PM CYP2D6 | Steady-state Cmin (ng/mL) | 215.23 (103.83) | 267.09 (120.31) | 307.70 (138.54) | 335.79 (150.74) | 354.74 (158.57) | |
| % change from GW-20 | 24.09 | 42.96 | 56.01 | 64.82 | |||
| p-value * | 0.0274 | <0.0001 | <0.0001 | <0.0001 | |||
| Steady-state Cmax (ng/mL) | 239.62 (110.43) | 296.94 (127.87) | 340.49 (146.82) | 369.09 (159.08) | 387.32 (166.69) | ||
| % change from GW-20 | 23.92 | 42.10 | 54.03 | 61.64 | |||
| p-value * | 0.0194 | <0.0001 | <0.0001 | <0.0001 | |||
| Phenotype | Dose | Pharmacokinetic Metric | Gestational Week | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 5 | 10 | 15 | 20 | 25 | 30 | 35 | 40 | |||
| UM CYP2D6 | 50 mg | Cmin < 60 ng/mL | 100 | 98 | 98 | 99 | 100 | 100 | 100 | 100 | 100 |
| Cmax > 230 ng/mL | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 75 mg | Cmin < 60 ng/mL | 94 | 94 | 96 | 97 | 93 | 97 | 98 | 100 | 100 | |
| Cmax > 230 ng/mL | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 100 mg | Cmin < 60 ng/mL | 78 | 78 | 84 | 92 | 90 | 94 | 94 | 97 | 99 | |
| Cmax > 230 ng/mL | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 125 mg | Cmin < 60 ng/mL | 67 | 67 | 72 | 79 | 81 | 89 | 94 | 94 | 95 | |
| Cmax > 230 ng/mL | 0 | 2 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | ||
| 150 mg | Cmin < 60 ng/mL | 55 | 61 | 66 | 70 | 72 | 82 | 89 | 94 | 94 | |
| Cmax > 230 ng/mL | 1 | 2 | 2 | 2 | 1 | 1 | 1 | 0 | 0 | ||
| 175 mg | Cmin < 60 ng/mL | 40 | 46 | 50 | 58 | 55 | 67 | 74 | 80 | 88 | |
| Cmax > 230 ng/mL | 1 | 2 | 2 | 2 | 2 | 1 | 0 | 0 | 0 | ||
| 200 mg | Cmin < 60 ng/mL | 29 | 32 | 37 | 49 | 40 | 53 | 67 | 74 | 80 | |
| Cmax > 230 ng/mL | 7 | 5 | 3 | 2 | 5 | 2 | 1 | 0 | 0 | ||
| 225 mg | Cmin < 60 ng/mL | 27 | 27 | 32 | 43 | 37 | 49 | 58 | 68 | 77 | |
| Cmax > 230 ng/mL | 8 | 8 | 6 | 3 | 7 | 4 | 2 | 1 | 0 | ||
| 250 mg | Cmin < 60 ng/mL | 15 | 16 | 25 | 32 | 33 | 38 | 51 | 64 | 72 | |
| Cmax > 230 ng/mL | 15 | 14 | 8 | 7 | 9 | 6 | 2 | 2 | 1 | ||
| 275 mg | Cmin < 60 ng/mL | 11 | 15 | 20 | 28 | 30 | 37 | 43 | 55 | 68 | |
| Cmax > 230 ng/mL | 24 | 22 | 14 | 8 | 11 | 6 | 6 | 2 | 1 | ||
| 300 mg | Cmin < 60 ng/mL | 9 | 13 | 15 | 21 | 28 | 32 | 38 | 51 | 65 | |
| Cmax > 230 ng/mL | 28 | 31 | 22 | 13 | 15 | 10 | 6 | 6 | 2 | ||
| EM CYP2D6 | 50 mg | Cmin < 60 ng/mL | 93 | 95 | 98 | 98 | 97 | 98 | 99 | 100 | 100 |
| Cmax > 230 ng/mL | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 75 mg | Cmin < 60 ng/mL | 80 | 86 | 90 | 92 | 91 | 96 | 97 | 97 | 98 | |
| Cmax > 230 ng/mL | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 100 mg | Cmin < 60 ng/mL | 60 | 62 | 72 | 78 | 78 | 83 | 89 | 96 | 97 | |
| Cmax > 230 ng/mL | 2 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | ||
| 125 mg | Cmin < 60 ng/mL | 40 | 46 | 53 | 61 | 61 | 74 | 80 | 84 | 93 | |
| Cmax > 230 ng/mL | 4 | 3 | 3 | 2 | 3 | 2 | 0 | 0 | 0 | ||
| 150 mg | Cmin < 60 ng/mL | 32 | 34 | 41 | 48 | 50 | 58 | 73 | 79 | 83 | |
