1H-NMR-Based Metabonomics Study to Reveal the Progressive Metabolism Regulation of SAP Deficiency on ApoE−/− Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Laboratory Animals
2.2. Blood Collection
2.3. 1H-NMR Spectroscopy
2.4. Pattern Recognition and Statistical Analysis
3. Results
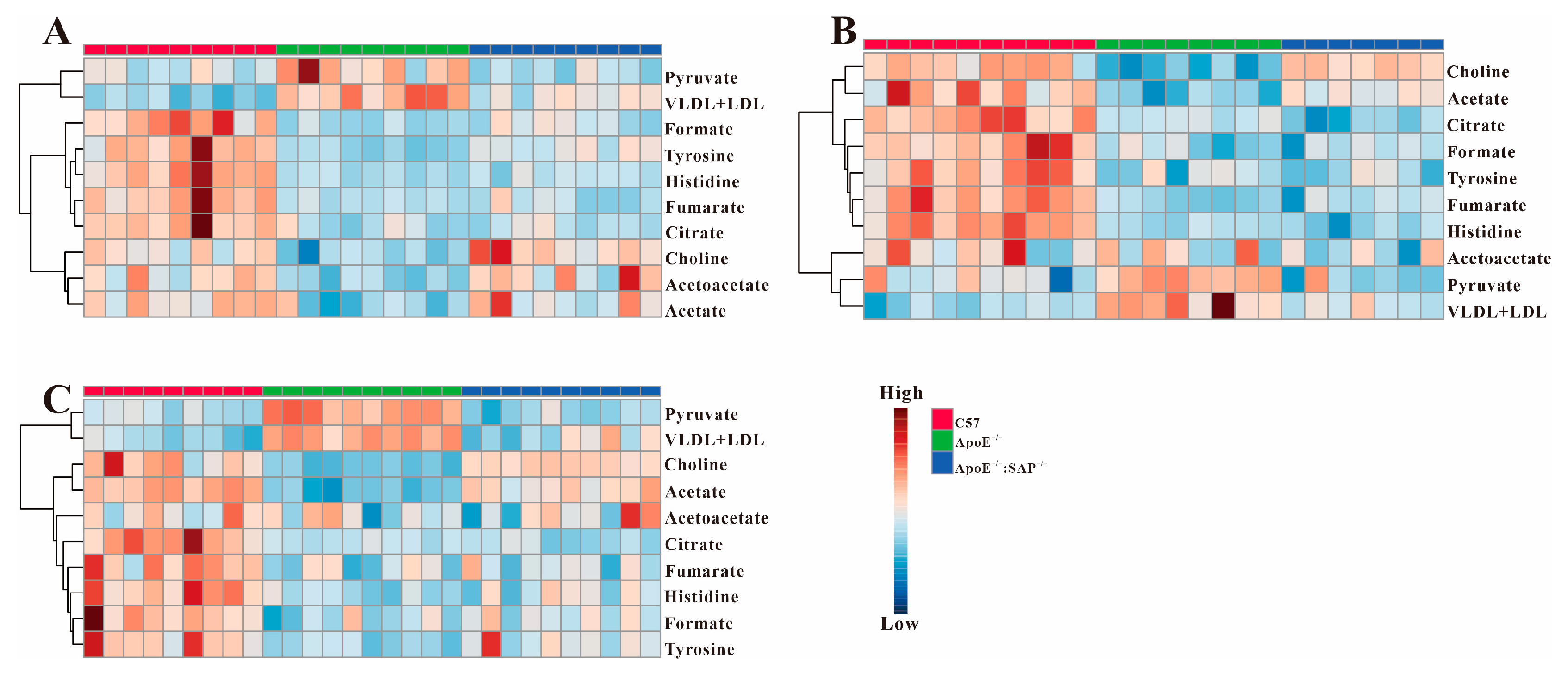
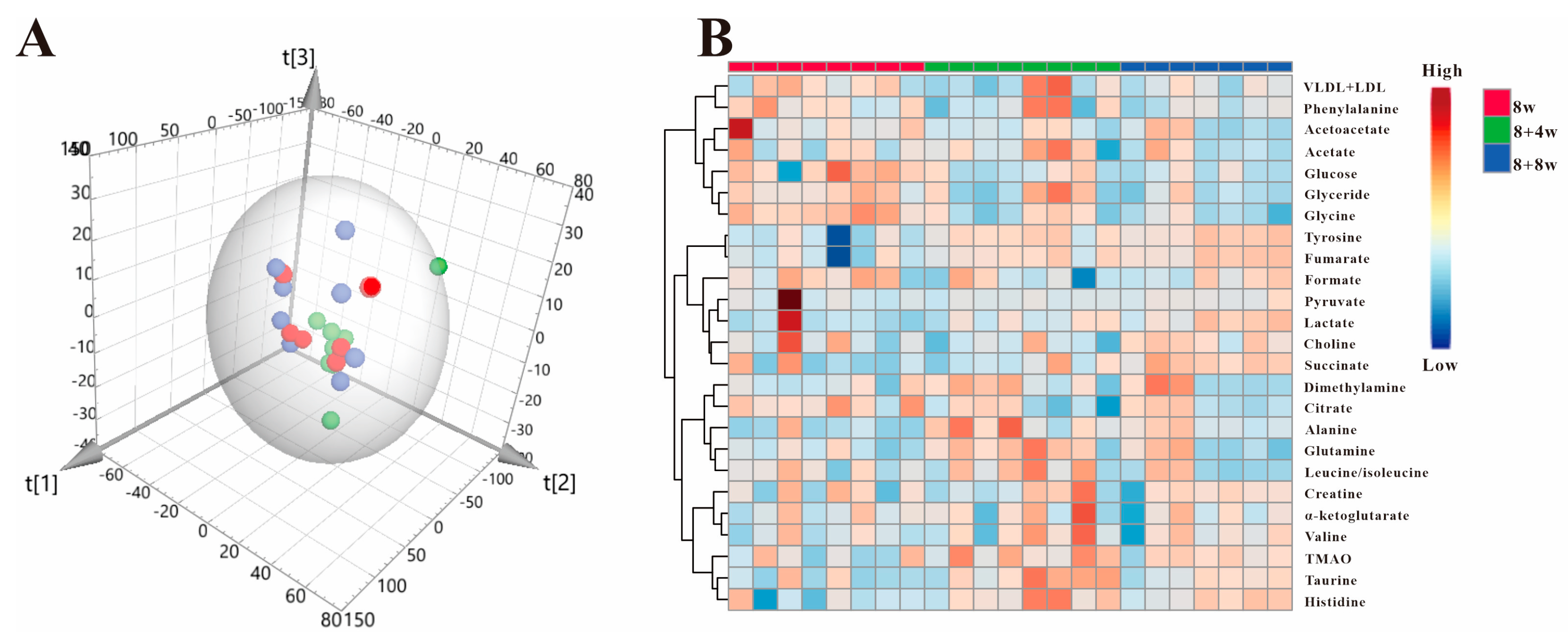
3.1. Metabolite Profiling of Serum Samples
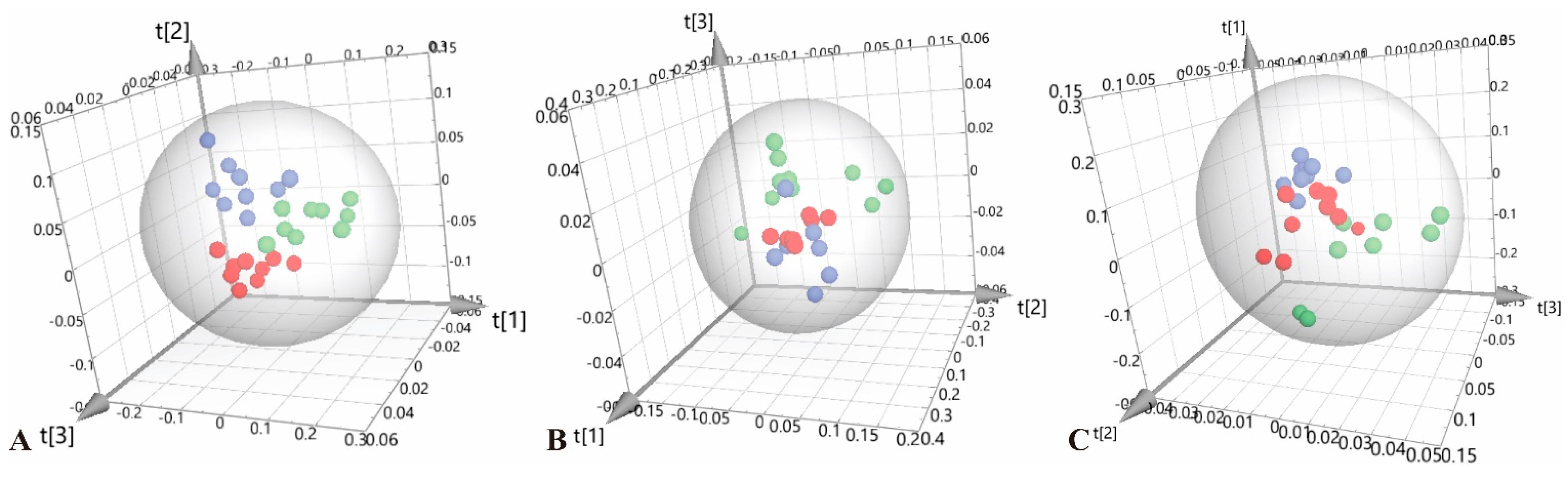
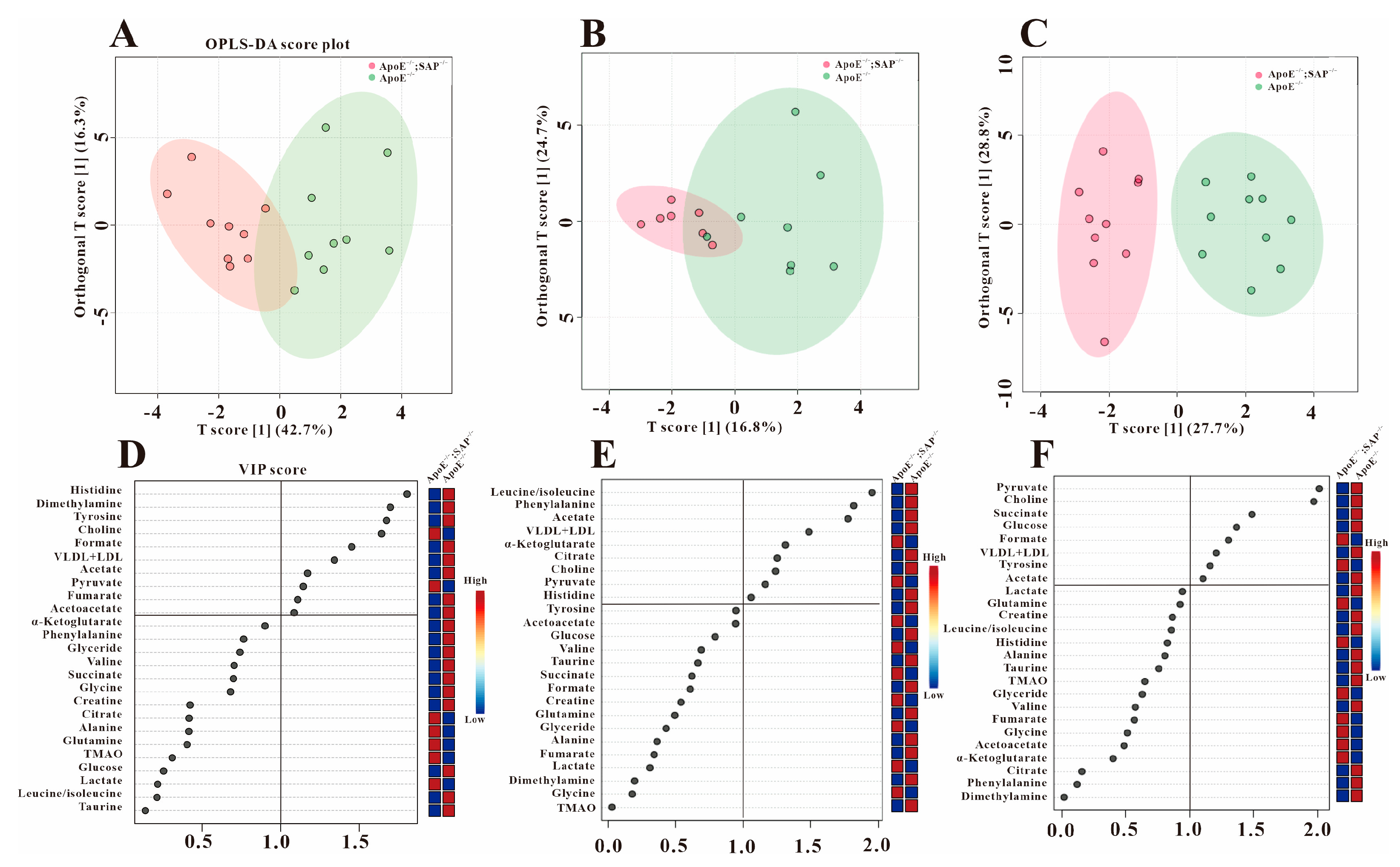
3.2. Multivariate Statistical Analysis
3.3. Analysis of Serum Differential Metabolites in ApoE−/− Mice
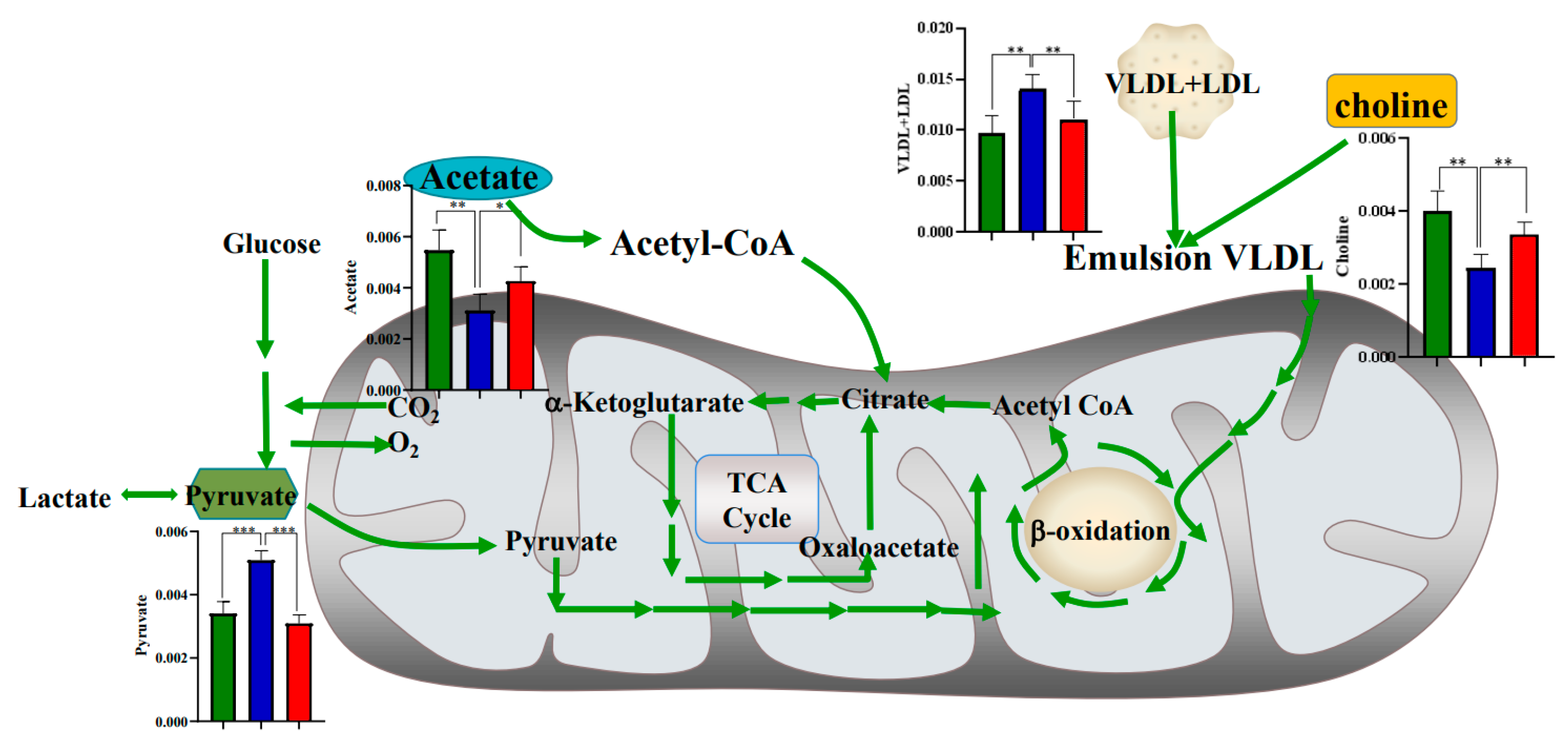
3.4. Metabolic Regulation of SAP Deficiency in ApoE−/− Mice
3.5. High Fat Diet Has No Effect on the Metabolic Regulation of SAP Deficiency
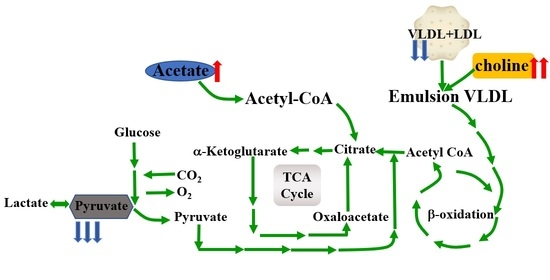
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Topol, E.J.; Yadav, J.S. Recognition of the importance of embolization in atherosclerotic vascular disease. Circulation 2000, 101, 570–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenny, N.S.; Arnold, A.M.; Kuller, L.H.; Tracy, R.P.; Psaty, B.M. Serum amyloid P and cardiovascular disease in older men and women:results from the Cardiovascular Health Study. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 352–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.A.; Hatanaka, K.; Ishibashi-Ueda, H.; Yutani, C.; Yamamoto, A. Characterization of serum amyloid P component from human aortic atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 1995, 15, 252–257. [Google Scholar] [CrossRef]
