Lipid Alterations in Glioma: A Systematic Review
Abstract
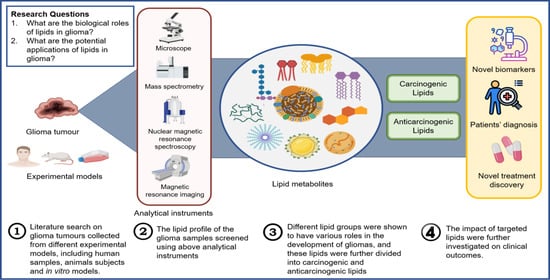
1. Introduction
2. Materials and Methods
3. Results
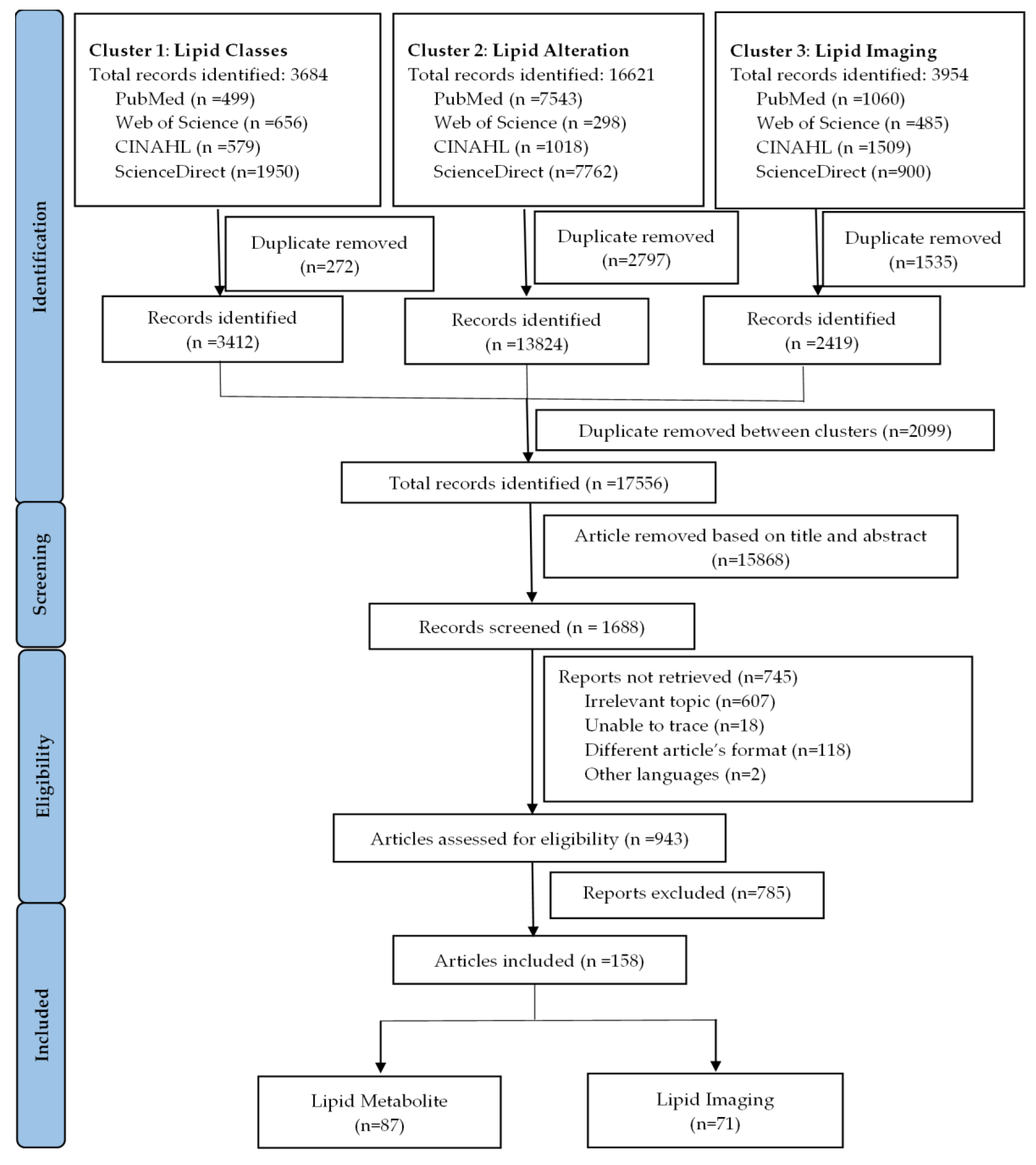
3.1. Eligible Studies
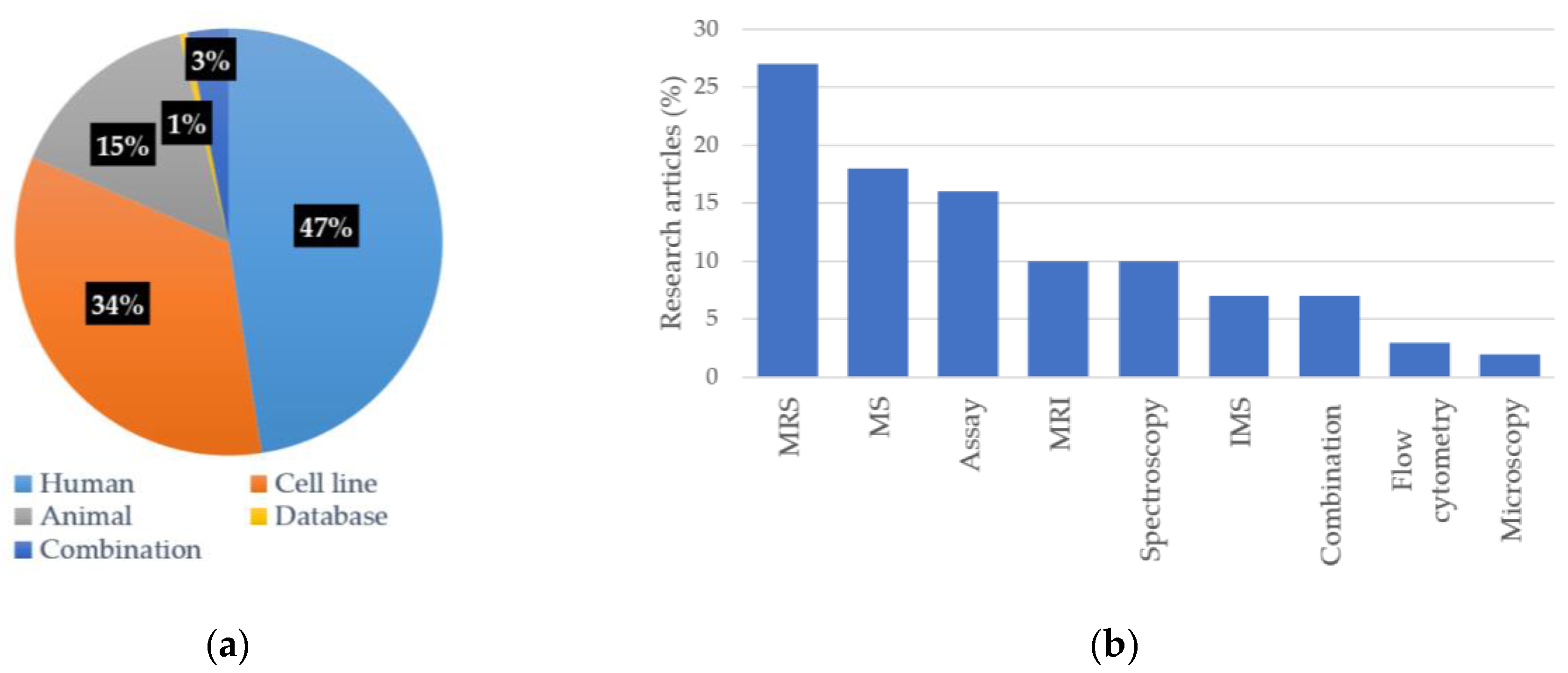
3.2. Characteristics of Included Studies
3.3. Lipids Metabolites Alteration in Glioma
3.3.1. Carcinogenic Lipids in Glioma
3.3.2. Anti-Carcinogenic Lipids in Glioma
3.4. Lipids Signal Intensities in Glioma
| Reference | Children/Adult | Histopathological Type | Lipid Metabolites Detected | |||||
|---|---|---|---|---|---|---|---|---|
| Lipid | Lip0.9 | Lip1.3 | Lip2.8 | Cho | GPC | |||
| [106] | Children | Medulloblastoma | ↑ | ↑ | ||||
| [135] | Children | LGG HGG | ||||||
| [121] | Children | Optic pathway glioma | ↑ | ↑ | ||||
| [122] | Children | Glioma (Grade III) | ↑ | |||||
| [107] | Children | HGG | ↑ | |||||
| [115] | Children | Pilocytic | ↓ | ↓ | ↑ | |||
| [108,109,110,111,112,136,137,138] | Adult | GBM | ↑ | |||||
| [123,124] | Adult | GBM | ↑ | ↑ | ||||
| [132] | Adult | GBM | ↑ | ↓ | ||||
| [114] | Adult | LGG HGG | ↓ ↑ | |||||
| [120] | Adult (Rat) | GBM | ↑ | ↑ | ||||
| [127] | Adult | GBM | ↑ | ↑ | ||||
| [128] | Adult | Grade II Grade III | ↑ ↑ | ↑ ↑ | ||||
| [116] | Adult | GBM | ↑ | ↑ | ↑ | ↑ | ||
| [129] | Adult | Grade III (Enhancing area) Grade III (Non-enhancing area) | ↑ | ↑ | ||||
| [133] | Adult | LGG HGG | ↑ | ↓ | ||||
| [130,131] | Adult | LGG | ↑ | |||||
| [125] | Adult | Fibrillary Astrocytoma Astrocytoma (Grade III) GBM | ↑ ↑ | ↑ ↑ ↑ | ||||
| [134] | Adult | LGG HGG | ↓ ↑ | |||||
| [139] | Adult | Astrocytoma (Grade III) GBM | ↑ | |||||
| [126] | Adult | Medulloblastoma Haemangioblastoma | ↑ | ↑ | ||||
| [117] | Adult | GBM | ↑ | ↑ | ↑ | |||
| [113] | Adult | GBM (Pseudoprogression) GBM (Recurrence) | ↑ ↓ | ↓ ↑ | ||||
| [119] | Adult | GBM | ↑ | ↑ | ||||
| [118] | Adult | Grade II Grade III Grade IV | ↑ | ↑ | ↑ | ↑ ↑ | ↑ ↑ ↓ | |
4. Discussion
4.1. Carcinogenic Lipids in Glioma
4.1.1. Fatty acyl (FA)
4.1.2. Glycolipid (GL)
4.1.3. Glycerophospholipids (GP)
4.1.4. Sphingolipid (SP)
4.1.5. Sterol Lipids (ST)
4.2. Anti-Carcinogenic Lipids in Glioma
4.2.1. Fatty Acyls (FA)
4.2.2. Sphingolipid (SP)
4.2.3. Sterol Lipids (ST)
4.2.4. Prenol Lipid (PR)
4.3. Lipid Metabolites Alteration on MRS
4.3.1. Choline
4.3.2. Lipids Signal
5. Conclusions
- (1)
- Glioma shifted metabolic plasticity; exert lipid metabolic differences producing lipogenic phenotypes.
- (2)
- Paediatric and adult gliomas have distinct lipid molecular profiles, where glycerophospholipids and fatty acids were among the most affected lipid classes.
- (3)
- The highlighted carcinogenic lipids were recognised to provide a favourable environment for glioma cells growth, proliferation, metastases and survival.
- (4)
- Conversely, the anti-carcinogenic lipids offer promising lipids compounds as possible innovative targets to be further investigated and developed as an innovative treatment strategy for glioma.
