Metabolomics and Lipidomics Signatures of Insulin Resistance and Abdominal Fat Depots in People Living with Obesity
Abstract
1. Introduction
2. Experimental Design, Materials and Methods
2.1. Study Participants
2.2. Measurements of Glucose Regulation
2.3. Oral Glucose Tolerance Test
2.4. Measurement of Muscle, Liver, and Adipose Tissue Insulin Resistance
2.5. Body Fat Composition, Abdominal Fat Distribution and Liver Fat
2.6. Biochemical Measurements in Blood
2.7. Targeted Metabolomics
2.8. Untargeted Lipidomics
2.9. Lipid Identification
2.10. Data Processing and Analysis
3. Results
3.1. Cohort Characteristics
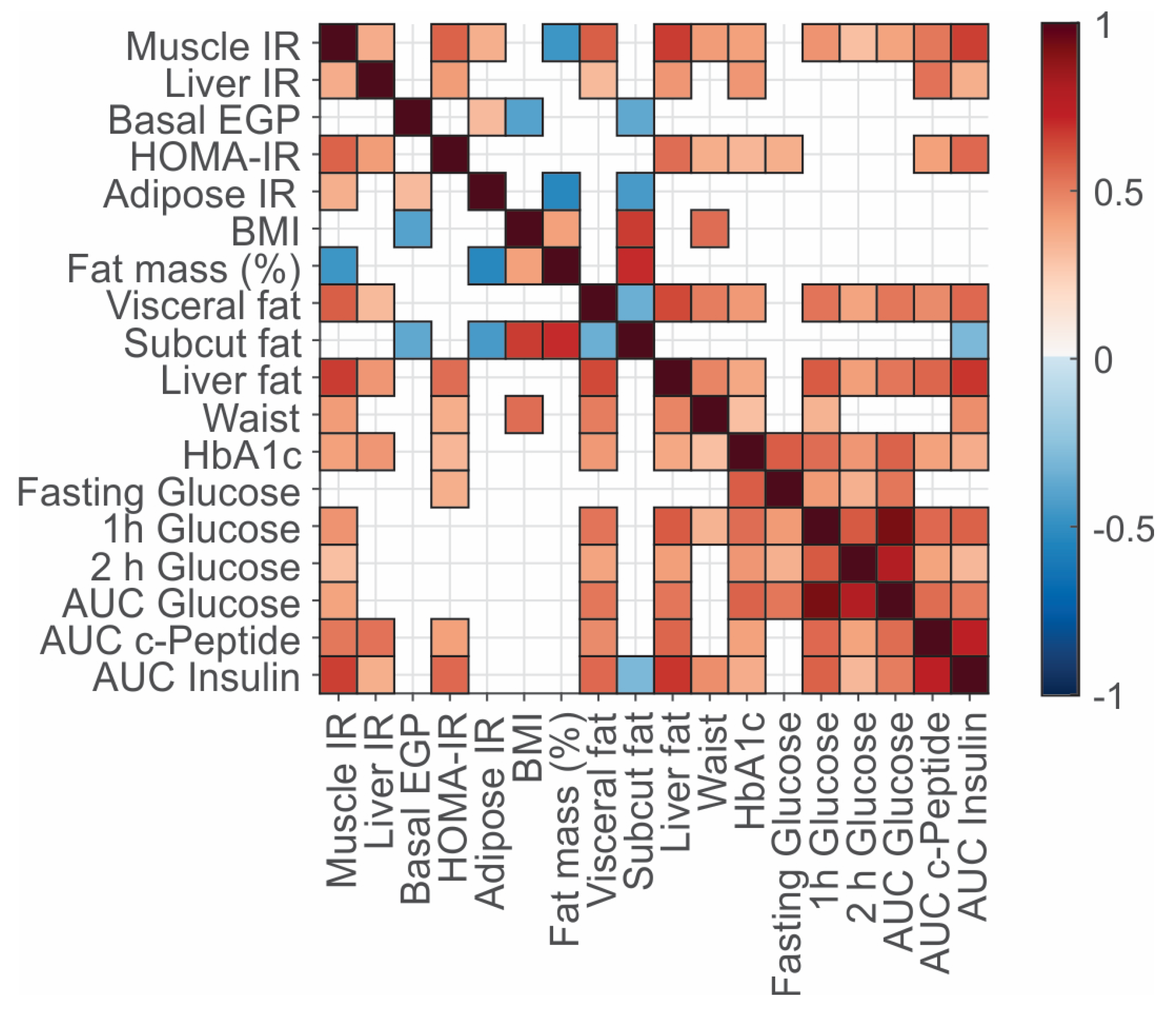
3.2. Associations between Clinical Variables, Fat Deposition, and Insulin Resistance Phenotypes
3.3. Plasma Omics Signature of Clinical and Metabolic Traits
3.4. Metabolomics Correlates of Clinical Phenotypes of Obesity
4. Discussion
4.1. Biomarkers Aligning with Muscle Insulin Resistance
4.2. Biomarkers Aligning with Liver Insulin Resistance
4.3. Biomarkers Aligning with Adipose Tissue Insulin Resistance
4.4. Biomarkers Aligning with Abdominal Fat Depots
4.5. Study Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Korenblat, K.M.; Fabbrini, E.; Mohammed, B.S.; Klein, S. Liver, Muscle and Adipose Tissue Insulin Action is Directly Related to Intrahepatic Triglyceride Content in Obese Subjects. Gastroenterology 2008, 134, 1369–1375. [Google Scholar] [CrossRef] [PubMed]
- Groop, L.C.; Bonadonna, R.C.; DelPrato, S.; Ratheiser, K.; Zyck, K.; Ferrannini, E.; DeFronzo, R.A. Glucose and free fatty acid metabolism in non-insulin-dependent diabetes mellitus. Evidence for multiple sites of insulin resistance. J. Clin. Investig. 1989, 84, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Ferrannini, E.; Simonson, D.C.; Katz, L.D.; Reichard, G., Jr.; Bevilacqua, S.; Barrett, E.J.; Olsson, M.; DeFronzo, R.A. The disposal of an oral glucose load in patients with non-insulin-dependent diabetes. Metabolism 1988, 37, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Trouwborst, I.; Bowser, S.M.; Goossens, G.H.; Blaak, E.E. Ectopic Fat Accumulation in Distinct Insulin Resistant Phenotypes; Targets for Personalized Nutritional Interventions. Front. Nutr. 2018, 5, 77. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Rojo, R.; Alcala-Diaz, J.F.; Wopereis, S.; Perez-Martinez, P.; Quintana-Navarro, G.M.; Marin, C.; Ordovas, J.M.; van Ommen, B.; Perez-Jimenez, F.; Delgado-Lista, J.; et al. The insulin resistance phenotype (muscle or liver) interacts with the type of diet to determine changes in disposition index after 2 years of intervention: The CORDIOPREV-DIAB randomised clinical trial. Diabetologia 2016, 59, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.L.; Liess, C.; Poljak, A.; Xu, A.; Zhang, J.; Thoma, C.; Trenell, M.; Milner, B.; Jenkins, A.B.; Chisholm, D.J.; et al. Phenotypic Characterization of Insulin-Resistant and Insulin-Sensitive Obesity. J. Clin. Endocrinol. Metab. 2015, 100, 4082–4091. [Google Scholar] [CrossRef] [PubMed]
- Samocha-Bonet, D.; Debs, S.; Greenfield, J.R. Prevention and Treatment of Type 2 Diabetes: A Pathophysiological-Based Approach. Trends Endocrinol. Metab. 2018, 29, 370–379. [Google Scholar] [CrossRef]
- Wagner, R.; Heni, M.; Tabak, A.G.; Machann, J.; Schick, F.; Randrianarisoa, E.; de Angelis, M.H.; Birkenfeld, A.L.; Stefan, N.; Peter, A.; et al. Pathophysiology-based subphenotyping of individuals at elevated risk for type 2 diabetes. Nat. Med. 2021, 27, 49–57. [Google Scholar] [CrossRef]
- Marshall, S.M. 60 years of metformin use: A glance at the past and a look to the future. Diabetologia 2017, 60, 1561–1565. [Google Scholar] [CrossRef]
- Davies, M.J.; Drexel, H.; Jornayvaz, F.R.; Pataky, Z.; Seferovic, P.M.; Wanner, C. Cardiovascular outcomes trials: A paradigm shift in the current management of type 2 diabetes. Cardiovasc. Diabetol. 2022, 21, 144. [Google Scholar] [CrossRef]
- Bergman, R.N.; Finegood, D.T.; Ader, M. Assessment of insulin sensitivity in vivo. Endocr. Rev. 1985, 6, 45–86. [Google Scholar] [CrossRef] [PubMed]
- Arakaki, A.K.; Skolnick, J.; McDonald, J.F. Marker metabolites can be therapeutic targets as well. Nature 2008, 456, 443. [Google Scholar] [CrossRef] [PubMed]
- Wesolowska-Andersen, A.; Brorsson, C.A.; Bizzotto, R.; Mari, A.; Tura, A.; Koivula, R.; Mahajan, A.; Vinuela, A.; Tajes, J.F.; Sharma, S.; et al. Four groups of type 2 diabetes contribute to the etiological and clinical heterogeneity in newly diagnosed individuals: An IMI DIRECT study. Cell Rep. Med. 2022, 3, 100477. [Google Scholar] [CrossRef] [PubMed]
- Rhee, E.P.; Cheng, S.; Larson, M.G.; Walford, G.A.; Lewis, G.D.; McCabe, E.; Yang, E.; Farrell, L.; Fox, C.S.; O’Donnell, C.J.; et al. Lipid profiling identifies a triacylglycerol signature of insulin resistance and improves diabetes prediction in humans. J. Clin. Investig. 2011, 121, 1402–1411. [Google Scholar] [CrossRef]
- American Diabetes Association: Standards of Medical Care in Diabetes (2022). Diabetes Care 2022, 45. [CrossRef]