- Stewart, C.R.; Haw, A., 3rd; Lopez, R.; McDonald, T.O.; Callaghan, J.M.; McConville, M.J.; Moore, K.J.; Howlett, G.J.; O’Brien, K.D. Serum amyloid P colocalizes with apolipoproteins in human atheroma: Functional implications. J. Lipid Res. 2007, 48, 2162–2171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.A.; Yutani, C.; Shimokado, K. Serum amyloid P component associates with high density lipoprotein as well as very low density lipoprotein but not with low density lipoprotein. Biochem. Biophys Res. Commun. 1998, 244, 249–252. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Cai, L.; Guo, L.; Tsukamoto, Y.; Yutani, C.; Li, X.A. Accumulation and expression of serum amyloid P component in human atherosclerotic lesions. Atherosclerosis 2010, 211, 90–95. [Google Scholar] [CrossRef] [Green Version]
- Xi, D.; Zhao, J.; Liu, J.; Xiong, H.; He, W.; Hu, J.; Lai, W.; Guo, Z. The Impact of Serum Amyloid P-Component on Gene Expression in RAW264.7 Mouse Macrophages. Biomed. Res. Int. 2016, 2016, 9380290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, L.; Wu, T.; Zeng, C.; Li, X.; Li, X.; Wen, D.; Ji, T.; Lan, T.; Xing, L.; Li, J.; et al. SAP deficiency mitigated atherosclerotic lesions in ApoE(-/-) mice. Atherosclerosis 2016, 244, 179–187. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Palacios, G.; Gao, J.; Zhang, N.; Li, G.; Lu, J.; Song, T.; Zhang, Y.; Lv, H. Plasma metabolomics reveals biomarkers of the atherosclerosis. J. Sep. Sci. 2010, 33, 2776–2783. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, C.; Nambi, V.; Morrison, A.C.; Folsom, A.R.; Ballantyne, C.M.; Boerwinkle, E.; Yu, B. Metabolomic Pattern Predicts Incident Coronary Heart Disease. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1475–1482. [Google Scholar] [CrossRef]