- (5)
- The advances of emerging in lipid characterisation techniques, both lipid molecular and imaging techniques expand our fundamental knowledge and perception of bioactive lipid metabolite in glioma tumour aetiology.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ostrom, Q.T.; Bauchet, L.; Davis, F.G.; Deltour, I.; Fisher, J.L.; Langer, C.E.; Pekmezci, M.; Schwartzbaum, J.A.; Turner, M.C.; Walsh, K.M.; et al. The epidemiology of glioma in adults: A “state of the science” review. Neuro-Oncology 2014, 16, 896–913. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Cioffi, G.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2014-2018. Neuro-Oncology 2021, 23, iii1–iii105. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Ohgaki, H.; Wiestler, O.D.; Cavenee, W.K.; Burger, P.C.; Jouvet, A.; Scheithauer, B.W.; Kleihues, P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007, 114, 97–109. [Google Scholar] [CrossRef]
- Shankar, G.M.; Francis, J.M.; Rinne, M.L.; Ramkissoon, S.H.; Huang, F.W.; Venteicher, A.S.; Akama-Garren, E.H.; Kang, Y.J.; Lelic, N.; Kim, J.C.; et al. Rapid Intraoperative Molecular Characterization of Glioma. JAMA Oncol. 2015, 1, 662–667. [Google Scholar] [CrossRef]
- Marien, E.; Meister, M.; Muley, T.; Fieuws, S.; Bordel, S.; Derua, R.; Spraggins, J.; van de Plas, R.; Dehairs, J.; Wouters, J.; et al. Non-small cell lung cancer is characterized by dramatic changes in phospholipid profiles. Int. J. Cancer 2015, 137, 1539–1548. [Google Scholar] [CrossRef]
- Pavlova, N.N.; Thompson, C.B. The emerging hallmarks of cancer metabolism. Cell Metab. 2016, 23, 27–47. [Google Scholar] [CrossRef]
- Krycer, J.R.; Sharpe, L.J.; Luu, W.; Brown, A.J. The Akt-SREBP nexus: Cell signaling meets lipid metabolism. Trends Endocrinol. Metab. 2010, 21, 268–276. [Google Scholar] [CrossRef]
- Menendez, J.A.; Lupu, R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat. Rev. Cancer 2007, 7, 763–777. [Google Scholar] [CrossRef]
- Bensaad, K.; Favaro, E.; Lewis, C.A.; Peck, B.; Lord, S.; Collins, J.M.; Pinnick, K.E.; Wigfield, S.; Buffa, F.M.; Li, J.L.; et al. Fatty acid uptake and lipid storage induced by HIF-1α contribute to cell growth and survival after hypoxia-reoxygenation. Cell Rep. 2014, 9, 349–365. [Google Scholar] [CrossRef]
- Butler, L.M.; Perone, Y.; Dehairs, J.; Lupien, L.E.; de Laat, V.; Talebi, A.; Loda, M.; Kinlaw, W.B.; Swinnen, J.V. Lipids and cancer: Emerging roles in pathogenesis, diagnosis and therapeutic intervention. Adv. Drug Deliv. Rev. 2020, 159, 245–293. [Google Scholar] [CrossRef]
- Srivastava, N.K.; Pradhan, S.; Gowda, G.A.; Kumar, R. In vitro, high-resolution 1H and 31P NMR based analysis of the lipid components in the tissue, serum, and CSF of the patients with primary brain tumors: One possible diagnostic view. NMR Biomed. 2010, 23, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Bell, E.H.; Chakravarti, A. Lipid metabolism emerges as a promising target for malignant glioma therapy. CNS Oncol. 2013, 2, 289–299. [Google Scholar] [CrossRef] [PubMed]
- El Khayari, A.; Bouchmaa, N.; Taib, B.; Wei, Z.; Zeng, A.; El Fatimy, R. Metabolic Rewiring in Glioblastoma Cancer: EGFR, IDH and Beyond. Front. Oncol. 2022, 12, 901951. [Google Scholar] [CrossRef] [PubMed]
- Fack, F.; Tardito, S.; Hochart, G.; Oudin, A.; Zheng, L.; Fritah, S.; Golebiewska, A.; Nazarov, P.V.; Bernard, A.; Hau, A.C.; et al. Altered metabolic landscape in IDH-mutant gliomas affects phospholipid, energy, and oxidative stress pathways. EMBO Mol. Med. 2017, 9, 1681–1695. [Google Scholar] [CrossRef]
- Wang, F.; Bhat, K.; Doucette, M.; Zhou, S.; Gu, Y.; Law, B.; Liu, X.; Wong, E.T.; Kang, J.X.; Hsieh, T.C.; et al. Docosahexaenoic acid (DHA) sensitizes brain tumor cells to etoposide-induced apoptosis. Curr. Mol. Med. 2011, 11, 503–511. [Google Scholar] [CrossRef]
- Hajimohammadebrahim-Ketabforoush, M.; Shahmohammadi, M.; Keikhaee, M.; Eslamian, G.; Vahdat Shariatpanahi, Z. Single high-dose vitamin D3 injection and clinical outcomes in brain tumor resection: A randomized, controlled clinical trial. Clin. Nutr. ESPEN 2021, 41, 153–159. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. J. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef]
- Mörén, L.; Wibom, C.; Bergström, P.; Johansson, M.; Antti, H.; Bergenheim, A.T. Characterization of the serum metabolome following radiation treatment in patients with high-grade gliomas. Radiat. Oncol. 2016, 11, 51. [Google Scholar] [CrossRef]
- Damiano, F.; De Benedetto, G.E.; Longo, S.; Giannotti, L.; Fico, D.; Siculella, L.; Giudetti, A.M. Decanoic acid and not octanoic acid stimulates fatty acid synthesis in U87MG glioblastoma cells: A metabolomics study. Front. Neurosci. 2020, 14, 783. [Google Scholar] [CrossRef] [PubMed]
- Mörén, L.; Bergenheim, A.T.; Ghasimi, S.; Brännström, T.; Johansson, M.; Antti, H. Metabolomic ccreening of tumor tissue and serum in glioma patients reveals diagnostic and prognostic information. Metabolites 2015, 5, 502–520. [Google Scholar] [CrossRef] [PubMed]
- Gastón, R.; María Eugenia, P.; Das, U.N.; Eynard, A.R. Polyunsaturated Fatty Acids Differentially Modulate Cell Proliferation and Endocannabinoid System in Two Human Cancer Lines. Arch. Med. Res. 2017, 48, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Lita, A.; Pliss, A.; Kuzmin, A.; Yamasaki, T.; Zhang, L.; Dowdy, T.; Burks, C.; de Val, N.; Celiku, O.; Ruiz-Rodado, V.; et al. IDH1 mutations induce organelle defects via dysregulated phospholipids. Nat. Commun. 2021, 12, 614. [Google Scholar] [CrossRef]
- Taïb, B.; Aboussalah, A.M.; Moniruzzaman, M.; Chen, S.; Haughey, N.J.; Kim, S.F.; Ahima, R.S. Lipid accumulation and oxidation in glioblastoma multiforme. Sci. Rep. 2019, 9, 19593. [Google Scholar] [CrossRef]
- Eberlin, L.S.; Liu, X.; Ferreira, C.R.; Santagata, S.; Agar, N.Y.; Cooks, R.G. Desorption electrospray ionization then MALDI mass spectrometry imaging of lipid and protein distributions in single tissue sections. Anal. Chem. 2011, 83, 8366–8371. [Google Scholar] [CrossRef]
- Wood, P.L. Endogenous Anti-Inflammatory Very-Long-Chain Dicarboxylic Acids: Potential Chemopreventive Lipids. Metabolites 2018, 8, 76. [Google Scholar] [CrossRef]
- Huang, J.; Weinstein, S.J.; Kitahara, C.M.; Karoly, E.D.; Sampson, J.N.; Albanes, D. A prospective study of serum metabolites and glioma risk. Oncotarget 2017, 8, 70366–70377. [Google Scholar] [CrossRef]
- Ferreira, M.T.; Gomes, R.N.; Panagopoulos, A.T.; de Almeida, F.G.; Veiga, J.C.E.; Colquhoun, A. Opposing roles of PGD(2) in GBM. Prostaglandins Other Lipid Mediat. 2018, 134, 66–76. [Google Scholar] [CrossRef]
- Brocard, E.; Oizel, K.; Lalier, L.; Pecqueur, C.; Paris, F.; Vallette, F.M.; Oliver, L. Radiation-induced PGE2 sustains human glioma cells growth and survival through EGF signaling. Oncotarget 2015, 6, 6840–6849. [Google Scholar] [CrossRef]
- Cook, P.J.; Thomas, R.; Kingsley, P.J.; Shimizu, F.; Montrose, D.C.; Marnett, L.J.; Tabar, V.S.; Dannenberg, A.J.; Benezra, R. Cox-2-derived PGE2 induces Id1-dependent radiation resistance and self-renewal in experimental glioblastoma. Neuro Oncol. 2016, 18, 1379–1389. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Han, L.; Zhang, X.; Li, L.; Jiang, C.; Qiu, Y.; Huang, R.; Xie, B.; Lin, Z.; Ren, J.; et al. Alteration of endocannabinoid system in human gliomas. J. Neurochem. 2012, 120, 842–849. [Google Scholar] [CrossRef] [PubMed]
- Im, J.H.; Yoo, B.C.; Lee, J.H.; Kim, K.H.; Kim, T.H.; Lee, K.Y.; Kim, J.H.; Park, J.B.; Kwon, J.W.; Shin, S.H.; et al. Comparative cerebrospinal fluid metabolites profiling in glioma patients to predict malignant transformation and leptomeningeal metastasis with a potential for preventive personalized medicine. EPMA J. 2020, 11, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Shakya, S.; Gromovsky, A.D.; Hale, J.S.; Knudsen, A.M.; Prager, B.; Wallace, L.C.; Penalva, L.O.F.; Brown, H.A.; Kristensen, B.W.; Rich, J.N.; et al. Altered lipid metabolism marks glioblastoma stem and non-stem cells in separate tumor niches. Acta Neuropathol. Commun. 2021, 9, 101. [Google Scholar] [CrossRef]
- Wu, X.; Geng, F.; Cheng, X.; Guo, Q.; Zhong, Y.; Cloughesy, T.F.; Yong, W.H.; Chakravarti, A.; Guo, D. Lipid droplets maintain energy homeostasis and glioblastoma growth via autophagic release of stored fatty acids. iScience 2020, 23, 101569. [Google Scholar] [CrossRef]
- Cabodevilla, A.G.; Sánchez-Caballero, L.; Nintou, E.; Boiadjieva, V.G.; Picatoste, F.; Gubern, A.; Claro, E. Cell survival during complete nutrient deprivation depends on lipid droplet-fueled β-oxidation of fatty acids. J. Biol. Chem. 2013, 288, 27777–27788. [Google Scholar] [CrossRef]
- Anna, I.; Bartosz, P.; Lech, P.; Halina, A. Novel strategies of Raman imaging for brain tumor research. Oncotarget 2017, 8, 85290–85310. [Google Scholar] [CrossRef]
- Bruntz, R.C.; Taylor, H.E.; Lindsley, C.W.; Brown, H.A. Phospholipase D2 mediates survival signaling through direct regulation of Akt in glioblastoma cells. J. Biol. Chem. 2014, 289, 600–616. [Google Scholar] [CrossRef]
- Mathews, T.P.; Hill, S.; Rose, K.L.; Ivanova, P.T.; Lindsley, C.W.; Brown, H.A. Human phospholipase D activity transiently regulates pyrimidine biosynthesis in malignant gliomas. ACS Chem. Biol. 2015, 10, 1258–1268. [Google Scholar] [CrossRef]
- Wildburger, N.C.; Wood, P.L.; Gumin, J.; Lichti, C.F.; Emmett, M.R.; Lang, F.F.; Nilsson, C.L. ESI-MS/MS and MALDI-IMS Localization Reveal Alterations in Phosphatidic Acid, Diacylglycerol, and DHA in Glioma Stem Cell Xenografts. J. Proteome Res. 2015, 14, 2511–2519. [Google Scholar] [CrossRef]
- Viswanath, P.; Radoul, M.; Izquierdo-Garcia, J.L.; Luchman, H.A.; Gregory Cairncross, J.; Pieper, R.O.; Phillips, J.J.; Ronen, S.M. Mutant IDH1 gliomas downregulate phosphocholine and phosphoethanolamine synthesis in a 2-hydroxyglutarate-dependent manner. Cancer Metab. 2018, 6, 3. [Google Scholar] [CrossRef] [PubMed]
- Viswanath, P.; Radoul, M.; Izquierdo-Garcia, J.L.; Ong, W.Q.; Luchman, H.A.; Cairncross, J.G.; Huang, B.; Pieper, R.O.; Phillips, J.J.; Ronen, S.M. 2-hydroxyglutarate-mediated autophagy of the endoplasmic reticulum leads to an unusual downregulation of phospholipid biosynthesis in mutant IDH1 gliomas. Cancer Res. 2018, 78, 2290–2304. [Google Scholar] [CrossRef] [PubMed]