- Petersons, C.J.; Mangelsdorf, B.L.; Jenkins, A.B.; Poljak, A.; Smith, M.D.; Greenfield, J.R.; Thompson, C.H.; Burt, M.G. Effects of low-dose prednisolone on hepatic and peripheral insulin sensitivity, insulin secretion, and abdominal adiposity in patients with inflammatory rheumatologic disease. Diabetes Care 2013, 36, 2822–2829. [Google Scholar] [CrossRef]
- Steele, R.; Wall, J.S.; De Bodo, R.C.; Altszuler, N. Measurement of size and turnover rate of body glucose pool by the isotope dilution method. Am. J. Physiol. 1956, 187, 15–24. [Google Scholar] [CrossRef]
- Finegood, D.T.; Bergman, R.N.; Vranic, M. Estimation of endogenous glucose production during hyperinsulinemic-euglycemic glucose clamps. Comparison of unlabeled and labeled exogenous glucose infusates. Diabetes 1987, 36, 914–924. [Google Scholar] [CrossRef]
- Koay, Y.C.; Stanton, K.; Kienzle, V.; Li, M.; Yang, J.; Celermajer, D.S.; O’Sullivan, J.F. Effect of chronic exercise in healthy young male adults: A metabolomic analysis. Cardiovasc. Res. 2021, 117, 613–622. [Google Scholar] [CrossRef]
- Matyash, V.; Liebisch, G.; Kurzchalia, T.V.; Shevchenko, A.; Schwudke, D. Lipid extraction by methyl-tert-butyl ether for high-throughput lipidomics. J. Lipid Res. 2008, 49, 1137–1146. [Google Scholar] [CrossRef]
- Adeva, M.M.; Calvino, J.; Souto, G.; Donapetry, C. Insulin resistance and the metabolism of branched-chain amino acids in humans. Amino Acids 2012, 43, 171–181. [Google Scholar] [CrossRef]
- Taya, N.; Katakami, N.; Omori, K.; Hosoe, S.; Watanabe, H.; Takahara, M.; Miyashita, K.; Nishizawa, H.; Konya, Y.; Obara, S.; et al. Change in fatty acid composition of plasma triglyceride caused by a 2 week comprehensive risk management for diabetes: A prospective observational study of type 2 diabetes patients with supercritical fluid chromatography/mass spectrometry-based semi-target lipidomic analysis. J. Diabetes Investig. 2022. [Google Scholar] [CrossRef]
- Moore, M.C.; Coate, K.C.; Winnick, J.J.; An, Z.; Cherrington, A.D. Regulation of hepatic glucose uptake and storage in vivo. Adv. Nutr. 2012, 3, 286–294. [Google Scholar] [CrossRef]
- Kowalski, G.M.; Moore, S.M.; Hamley, S.; Selathurai, A.; Bruce, C.R. The Effect of Ingested Glucose Dose on the Suppression of Endogenous Glucose Production in Humans. Diabetes 2017, 66, 2400–2406. [Google Scholar] [CrossRef]
- Gu, X.; Al Dubayee, M.; Alshahrani, A.; Masood, A.; Benabdelkamel, H.; Zahra, M.; Li, L.; Abdel Rahman, A.M.; Aljada, A. Distinctive Metabolomics Patterns Associated With Insulin Resistance and Type 2 Diabetes Mellitus. Front. Mol. Biosci. 2020, 7, 609806. [Google Scholar] [CrossRef] [PubMed]