- Jung, S.; Song, S.W.; Lee, S.; Kim, S.H.; Ann, S.-J.; Cheon, E.J.; Yi, G.; Choi, E.-Y.; Lee, S.H.; Joo, H.-C.; et al. Metabolic phenotyping of human atherosclerotic plaques: Metabolic alterations and their biological relevance in plaque-containing aorta. Atherosclerosis 2018, 269, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Izidoro, M.A.; Cecconi, A.; Panadero, M.I.; Mateo, J.; Godzien, J.; Vilchez, J.P.; López-Gonzálvez, Á.; Ruiz-Cabello, J.; Ibañez, B.; Barbas, C.; et al. Plasma Metabolic Signature of Atherosclerosis Progression and Colchicine Treatment in Rabbits. Sci. Rep. 2020, 10, 7072. [Google Scholar] [CrossRef] [PubMed]
- Teul, J.; Rupérez, F.J.; Garcia, A.; Vaysse, J.; Balayssac, S.; Gilard, V.; Malet-Martino, M.; Martin-Ventura, J.L.; Blanco-Colio, L.M.; Tuñón, J.; et al. Improving metabolite knowledge in stable atherosclerosis patients by association and correlation of GC-MS and 1H-NMR fingerprints. J. Proteome Res. 2009, 8, 5580–5589. [Google Scholar] [CrossRef] [PubMed]
- Guillermier, C.; Doherty, S.P.; Whitney, A.G.; Babaev, V.R.; Linton, M.; Steinhauser, M.L.; Brown, J.D. Imaging mass spectrometry reveals heterogeneity of proliferation and metabolism in atherosclerosis. JCI Insight 2019, 4, 11. [Google Scholar] [CrossRef]
- Iida, M.; Harada, S.; Takebayashi, T. Application of Metabolomics to Epidemiological Studies of Atherosclerosis and Cardiovascular Disease. J. Atheroscler. Thromb. 2019, 26, 747–757. [Google Scholar] [CrossRef] [Green Version]
