Effect of Binding Linkers on the Efficiency and Metabolite Profile of Biomimetic Reactions Catalyzed by Immobilized Metalloporphyrin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Metabolism of Chloroquine by Human, Rat, and Mouse Microsomal Reaction
2.2.2. HPLC-DAD-MS Measurement
2.2.3. Biomimetic Oxidation of Chloroquine Catalyzed by Dissolved FeTPPS Metalloporphyrin
2.2.4. Surface Functionalization of Silica Particles
2.2.5. Immobilization of FeTPPS Metalloporphyrin on Functionalized Silica Particles in Batch Mode
2.2.6. Determination of Immobilization Yield (YI)
2.2.7. SEM/EDAX Analysis
2.2.8. Biomimetic Oxidation of Chloroquine Catalyzed by Dissolved FeTPPS in Batch Reaction Mode
2.2.9. Biomimetic Oxidation of Chloroquine Catalyzed by Immobilized FeTPPS in Batch Mode
2.2.10. Immobilization of FeTPPS Metalloporphyrin on Functionalized Silica Particles in Continuous-Flow Mode
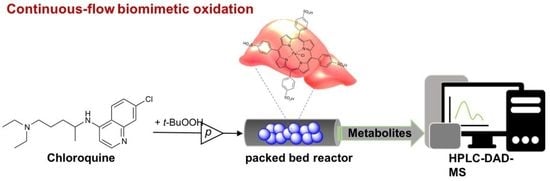
2.2.11. Biomimetic Oxidation of Chloroquine Catalyzed by Immobilized FeTPPS in Continuous-Flow Mode
2.2.12. Calculation of Biomimetic Reaction Parameters
3. Results
3.1. Metabolic Stability Study for Chloroquine by Liver Microsome from Different Origins
3.2. Immobilization of Metalloporphyirin onto Amino-Functionalized Silica Particles
3.3. Biomimetic Oxidation of Chloroquine by Dissolved and Immobilized Metalloporphyrin in Batch Mode
3.4. Biomimetic Oxidation of Chloroquine by Dissolved and Immobilized Metalloporphyirin in Continuos-Flow Mode
3.5. Comparison of the Effectivity of Liver Microsomal and Biomimetic Systems for the Investigation on Chloroquine Metabolism
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fatunde, O.A.; Brown, S.A. The Role of CYP450 Drug Metabolism in Precision Cardio-Oncology. Int. J. Mol. Sci. 2020, 21, 604. [Google Scholar] [CrossRef] [Green Version]
- Bowman, C.M.; Benet, L.Z. In Vitro-In Vivo Extrapolation and Hepatic Clearance-Dependent Underprediction. J. Pharm. Sci. 2019, 108, 2500–2504. [Google Scholar] [CrossRef]
- Sun, H.; Scott, D.O. Structure-based Drug Metabolism Predictions for Drug Design. Chem. Biol. Drug Des. 2010, 75, 3–17. [Google Scholar] [CrossRef]
- Fasinu Pius, J.; Bouic, P.; Rosenkranz, B. Liver-Based In Vitro Technologies for Drug Biotransformation Studies—A Review. Curr. Drug Metab. 2012, 13, 215–224. [Google Scholar] [CrossRef] [PubMed]
- van de Kerkhof, G.E.; de Graaf, A.M.; Groothuis, I.; Geny, M.M. In Vitro Methods to Study Intestinal Drug Metabolism. Curr. Drug Metab. 2007, 8, 658–675. [Google Scholar] [CrossRef] [PubMed]
- Lohmann, W.; Karst, U. Biomimetic modeling of oxidative drug metabolism. Anal. Bioanal. Chem. 2008, 391, 79–96. [Google Scholar] [CrossRef] [PubMed]
- Gupta, C.P. Role of iron (Fe) in body. IOSR J. of Appl. Chem. 2014, 7, 38–46. [Google Scholar] [CrossRef]
- Shelnutt, J.A.; Song, X.Z.; Ma, J.G.; Jia, S.L.; Jentzen, W.; Medforth, C.J. Nonplanar porphyrins and their significance in proteins. Chem. Soc. Rev. 1998, 27, 31–42. [Google Scholar] [CrossRef]
- de Montellano, P.R.O.; de Voss, J.J. Cytochrome P450: Structure, Mechanism, and Biochemistry; Plenum Publishers: New York, NY, USA, 2005; pp. 183–245. [Google Scholar] [CrossRef]
- Paludetto, M.N.; Bijani, C.; Puisset, F.; Bernardes-Génisson, V.; Arellano, C.; Robert, A. Metalloporphyrin-Catalyzed Oxidation of Sunitinib and Pazopanib, Two Anticancer Tyrosine Kinase Inhibitors: Evidence for New Potentially Toxic Metabolites. J. Med. Chem. 2018, 61, 7849–7860. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Lu, N.; Gu, Y.; Li, C.; Zhang, T.; Liu, H.; Zhang, Z.; Zhai, S. Catalytic activity of biomimetic model of cytochrome P450 in oxidation of dopamine. Talanta 2018, 179, 401–408. [Google Scholar] [CrossRef]