- Soltani, N.; Qiu, H.; Aleksic, M.; Glinka, Y.; Zhao, F.; Liu, R.; Li, Y.; Zhang, N.; Chakrabarti, R.; Ng, T.; et al. GABA exerts protective and regenerative effects on islet beta cells and reverses diabetes. Proc. Natl. Acad. Sci. USA 2011, 108, 11692–11697. [Google Scholar] [CrossRef]
- Costello, L.C.; Franklin, R.B. Plasma Citrate Homeostasis: How It Is Regulated; And Its Physiological and Clinical Implications. An Important, But Neglected, Relationship in Medicine. HSOA J. Hum. Endocrinol. 2016, 1. [Google Scholar] [CrossRef]
- Cavuoto, P.; Wittert, G.A. The role of the endocannabinoid system in the regulation of energy expenditure. Best Pract. Res. Clin. Endocrinol. Metab. 2009, 23, 79–86. [Google Scholar] [CrossRef]
- Di Marzo, V.; Matias, I. Endocannabinoid control of food intake and energy balance. Nat. Neurosci. 2005, 8, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, V.; Verrijken, A.; Hakkarainen, A.; Petrosino, S.; Mertens, I.; Lundbom, N.; Piscitelli, F.; Westerbacka, J.; Soro-Paavonen, A.; Matias, I.; et al. Role of insulin as a negative regulator of plasma endocannabinoid levels in obese and nonobese subjects. Eur. J. Endocrinol. 2009, 161, 715–722. [Google Scholar] [CrossRef]
- Cornier, M.A.; Bergman, B.C.; Bessesen, D.H. The effects of short-term overfeeding on insulin action in lean and reduced-obese individuals. Metabolism 2006, 55, 1207–1214. [Google Scholar] [CrossRef]
- Wang, T.J.; Larson, M.G.; Vasan, R.S.; Cheng, S.; Rhee, E.P.; McCabe, E.; Lewis, G.D.; Fox, C.S.; Jacques, P.F.; Fernandez, C.; et al. Metabolite profiles and the risk of developing diabetes. Nat. Med. 2011, 17, 448–453. [Google Scholar] [CrossRef]
- Wurtz, P.; Soininen, P.; Kangas, A.J.; Ronnemaa, T.; Lehtimaki, T.; Kahonen, M.; Viikari, J.S.; Raitakari, O.T.; Ala-Korpela, M. Branched-chain and aromatic amino acids are predictors of insulin resistance in young adults. Diabetes Care 2013, 36, 648–655. [Google Scholar] [CrossRef]
- Oxenkrug, G. Insulin resistance and dysregulation of tryptophan-kynurenine and kynurenine-nicotinamide adenine dinucleotide metabolic pathways. Mol Neurobiol 2013, 48, 294–301. [Google Scholar] [CrossRef]
- Vangipurapu, J.; Stancakova, A.; Smith, U.; Kuusisto, J.; Laakso, M. Nine Amino Acids Are Associated With Decreased Insulin Secretion and Elevated Glucose Levels in a 7.4-Year Follow-up Study of 5,181 Finnish Men. Diabetes 2019, 68, 1353–1358. [Google Scholar] [CrossRef]