- Tzoulaki, I.; Castagné, R.; Boulangé, C.L.; Karaman, I.; Chekmeneva, E.; Evangelou, E.; Ebbels, T.M.D.; Kaluarachchi, M.R.; Chadeau-Hyam, M.; Mosen, D.; et al. Serum metabolic signatures of coronary and carotid atherosclerosis and subsequent cardiovascular disease. Eur. Heart J. 2019, 40, 2883–2896. [Google Scholar] [CrossRef] [Green Version]
- Brindle, J.T.; Antti, H.; Holmes, E.; Tranter, G.; Nicholson, J.K.; Bethell, H.W.; Clarke, S.; Schofield, P.M.; McKilligin, E.; Mosedale, D.E.; et al. Rapid and noninvasive diagnosis of the presence and severity of coronary heart disease using 1H-NMR-based metabonomics. Nat. Med. 2002, 8, 1439–1444. [Google Scholar] [CrossRef]
- Li, B.; Lu, X.; Wang, J.; He, X.; Gu, Q.; Wang, L.; Yang, Y. The metabonomics study of P-selectin glycoprotein ligand-1 (PSGL-1) deficiency inhibiting the progression of atherosclerosis in LDLR-/- mice. Int. J. Biol. Sci. 2018, 14, 36–46. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Li, Q.; Hou, R.; Liang, H.; Zhang, Y.; Yang, Y. An integrated metabonomics study to reveal the inhibitory effect and metabolism regulation of taurine on breast cancer. J. Pharm. Biomed. Anal. 2022, 214, 114711. [Google Scholar] [CrossRef]
- Mora-Ortiz, M.; Nuñez Ramos, P.; Oregioni, A.; Claus, S.P. NMR metabolomics identifies over 60 biomarkers associated with Type II Diabetes impairment in db/db mice. Metabolomics 2019, 15, 89. [Google Scholar] [CrossRef]
- Meng, Z.; Tian, S.; Yan, J.; Jia, M.; Yan, S.; Li, R.; Zhang, R.; Zhu, W.; Zhou, Z. Effects of perinatal exposure to BPA, BPF and BPAF on liver function in male mouse offspring involving in oxidative damage and metabolic disorder. Environ. Pollut. 