- Zhao, M.; Wu, C.-D. Biomimetic Activation of Molecular Oxygen with a Combined Metalloporphyrinic Framework and Co-catalyst Platform. ChemCatChem 2017, 9, 1192–1196. [Google Scholar] [CrossRef]
- Zanardi, F.B.; Barbosa, I.A.; de Sousa Filho, P.C.; Zanatta, L.D.; da Silva, D.L.; Serra, O.A.; Iamamoto, Y. Manganese porphyrin functionalized on Fe3O4@nSiO2@MCM-41 magnetic composite: Structural characterization and catalytic activity as cytochrome P450 model. Micropor. Mesopor. Mater. 2016, 219, 161–171. [Google Scholar] [CrossRef]
- Lassila, T.; Mattila, S.; Turpeinen, M.; Tolonen, A. Glutathione trapping of reactive drug metabolites produced by biomimetic metalloporphyrin catalysts. Rapid Commun. Mass Spectrom. 2015, 29, 521–532. [Google Scholar] [CrossRef]
- Nappa, M.J.; Tolman, C.A. Steric and electronic control of iron porphyrin catalyzed hydrocarbon oxidations. Inorg. Chem. 1985, 24, 4711–4719. [Google Scholar] [CrossRef]
- Lente, G.; Fábián, I. Kinetics and mechanism of the oxidation of water soluble porphyrin Fe(III)TPPS with hydrogen peroxide and the peroxomonosulfate ion. Dalton Trans. 2007, 38, 4268–4275. [Google Scholar] [CrossRef]
- Garcia-Bosch, I.; Sharma, S.K.; Karlin, K.D. A Selective Stepwise Heme Oxygenase Model System: An Iron(IV)-Oxo Porphyrin π-Cation Radical Leads to a Verdoheme-Type Compound via an Isoporphyrin Intermediate. J. Am. Chem. Soc. 2013, 135, 16248–16251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brulé, E.; de Miguel, Y.R. Supported manganese porphyrin catalysts as P450 enzyme mimics for alkene epoxidation. Tetrahedron Lett. 2002, 43, 8555–8558. [Google Scholar] [CrossRef]
- Fődi, T.; Ignácz, G.; Decsi, B.; Béni, Z.; Túrós, G.I.; Kupai, J.; Balogh-Weiser, D.; Greiner, I.; Huszthy, P.; Balogh, G.T. Biomimetic Synthesis of Drug Metabolites in Batch and Continuous-Flow Reactors. Chemistry 2018, 24, 9385–9392. [Google Scholar] [CrossRef] [PubMed]
- Decsi, B.; Krammer, R.; Hegedűs, K.; Ender, F.; Gyarmati, B.; Szilágyi, A.; Tőtős, R.; Katona, G.; Paizs, C.; Balogh, G.T.; et al. Liver-on-a-Chip‒Magnetic Nanoparticle Bound Synthetic Metalloporphyrin-Catalyzed Biomimetic Oxidation of a Drug in a Magnechip Reactor. Micromachines 2019, 10, 668. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. World Malaria Report 2021; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Achan, J.; Talisuna, A.O.; Erhart, A.; Yeka, A.; Tibenderana, J.K.; Baliraine, F.N.; Rosenthal, P.J.; D’Alessandro, U. Quinine, an old anti-malarial drug in a modern world: Role in the treatment of malaria. Malar. J. 2011, 10, 144. [Google Scholar] [CrossRef] [Green Version]
- Krafts, K.; Hempelmann, E.; Skórska-Stania, A. From methylene blue to chloroquine: A brief review of the development of an antimalarial therapy. Parasitol. Res. 2012, 111, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Surrey, A.R.; Hammer, H.F. The Preparation of 7-Chloro-4-(4-(N-ethyl-N-β-hydroxyethylamino)-1-methylbutylamino)-quinoline and Related Compounds. J. Am. Chem. Soc. 1950, 72, 1814–1815. [Google Scholar] [CrossRef]