- Bender, D.A. NIACIN|Physiology. In Encyclopedia of Food Sciences and Nutrition; Caballero, B., Ed.; Academic Press: Oxford, UK, 2003; pp. 4119–4128. [Google Scholar] [CrossRef]
- Abdel-Halim, S.M.; Al Madhoun, A.; Nizam, R.; Melhem, M.; Cherian, P.; Al-Khairi, I.; Haddad, D.; Abu-Farha, M.; Abubaker, J.; Bitar, M.S.; et al. Increased Plasma Levels of Adenylate Cyclase 8 and cAMP Are Associated with Obesity and Type 2 Diabetes: Results from a Cross-Sectional Study. Biology 2020, 9, 244. [Google Scholar] [CrossRef]
- Newsom, S.A.; Brozinick, J.T.; Kiseljak-Vassiliades, K.; Strauss, A.N.; Bacon, S.D.; Kerege, A.A.; Bui, H.H.; Sanders, P.; Siddall, P.; Wei, T.; et al. Skeletal muscle phosphatidylcholine and phosphatidylethanolamine are related to insulin sensitivity and respond to acute exercise in humans. J. Appl. Physiol. 1985 2016, 120, 1355–1363. [Google Scholar] [CrossRef]
- Magkos, F.; Su, X.; Bradley, D.; Fabbrini, E.; Conte, C.; Eagon, J.C.; Varela, J.E.; Brunt, E.M.; Patterson, B.W.; Klein, S. Intrahepatic diacylglycerol content is associated with hepatic insulin resistance in obese subjects. Gastroenterology 2012, 142, 1444–1446. [Google Scholar] [CrossRef]
- Sheetz, M.J.; King, G.L. Molecular understanding of hyperglycemia’s adverse effects for diabetic complications. JAMA 2002, 288, 2579–2588. [Google Scholar] [CrossRef]
- Green, C.R.; Wallace, M.; Divakaruni, A.S.; Phillips, S.A.; Murphy, A.N.; Ciaraldi, T.P.; Metallo, C.M. Branched-chain amino acid catabolism fuels adipocyte differentiation and lipogenesis. Nat. Chem. Biol. 2016, 12, 15–21. [Google Scholar] [CrossRef]
- Herman, M.A.; She, P.; Peroni, O.D.; Lynch, C.J.; Kahn, B.B. Adipose tissue branched chain amino acid (BCAA) metabolism modulates circulating BCAA levels. J. Biol. Chem. 2010, 285, 11348–11356. [Google Scholar] [CrossRef]
- Hellmuth, C.; Kirchberg, F.F.; Lass, N.; Harder, U.; Peissner, W.; Koletzko, B.; Reinehr, T. Tyrosine Is Associated with Insulin Resistance in Longitudinal Metabolomic Profiling of Obese Children. J. Diabetes. Res. 2016, 2016, 2108909. [Google Scholar] [CrossRef]
- Floegel, A.; Stefan, N.; Yu, Z.; Muhlenbruch, K.; Drogan, D.; Joost, H.G.; Fritsche, A.; Haring, H.U.; de Angelis, M.H.; Peters, A.; et al. Identification of serum metabolites associated with risk of type 2 diabetes using a targeted metabolomic approach. Diabetes 2013, 62, 639–648. [Google Scholar] [CrossRef]