- St-Coeur, P.D.; Poitras, J.J.; Cuperlovic-Culf, M.; Touaibia, M.; Morin, P., Jr. Investigating a signature of temozolomide resistance in GBM cell lines using metabolomics. J. Neuro-Oncol. 2015, 125, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Gilard, V.; Ferey, J.; Marguet, F.; Fontanilles, M.; Ducatez, F.; Pilon, C.; Lesueur, C.; Pereira, T.; Basset, C.; Schmitz-Afonso, I.; et al. Integrative metabolomics reveals deep tissue and systemic metabolic remodeling in glioblastoma. Cancers 2021, 13, 5157. [Google Scholar] [CrossRef]
- Koch, K.; Hartmann, R.; Schröter, F.; Suwala, A.K.; Maciaczyk, D.; Krüger, A.C.; Willbold, D.; Kahlert, U.D.; Maciaczyk, J. Reciprocal regulation of the cholinic phenotype and epithelial-mesenchymal transition in glioblastoma cells. Oncotarget 2016, 7, 73414–73431. [Google Scholar] [CrossRef]
- Shao, W.; Gu, J.; Huang, C.; Liu, D.; Huang, H.; Huang, Z.; Lin, Z.; Yang, W.; Liu, K.; Lin, D.; et al. Malignancy-associated metabolic profiling of human glioma cell lines using 1H NMR spectroscopy. Mol. Cancer 2014, 13, 197. [Google Scholar] [CrossRef]
- Cuperlovic-Culf, M.; Ferguson, D.; Culf, A.; Morin, P., Jr.; Touaibia, M. 1H NMR metabolomics analysis of glioblastoma subtypes: Correlation between metabolomics and gene expression characteristics. J. Biol. Chem. 2012, 287, 20164–20175. [Google Scholar] [CrossRef]
- Pirro, V.; Llor, R.S.; Jarmusch, A.K.; Alfaro, C.M.; Cohen-Gadol, A.A.; Hattab, E.M.; Cooks, R.G. Analysis of human gliomas by swab touch spray-mass spectrometry: Applications to intraoperative assessment of surgical margins and presence of oncometabolites. Analyst 2017, 142, 4058–4066. [Google Scholar] [CrossRef]
- Jarmusch, A.; Pirro, V.; Baird, Z.; Hattab, E.; Cohen-Gadol, A.; Cooks, R. Lipid and metabolite profiles of human brain tumors by desorption electrospray ionization-MS. Proc. Natl. Acad. Sci. USA 2016, 113, 1486–1491. [Google Scholar] [CrossRef]
- Lee, J.E.; Jeun, S.S.; Kim, S.H.; Yoo, C.Y.; Baek, H.M.; Yang, S.H. Metabolic profiling of human gliomas assessed with NMR. J. Clin. Neurosci. 2019, 68, 275–280. [Google Scholar] [CrossRef]
- Jothi, J.; Janardhanam, V.A.; Krishnaswamy, R. Metabolic variations between low-grade and high-grade gliomas-profiling by (1)H NMR spectroscopy. J. Proteome Res. 2020, 19, 2483–2490. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Jia, H.; Li, Q.; Cui, J.; Li, R.; Zou, Z.; Hong, X. Glycerophosphatidylcholine PC(36:1) absence and 3’-phosphoadenylate (pAp) accumulation are hallmarks of the human glioma metabolome. Sci. Rep. 2018, 8, 14783. [Google Scholar] [CrossRef]
- Osawa, T.; Shimamura, T.; Saito, K.; Hasegawa, Y.; Ishii, N.; Nishida, M.; Ando, R.; Kondo, A.; Anwar, M.; Tsuchida, R.; et al. Phosphoethanolamine Accumulation protects cancer cells under glutamine starvation through downregulation of PCYT2. Cell Rep. 2019, 29, 89–103.e107. [Google Scholar] [CrossRef] [PubMed]
- Loskutov, Y.V.; Griffin, C.L.; Marinak, K.M.; Bobko, A.; Margaryan, N.V.; Geldenhuys, W.J.; Sarkaria, J.N.; Pugacheva, E.N. LPA signaling is regulated through the primary cilium: A novel target in glioblastoma. Oncogene 2018, 37, 1457–1471. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Xuan, Q.; Zhang, C.; Hu, C.; Li, Y.; Zhao, X.; Liu, S.; Ren, F.; Zhang, Y.; Zhou, L.; et al. Metabolic alterations related to glioma grading based on metabolomics and lipidomics analyses. Metabolites 2020, 10, 478. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Hu, P.; Zang, Q.; Yue, X.; Zhou, Z.; Xu, X.; Xu, J.; Li, S.; Chen, Y.; Qiang, B.; et al. LC-MS-based metabolomics reveals metabolic signatures related to glioma stem-like cell self-renewal and differentiation. RSC Adv 2017, 7, 24221–24232. [Google Scholar] [CrossRef]
- Li, M.H.; Swenson, R.; Harel, M.; Jana, S.; Stolarzewicz, E.; Hla, T.; Shapiro, L.H.; Ferrer, F. Antitumor Activity of a Novel Sphingosine-1-Phosphate 2 Antagonist, AB1, in Neuroblastoma. J. Pharmacol. Exp. Ther. 2015, 354, 261–268. [Google Scholar] [CrossRef]
- Abdel Hadi, L.; Anelli, V.; Guarnaccia, L.; Navone, S.; Beretta, M.; Moccia, F.; Tringali, C.; Urechie, V.; Campanella, R.; Marfia, G.; et al. A bidirectional crosstalk between glioblastoma and brain endothelial cells potentiates the angiogenic and proliferative signaling of sphingosine-1-phosphate in the glioblastoma microenvironment. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2018, 1863, 1179–1192. [Google Scholar] [CrossRef]
- Oancea-Castillo, L.R.; Klein, C.; Abdollahi, A.; Weber, K.J.; Régnier-Vigouroux, A.; Dokic, I. Comparative analysis of the effects of a sphingosine kinase inhibitor to temozolomide and radiation treatment on glioblastoma cell lines. Cancer Biol. Ther. 2017, 18, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Bien-Möller, S.; Lange, S.; Holm, T.; Böhm, A.; Paland, H.; Küpper, J.; Herzog, S.; Weitmann, K.; Havemann, C.; Vogelgesang, S.; et al. Expression of S1P metabolizing enzymes and receptors correlate with survival time and regulate cell migration in glioblastoma multiforme. Oncotarget 2016, 7, 13031–13046. [Google Scholar] [CrossRef]
- Abuhusain, H.J.; Matin, A.; Qiao, Q.; Shen, H.; Kain, N.; Day, B.W.; Stringer, B.W.; Daniels, B.; Laaksonen, M.A.; Teo, C.; et al. A metabolic shift favoring sphingosine 1-phosphate at the expense of ceramide controls glioblastoma angiogenesis. J. Biol. Chem. 2013, 288, 37355–37364. [Google Scholar] [CrossRef] [PubMed]
- Bassi, R.; Brambilla, S.; Tringali, C.; Giussani, P. Extracellular Sphingosine-1-Phosphate Downstream of EGFR Increases Human Glioblastoma Cell Survival. Int. J. Mol. Sci. 2021, 22, 6824. [Google Scholar] [CrossRef] [PubMed]
- Doan, N.B.; Nguyen, H.S.; Al-Gizawiy, M.M.; Mueller, W.M.; Sabbadini, R.A.; Rand, S.D.; Connelly, J.M.; Chitambar, C.R.; Schmainda, K.M.; Mirza, S.P. Acid ceramidase confers radioresistance to glioblastoma cells. Oncol. Rep. 2017, 38, 1932–1940. [Google Scholar] [CrossRef] [PubMed]
- Giussani, P.; Bassi, R.; Anelli, V.; Brioschi, L.; De Zen, F.; Riccitelli, E.; Caroli, M.; Campanella, R.; Gaini, S.M.; Viani, P.; et al. Glucosylceramide synthase protects glioblastoma cells against autophagic and apoptotic death induced by temozolomide and Paclitaxel. Cancer Investig. 2012, 30, 27–37. [Google Scholar] [CrossRef]
- Riccitelli, E.; Giussani, P.; Di Vito, C.; Condomitti, G.; Tringali, C.; Caroli, M.; Galli, R.; Viani, P.; Riboni, L. Extracellular sphingosine-1-phosphate: A novel actor in human glioblastoma stem cell survival. PLoS ONE 2013, 8, e68229. [Google Scholar] [CrossRef]
- Bernhart, E.; Damm, S.; Wintersperger, A.; Nusshold, C.; Brunner, A.M.; Plastira, I.; Rechberger, G.; Reicher, H.; Wadsack, C.; Zimmer, A.; et al. Interference with distinct steps of sphingolipid synthesis and signaling attenuates proliferation of U87MG glioma cells. Biochem. Pharmacol. 2015, 96, 119–130. [Google Scholar] [CrossRef]
- Depciuch, J.; Tołpa, B.; Witek, P.; Szmuc, K.; Kaznowska, E.; Osuchowski, M.; Król, P.; Cebulski, J. Raman and FTIR spectroscopy in determining the chemical changes in healthy brain tissues and glioblastoma tumor tissues. Spectrochim. Acta. Part A Mol. Biomol. Spectrosc. 2020, 225, 117526. [Google Scholar] [CrossRef]
- Dowdy, T.; Zhang, L.; Celiku, O.; Movva, S.; Lita, A.; Ruiz-Rodado, V.; Gilbert, M.R.; Larion, M. Sphingolipid pathway as a source of vulnerability in IDH1(mut) glioma. Cancers 2020, 12, 2910. [Google Scholar] [CrossRef]
- Zhai, X.H.; Xiao, J.; Yu, J.K.; Sun, H.; Zheng, S. Novel sphingomyelin biomarkers for brain glioma and associated regulation research on the PI3K/Akt signaling pathway. Oncol. Lett. 2019, 18, 6207–6213. [Google Scholar] [CrossRef]
- Fleurence, J.; Cochonneau, D.; Fougeray, S.; Oliver, L.; Geraldo, F.; Terme, M.; Dorvillius, M.; Loussouarn, D.; Vallette, F.; Paris, F.; et al. Targeting and killing glioblastoma with monoclonal antibody to O-acetyl GD2 ganglioside. Oncotarget 2016, 7, 41172–41185. [Google Scholar] [CrossRef]
- Wingerter, A.; El Malki, K.; Sandhoff, R.; Seidmann, L.; Wagner, D.C.; Lehmann, N.; Vewinger, N.; Frauenknecht, K.B.M.; Sommer, C.J.; Traub, F.; et al. Exploiting gangliosides for the therapy of Ewing’s Sarcoma and H3K27M-mutant diffuse midline glioma. Cancers 2021, 13, 520. [Google Scholar] [CrossRef] [PubMed]
- Yeh, S.C.; Wang, P.Y.; Lou, Y.W.; Khoo, K.H.; Hsiao, M.; Hsu, T.L.; Wong, C.H. Glycolipid GD3 and GD3 synthase are key drivers for glioblastoma stem cells and tumorigenicity. Proc. Natl. Acad. Sci. USA 2016, 113, 5592–5597. [Google Scholar] [CrossRef] [PubMed]
- Fabris, D.; Rožman, M.; Sajko, T.; Vukelić, Ž. Aberrant ganglioside composition in glioblastoma multiforme and peritumoral tissue: A mass spectrometry characterization. Biochimie 2017, 137, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Fabris, D.; Karmelić, I.; Muharemović, H.; Sajko, T.; Jurilj, M.; Potočki, S.; Novak, R.; Vukelić, Ž. Ganglioside composition distinguishes anaplastic ganglioglioma tumor tissue from peritumoral brain tissue: Complementary mass spectrometry and thin-layer chromatography evidence. Int. J. Mol. Sci. 2021, 22, 8844. [Google Scholar] [CrossRef] [PubMed]
- Kundu, M.; Mahata, B.; Banerjee, A.; Chakraborty, S.; Debnath, S.; Ray, S.S.; Ghosh, Z.; Biswas, K. Ganglioside GM2 mediates migration of tumor cells by interacting with integrin and modulating the downstream signaling pathway. Biochim. Biophys. Acta 2016, 1863, 1472–1489. [Google Scholar] [CrossRef]
- Ermini, L.; Morganti, E.; Post, A.; Yeganeh, B.; Caniggia, I.; Leadley, M.; Faria, C.C.; Rutka, J.T.; Post, M. Imaging mass spectrometry identifies prognostic ganglioside species in rodent intracranial transplants of glioma and medulloblastoma. PLoS ONE 2017, 12, e0176254. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Geng, F.; Cheng, X.; Wu, X.; Yoo, J.Y.; Cheng, C.; Guo, J.Y.; Mo, X.; Ru, P.; Hurwitz, B.; Kim, S.H.; et al. Inhibition of SOAT1 suppresses glioblastoma growth via blocking SREBP-1-mediated lipogenesis. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2016, 22, 5337–5348. [Google Scholar] [CrossRef] [PubMed]
- Eibinger, G.; Fauler, G.; Bernhart, E.; Frank, S.; Hammer, A.; Wintersperger, A.; Eder, H.; Heinemann, A.; Mischel, P.S.; Malle, E.; et al. On the role of 25-hydroxycholesterol synthesis by glioblastoma cell lines. Implications for chemotactic monocyte recruitment. Exp. Cell Res. 2013, 319, 1828–1838. [Google Scholar] [CrossRef]
- Cigliano, L.; Spagnuolo, M.S.; Napolitano, G.; Iannotta, L.; Fasciolo, G.; Barone, D.; Venditti, P. 24S-hydroxycholesterol affects redox homeostasis in human glial U-87 MG cells. Mol. Cell. Endocrinol. 2019, 486, 25–33. [Google Scholar] [CrossRef]