| Cmax > 230 ng/mL | 8 | 4 | 4 | 3 | 3 | 3 | 3 | 1 | 0 | ||
| 175 mg | Cmin < 60 ng/mL | 19 | 13 | 19 | 24 | 35 | 41 | 46 | 55 | 74 | |
| Cmax > 230 ng/mL | 9 | 6 | 5 | 4 | 3 | 3 | 3 | 2 | 0 | ||
| 200 mg | Cmin < 60 ng/mL | 12 | 7 | 10 | 16 | 22 | 34 | 42 | 46 | 55 | |
| Cmax > 230 ng/mL | 16 | 12 | 8 | 6 | 5 | 3 | 3 | 3 | 2 | ||
| 225 mg | Cmin < 60 ng/mL | 10 | 5 | 7 | 10 | 20 | 24 | 36 | 42 | 48 | |
| Cmax > 230 ng/mL | 22 | 15 | 12 | 9 | 10 | 5 | 3 | 3 | 3 | ||
| 250 mg | Cmin < 60 ng/mL | 7 | 5 | 5 | 7 | 15 | 20 | 25 | 39 | 43 | |
| Cmax > 230 ng/mL | 34 | 24 | 17 | 14 | 17 | 13 | 5 | 3 | 3 | ||
| 275 mg | Cmin < 60 ng/mL | 6 | 4 | 5 | 5 | 12 | 16 | 22 | 36 | 42 | |
| Cmax > 230 ng/mL | 45 | 34 | 26 | 17 | 21 | 15 | 11 | 3 | 3 | ||
| 300 mg | Cmin < 60 ng/mL | 5 | 3 | 4 | 5 | 11 | 13 | 20 | 25 | 38 | |
| Cmax > 230 ng/mL | 50 | 47 | 37 | 27 | 25 | 21 | 16 | 9 | 3 | ||
| PM CYP2D6 | 50 mg | Cmin < 60 ng/mL | 44 | 45 | 48 | 50 | 58 | 60 | 65 | 65 | 69 |
| Cmax > 230 ng/mL | 2 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | ||
| 75 mg | Cmin < 60 ng/mL | 18 | 19 | 20 | 21 | 23 | 26 | 30 | 33 | 37 | |
| Cmax > 230 ng/mL | 4 | 4 | 4 | 4 | 3 | 3 | 3 | 3 | 2 | ||
| 100 mg | Cmin < 60 ng/mL | 7 | 7 | 7 | 7 | 11 | 11 | 13 | 17 | 19 | |
| Cmax > 230 ng/mL | 22 | 18 | 15 | 13 | 16 | 7 | 4 | 4 | 4 | ||
| 125 mg | Cmin < 60 ng/mL | 2 | 2 | 2 | 3 | 4 | 5 | 6 | 8 | 9 | |
| Cmax > 230 ng/mL | 45 | 47 | 44 | 43 | 29 | 24 | 21 | 19 | 16 | ||
| 150 mg | Cmin < 60 ng/mL | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 3 | 4 | |
| Cmax > 230 ng/mL | 64 | 65 | 59 | 57 | 46 | 45 | 44 | 36 | 28 | ||
| 175 mg | Cmin < 60 ng/mL | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | |
| Cmax > 230 ng/mL | 65 | 68 | 68 | 65 | 56 | 47 | 45 | 41 | 37 | ||
| 200 mg | Cmin < 60 ng/mL | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Cmax > 230 ng/mL | 76 | 73 | 72 | 72 | 67 | 65 | 60 | 57 | 47 | ||
| 225 mg | Cmin < 60 ng/mL | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Cmax > 230 ng/mL | 81 | 78 | 77 | 76 | 73 | 69 | 69 | 66 | 61 | ||
| 250 mg | Cmin < 60 ng/mL | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Cmax > 230 ng/mL | 85 | 87 | 86 | 82 | 81 | 79 | 76 | 72 | 69 | ||
| 275 mg | Cmin < 60 ng/mL | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Cmax > 230 ng/mL | 91 | 93 | 91 | 89 | 86 | 86 | 82 | 79 | 74 | ||
| 300 mg | Cmin < 60 ng/mL | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Cmax > 230 ng/mL | 94 | 93 | 93 | 93 | 90 | 89 | 87 | 86 | 81 | ||
| Gestational Week (GW) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Phenotype | Pharmacokinetic Metric | 0 (Baseline) | 5 | 10 | 15 | 20↓ | 25 | 30 | 35 | 40 (Full Term) | |
| UM CYP2D6 | Recommended daily dose (mg) | 250 | 250 | 275 | 300 | 300 | 300 | 300 | 300 | 300 | |
| CL (L/h) | 95.69 (51.51) | 101.60 (59.00) | 104.50 (62.05) | 128.70 (77.43) | 144.40 (113.00) | 166.70 (131.00) | 196.90 (157.80) | 234.20 (190.80) | 279.90 (231.30) | ||