- Hocking, S.; Samocha-Bonet, D.; Milner, K.L.; Greenfield, J.R.; Chisholm, D.J. Adiposity and insulin resistance in humans: The role of the different tissue and cellular lipid depots. Endocr. Rev. 2013, 34, 463–500. [Google Scholar] [CrossRef]
- Tamasawa, N.; Tamasawa, A.; Takebe, K. Higher levels of plasma cholesterol sulfate in patients with liver cirrhosis and hypercholesterolemia. Lipids 1993, 28, 833–836. [Google Scholar] [CrossRef]
- Ostojic, S.M.; Ratgeber, L.; Olah, A.; Betlehem, J.; Acs, P. Guanidinoacetic acid deficiency: A new entity in clinical medicine? Int. J. Med. Sci. 2020, 17, 2544–2550. [Google Scholar] [CrossRef]
- Ostojic, S.M.; Vranes, M.; Loncar, D.; Zenic, N.; Sekulic, D. Guanidinoacetic Acid and Creatine are Associated with Cardiometabolic Risk Factors in Healthy Men and Women: A Cross-Sectional Study. Nutrients 2018, 10, 87. [Google Scholar] [CrossRef]
- Kawanaka, M.; Nishino, K.; Oka, T.; Urata, N.; Nakamura, J.; Suehiro, M.; Kawamoto, H.; Chiba, Y.; Yamada, G. Tyrosine levels are associated with insulin resistance in patients with nonalcoholic fatty liver disease. Hepatic Med. 2015, 7, 29–35. [Google Scholar] [CrossRef]
- Wishart, D.S. Metabolomics for Investigating Physiological and Pathophysiological Processes. Physiol. Rev. 2019, 99, 1819–1875. [Google Scholar] [CrossRef]
- Kiyonami, R.; Peake, D.A.; Liu, X.; Huang, Y. Large-scale lipid profiling of a human serum lipidome using a high-resolution, accurate-mass LC/MS/MS approach. In Proceedings of the LIPID MAPS Annual Meeting, La Jolla, CA, USA, 17–18 May 2016. [Google Scholar]
- Peake, D.A.; Kiyonami, R.; Yokoi, Y.; Fukamachi, Y.; Huang, Y. Processing of a complex lipid dataset for the NIST inter-laboratory comparison exercise for lipidomics measurements in human serum and plasma. In Proceedings of the ASMS Annual Meeting, Saint Louis, MO, USA, 31st May–4th June 2015. [Google Scholar]


| Class of Measurement | Characteristic | Mean/Median (± SD or IQR) | Min-Max Values |
|---|---|---|---|
| N (M/F) | 62 (27/35) | ||
| Age (years) | 50.5 ± 11.4 | 21–69 | |
| Body mass, fat composition and distribution | BMI (kg.m−2) | 36.3 ± 4.5 | 30.9–48.5 |
| Waist circumference (cm) | 111 ± 14 | 85–155 | |
| Body fat (% of total mass) | 48 (40, 52) | 31–61 | |
| Visceral fat (cm3) | 249 (212, 320) | 139–492 | |
| Subcutaneous fat (cm2) | 501 (418, 596) | 265–835 | |
| Liver fat (%) | 10.6 (5.9, 21.3) | 0.7–63.5 | |
| Blood pressure | Systolic (mm Hg) | 125 ± 13 | 100–169 |
| Diastolic (mm Hg) | 82 ± 9 | 59–106 | |
| Serum lipids | Total cholesterol (mmol.L−1) | 4.9 ± 0.8 | 3.1–7.1 |