- Liang, R.; Li, J.; Li, M.; Yang, Y.; Wang, X.; Mao, Q.; Liu, Y. Clinical significance of pre-surgical serum lipid levels in patients with glioblastoma. Oncotarget 2017, 8, 85940–85948. [Google Scholar] [CrossRef]
- Ng, Y.W.; Say, Y.H. Palmitic acid induces neurotoxicity and gliatoxicity in SH-SY5Y human neuroblastoma and T98G human glioblastoma cells. PeerJ 2018, 6, e4696. [Google Scholar] [CrossRef] [PubMed]
- Antal, O.; Hackler, L.; Shen, J.; Mán, I.; Hideghéty, K.; Kitajka, K.; Puskás, L.G. Combination of unsaturated fatty acids and ionizing radiation on human glioma cells: Cellular, biochemical and gene expression analysis. Lipids Health Dis. 2014, 13, 142. [Google Scholar] [CrossRef] [PubMed]
- Antal, O.; Péter, M.; Hackler, L., Jr.; Mán, I.; Szebeni, G.; Ayaydin, F.; Hideghéty, K.; Vigh, L.; Kitajka, K.; Balogh, G.; et al. Lipidomic analysis reveals a radiosensitizing role of gamma-linolenic acid in glioma cells. Biochim. Biophys. Acta 2015, 1851, 1271–1282. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, Y.; Choi, W.-S.; Sun, X.; Godbout, R. Super resolution microscopy reveals DHA-dependent alterations in glioblastoma membrane remodelling and cell migration. Nanoscale 2021, 13, 9706–9722. [Google Scholar] [CrossRef]
- Zhu, Z.; Tan, Z.; Li, Y.; Luo, H.; Hu, X.; Tang, M.; Hescheler, J.; Mu, Y.; Zhang, L. Docosahexaenoic acid alters Gsα localization in lipid raft and potentiates adenylate cyclase. Nutrition 2015, 31, 1025–1030. [Google Scholar] [CrossRef]
- Yuan, Y.; Shah, N.; Almohaisin, M.I.; Saha, S.; Lu, F. Assessing fatty acid-induced lipotoxicity and its therapeutic potential in glioblastoma using stimulated Raman microscopy. Sci. Rep. 2021, 11, 7422. [Google Scholar] [CrossRef]
- McConnell, D.D.; McGreevy, J.W.; Williams, M.N.; Litofsky, N.S. Do Anti-Oxidants Vitamin D(3,) Melatonin, and Alpha-Lipoic Acid Have Synergistic Effects with Temozolomide on Cultured Glioblastoma Cells? Medicines 2018, 5, 58. [Google Scholar] [CrossRef]
- Jung, J.S.; Ahn, Y.H.; Moon, B.I.; Kim, H.S. Exogenous C2 ceramide suppresses matrix metalloproteinase gene expression by inhibiting ROS production and MAPK signaling pathways in PMA-stimulated human astroglioma cells. Int. J. Mol. Sci. 2016, 17, 477. [Google Scholar] [CrossRef]
- Wang, Z.; Wen, L.; Zhu, F.; Wang, Y.; Xie, Q.; Chen, Z.; Li, Y. Overexpression of ceramide synthase 1 increases C18-ceramide and leads to lethal autophagy in human glioma. Oncotarget 2017, 8, 104022–104036. [Google Scholar] [CrossRef]
- Noack, J.; Choi, J.; Richter, K.; Kopp-Schneider, A.; Régnier-Vigouroux, A. A sphingosine kinase inhibitor combined with temozolomide induces glioblastoma cell death through accumulation of dihydrosphingosine and dihydroceramide, endoplasmic reticulum stress and autophagy. Cell Death Dis. 2014, 5, e1425. [Google Scholar] [CrossRef]
- Romero-Ramírez, L.; García-Álvarez, I.; Casas, J.; Barreda-Manso, M.A.; Yanguas-Casás, N.; Nieto-Sampedro, M.; Fernández-Mayoralas, A. New oleyl glycoside as anti-cancer agent that targets on neutral sphingomyelinase. Biochem. Pharmacol. 2015, 97, 158–172. [Google Scholar] [CrossRef] [PubMed]
- Clarion, L.; Schindler, M.; de Weille, J.; Lolmède, K.; Laroche-Clary, A.; Uro-Coste, E.; Robert, J.; Mersel, M.; Bakalara, N. 7β-Hydroxycholesterol-induced energy stress leads to sequential opposing signaling responses and to death of C6 glioblastoma cells. Biochem. Pharmacol. 2012, 83, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Emanuelsson, I.; Wikvall, K.; Friman, T.; Norlin, M. Vitamin D analogues tacalcitol and calcipotriol inhibit proliferation and migration of T98G human glioblastoma cells. Basic Clin. Pharmacol. Toxicol. 2018, 123, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Cataldi, S.; Arcuri, C.; Lazzarini, A.; Nakashidze, I.; Ragonese, F.; Fioretti, B.; Ferri, I.; Conte, C.; Codini, M.; Beccari, T.; et al. Effect of 1α,25(OH)(2) vitamin D(3) in mutant P53 glioblastoma cells: Involvement of neutral sphingomyelinase1. Cancers 2020, 12, 3163. [Google Scholar] [CrossRef]
- Zigmont, V.; Garrett, A.; Peng, J.; Seweryn, M.; Rempala, G.A.; Harris, R.; Holloman, C.; Gundersen, T.E.; Ahlbom, A.; Feychting, M.; et al. Association between prediagnostic serum 25-Hydroxyvitamin D concentration and glioma. Nutr. Cancer 2015, 67, 1120–1130. [Google Scholar] [CrossRef]
- Samadi, M.; Nury, T.; Khalafi-Nezhad, A.; Lizard, G. Protecting group-free radical decarboxylation of bile acids: Synthesis of novel steroidal substituted maleic anhydrides and maleimides and evaluation of their cytotoxicity on C6 rat glioma cells. Steroids 2017, 125, 124–130. [Google Scholar] [CrossRef]
- Guo, G.; Yao, W.; Zhang, Q.; Bo, Y. Oleanolic acid suppresses migration and invasion of malignant glioma cells by inactivating MAPK/ERK signaling pathway. PLoS ONE 2013, 8, e72079. [Google Scholar] [CrossRef]
- Hait, N.C.; Maiti, A. The role of sphingosine-1-phosphate and ceramide-1-phosphate in inflammation and cancer. Mediat. Inflamm. 2017, 2017, 4806541. [Google Scholar] [CrossRef]
- Fenn, M.B.; Xanthopoulos, P.; Pyrgiotakis, G.; Grobmyer, S.R.; Pardalos, P.M.; Hench, L.L. Raman spectroscopy for clinical oncology. Adv. Opt. Technol. 2011, 2011, 213783. [Google Scholar] [CrossRef][Green Version]
- Ricci, M.; Ragonese, F.; Gironi, B.; Paolantoni, M.; Morresi, A.; Latterini, L.; Fioretti, B.; Sassi, P. Glioblastoma single-cell microRaman analysis under stress treatments. Sci. Rep. 2018, 8, 7979. [Google Scholar] [CrossRef]
- Uckermann, O.; Galli, R.; Tamosaityte, S.; Leipnitz, E.; Geiger, K.D.; Schackert, G.; Koch, E.; Steiner, G.; Kirsch, M. Label-free delineation of brain tumors by coherent anti-Stokes Raman scattering microscopy in an orthotopic mouse model and human glioblastoma. PLoS ONE 2014, 9, e107115. [Google Scholar] [CrossRef]
- Kaur, E.; Sahu, A.; Hole, A.R.; Rajendra, J.; Chaubal, R.; Gardi, N.; Dutt, A.; Moiyadi, A.; Krishna, C.M.; Dutt, S. Unique spectral markers discern recurrent Glioblastoma cells from heterogeneous parent population. Sci. Rep. 2016, 6, 26538. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, H.N.; Banerji, A.; Banerjee, A.N.; Riddick, E.; Petis, J.; Evans, S.; Patel, M.; Parson, C.; Smith, V.; Gwebu, E.; et al. Deciphering the finger prints of brain cancer glioblastoma multiforme from four different patients by using near infrared Raman spectroscopy. J. Cancer Sci. Ther. 2015, 7, 44–47. [Google Scholar] [CrossRef] [PubMed]
- Imiela, A.; Surmacki, J.; Abramczyk, H. Novel strategies of Raman imaging for monitoring the therapeutic benefit of temozolomide in glioblastoma. J. Mol. Struct. 2020, 1217, 128381. [Google Scholar] [CrossRef]
- Uckermann, O.; Juratli, T.A.; Galli, R.; Conde, M.; Wiedemuth, R.; Krex, D.; Geiger, K.; Temme, A.; Schackert, G.; Koch, E.; et al. Optical analysis of glioma: Fourier-Transform Infrared Spectroscopy reveals the IDH1 mutation status. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2018, 24, 2530–2538. [Google Scholar] [CrossRef] [PubMed]
- Blüml, S.; Margol, A.S.; Sposto, R.; Kennedy, R.J.; Robison, N.J.; Vali, M.; Hung, L.T.; Muthugounder, S.; Finlay, J.L.; Erdreich-Epstein, A.; et al. Molecular subgroups of medulloblastoma identification using noninvasive magnetic resonance spectroscopy. Neuro-Oncology 2016, 18, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.; Cummins, C.L.; Macpherson, L.; Sun, Y.; Natarajan, K.; Grundy, R.G.; Arvanitis, T.N.; Kauppinen, R.A.; Peet, A.C. Magnetic resonance spectroscopy metabolite profiles predict survival in paediatric brain tumours. Eur. J. Cancer 2013, 49, 457–464. [Google Scholar] [CrossRef]
- Li, Y.; Lupo, J.M.; Parvataneni, R.; Lamborn, K.R.; Cha, S.; Chang, S.M.; Nelson, S.J. Survival analysis in patients with newly diagnosed glioblastoma using pre- and postradiotherapy MR spectroscopic imaging. Neuro-Oncology 2013, 15, 607–617. [Google Scholar] [CrossRef]
- Craveiro, M.; Clément-Schatlo, V.; Marino, D.; Gruetter, R.; Cudalbu, C. In vivo brain macromolecule signals in healthy and glioblastoma mouse models: 1H magnetic resonance spectroscopy, post-processing and metabolite quantification at 14.1 T. J. Neurochem. 2014, 129, 806–815. [Google Scholar] [CrossRef]
- Toussaint, M.; Pinel, S.; Auger, F.; Durieux, N.; Thomassin, M.; Thomas, E.; Moussaron, A.; Meng, D.; Plénat, F.; Amouroux, M.; et al. Proton MR spectroscopy and diffusion MR imaging monitoring to predict tumor response to interstitial photodynamic therapy for glioblastoma. Theranostics 2017, 7, 436–451. [Google Scholar] [CrossRef]
- Durmo, F.; Rydelius, A.; Cuellar Baena, S.; Askaner, K.; Lätt, J.; Bengzon, J.; Englund, E.; Chenevert, T.L.; Björkman-Burtscher, I.M.; Sundgren, P.C. Multivoxel (1)H-MR spectroscopy biometrics for preoprerative differentiation between brain tumors. Tomography 2018, 4, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Palma, A.; Grande, S.; Ricci-Vitiani, L.; Luciani, A.M.; Buccarelli, M.; Biffoni, M.; Dini, V.; Cirrone, G.A.P.; Ciocca, M.; Guidoni, L.; et al. Different mechanisms underlie the metabolic response of GBM stem-like cells to ionizing radiation: Biological and MRS studies on effects of photons and carbon ions. Int. J. Mol. Sci. 2020, 21, 5167. [Google Scholar] [CrossRef] [PubMed]
- Sawlani, V.; Taylor, R.; Rowley, K.; Redfern, R.; Martin, J.; Poptani, H. Magnetic resonance spectroscopy for differentiating pseudo-progression from true progression in GBM on concurrent chemoradiotherapy. Neuroradiol. J. 2012, 25, 575–586. [Google Scholar] [CrossRef]
- Nakamura, H.; Doi, M.; Suzuki, T.; Yoshida, Y.; Hoshikawa, M.; Uchida, M.; Tanaka, Y.; Takagi, M.; Nakajima, Y. The significance of lactate and lipid peaks for predicting primary neuroepithelial tumor grade with proton MR spectroscopy. Magn. Reson. Med. Sci. 2018, 17, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Orphanidou-Vlachou, E.; Auer, D.; Brundler, M.A.; Davies, N.P.; Jaspan, T.; MacPherson, L.; Natarajan, K.; Sun, Y.; Arvanitis, T.N.; Grundy, R.G.; et al. 1H magnetic resonance spectroscopy in the diagnosis of paediatric low grade brain tumours. Eur. J. Radiol. 2013, 82, e295–e301. [Google Scholar] [CrossRef]
- Delgado-Goñi, T.; Ortega-Martorell, S.; Ciezka, M.; Olier, I.; Candiota, A.P.; Julià-Sapé, M.; Fernández, F.; Pumarola, M.; Lisboa, P.J.; Arús, C. MRSI-based molecular imaging of therapy response to temozolomide in preclinical glioblastoma using source analysis. NMR Biomed 2016, 29, 732–743. [Google Scholar] [CrossRef] [PubMed]