- Conan, N.J. The treatment of hepatic amebiasis with chloroquine. Am. J. Med. 1949, 6, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Schrezenmeier, E.; Dörner, T. Mechanisms of action of hydroxychloroquine and chloroquine: Implications for rheumatology. Nat. Rev. Rheumatol. 2020, 16, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Farias, K.J.S.; Machado, P.R.L.; Muniz, J.A.P.C.; Imbeloni, A.A.; da Fonseca, B.A.L. Antiviral Activity of Chloroquine Against Dengue Virus Type 2 Replication in Aotus Monkeys. Viral Immunol. 2015, 28, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Savarino, A.; Di Trani, L.; Donatelli, I.; Cauda, R.; Cassone, A. New insights into the antiviral effects of chloroquine. Lancet Infect. Dis. 2006, 6, 67–69. [Google Scholar] [CrossRef]
- Keyaerts, E.; Vijgen, L.; Maes, P.; Neyts, J.; Van Ranst, M. In vitro inhibition of severe acute respiratory syndrome coronavirus by chloroquine. Biochem. Biophys. Res. Commun. 2004, 323, 264–268. [Google Scholar] [CrossRef]
- Sahraei, Z.; Shabani, M.; Shokouhi, S.; Saffaei, A. Aminoquinolines against coronavirus disease 2019 (COVID-19): Chloroquine or hydroxychloroquine. Int. J. Antimicrob. Agents 2020, 55, 105945. [Google Scholar] [CrossRef]
- Shah, S.; Das, S.; Jain, A.; Misra, D.P.; Negi, V.S. A systematic review of the prophylactic role of chloroquine and hydroxychloroquine in coronavirus disease-19 (COVID-19). Int. J. Rheum. Dis. 2020, 23, 613–619. [Google Scholar] [CrossRef]
- Hashem, A.M.; Alghamdi, B.S.; Abdullah, A.A.; Alshehri, F.S.; Bukhari, A.; Alfaleh, M.A.; Memish, Z.A. Therapeutic use of chloroquine and hydroxychloroquine in COVID-19 and other viral infections: A narrative review. Trav. Med. Infect. Dis. 2020, 35, 101735. [Google Scholar] [CrossRef]
- Cortegiani, A.; Ingoglia, G.; Ippolito, M.; Giarratano, A.; Einav, S. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J. Crit. Care 2020, 57, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Ducharme, J.; Farinotti, R. Clinical pharmacokinetics and metabolism of chloroquine. Focus on recent advancements. Clin. Pharmacokinet. 1996, 4, 257–274. [Google Scholar] [CrossRef] [PubMed]
- Projean, D.; Baune, B.; Farinotti, R.; Flinois, J.P.; Beaune, P.; Taburet, A.M.; Ducharme, J. In vitro metabolism of chloroquine: Identification of cyp2c8, cyp3a4, and cyp2d6 as the main isoforms catalyzing n-desethylchloroquine formation. Drug Metab. Dispos. 2003, 31, 748–754. [Google Scholar] [CrossRef] [Green Version]
- Frisk-Holmberg, M.; Berggvist, Y.; Termond, E.; Domeij-Nyberg, B. The single dose kinetics of chloroquine and its major metabolite desethylchloroquine in healthy subjects. Eur. J. Clin. Pharmacol. 1984, 26, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, L.L.; Walker, O.; Alván, G.; Beermann, B.; Estevez, F.; Gleisner, L.; Lindström, B.; Sjöqvist, F. Disposition of chloroquine in man after single intravenous and oral doses. Br. J. Clin. Pharmacol. 1983, 15, 471–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.A.; Park, J.Y.; Lee, J.S.; Lim, S. Cytochrome P450 2C8 and CYP3A4/5 are involved in chloroquine metabolism in human liver microsomes. Arch. Pharmacal Res. 2003, 26, 631–637. [Google Scholar] [CrossRef]
- Ette, E.I.; Essien, E.E.; Wilkinson, O.A.; Thomas, E.A.; Brown-Awala, A. Pharmacokinetics of Chloroquine and Some of Its Metabolites in Healthy Volunteers: A Single Dose Study. Clin. Pharmacol. 1989, 29, 457–462. [Google Scholar] [CrossRef]


 ) and FeTPPs immobilized onto amino-functionalized silica particles ((a) Silica-Am-1-FeTPPS, (b) Silica-Am-2-FeTPPS and (c) Silica-Am-3-FeTPPS marked with
) and FeTPPs immobilized onto amino-functionalized silica particles ((a) Silica-Am-1-FeTPPS, (b) Silica-Am-2-FeTPPS and (c) Silica-Am-3-FeTPPS marked with  ) by SEM-EDAX. * atomic% < 0.1%).
) by SEM-EDAX. * atomic% < 0.1%).
