The Role of the GH/IGF1 Axis on the Development of MAFLD in Pediatric Patients with Obesity
Abstract
1. Introduction
2. Materials and Methods
3. Statistical Analysis
4. Results
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Liang, S.; Yu, Z.; Song, X.; Wang, Y.; Li, M.; Xue, J. Reduced Growth Hormone Secretion is Associated with Nonalcoholic Fatty Liver Disease in Obese Children. Horm. Metab. Res. 2018, 50, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, D.; Cabello-Verrugio, C.; Solís, N.; San Martín, D.; Cofré, C.; Pizarro, M.; Arab, J.P.; Abrigo, J.; Campos, F.; Irigoyen, B.; et al. Somatotropic Axis Dysfunction in Non-Alcoholic Fatty Liver Disease: Beneficial Hepatic and Systemic Effects of Hormone Supplementation. Int. J. Mol. Sci. 2018, 19, 1339. [Google Scholar] [CrossRef] [PubMed]
- Stanley, T.L.; Fourman, L.T.; Zheng, I.; McClure, C.M.; Feldpausch, M.N.; Torriani, M.; Corey, K.E.; Chung, R.T.; Lee, H.; Kleiner, D.E.; et al. Relationship of IGF-1 and IGF-Binding Proteins to Disease Severity and Glycemia in Nonalcoholic Fatty Liver Disease. J. Clin. Endocrinol. Metab. 2021, 106, e520–e533. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, T.; Nakao, K.; Hamasaki, K.; Furukawa, R.; Tsuruta, S.; Ueda, Y.; Taura, N.; Shibata, H.; Fujimoto, M.; Toriyama, K.; et al. Role of growth hormone, insulin-like growth factor 1 and insulin-like growth factor-binding protein 3 in development of non-alcoholic fatty liver disease. Hepatol. Int. 2007, 1, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Clemmons, D.R. The relative roles of growth hormone and IGF-1 in controlling insulin sensitivity. J. Clin. Investig. 2004, 113, 25–27. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Menon, R.K.; Cohen, P.; Hwang, D.; Clemens, T.; DiGirolamo, D.J.; Kopchick, J.J.; Le Roith, D.; Trucco, M.; Sperling, M.A. Liver-specific deletion of the growth hormone receptor reveals essential role of growth hormone signaling in hepatic lipid metabolism. J. Biol. Chem. 2009, 284, 19937–19944. [Google Scholar] [CrossRef] [PubMed]
- Fusco, A.; Miele, L.; D’Uonnolo, A.; Forgione, A.; Riccardi, L.; Cefalo, C.; Barini, A.; Bianchi, A.; Giampietro, A.; Cimino, V.; et al. Nonalcoholic fatty liver disease is associated with increased GHBP and reduced GH/IGF-I levels. Clin. Endocrinol. 2012, 77, 531–536. [Google Scholar] [CrossRef]
- Bredella, M.A.; Torriani, M.; Thomas, B.J.; Ghomi, R.H.; Brick, D.J.; Gerweck, A.V.; Miller, K.K. Peak growth hormone-releasing hormone-arginine-stimulated growth hormone is inversely associated with intramyocellular and intrahepatic lipid content in premenopausal women with obesity. J. Clin. Endocrinol. Metab. 2009, 94, 3995–4002. [Google Scholar] [CrossRef][Green Version]
- Clemmons, D.R. Role of IGF-binding proteins in regulating IGF responses to changes in metabolism. J. Mol. Endocrinol. 2018, 61, T139–T169. [Google Scholar] [CrossRef]
- Martinez-Castillo, M.; Rosique-Oramas, D.; Medina-Avila, Z.; Perez-Hernandez, J.L.; Higuera-De la Tijera, F.; Santana-Vargas, D.; Montalvo-Jave, E.E.; Sanchez-Avila, F.; Torre, A.; Kershenobich, D.; et al. Differential production of insulin-like growth factor-binding proteins in liver fibrosis progression. Mol. Cell. Biochem. 2020, 469, 65–75. [Google Scholar] [CrossRef]
- Petaja, E.M.; Zhou, Y.; Havana, M.; Hakkarainen, A.; Lundbom, N.; Ihalainen, J.; Yki-Jarvinen, H. Phosphorylated IGFBP 1 as a non-invasive predictor of liver fat in NAFLD. Sci. Rep. 2016, 6, 24740. [Google Scholar] [CrossRef]
- Kotronen, A.; Lewitt, M.; Hall, K.; Brismar, K.; Yki-Jarvinen, H. Insulin-like growth factor binding protein 1 as a novel specific marker of hepatic insulin sensitivity. J. Clin. Endocrinol. Metab. 2008, 93, 4867–4872. [Google Scholar] [CrossRef] [PubMed]
- Fahlbusch, P.; Knebel, B.; Horbelt, T.; Barbosa, D.M.; Nikolic, A.; Jacob, S.; Al-Hasani, H.; Van de Velde, F.; Van Nieuwenhove, Y.; Muller-Wieland, D.; et al. Physiological Disturbance in Fatty Liver Energy Metabolism Converges on IGFBP2 Abundance and Regulation in Mice and Men. Int. J. Mol. Sci. 2020, 21, 4144. [Google Scholar] [CrossRef] [PubMed]