- Hulsey, K.M.; Mashimo, T.; Banerjee, A.; Soesbe, T.C.; Spence, J.S.; Vemireddy, V.; Maher, E.A.; Bachoo, R.M.; Choi, C. ¹H MRS characterization of neurochemical profiles in orthotopic mouse models of human brain tumors. NMR Biomed. 2015, 28, 108–115. [Google Scholar] [CrossRef]
- Martín-Sitjar, J.; Delgado-Goñi, T.; Cabañas, M.E.; Tzen, J.; Arús, C. Influence of the spinning rate in the HR-MAS pattern of mobile lipids in C6 glioma cells and in artificial oil bodies. Magma 2012, 25, 487–496. [Google Scholar] [CrossRef]
- Ramm, P.; Bettscheider, M.; Beier, D.; Kalbitzer, H.R.; Kremer, W.; Bogdahn, U.; Hau, P.; Aigner, L.; Beier, C.P. 1H-nuclear magnetic resonance spectroscopy of glioblastoma cancer stem cells. Stem Cells Dev. 2011, 20, 2189–2195. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, H.; Makaryus, R.; Yu, M.; Smith, S.D.; Sayed, K.; Feng, T.; Holland, E.; Van der Linden, A.; Bolwig, T.G.; et al. Metabolic profiling of dividing cells in live rodent brain by proton magnetic resonance spectroscopy (1HMRS) and LCModel analysis. PLoS ONE 2014, 9, e94755. [Google Scholar] [CrossRef]
- Novak, J.; Wilson, M.; Macpherson, L.; Arvanitis, T.N.; Davies, N.P.; Peet, A.C. Clinical protocols for ³¹P MRS of the brain and their use in evaluating optic pathway gliomas in children. Eur. J. Radiol. 2014, 83, e106–e112. [Google Scholar] [CrossRef] [PubMed]
- Porto, L.; Kieslich, M.; Franz, K.; Lehrnbecher, T.; Zanella, F.; Pilatus, U.; Hattingen, E. MR spectroscopy differentiation between high and low grade astrocytomas: A comparison between paediatric and adult tumours. Eur. J. Paediatr. Neurol. 2011, 15, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Nelson, S.J.; Kadambi, A.K.; Park, I.; Li, Y.; Crane, J.; Olson, M.; Molinaro, A.; Roy, R.; Butowski, N.; Cha, S.; et al. Association of early changes in 1H MRSI parameters with survival for patients with newly diagnosed glioblastoma receiving a multimodality treatment regimen. Neuro-Oncology 2017, 19, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Bernabéu-Sanz, Á.; Fuentes-Baile, M.; Alenda, C. Main genetic differences in high-grade gliomas may present different MR imaging and MR spectroscopy correlates. Eur. Radiol. 2021, 31, 749–763. [Google Scholar] [CrossRef]
- Jaskólski, D.J.; Fortuniak, J.; Majos, A.; Gajewicz, W.; Papierz, W.; Liberski, P.P.; Sikorska, B.; Stefańczyk, L. Magnetic resonance spectroscopy in intracranial tumours of glial origin. Neurol. Neurochir. Pol. 2013, 47, 438–449. [Google Scholar] [CrossRef]
- Mora, P.; Pons, A.; Cos, M.; Camins, A.; Muntané, A.; Aguilera, C.; Arús, C.; Majós, C. Magnetic resonance spectroscopy in posterior fossa tumours: The tumour spectroscopic signature may improve discrimination in adults among haemangioblastoma, ependymal tumours, medulloblastoma, and metastasis. Eur. Radiol. 2019, 29, 2792–2801. [Google Scholar] [CrossRef]
- Madhu, B.; Jauhiainen, A.; McGuire, S.; Griffiths, J.R. Exploration of human brain tumour metabolism using pairwise metabolite-metabolite correlation analysis (MMCA) of HR-MAS 1H NMR spectra. PLoS ONE 2017, 12, e0185980. [Google Scholar] [CrossRef]
- Jalbert, L.E.; Elkhaled, A.; Phillips, J.J.; Neill, E.; Williams, A.; Crane, J.C.; Olson, M.P.; Molinaro, A.M.; Berger, M.S.; Kurhanewicz, J.; et al. Metabolic profiling of IDH mutation and malignant progression in infiltrating glioma. Sci. Rep. 2017, 7, 44792. [Google Scholar] [CrossRef]
- Ozturk-Isik, E.; Pirzkall, A.; Lamborn, K.R.; Cha, S.; Chang, S.M.; Nelson, S.J. Spatial characteristics of newly diagnosed grade 3 glioma assessed by magnetic resonance metabolic and diffusion tensor imaging. Transl. Oncol. 2012, 5, 10–18. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Wei, F.; Sima, D.M.; van Cauter, S.; Himmelreich, U.; Pi, Y.; Hu, G.; Yao, Y.; van Huffel, S. An advanced MRI and MRSI data fusion scheme for enhancing unsupervised brain tumor differentiation. Comput. Biol. Med. 2017, 81, 121–129. [Google Scholar] [CrossRef]
- Javid, D.; Habiba, U.; Rashid, Q.; Muhammad, B. Age-related metabolic study of glioma brain using magnetic resonance spectroscopy. Materialstoday Proc. 2020, 47, S116–S120. [Google Scholar] [CrossRef]
- Li, Y.; Pi, Y.; Liu, X.; Liu, Y.; Van Cauter, S. Data analysis and tissue type assignment for glioblastoma multiforme. BioMed Res. Int. 2014, 2014, 762126. [Google Scholar] [CrossRef] [PubMed]
- Postma, G.J.; Luts, J.; Idema, A.J.; Julià-Sapé, M.; Moreno-Torres, A.; Gajewicz, W.; Suykens, J.A.; Heerschap, A.; Van Huffel, S.; Buydens, L.M. On the relevance of automatically selected single-voxel MRS and multimodal MRI and MRSI features for brain tumour differentiation. Comput. Biol. Med. 2011, 41, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Aprile, I.; Torni, C.; Fiaschini, P.; Muti, M. High-grade cerebral glioma characterization: Usefulness of MR spectroscopy and perfusion imaging associated evaluation. Neuroradiol. J. 2012, 25, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Yao, R.; Cheng, A.; Liu, M.; Zhang, Z.; Jin, B.; Yu, H. The diagnostic value of apparent diffusion coefficient and proton magnetic resonance spectroscopy in the grading of pediatric gliomas. J. Comput. Assist. Tomogr. 2021, 45, 269–276. [Google Scholar] [CrossRef]
- Hnilicová, P.; Richterová, R.; Kantorová, E.; Bittšanský, M.; Baranovičová, E.; Dobrota, D. Proton MR spectroscopic imaging of human glioblastomas at 1.5 Tesla. Gen. Physiol. Biophys. 2017, 36, 531–537. [Google Scholar] [CrossRef]
- Mlynárik, V.; Cudalbu, C.; Clément, V.; Marino, D.; Radovanovic, I.; Gruetter, R. In vivo metabolic profiling of glioma-initiating cells using proton magnetic resonance spectroscopy at 14.1 Tesla. NMR Biomed. 2012, 25, 506–513. [Google Scholar] [CrossRef]
- Wang, A.M.; Leung, G.K.; Kiang, K.M.; Chan, D.; Cao, P.; Wu, E.X. Separation and quantification of lactate and lipid at 1.3 ppm by diffusion-weighted magnetic resonance spectroscopy. Magn. Reson. Med. 2017, 77, 480–489. [Google Scholar] [CrossRef]
- Yamasaki, F.; Takaba, J.; Ohtaki, M.; Abe, N.; Kajiwara, Y.; Saito, T.; Yoshioka, H.; Hama, S.; Akimitsu, T.; Sugiyama, K.; et al. Detection and differentiation of lactate and lipids by single-voxel proton MR spectroscopy. Neurosurg. Rev. 2005, 28, 267–277. [Google Scholar] [CrossRef]
- Chen, M.; Huang, J. The expanded role of fatty acid metabolism in cancer: New aspects and targets. Precis. Clin. Med. 2019, 2, 183–191. [Google Scholar] [CrossRef]
- Kant, S.; Kesarwani, P.; Prabhu, A.; Graham, S.F.; Buelow, K.L.; Nakano, I.; Chinnaiyan, P. Enhanced fatty acid oxidation provides glioblastoma cells metabolic plasticity to accommodate to its dynamic nutrient microenvironment. Cell Death Dis. 2020, 11, 253. [Google Scholar] [CrossRef] [PubMed]
- Sperry, J.; Condro, M.C.; Guo, L.; Braas, D.; Vanderveer-Harris, N.; Kim, K.K.O.; Pope, W.B.; Divakaruni, A.S.; Lai, A.; Christofk, H.; et al. Glioblastoma utilizes fatty acids and ketone bodies for growth allowing progression during ketogenic diet therapy. iScience 2020, 23, 101453. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.H.; Ren, X.H.; Liang, X.H.; Tang, Y.L. Roles of fatty acid metabolism in tumourigenesis: Beyond providing nutrition (Review). Mol. Med. Rep. 2018, 18, 5307–5316. [Google Scholar] [CrossRef] [PubMed]
- Konerding, M.A.; Fait, E.; Gaumann, A. 3D microvascular architecture of pre-cancerous lesions and invasive carcinomas of the colon. Br. J. Cancer 2001, 84, 1354–1362. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, S.; Zhan, R.; Wang, Y.; Pai, S.K.; Hirota, S.; Hosobe, S.; Takano, Y.; Saito, K.; Furuta, E.; Iiizumi, M.; et al. Mechanism of apoptosis induced by the inhibition of fatty acid synthase in breast cancer cells. Cancer Res. 2006, 66, 5934–5940. [Google Scholar] [CrossRef]
- Chen, X.; Qian, Y.; Wu, S. The Warburg effect: Evolving interpretations of an established concept. Free. Radic. Biol. Med. 2015, 79, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Dueregger, A.; Schöpf, B.; Eder, T.; Höfer, J.; Gnaiger, E.; Aufinger, A.; Kenner, L.; Perktold, B.; Ramoner, R.; Klocker, H.; et al. Differential utilization of dietary fatty acids in benign and malignant cells of the prostate. PLoS ONE 2015, 10, e0135704. [Google Scholar] [CrossRef]
- McCarty, M.F.; DiNicolantonio, J.J. Lauric acid-rich medium-chain triglycerides can substitute for other oils in cooking applications and may have limited pathogenicity. Openheart 2016, 3, e000467. [Google Scholar] [CrossRef]
- Roopashree, P.G.; Shetty, S.S.; Suchetha Kumari, N. Effect of medium chain fatty acid in human health and disease. J. Funct. Foods 2021, 87, 104724. [Google Scholar] [CrossRef]
- Das, U.N. Essential fatty acids-A review. Curr. Pharm. Biotechnol. 2006, 7, 467–482. [Google Scholar] [CrossRef]
- Ruiying, C.; Zeyun, L.; Yongliang, Y.; Zijia, Z.; Ji, Z.; Xin, T.; Xiaojian, Z. A comprehensive analysis of metabolomics and transcriptomics in non-small cell lung cancer. PLoS ONE 2020, 15, e0232272. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.; Zhong, X.; Tian, X. Metabolomics of papillary thyroid carcinoma tissues: Potential biomarkers for diagnosis and promising targets for therapy. Tumour Biol. 2016, 37, 11163–11175. [Google Scholar] [CrossRef] [PubMed]
- Fhu, C.W.; Ali, A. Protein lipidation by palmitoylation and myristoylation in cancer. Front. Cell Dev. Biol. 2021, 9, 673647. [Google Scholar] [CrossRef]
- Pascual, G.; Domínguez, D.; Elosúa-Bayes, M.; Beckedorff, F.; Laudanna, C.; Bigas, C.; Douillet, D.; Greco, C.; Symeonidi, A.; Hernández, I.; et al. Dietary palmitic acid promotes a prometastatic memory via Schwann cells. Nature 2021, 599, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Fan, Z.; Wang, Z.; Dai, Q.; Xiang, Z.; Yuan, F.; Yan, M.; Zhu, Z.; Liu, B.; Li, C. CD36 mediates palmitate acid-induced metastasis of gastric cancer via AKT/GSK-3β/β-catenin pathway. J. Exp. Clin. Cancer Res. 2019, 38, 52. [Google Scholar] [CrossRef] [PubMed]
- Evans, L.M.; Cowey, S.L.; Siegal, G.P.; Hardy, R.W. Stearate preferentially induces apoptosis in human breast cancer cells. Nutr. Cancer 2009, 61, 746–753. [Google Scholar] [CrossRef]
- Nakajima, S.; Gotoh, M.; Fukasawa, K.; Murakami-Murofushi, K.; Kunugi, H. Oleic acid is a potent inducer for lipid droplet accumulation through its esterification to glycerol by diacylglycerol acyltransferase in primary cortical astrocytes. Brain Res. 2019, 1725, 146484. [Google Scholar] [CrossRef]