 ) and FeTPPs immobilized onto amino-functionalized silica particles ((a) Silica-Am-1-FeTPPS, (b) Silica-Am-2-FeTPPS and (c) Silica-Am-3-FeTPPS marked with
) and FeTPPs immobilized onto amino-functionalized silica particles ((a) Silica-Am-1-FeTPPS, (b) Silica-Am-2-FeTPPS and (c) Silica-Am-3-FeTPPS marked with  ) by SEM-EDAX. * atomic% < 0.1%).
) by SEM-EDAX. * atomic% < 0.1%).

| Metabolite Profile | |||
|---|---|---|---|
| HLM | RLM | MLM | |
 |  |  | |
| Chloroquine (CQ) | 97.4 | 97.5 | 94.8 |
| M1 (CQ − 28u, −Et) | 2.6 | 2.1 | 3.4 |
| M2 (CQ + 16, +O) | – | 0.5 | 1.8 |
| c1 (%) | 2.6 | 2.5 | 5.2 |
| Metabolite Profile (%) | ||||
|---|---|---|---|---|
| FeTPPS | Silica-Am-1-FeTPPS | Silica-Am-2-FeTPPS | Silica-Am-3-FeTPPS | |
 |  |  |  | |
| CQ | 60.5 | 47.4 | 73.6 | 81.4 |
| M1 (CQ − 28u) | 28.4 | 39.6 | 19.8 | 13.8 |
| M2 (CQ + 16) | – | – | – | – |
| M3 (M1 − 28u) | – | 12.5 | 6.6 | – |
| M4 (M3 + 16u) | 0.2 | 0.2 | 0.1 | <0.1 |
| M5 (M1 + 30u) | 0.1 | – | – | <0.1 |
| M6 (M5 − 2u) | 0.2 | 0.1 | – | – |
| Other | 0.4 | 0.4 | – | – |
| c1 (%) | 39.5 | 52.6 | 26.4 | 18.6 |
| Silica-Am-1-FeTPPS | Silica-Am-2-FeTPPS | Silica-Am-3-FeTPPS | |
|---|---|---|---|
 |  |  | |
| CQ | 98.0 | 23.6 | 49.6 |
| M1 (DCQ) | 2.0 | 29.3 | 29.9 |
| M3 (DDCQ) | – | 36.6 | 19.3 |
| Other | – | 10.4 | 2.1 |
| c1 (%) | 2.0 | 76.4 | 50.4 |
| Catalyst | Reaction Mode | TON (–) | STY (mg L−1 h−1) |
|---|---|---|---|
| HLM | batch | – | 0.32 |
| RLM | – | 0.31 | |
| MLM | – | 0.64 | |
| FeTPPS | 6.56 | 184 | |
| Silica-Am-1-FeTPPS | batch | 8.73 | 296 |
| Silica-Am-2-FeTPPS | 4.38 | 119 | |
| Silica-Am-3-FeTPPS | 3.09 | 84 | |
| Silica-Am-1-FeTPPS | continuous-flow | 0.04 | 2309 |
| Silica-Am-2-FeTPPS | 1.62 | 88,213 | |
| Silica-Am-3-FeTPPS | 1.07 | 58,193 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balogh, G.T.; Decsi, B.; Krammer, R.; Kenéz, B.; Ender, F.; Hergert, T.; Balogh-Weiser, D. Effect of Binding Linkers on the Efficiency and Metabolite Profile of Biomimetic Reactions Catalyzed by Immobilized Metalloporphyrin. Metabolites 2022, 12, 1269. https://doi.org/10.3390/metabo12121269
Balogh GT, Decsi B, Krammer R, Kenéz B, Ender F, Hergert T, Balogh-Weiser D. Effect of Binding Linkers on the Efficiency and Metabolite Profile of Biomimetic Reactions Catalyzed by Immobilized Metalloporphyrin. Metabolites. 2022; 12(12):1269. https://doi.org/10.3390/metabo12121269
Chicago/Turabian StyleBalogh, György T., Balázs Decsi, Réka Krammer, Balázs Kenéz, Ferenc Ender, Tamás Hergert, and Diána Balogh-Weiser. 2022. "Effect of Binding Linkers on the Efficiency and Metabolite Profile of Biomimetic Reactions Catalyzed by Immobilized Metalloporphyrin" Metabolites 12, no. 12: 1269. https://doi.org/10.3390/metabo12121269
APA StyleBalogh, G. T., Decsi, B., Krammer, R., Kenéz, B., Ender, F., Hergert, T., & Balogh-Weiser, D. (2022). Effect of Binding Linkers on the Efficiency and Metabolite Profile of Biomimetic Reactions Catalyzed by Immobilized Metalloporphyrin. Metabolites, 12(12), 1269. https://doi.org/10.3390/metabo12121269