2019, 247, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, Y.; Zheng, L.; Wu, T.; Li, J.; Zhang, Q.; Li, X.; Yuan, F.; Wang, L.; Guo, J. Serum metabonomic analysis of apoE(-/-) mice reveals progression axes for atherosclerosis based on NMR spectroscopy. Mol. Biosyst. 2014, 10, 3170–3178. [Google Scholar] [CrossRef] [PubMed]
- Breslow, J.L. Mouse models of atherosclerosis. Science 1996, 272, 685–688. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Zang, X.; Cui, X.; Zhang, J. Autophagy, Hyperlipidemia, and Atherosclerosis. Adv. Exp. Med. Biol. 2020, 1207, 237–264. [Google Scholar]
- Jin, M.; Pan, T.; Tocher, D.R.; Betancor, M.B.; Monroig, Ó.; Shen, Y.; Zhu, T.; Sun, P.; Jiao, L.; Zhou, Q. Dietary choline supplementation attenuated high-fat diet-induced inflammation through regulation of lipid metabolism and suppression of NFκB activation in juvenile black seabream (Acanthopagrus schlegelii). J. Nutr Sci. 2019, 8, e38. [Google Scholar] [CrossRef] [Green Version]
- Al Rajabi, A.; Castro, G.S.; da Silva, R.P.; Nelson, R.C.; Thiesen, A.; Vannucchi, H.; Vine, D.F.; Proctor, S.D.; Field, C.J.; Curtis, J.M.; et al. Choline supplementation protects against liver damage by normalizing cholesterol metabolism in Pemt/Ldlr knockout mice fed a high-fat diet. J. Nutr. 2014, 144, 252–257. [Google Scholar] [CrossRef] [Green Version]
- Lynch, R.M.; Paul, R.J. Compartmentation of glycolytic and glycogenolytic metabolism in vascular smooth muscle. Science 1983, 222, 1344–1346. [Google Scholar] [CrossRef]
- Grassi, I.; Nanni, C.; Allegri, V.; Morigi, J.J.; Montini, G.C.; Castellucci, P.; Fanti, S. The clinical use of PET with (11)C-acetate. Am. J. Nucl. Med. Mol. Imaging 2012, 2, 33–47. [Google Scholar]