- Yang, P.; Su, C.; Luo, X.; Zeng, H.; Zhao, L.; Wei, L.; Zhang, X.; Varghese, Z.; Moorhead, J.F.; Chen, Y.; et al. Dietary oleic acid-induced CD36 promotes cervical cancer cell growth and metastasis via up-regulation Src/ERK pathway. Cancer Lett. 2018, 438, 76–85. [Google Scholar] [CrossRef]
- Allaj, V.; Guo, C.; Nie, D. Non-steroid anti-inflammatory drugs, prostaglandins, and cancer. Cell Biosci. 2013, 3, 8. [Google Scholar] [CrossRef]
- Cheng, X.; Geng, F.; Pan, M.; Wu, X.; Zhong, Y.; Wang, C.; Tian, Z.; Cheng, C.; Zhang, R.; Puduvalli, V.; et al. Targeting DGAT1 ameliorates glioblastoma by increasing fat catabolism and oxidative stress. Cell Metab. 2020, 32, 229–242.e228. [Google Scholar] [CrossRef]
- Baron, C.L.; Malhotra, V. Role of diacylglycerol in PKD recruitment to the TGN and protein transport to the plasma membrane. Science 2002, 295, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Yeaman, C.; Ayala, M.I.; Wright, J.R.; Bard, F.; Bossard, C.; Ang, A.; Maeda, Y.; Seufferlein, T.; Mellman, I.; Nelson, W.J.; et al. Protein kinase D regulates basolateral membrane protein exit from trans-Golgi network. Nat. Cell Biol. 2004, 6, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Welte, M.A. Expanding roles for lipid droplets. Curr. Biol. 2015, 25, R470–R481. [Google Scholar] [CrossRef] [PubMed]
- Gross, D.A.; Silver, D.L. Cytosolic lipid droplets: From mechanisms of fat storage to disease. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 304–326. [Google Scholar] [CrossRef] [PubMed]
- Petan, T.; Jarc, E.; Jusović, M. Lipid droplets in cancer: Guardians of fat in a stressful world. Molecules 2018, 23, 1941. [Google Scholar] [CrossRef] [PubMed]
- Corbet, C.; Pinto, A.; Martherus, R.; Santiago de Jesus, J.P.; Polet, F.; Feron, O. Acidosis drives the reprogramming of fatty acid metabolism in cancer cells through changes in mitochondrial and histone acetylation. Cell Metab. 2016, 24, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Cruz, A.L.S.; Barreto, E.A.; Fazolini, N.P.B.; Viola, J.P.B.; Bozza, P.T. Lipid droplets: Platforms with multiple functions in cancer hallmarks. Cell Death Dis. 2020, 11, 105. [Google Scholar] [CrossRef]
- Sugiura, T.; Kondo, S.; Sukagawa, A.; Nakane, S.; Shinoda, A.; Itoh, K.; Yamashita, A.; Waku, K. 2-Arachidonoylglycerol: A possible endogenous cannabinoid receptor ligand in brain. Biochem. Biophys. Res. Commun. 1995, 215, 89–97. [Google Scholar] [CrossRef]
- Kohnz, R.A.; Nomura, D.K. Chemical approaches to therapeutically target the metabolism and signaling of the endocannabinoid 2-AG and eicosanoids. Chem. Soc. Rev. 2014, 43, 6859–6869. [Google Scholar] [CrossRef]
- Khunluck, T.; Lertsuwan, K.; Chutoe, C.; Sooksawanwit, S.; Inson, I.; Teerapornpuntakit, J.; Tohtong, R.; Charoenphandhu, N. Activation of cannabinoid receptors in breast cancer cells improves osteoblast viability in cancer-bone interaction model while reducing breast cancer cell survival and migration. Sci. Rep. 2022, 12, 7398. [Google Scholar] [CrossRef]
- Jacobsson, S.O.; Wallin, T.; Fowler, C.J. Inhibition of rat C6 glioma cell proliferation by endogenous and synthetic cannabinoids. Relative involvement of cannabinoid and vanilloid receptors. J. Pharmacol. Exp. Ther. 2001, 299, 951–959. [Google Scholar] [PubMed]
- Bhattacharya, A. Lipid Metabolism in plants under low-temperature stress: A review. Springer: Singapore, 2022; pp. 409–516. [Google Scholar]
- Dolce, V.; Cappello, A.R.; Lappano, R.; Maggiolini, M. Glycerophospholipid synthesis as a novel drug target against cancer. Curr. Mol. Pharmacol. 2011, 4, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Hirt, H.; Lee, Y. Phosphatidic acid activates a wound-activated MAPK in Glycine max. Plant J. 2001, 26, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Papandreou, I.; Goliasova, T.; Denko, N.C. Anticancer drugs that target metabolism: Is dichloroacetate the new paradigm? Int. J. Cancer 2011, 128, 1001–1008. [Google Scholar] [CrossRef]
- Han, S.; Huh, J.; Kim, W.; Jeong, S.; Min do, S.; Jung, Y. Phospholipase D activates HIF-1-VEGF pathway via phosphatidic acid. Exp. Mol. Med. 2014, 46, e126. [Google Scholar] [CrossRef]
- Beckonert, O.; Monnerjahn, J.; Bonk, U.; Leibfritz, D. Visualizing metabolic changes in breast-cancer tissue using 1H-NMR spectroscopy and self-organizing maps. NMR Biomed. 2003, 16, 1–11. [Google Scholar] [CrossRef]
- Sonkar, K.; Ayyappan, V.; Tressler, C.M.; Adelaja, O.; Cai, R.; Cheng, M.; Glunde, K. Focus on the glycerophosphocholine pathway in choline phospholipid metabolism of cancer. NMR Biomed. 2019, 32, e4112. [Google Scholar] [CrossRef]
- Patel, D.; Witt, S.N. Ethanolamine and phosphatidylethanolamine: Partners in health and disease. Oxidative Med. Cell. Longev. 2017, 2017, 4829180. [Google Scholar] [CrossRef]
- Marsh, D. Lateral pressure profile, spontaneous curvature frustration, and the incorporation and conformation of proteins in membranes. Biophys. J. 2007, 93, 3884–3899. [Google Scholar] [CrossRef]
- Owusu Obeng, E.; Rusciano, I.; Marvi, M.V.; Fazio, A.; Ratti, S.; Follo, M.Y.; Xian, J.; Manzoli, L.; Billi, A.M.; Mongiorgi, S.; et al. Phosphoinositide-dependent signaling in cancer: A focus on phospholipase C isozymes. Int. J. Mol. Sci. 2020, 21, 2581. [Google Scholar] [CrossRef]
- Mullen, T.D.; Obeid, L.M. Ceramide and apoptosis: Exploring the enigmatic connections between sphingolipid metabolism and programmed cell death. Anti-Cancer Agents Med. Chem. 2012, 12, 340–363. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, G.; Uemura, T.; Chuang, C.C.; Polishchuk, E.; Santoro, M.; Ohvo-Rekilä, H.; Sato, T.; Di Tullio, G.; Varriale, A.; D’Auria, S.; et al. Vesicular and non-vesicular transport feed distinct glycosylation pathways in the Golgi. Nature 2013, 501, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Siskind, L.J. Mitochondrial ceramide and the induction of apoptosis. J. Bioenerg. Biomembr. 2005, 37, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Regina Todeschini, A.; Hakomori, S.I. Functional role of glycosphingolipids and gangliosides in control of cell adhesion, motility, and growth, through glycosynaptic microdomains. Biochim. Biophys. Acta 2008, 1780, 421–433. [Google Scholar] [CrossRef]
- Sezgin, E.; Levental, I.; Mayor, S.; Eggeling, C. The mystery of membrane organization: Composition, regulation and roles of lipid rafts. Nat. Rev. Mol. Cell Biol. 2017, 18, 361–374. [Google Scholar] [CrossRef]
- Giacomini, I.; Gianfanti, F.; Desbats, M.A.; Orso, G.; Berretta, M.; Prayer-Galetti, T.; Ragazzi, E.; Cocetta, V. Cholesterol metabolic reprogramming in cancer and its pharmacological modulation as therapeutic strategy. Front. Oncol. 2021, 11, 682911. [Google Scholar] [CrossRef]
- Russo, D.; Parashuraman, S.; D’Angelo, G. Glycosphingolipid-protein interaction in signal transduction. Int. J. Mol. Sci. 2016, 17, 1723. [Google Scholar] [CrossRef]
- Nazha, B.; Inal, C.; Owonikoko, T.K. Disialoganglioside GD2 expression in solid tumors and role as a target for cancer therapy. Front. Oncol. 2020, 10, 1000. [Google Scholar] [CrossRef]
- Navid, F.; Santana, V.M.; Barfield, R.C. Anti-GD2 antibody therapy for GD2-expressing tumors. Curr. Cancer Drug Targets 2010, 10, 200–209. [Google Scholar] [CrossRef]
- Lang, Z.; Guerrera, M.; Li, R.; Ladisch, S. Ganglioside GD1a enhances VEGF-induced endothelial cell proliferation and migration. Biochem. Biophys. Res. Commun. 2001, 282, 1031–1037. [Google Scholar] [CrossRef]
- Sasaki, N.; Toyoda, M.; Ishiwata, T. Gangliosides as signaling regulators in cancer. Int. J. Mol. Sci. 2021, 22, 5076. [Google Scholar] [CrossRef] [PubMed]
- Dufourc, E.J. Sterols and membrane dynamics. J. Chem. Biol. 2008, 1, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, Y.; Rogers, M.A. Sterol metabolism and transport in atherosclerosis and cancer. Front. Endocrinol. 2018, 9, 509. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.; Ahmad, F.; Kambach, D.M.; Sun, Q.; Halim, A.S.; Kramp, T.; Camphausen, K.A.; Stommel, J.M. LXRβ controls glioblastoma cell growth, lipid balance, and immune modulation independently of ABCA1. Sci. Rep. 2019, 9, 15458. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Li, S.; Xue, A.Z.; Smith Callahan, L.A.; Liu, Y. Expression of SREBP2 and cholesterol metabolism related genes in TCGA glioma cohorts. Medicine 2020, 99, e18815. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Hao, D.; Wang, L.; Li, J.; Meng, Y.; Li, P.; Wang, Y.; Zhang, C.; Zhou, H.; Gardner, K.; et al. CtBP promotes metastasis of breast cancer through repressing cholesterol and activating TGF-β signaling. Oncogene 2019, 38, 2076–2091. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.Y.; Li, B.L.; Chang, C.C.; Urano, Y. Acyl-coenzyme A:Ccholesterol acyltransferases. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E1–E9. [Google Scholar] [CrossRef]
- Gamba, P.; Giannelli, S.; Staurenghi, E.; Testa, G.; Sottero, B.; Biasi, F.; Poli, G.; Leonarduzzi, G. The controversial role of 24-S-hydroxycholesterol in Alzheimer’s disease. Antioxidants 2021, 10, 740. [Google Scholar] [CrossRef]
- Han, M.; Wang, S.; Yang, N.; Wang, X.; Zhao, W.; Saed, H.S.; Daubon, T.; Huang, B.; Chen, A.; Li, G.; et al. Therapeutic implications of altered cholesterol homeostasis mediated by loss of CYP46A1 in human glioblastoma. EMBO Mol. Med. 2020, 12, e10924. [Google Scholar] [CrossRef]
- Ding, X.; Zhang, W.; Li, S.; Yang, H. The role of cholesterol metabolism in cancer. Am. J. Cancer Res. 2019, 9, 219–227. [Google Scholar]
- Björkhem, I. Crossing the barrier: Oxysterols as cholesterol transporters and metabolic modulators in the brain. J. Intern. Med. 2006, 260, 493–508. [Google Scholar] [CrossRef] [PubMed]
- Peetla, C.; Vijayaraghavalu, S.; Labhasetwar, V. Biophysics of cell membrane lipids in cancer drug resistance: Implications for drug transport and drug delivery with nanoparticles. Adv. Drug Deliv. Rev. 2013, 65, 1686–1698. [Google Scholar] [CrossRef] [PubMed]
- Bogdanovic, N.; Bretillon, L.; Lund, E.G.; Diczfalusy, U.; Lannfelt, L.; Winblad, B.; Russell, D.W.; Björkhem, I. On the turnover of brain cholesterol in patients with Alzheimer’s disease. Abnormal induction of the cholesterol-catabolic enzyme CYP46 in glial cells. Neurosci. Lett. 2001, 314, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, J.; Rao, K.; Pastorino, S.; Kesari, S. Corticosteroids in brain cancer patients: Benefits and pitfalls. Expert Rev. Clin. Pharmacol. 2011, 4, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Radwan, A.A.; Alanazi, F.K. Targeting cancer using cholesterol conjugates. Saudi Pharm. J. 2014, 22, 3–16. [Google Scholar] [CrossRef]