| LDL cholesterol (mmol.L−1) | 3.0 ± 0.7 | 1.5–5.0 | |
| HDL cholesterol (mmol.L−1) | 1.3 ± 0.3 | 0.8–2.0 | |
| Triglycerides (mmol.L−1) | 1.1 ± 0.4 | 0.4–2.5 | |
| NEFA (mmol.L−1) | 0.4 ± 0.1 | 0.1–0.7 | |
| Glucose regulation | HbA1c (mmol/mol) | 36 ± 3 | 29–43 |
| HbA1c (%) | 5.5 ± 0.3 | 4.8–6.1 | |
| Fasting blood glucose (mmol.L−1) | 4.8 ± 0.4 | 3.5–6.0 | |
| 2-hour blood glucose (mmol.L−1) | 6.3 ± 1.6 | 2.7–10.1 | |
| Individuals with IFG (n) | 1 | ||
| Individuals with IGT (n) | 12 | ||
| Individuals with IFG and IGT (n) | 2 | ||
| Individuals with HbA1c ≥ 5.7% | 21 | ||
| Fasting insulin (mU.L−1) | 16.5 (11.3, 27.4) | 5.9–73.4 | |
| HOMA-IR | 3.4 (2.2, 6.2) | 1.2–19.6 | |
| EGP (mg.kg−1.min−1) | 2.1 ± 0.3 | 1.4–2.8 | |
| EGP suppression (%) | 65 ± 14 | 30–93 | |
| GIR/FFM (M-value, µmol.min−1.kg−1) | 90 ± 30 | 24–186 | |
| NEFA suppression (%) | 32 ± 13 | 10–62 | |
| Serum insulin during the clamp (mU.L−1) | Low dose clamp | 42 ± 13 | 19–86 |
| High dose clamp | 212 ± 44 | 131–296 | |
| Medication use | Individuals treated with anti-hypertensive medications (n (%)) | 13 (21) | |
| Individuals treated with lipid reducing agents (n (%)) | 8 (13) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koay, Y.C.; Coster, A.C.F.; Chen, D.L.; Milner, B.; Batarseh, A.; O’Sullivan, J.F.; Greenfield, J.R.; Samocha-Bonet, D. Metabolomics and Lipidomics Signatures of Insulin Resistance and Abdominal Fat Depots in People Living with Obesity. Metabolites 2022, 12, 1272. https://doi.org/10.3390/metabo12121272
Koay YC, Coster ACF, Chen DL, Milner B, Batarseh A, O’Sullivan JF, Greenfield JR, Samocha-Bonet D. Metabolomics and Lipidomics Signatures of Insulin Resistance and Abdominal Fat Depots in People Living with Obesity. Metabolites. 2022; 12(12):1272. https://doi.org/10.3390/metabo12121272
Chicago/Turabian StyleKoay, Yen Chin, Adelle C. F. Coster, Daniel L. Chen, Brad Milner, Amani Batarseh, John F. O’Sullivan, Jerry R. Greenfield, and Dorit Samocha-Bonet. 2022. "Metabolomics and Lipidomics Signatures of Insulin Resistance and Abdominal Fat Depots in People Living with Obesity" Metabolites 12, no. 12: 1272. https://doi.org/10.3390/metabo12121272
APA StyleKoay, Y. C., Coster, A. C. F., Chen, D. L., Milner, B., Batarseh, A., O’Sullivan, J. F., Greenfield, J. R., & Samocha-Bonet, D. (2022). Metabolomics and Lipidomics Signatures of Insulin Resistance and Abdominal Fat Depots in People Living with Obesity. Metabolites, 12(12), 1272. https://doi.org/10.3390/metabo12121272