- Kerbs, H.A. The history of the tricarboxylic acid cycle. Perspect Biol. Med. 1970, 14, 154–170. [Google Scholar] [CrossRef]
- Rathod, R.; Gajera, B.; Nazir, K.; Wallenius, J.; Velagapudi, V. Simultaneous Measurement of Tricarboxylic Acid Cycle Intermediates in Different Biological Matrices Using Liquid Chromatography-Tandem Mass Spectrometry; Quantitation and Comparison of TCA Cycle Intermediates in Human Serum, Plasma, Kasumi-1 Cell and Murine Liver Tissue. Metabolites 2020, 10, 103. [Google Scholar]
- Eniafe, J.; Jiang, S. The functional roles of TCA cycle metabolites in cancer. Oncogene 2021, 40, 3351–3363. [Google Scholar] [CrossRef] [PubMed]
- Shiomi, M.; Takeda, H.; Irino, Y.; Kimura, N.; Yamada, S.; Kuniyoshi, N.; Kikumori, A.; Koike, Y.; Koike, T.; Yoshida, M. Identification of novel serum markers for the progression of coronary atherosclerosis in WHHLMI rabbits, an animal model of familial hypercholesterolemia. Atherosclerosis 2019, 284, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.B.; Jia, H.M.; Xu, T.; Liu, Y.T.; Zhang, H.W.; Yu, L.L.; Cai, D.Y.; Zou, Z.M. A 1H-NMR based metabonomics approach to progression of coronary atherosclerosis in a rabbit model. Process. Biochem. 2011, 46, 2240–2247. [Google Scholar] [CrossRef]
- Shirai, T.; Nazarewicz, R.R.; Wallis, B.B.; Yanes, R.E.; Watanabe, R.; Hilhorst, M.; Tian, L.; Harrison, D.G.; Giacomini, J.C.; Assimes, T.L.; et al. The glycolytic enzyme PKM2 bridges metabolic and inflammatory dysfunction in coronary artery disease. J. Exp. Med. 2016, 213, 337–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baragetti, A.; Bonacina, F.; Catapano, A.L.; Norata, G.D. Effect of Lipids and Lipoproteins on Hematopoietic Cell Metabolism and Commitment in Atherosclerosis. Immunometabolism 2021, 3, e210014. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Zhang, Y.; Chang, Y.; Li, X.; Zhao, W.; An, S. Pyruvate kinase is required for sex pheromone biosynthesis in Helicoverpa armigera. Front. Physio. 2021, 12, 707389. [Google Scholar] [CrossRef] [PubMed]
- Prochownik, E.V.; Wang, H. The metabolic fates of pyruvate in normal and neoplastic cells. Cells 2021, 10, 762. [Google Scholar] [CrossRef]
- Tian, F.; Gu, L.; Si, A.; Yao, Q.; Zhang, X.; Zhao, J.; Hu, D. The metabolomic study on atherosclerosis mice and its application in a traditional Chinese medicine Sishen granule. Biomed. Chromatogr. 2016, 30, 969–975. [Google Scholar] [CrossRef]



| Metabolites | δ 1H (ppm) | Pathway | 8 w | 8 + 4 w | 8 + 8 w | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ApoE−/− vs. C57 | ApoE−/−;SAP−/− vs. ApoE−/− | ApoE−/−;SAP−/− vs. C57 | ApoE−/− vs. C57 | ApoE−/−;SAP−/− vs. ApoE−/− | ApoE−/−;SAP−/− vs. C57 | ApoE−/− vs. C57 | ApoE−/−;SAP−/− vs. ApoE−/− | ApoE−/−;SAP−/− vs. C57 | |||
| Formate | 8.46 | Ketone metabolism | ↓ ** | - | ↓ * | ↓ * | - | ↓ * | ↓ ** | - | ↓ * |
| Tyrosine | 6.89, 7.81 | Amino acid metabolism | ↓ *** | ↑ ** | ↓ * | ↓ *** | - | ↓ ** | ↓ ** | - | ↓ * |
| Fumarate | 6.51 | Energy metabolism | ↓ *** | - | ↓ ** | ↓ *** | - | ↓ ** | ↓ ** | - | ↓ ** |
| Histidine | 7.04, 7.74 | Amino acid metabolism | ↓ *** | - | ↓ ** | ↓ *** | - | ↓ ** | ↓ *** | - | ↓ ** |
| Citrate | 2.54 | Energy metabolism | - | - | - | ↓ ** | - | ↓ * | ↓ * | - | ↓ ** |