- Das, U.N. From bench to the clinic: Gamma-linolenic acid therapy of human gliomas. Prostaglandins Leukot. Essent. Fat. Acids 2004, 70, 539–552. [Google Scholar] [CrossRef]
- Sandrone, S.S.; Repossi, G.; Candolfi, M.; Eynard, A.R. Polyunsaturated fatty acids and gliomas: A critical review of experimental, clinical, and epidemiologic data. Nutrition 2014, 30, 1104–1109. [Google Scholar] [CrossRef]
- Priore, P.; Gnoni, A.; Natali, F.; Testini, M.; Gnoni, G.V.; Siculella, L.; Damiano, F. Oleic Acid and Hydroxytyrosol inhibit cholesterol and fatty acid synthesis in C6 glioma cells. Oxidative Med. Cell. Longev. 2017, 2017, 9076052. [Google Scholar] [CrossRef]
- de Oliveira, F.S.; de Oliveira, P.M.; Farias, L.M.; Brinkerhoff, R.C.; Sobrinho, R.; Treptow, T.M.; Montes D’Oca, C.R.; Marinho, M.A.G.; Hort, M.A.; Horn, A.P.; et al. Synthesis and antitumoral activity of novel analogues monastrol-fatty acids against glioma cells. MedChemComm 2018, 9, 1282–1288. [Google Scholar] [CrossRef]
- Jia, S.N.; Lin, C.; Chen, D.F.; Li, A.Q.; Dai, L.; Zhang, L.; Zhao, L.L.; Yang, J.S.; Yang, F.; Yang, W.J. The transcription factor p8 regulates autophagy in response to palmitic acid stress via a mammalian target of rapamycin (mTOR)-independent signaling pathway. J. Biol. Chem. 2016, 291, 4462–4472. [Google Scholar] [CrossRef]
- Dyall, S.C. Long-chain omega-3 fatty acids and the brain: A review of the independent and shared effects of EPA, DPA and DHA. Front. Aging Neurosci. 2015, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- Göder, A.; Nagel, G.; Kraus, A.; Dörsam, B.; Seiwert, N.; Kaina, B.; Fahrer, J. Lipoic acid inhibits the DNA repair protein O 6-methylguanine-DNA methyltransferase (MGMT) and triggers its depletion in colorectal cancer cells with concomitant autophagy induction. Carcinogenesis 2015, 36, 817–831. [Google Scholar] [CrossRef] [PubMed]
- Deveci, H.A.; Akyuva, Y.; Nur, G.; Nazıroğlu, M. Alpha lipoic acid attenuates hypoxia-induced apoptosis, inflammation and mitochondrial oxidative stress via inhibition of TRPA1 channel in human glioblastoma cell line. Biomed. Pharmacother. 2019, 111, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Eyles, D.W.; Smith, S.; Kinobe, R.; Hewison, M.; McGrath, J.J. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J. Chem. Neuroanat. 2005, 29, 21–30. [Google Scholar] [CrossRef]
- Fife, R.S.; Sledge, G.W., Jr.; Proctor, C. Effects of vitamin D3 on proliferation of cancer cells in vitro. Cancer Lett. 1997, 120, 65–69. [Google Scholar] [CrossRef]
- Liu, L.; Hu, Z.; Zhang, H.; Hou, Y.; Zhang, Z.; Zhou, G.; Li, B. Vitamin D postpones the progression of epithelial ovarian cancer induced by 7, 12-dimethylbenz [a] anthracene both in vitro and in vivo. OncoTargets Ther. 2016, 9, 2365–2375. [Google Scholar] [CrossRef][Green Version]
- Salvador, J.A.; Carvalho, J.F.; Neves, M.A.; Silvestre, S.M.; Leitão, A.J.; Silva, M.M.; ML, S.e.M. Anticancer steroids: Linking natural and semi-synthetic compounds. Nat. Prod. Rep. 2013, 30, 324–374. [Google Scholar] [CrossRef]
- Sortino, M.; Garibotto, F.; Cechinel Filho, V.; Gupta, M.; Enriz, R.; Zacchino, S. Antifungal, cytotoxic and SAR studies of a series of N-alkyl, N-aryl and N-alkylphenyl-1,4-pyrrolediones and related compounds. Bioorg. Med. Chem. 2011, 19, 2823–2834. [Google Scholar] [CrossRef]
- Nakamura, N.; Hirakawa, A.; Gao, J.-J.; Kakuda, H.; Shiro, M.; Komatsu, Y.; Sheu, C.-c.; Hattori, M. Five new maleic and succinic acid derivatives from the mycelium of Antrodia camphorata and their cytotoxic effects on LLC tumor cell line. J. Nat. Prod. 2004, 67, 46–48. [Google Scholar] [CrossRef]
- Wójciak-Kosior, M.; Paduch, R.; Matysik-Woźniak, A.; Niedziela, P.; Donica, H. The effect of ursolic and oleanolic acids on human skin fibroblast cells. Folia Histochem. Cytobiol. 2011, 49, 664–669. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, Y.; Komohara, Y.; Kudo, R.; Tsurushima, K.; Ohnishi, K.; Ikeda, T.; Takeya, M. Oleanolic acid inhibits macrophage differentiation into the M2 phenotype and glioblastoma cell proliferation by suppressing the activation of STAT3. Oncol. Rep. 2011, 26, 1533–1537. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Deeb, D.; Jiang, H.; Liu, Y.; Dulchavsky, S.A.; Gautam, S.C. Synthetic triterpenoids inhibit growth and induce apoptosis in human glioblastoma and neuroblastoma cells through inhibition of prosurvival Akt, NF-kappaB and Notch1 signaling. J. Neuro-Oncol. 2007, 84, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, A.K.; DeBerardinis, R.J. Applications of metabolomics to study cancer metabolism. Biochim. Biophys. Acta Rev. Cancer 2018, 1870, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.M.; Parolia, A.; Dunphy, M.P.; Venneti, S. Non-invasive metabolic imaging of brain tumours in the era of precision medicine. Nat. Rev. Clin. Oncol. 2016, 13, 725–739. [Google Scholar] [CrossRef]
- Yamasaki, F.; Takayasu, T.; Nosaka, R.; Amatya, V.J.; Doskaliyev, A.; Akiyama, Y.; Tominaga, A.; Takeshima, Y.; Sugiyama, K.; Kurisu, K. Magnetic resonance spectroscopy detection of high lipid levels in intraaxial tumors without central necrosis: A characteristic of malignant lymphoma. J. Neurosurg. 2015, 122, 1370–1379. [Google Scholar] [CrossRef]
- Soares, D.P.; Law, M. Magnetic resonance spectroscopy of the brain: Review of metabolites and clinical applications. Clin. Radiol. 2009, 64, 12–21. [Google Scholar] [CrossRef]
- Elkhaled, A.; Jalbert, L.; Constantin, A.; Yoshihara, H.A.; Phillips, J.J.; Molinaro, A.M.; Chang, S.M.; Nelson, S.J. Characterization of metabolites in infiltrating gliomas using ex vivo ¹H high-resolution magic angle spinning spectroscopy. NMR Biomed. 2014, 27, 578–593. [Google Scholar] [CrossRef]
- Bolan, P.J. Magnetic resonance spectroscopy of the breast: Current status. Magn. Reson. Imaging Clin. 2013, 21, 625–639. [Google Scholar] [CrossRef]
- Kurhanewicz, J.; Swanson, M.G.; Nelson, S.J.; Vigneron, D.B. Combined magnetic resonance imaging and spectroscopic imaging approach to molecular imaging of prostate cancer. J. Magn. Reson. Imaging JMRI 2002, 16, 451–463. [Google Scholar] [CrossRef]
- Glunde, K.; Bhujwalla, Z.M.; Ronen, S.M. Choline metabolism in malignant transformation. Nat. Rev. Cancer 2011, 11, 835–848. [Google Scholar] [CrossRef]
- Wehrl, H.F.; Schwab, J.; Hasenbach, K.; Reischl, G.; Tabatabai, G.; Quintanilla-Martinez, L.; Jiru, F.; Chughtai, K.; Kiss, A.; Cay, F.; et al. Multimodal elucidation of choline metabolism in a murine glioma model using magnetic resonance spectroscopy and 11C-choline positron emission tomography. Cancer Res. 2013, 73, 1470–1480. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.L.; Chang, L.; Booth, R.; Ernst, T.; Cornford, M.; Nikas, D.; McBride, D.; Jenden, D.J. In vivo 1H MRS choline: Correlation with in vitro chemistry/histology. Life Sci. 1996, 58, 1929–1935. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.Y.; Kwock, L.; Castillo, M.; Smith, J.K. Association of choline levels and tumor perfusion in brain metastases assessed with proton MR spectroscopy and dynamic susceptibility contrast-enhanced perfusion weighted MRI. Technol. Cancer Res. Treat. 2010, 9, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Kaminogo, M.; Ishimaru, H.; Morikawa, M.; Ochi, M.; Ushijima, R.; Tani, M.; Matsuo, Y.; Kawakubo, J.; Shibata, S. Diagnostic potential of short echo time MR spectroscopy of gliomas with single-voxel and point-resolved spatially localised proton spectroscopy of brain. Neuroradiology 2001, 43, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Delikatny, E.J.; Chawla, S.; Leung, D.J.; Poptani, H. MR-visible lipids and the tumor microenvironment. NMR Biomed. 2011, 24, 592–611. [Google Scholar] [CrossRef]
- Mohan, S.; Verma, A.; Lim, C.C.; Hui, F.; Kumar, S. Lipid resonance on in vivo proton MR spectroscopy: Value of other metabolites in differential diagnosis. Neuroradiol. J. 2010, 23, 269–278. [Google Scholar] [CrossRef]
- Tang, Y.Z.; Booth, T.C.; Bhogal, P.; Malhotra, A.; Wilhelm, T. Imaging of primary central nervous system lymphoma. Clin. Radiol. 2011, 66, 768–777. [Google Scholar] [CrossRef]
- Astrakas, L.G.; Zurakowski, D.; Tzika, A.A.; Zarifi, M.K.; Anthony, D.C.; De Girolami, U.; Tarbell, N.J.; Black, P.M. Noninvasive magnetic resonance spectroscopic imaging biomarkers to predict the clinical grade of pediatric brain tumors. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2004, 10, 8220–8228. [Google Scholar] [CrossRef]
- Eggers, H.; Börnert, P. Chemical shift encoding-based water-fat separation methods. J. Magn. Reson. Imaging JMRI 2014, 40, 251–268. [Google Scholar] [CrossRef]
- Reeder, S.B.; Cruite, I.; Hamilton, G.; Sirlin, C.B. Quantitative assessment of liver fat with magnetic resonance imaging and spectroscopy. J. Magn. Reson. Imaging JMRI 2011, 34, 729–749. [Google Scholar] [CrossRef]
- Lindskog, M.; Spenger, C.; Klason, T.; Jarvet, J.; Gräslund, A.; Johnsen, J.I.; Ponthan, F.; Douglas, L.; Nordell, B.; Kogner, P. Proton magnetic resonance spectroscopy in neuroblastoma: Current status, prospects and limitations. Cancer Lett. 2005, 228, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Sibtain, N.A.; Howe, F.A.; Saunders, D.E. The clinical value of proton magnetic resonance spectroscopy in adult brain tumours. Clin. Radiol. 2007, 62, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Mountford, C.E.; Grossman, G.; Reid, G.; Fox, R.M. Characterization of transformed cells and tumors by proton nuclear magnetic resonance spectroscopy. Cancer Res. 1982, 42, 2270–2276. [Google Scholar] [PubMed]
- Rémy, C.; Fouilhé, N.; Barba, I.; Sam-Laï, E.; Lahrech, H.; Cucurella, M.G.; Izquierdo, M.; Moreno, A.; Ziegler, A.; Massarelli, R.; et al. Evidence that mobile lipids detected in rat brain glioma by 1H nuclear magnetic resonance correspond to lipid droplets. Cancer Res. 1997, 57, 407–414. [Google Scholar] [PubMed]
- Kuesel, A.C.; Donnelly, S.M.; Halliday, W.; Sutherland, G.R.; Smith, I.C. Mobile lipids and metabolic heterogeneity of brain tumours as detectable by ex vivo 1H MR spectroscopy. NMR Biomed. 1994, 7, 172–180. [Google Scholar] [CrossRef]
- Pérez, Y.; Lahrech, H.; Cabañas, M.E.; Barnadas, R.; Sabés, M.; Rémy, C.; Arús, C. Measurement by nuclear magnetic resonance diffusion of the dimensions of the mobile lipid compartment in C6 cells. Cancer Res. 2002, 62, 5672–5677. [Google Scholar]