| AUC (ng/mL·h) | 1691.00 (836.10) | 1665.00 (944.40) | 1640.00 (958.40) | 1611.00 (959.20) | 1603.00 (1025.00) | 1380.00 (877.50) | 1189.00 (772.30) | 1017.00 (673.40) | 863.70 (582.30) | ||
| Cord concentration | Cmin (ng/mL) | 61.49 (43.49) | 69.33 (49.85) | 71.62 (52.80) | 70.08 (52.74) | 66.45 (50.88) | |||||
| Cmax (ng/mL) | 77.97 (49.70) | 88.84 (57.58) | 91.86 (61.15) | 89.38 (60.98) | 84.16 (58.68) | ||||||
| EM CYP2D6 | Recommended daily dose (mg) | 175, 200 | 175, 200, 225 | 175, 200, 225, 250 | 200, 225, 250, 275 | 225, 250, 275 | 250, 275 | 275, 300 | 300 | 300 | |
| CL (L/h) | 66.97 (41.10) | 74.37 (43.17) | 72.33 (39.38) | 81.45 (45.32) | 91.63 (72.31) | 106.60 (86.12) | 123.40 (101.50) | 149.60 (124.70) | 175.90 (148.80) | ||
| AUC (ng/mL·h) | 1743.00 (994.60) | 1751.00 (905.60) | 1724.00 (922.30) | 1749.00 (944.70) | 1776.00 (1029.00) | 1664.00 (960.70) | 1595.00 (933.00) | 1453.00 (855.50) | 1251.00 (744.90) | ||
| Cord concentration | Cmin (ng/mL) | 69.28 (46.30) | 84.98 (56.66) | 97.57 (65.93) | 101.50 (68.97) | 97.40 (66.77) | |||||
| Cmax (ng/mL) | 83.83 (51.01) | 103.40 (62.61) | 118.80 (72.98) | 123.10 (76.28) | 117.50 (73.72) | ||||||
| PM CYP2D6 | Recommended daily dose (mg) | 75, 100 | 75, 100 | 75, 100 | 100, | 100 | 100 | 100 | 100, 125 | 100, 125 | |
| CL (L/h) | 31.44 (14.72) | 31.77 (15.88) | 32.48 (16.22) | 33.39 (16.68) | 35.11 (16.04) | 36.57 (16.46) | 38.12 (17.11) | 39.97 (17.85) | 42.18 (18.78) | ||
| AUC (ng/mL·h) | 3373.00 (1723.00) | 3331.00 (1564.00) | 3257.00 (1527.00) | 3618.00 (1598.00) | 3422.00 (1545.00) | 3251.00 (1367.00) | 3116.00 (1308.00) | 3340.00 (1452.00) | 3162.00 (1372.00) | ||
| Cord concentration | Cmin (ng/mL) | 65.60 (33.83) | 81.33 (39.24) | 93.67 (45.16) | 115.00 (56.94) | 121.50 (59.97) | |||||
| Cmax (ng/mL) | 86.10 (38.67) | 106.80 (44.91) | 122.40 (51.55) | 149.00 (65.13) | 155.90 (68.07) | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burhanuddin, K.; Badhan, R. Optimising Fluvoxamine Maternal/Fetal Exposure during Gestation: A Pharmacokinetic Virtual Clinical Trials Study. Metabolites 2022, 12, 1281. https://doi.org/10.3390/metabo12121281
Burhanuddin K, Badhan R. Optimising Fluvoxamine Maternal/Fetal Exposure during Gestation: A Pharmacokinetic Virtual Clinical Trials Study. Metabolites. 2022; 12(12):1281. https://doi.org/10.3390/metabo12121281
Chicago/Turabian StyleBurhanuddin, Khairulanwar, and Raj Badhan. 2022. "Optimising Fluvoxamine Maternal/Fetal Exposure during Gestation: A Pharmacokinetic Virtual Clinical Trials Study" Metabolites 12, no. 12: 1281. https://doi.org/10.3390/metabo12121281
APA StyleBurhanuddin, K., & Badhan, R. (2022). Optimising Fluvoxamine Maternal/Fetal Exposure during Gestation: A Pharmacokinetic Virtual Clinical Trials Study. Metabolites, 12(12), 1281. https://doi.org/10.3390/metabo12121281