| Acetoacetate | 2.28 | Energy metabolism | ↓ ** | ↑ ** | - | - | - | - | - | - | - |
| Acetate | 1.92 | Ketone metabolism | ↓ * | ↑ * | - | ↓ ** | ↑ ** | - | ↓ ** | ↑ * | - |
| Pyruvate | 2.37 | Energy metabolism | ↑ ** | ↓ ** | - | ↑ * | ↓ ** | - | ↑ *** | ↓ *** | - |
| Choline | 3.2 | Lipid metabolism | ↓ ** | ↑ * | - | ↓ ** | ↑ * | - | ↓ ** | ↑ ** | - |
| VLDL + LDL | 0.88, 1.28 | Lipid metabolism | ↑ ** | ↓ ** | - | ↑ ** | ↓ * | - | ↑ ** | ↓ ** | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Chen, W.; Huang, W.; Hou, R.; Huang, X.; Xu, M.; Que, L.; Wang, L.; Yang, Y. 1H-NMR-Based Metabonomics Study to Reveal the Progressive Metabolism Regulation of SAP Deficiency on ApoE−/− Mice. Metabolites 2022, 12, 1278. https://doi.org/10.3390/metabo12121278
Li Q, Chen W, Huang W, Hou R, Huang X, Xu M, Que L, Wang L, Yang Y. 1H-NMR-Based Metabonomics Study to Reveal the Progressive Metabolism Regulation of SAP Deficiency on ApoE−/− Mice. Metabolites. 2022; 12(12):1278. https://doi.org/10.3390/metabo12121278
Chicago/Turabian StyleLi, Qian, Wanting Chen, Wenbin Huang, Ranran Hou, Xinping Huang, Man Xu, Limei Que, Lijing Wang, and Yongxia Yang. 2022. "1H-NMR-Based Metabonomics Study to Reveal the Progressive Metabolism Regulation of SAP Deficiency on ApoE−/− Mice" Metabolites 12, no. 12: 1278. https://doi.org/10.3390/metabo12121278
APA StyleLi, Q., Chen, W., Huang, W., Hou, R., Huang, X., Xu, M., Que, L., Wang, L., & Yang, Y. (2022). 1H-NMR-Based Metabonomics Study to Reveal the Progressive Metabolism Regulation of SAP Deficiency on ApoE−/− Mice. Metabolites, 12(12), 1278. https://doi.org/10.3390/metabo12121278