- Milkevitch, M.; Shim, H.; Pilatus, U.; Pickup, S.; Wehrle, J.P.; Samid, D.; Poptani, H.; Glickson, J.D.; Delikatny, E.J. Increases in NMR-visible lipid and glycerophosphocholine during phenylbutyrate-induced apoptosis in human prostate cancer cells. Biochim. Biophys. Acta 2005, 1734, 1–12. [Google Scholar] [CrossRef]
- Seow, P.; Narayanan, V.; Hernowo, A.T.; Wong, J.H.D.; Ramli, N. Quantification and visualization of lipid landscape in glioma using in -and opposed-phase imaging. NeuroImage Clin. 2018, 20, 531–536. [Google Scholar] [CrossRef]
- Seow, P.; Narayanan, V.; Romelean, R.J.; Wong, J.H.D.; Win, M.T.; Chandran, H.; Chinna, K.; Rahmat, K.; Ramli, N. Lipid fraction derived from MRI in- and opposed-phase sequence as a novel biomarker for predicting survival outcome of glioma. Acad. Radiol. 2020, 27, 180–187. [Google Scholar] [CrossRef]

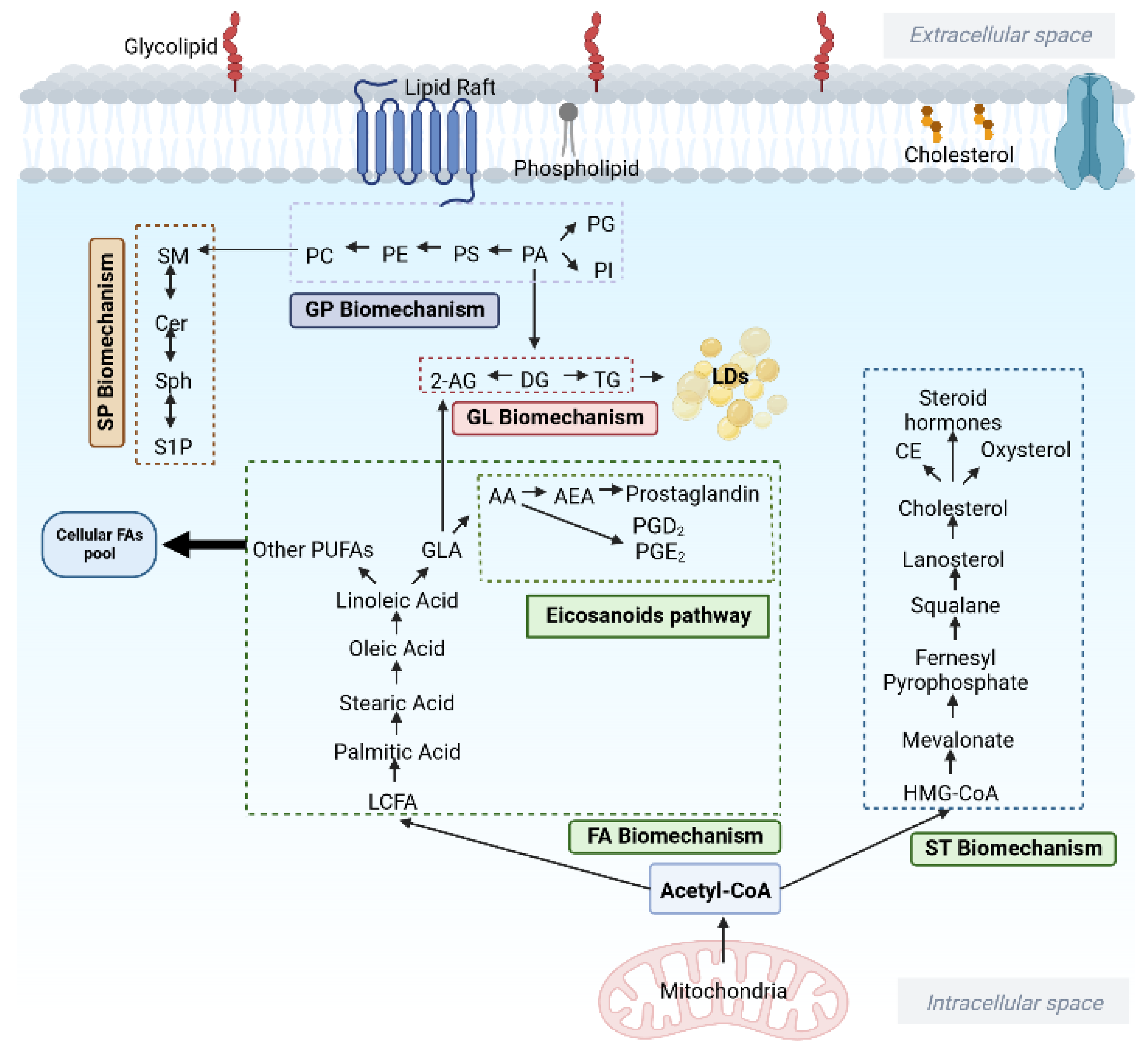
| Research | Diagnosis | Experimental Model | Sample Type | Analytical Platform | Lipid Species | Physiological Effect on Glioma |
|---|---|---|---|---|---|---|
| Fatty Acyls (FA) | ||||||
| [20] | GBM | Human | Serum | GC-TOFMS | ↑ Butyric acid (C4) | Provide substrate for glutamate metabolism |
| [21] | GBM | Cell line | Tissue | GCMS | ↑ Octanoic acid (C8) | Activated ketone body metabolism for glioma cell survival |
| [21] | GBM | Cell line | Tissue | GCMS | ↑ Decanoic acid (C10) | Stimulate fatty acyl production |
| [22] | GBM | Human | Tissue | GC-TOFMS | ↓ Lauric acid (C12) | Tumour malignancy |
| [23] | GBM | Cell line | Tissue | HPLC, Spectrophotometric | ↑ Palmitic acid (C16) | Enhanced glioma cell proliferation |
| [20] | GBM | Human | Serum | GC-TOFMS | ↑ Stearic acid (C18:0) | Provide substrate for glutamate metabolism |
| [24,25,26] | Oligodendroglioma Astrocytoma GBM, glioma | Cell line | Tissue | Raman spectroscopy MS | ↑ Oleic acid (C18:1) | Cellular apoptosis, Enhance proliferation of GBM cells |
| [20] | GBM | Human | Serum | GC-TOFMS | ↑ Linolenic acid (C18:3) | Provide substrate for glutamate metabolism |
| [20] | GBM | Human | Serum | GC-TOFMS | ↑ AA (C20:4) | Provide substrate for glutamate metabolism |
| [27] | GBM | Human | Serum | MS | ↓ VLCDCA | Anti-inflammatory and has chemopreventative properties |
| [28] | Glioma (Grade II, III), GBM | Human | Serum | LCMS/MS | ↑ Stearoylcarnitine (C18), margaroylcarnitine, Eicosenoylcarnitine (C20:1) | Supply substrate for the activation of fatty acyl metabolism |
| [29] | GBM | Cell line | Tissue | HPLC-MS/MS | ↑ PGD2 | Support glioma growth and invasion |
| [30] [31] | Glioma GBM | Cell line Human & animal | Tissue | Biochemical assay | ↑ PGE2 | Protect glioma cells against radiation treatment. Escalate self-renewal capacity and resistance to radiation-induced DNA damage |
| Glycerolipid (GL) | ||||||
| [32] | LGG, HGG | Human | Tissue | LCMS | ↑ 2-AG | Inhibit cell apoptosis, support cell proliferation and survival |
| [33] | Grade III | Human | CSF | MS/MS | ↑ DG | Malignant transformation |
| [21,34] | GBM | Cell line | Tissue | GCMS | ↓ DG (DG34:0, DG34:1, DG36:1, DG38:4, DG38:6, DG40:6) | Influence carcinogenesis signaling and inflammatory reaction in GBM |
| [35,36] | GBM | Cell line | Tissue | Microscopy | ↓ TG, LD | Mitochondria hydrolyzed lipid droplets and utilized triglycerides for energy production |
| [37] | Medulloblastoma | Human | Tissue | Raman Imaging | ↑ TG | Tumour development |
| Glycerophospholipid (GP) | ||||||
| [38,39] | GBM | Cell line | Tissue | Biochemical assay, UPLC-MS/MS | ↑ PA | Lipid signaling of autophagy and cell survival in glioma |
| [37,40] | GBM, glioma | Animal, human | Tissue | MALDI-IMS ESI-MS/MS, Raman imaging | ↓ PA (PA36:2, PA42:5, PA42.7) ↓ DHA | Influence carcinogenesis signaling and inflammatory reaction in GBM |
| [41] | IDHwt glioma | Cell culture | Tissue | NMR | ↑ PC | Increase cell turnover and tumour growth |
| [34,42,43,44,45,46,47] [28,48,49,50,51,52] | GBM Astrocytoma, GBM | Cell line Human | Tissue Tissue, Serum | NMR LCMS | ↑ PC ↑ PC PC14:2 | Malignant progression and aggressiveness in GBM |
| [26,34,48] [33] | Glioma Grade III | Human | Tissue CSF | Swab TS-MS, LCMS MS/MS | ↑ PI, PG | Enhanced tumour infiltration |
| [34,53] | GBM | Cell line | Tissue | MSI, LCMS | ↑ PE | Enhanced tumour growth |
| [41] | IDHwt glioma | Cell line | Tissue | NMR | ↑ PE | Increase cell turnover and tumour growth |
| [54] | GBM | Animal | Tissue | MRI | ↓ LPA | Support cell proliferation through the disassembling of primary cilia |
| [55,56] | Glioma Grade II, III GBM GBM | Human Cell line | Tissue | GCMS. LCMS | ↓ LPC, LPE | Provide substrates to mitochondria to generate energy |
| Sphingolipid (SP) | ||||||
| [57,58,59,60,61,62,63,64,65] | GBM | Cell line | Tissue | Biochemical assay | ↑ S1P | Resistant to chemo-therapeutic treatment and sustain the growth of glioma cells. Induced cell angiogenesis |
| [66,67] [33] | GBM Grade III | Cell line Human | Tissue CSF | Biochemical assay MS/MS | ↑ S1P, ↑ Cer, ↑ SM | GBM cell proliferation |
| [68] | Oligodendroglioma | Cell line | Tissue | LCMS | ↑ NDMS, ↑ Sphingosine-C18, ↑ Sphingosine C17, ↑ Sphinganine C17 | Signaling roles for proliferation and survival |
| [28,69] | Glioma Grade II-III and GBM | Human | Serum Tissue | LCMS/MS, MALDI-TOF-MS | ↑ SM (d16:1/23:0, d17:1/18:0, d18:1/17:0, d18:0/15:0, d18:1/16:0, d18:0/17:0, d19:1/16:0) | Involved in the regulation of sphingolipid metabolism and malignancy of glioma, glioma cell senescence and apoptosis |
| [33] | Grade III | Human | CSF | HPLC/MS | ↑ N-Lignoceroylsphingosine | Involved in lipid signaling and apoptosis |
| [70] | GBM | Human | Tissue | Biochemical assay | ↑ OAcGD2 | Increase tumour density and involvement in immunoresistance |
| [71] | Diffuse midline glioma | Cell line | Tissue | Biochemical assay | ↑ GD2 | Enhanced metastasis |
| [72,73,74] | GBM, anaplastic oligodendroglioma | Animal | Tissue | Biochemical assay | ↑ GD3 | Involved in cell transformation and malignancy |
| [75] | GBM | Cell line | Tissue | Biochemical assay | ↑ GM2 | Enhanced cell migration |
| [76] | Glioma, Medulloblastoma | Animal | Tissue | MALDI-IMS | ↑ GM3 | Induced malignancy, invasiveness and progression of tumour |
| Sterol Lipid (ST) | ||||||
| [77] | GBM | Cell line | Tissue | Biochemical assay | ↑ CE | Tumour progression and malignant |
| [33] | Grade III | Human | CSF | HPLC/MS | ↑ 1-Oleyl-cholesterol | Enhance tumour growth |
| [33] | Grade III | Human | CSF | HPLC/MS | ↑ Tetrahydrocorticosterone | Enhanced metastasis |
| [78,79] | GBM | Cell line | Tissue | GCMS Biochemical assay | ↑ 24S-OHC | Induced tumour growth by regulating proinflammatory immune cells. nduced pathogenesis of tumour cells |
| [80] | GBM | Human | Serum | Biochemical assay | LDL | Support proliferation and growth of glioma |
| Research | Diagnosis | Experimental Model | Sample Type | Analytical Platform | Lipid Species | Physiological Effect on Glioma |
|---|---|---|---|---|---|---|
| Fatty Acyl (FA) | ||||||
| [81] | GBM | Cell line | Tissue | Biochemical assay GCMS | Palmitic acid (C16), | Increase activity of neurotoxicity and gliatoxicity in glioma cells. |
| [81] | GBM | Cell line | Tissue | Biochemical assay GCMS | stearic acid (C18) | Increase activity of neurotoxicity and gliatoxicity in glioma cells. |
| [82] | Glioma | Cell line | Tissue | Biochemical assay | GLA (C18:3) | Enhanced radio sensitivity towards radiotherapy |
| [83] | GBM | Cell line | Tissue | MS | GLA (C18:3) | Reduced the number of lipid droplet formation and induced cell death to GBM cells |
| [23] | GBM | Cell line | Tissue | HPLC, Spectrophotometric | EPA (20:5) | Ceased growth of glioma cells. |
| [82] | Glioma | Cell line | Tissue | Biochemical assay | DHA (C22:6) | Enhanced radio sensitivity towards radiotherapy |
| [84] | GBM | Cell line | Tissue | STED microscopy | DHA (C22:6) | Affect the configuration of membrane lipid order, that link to cell migration |
| [85] | Glioma | Animal | Tissue | Biochemical assay | DHA (C22:6) | Preserve lipid domain in membrane bilayer. |
| [86] | GBM | Cell line | Tissue | SRS Microscopy | DHA (C22:6) | Decreased the survival rate of glioma cell by reducing the formation of lipid droplets |
| [87] | GBM | Cell line | Tissue | Biochemical assay | Lipoic acid | Retarded glioma growth by reduced cell proliferation |
| [82] | Glioma | Cell line | Tissue | Biochemical assay | Lipoic acid | Enhanced radio sensitivity towards radiotherapy |
| Sphingolipid (SP) | ||||||
| [88] | GBM | Cell line | Tissue | Biochemical assay | C2 ceramide | Prevent glioma invasion through inhibition of MMP expression |
| [89] | GBM | Cell line | Tissue | Biochemical assay | C18 ceramide | Inhibit cell viability and prevent glioma growth |
| [90] | GBM | Cell line | Tissue | Biochemical assay | dhCer, dhSph | Increase oxidative stress, endoplasmic reticulum stress autophagy in glioma cells |
| [91] | GBM | Animal | Tissue | Biochemical assay, LCMS | Glycosides | Induces endoplasmic reticulum stress and cell death |
| Sterol Lipid (ST) | ||||||
| [92] | GBM | Animal | Tissue | LCMS | 7B-hydroxycholesterol | Reduce level of cholesterol, cholesterol ester and cholesterol derivatives |
| [87,93,94,95] | GBM | Cell line | Tissue | Biochemical assay | Vitamin D3 | Reduced tumour growth and prevent proliferation |
| [96] | GBM | Animal | Tissue | Biochemical assay | Steroidal maleimides | Ceased tumour growth and highly cytotoxic to tumour cells |
| Prenol Lipid (PL) | ||||||
| [97] | GBM | Cell line | Tissue | Biochemical assay | Oleanoic acid | Reduced tumour cells migration and invasion |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdul Rashid, K.; Ibrahim, K.; Wong, J.H.D.; Mohd Ramli, N. Lipid Alterations in Glioma: A Systematic Review. Metabolites 2022, 12, 1280. https://doi.org/10.3390/metabo12121280
Abdul Rashid K, Ibrahim K, Wong JHD, Mohd Ramli N. Lipid Alterations in Glioma: A Systematic Review. Metabolites. 2022; 12(12):1280. https://doi.org/10.3390/metabo12121280
Chicago/Turabian StyleAbdul Rashid, Khairunnisa, Kamariah Ibrahim, Jeannie Hsiu Ding Wong, and Norlisah Mohd Ramli. 2022. "Lipid Alterations in Glioma: A Systematic Review" Metabolites 12, no. 12: 1280. https://doi.org/10.3390/metabo12121280
APA StyleAbdul Rashid, K., Ibrahim, K., Wong, J. H. D., & Mohd Ramli, N. (2022). Lipid Alterations in Glioma: A Systematic Review. Metabolites, 12(12), 1280. https://doi.org/10.3390/metabo12121280