- Dali-Youcef, N.; Vix, M.; Costantino, F.; El-Saghire, H.; Lhermitte, B.; Callari, C.; D’Agostino, J.; Perretta, S.; Paveliu, S.; Gualtierotti, M.; et al. Interleukin-32 Contributes to Human Nonalcoholic Fatty Liver Disease and Insulin Resistance. Hepatol. Commun. 2019, 3, 1205–1220. [Google Scholar] [CrossRef] [PubMed]
- Min, H.K.; Maruyama, H.; Jang, B.K.; Shimada, M.; Mirshahi, F.; Ren, S.; Oh, Y.; Puri, P.; Sanyal, A.J. Suppression of IGF binding protein-3 by palmitate promotes hepatic inflammatory responses. FASEB J. 2016, 30, 4071–4082. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Bermejo, A.; Khosravi, J.; Fernandez-Real, J.M.; Hwa, V.; Pratt, K.L.; Casamitjana, R.; Garcia-Gil, M.M.; Rosenfeld, R.G.; Ricart, W. Insulin resistance is associated with increased serum concentration of IGF-binding protein-related protein 1 (IGFBP-rP1/MAC25). Diabetes 2006, 55, 2333–2339. [Google Scholar] [CrossRef]
- Yan, H.; Li, T.; Wang, Y.; Li, H.; Xu, J.; Lu, X. Insulin-like growth factor binding protein 7 accelerates hepatic steatosis and insulin resistance in non-alcoholic fatty liver disease. Clin. Exp. Pharmacol. Physiol. 2019, 46, 1101–1110. [Google Scholar] [CrossRef]
- Takahashi, Y.; Iida, K.; Takahashi, K.; Yoshioka, S.; Fukuoka, H.; Takeno, R.; Imanaka, M.; Nishizawa, H.; Takahashi, M.; Seo, Y.; et al. Growth hormone reverses nonalcoholic steatohepatitis in a patient with adult growth hormone deficiency. Gastroenterology 2007, 132, 938–943. [Google Scholar] [CrossRef]
- Sobrevals, L.; Rodriguez, C.; Romero-Trevejo, J.L.; Gondi, G.; Monreal, I.; Paneda, A.; Juanarena, N.; Arcelus, S.; Razquin, N.; Guembe, L.; et al. Insulin-like growth factor I gene transfer to cirrhotic liver induces fibrolysis and reduces fibrogenesis leading to cirrhosis reversion in rats. Hepatology 2010, 51, 912–921. [Google Scholar]
- Vajro, P.; Lenta, S.; Socha, P.; Dhawan, A.; McKiernan, P.; Baumann, U.; Durmaz, O.; Lacaille, F.; McLin, V.; Nobili, V. Diagnosis of nonalcoholic fatty liver disease in children and adolescents: Position paper of the ESPGHAN Hepatology Committee. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 700–713. [Google Scholar] [CrossRef]
- Feldstein, A.E.; Charatcharoenwitthaya, P.; Treeprasertsuk, S.; Benson, J.T.; Enders, F.B.; Angulo, P. The natural history of non-alcoholic fatty liver disease in children: A follow-up study for up to 20 years. Gut 2009, 58, 1538–1544. [Google Scholar] [CrossRef]
- Rosenfeld, R.G.; Albertsson-Wikland, K.; Cassorla, F.; Frasier, S.D.; Hasegawa, Y.; Hintz, R.L.; LaFranchi, S.; Lippe, B.; Loriaux, L.; Melmed, S. Diagnostic controversy: The diagnosis of childhood growth hormone deficiency revisited. J. Clin. Endocrinol. Metab. 1995, 80, 1532–1540. [Google Scholar] [PubMed]
- Linea Guida Clinica Sulla Diagnosi del Deficit di GH. Available online: https://www.ospedalebambinogesu.it/ (accessed on 10 September 2022).
- Conwell, L.S.; Trost, S.G.; Brown, W.J.; Batch, J.A. Indexes of insulin resistance and secretion in obese children and adolescents: A validation study. Diabetes Care 2004, 27, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Brunt, E.M.; Kleiner, D.E.; Wilson, L.A.; Belt, P.; Neuschwander-Tetri, B.A.; NASH Clinical Research Network (CRN). Nonalcoholic fatty liver disease (NAFLD) activity score and the histopathologic diagnosis in NAFLD: Distinct clinicopathologic meanings. Hepatology 2011, 53, 810–820. [Google Scholar] [CrossRef]
- Santiago-Rolón, A.; Purcell, D.; Rosado, K.; Toro, D.H. A comparison of brunt’s criteria, the non-alcoholic fatty liver disease activity score (NAS), and a proposed NAS scoring that includes fibrosis in non-alcoholic fatty liver disease staging. Puerto Rico Health Sci. J. 2015, 34, 189–194. [Google Scholar]
- Rufinatscha, K.; Ress, C.; Folie, S.; Haas, S.; Salzmann, K.; Moser, P.; Dobner, J.; Weiss, G.; Iruzubieta, P.; Arias-Loste, M.T.; et al. Metabolic effects of reduced growth hormone action in fatty liver disease. Hepatol. Int. 2018, 12, 474–481. [Google Scholar] [CrossRef]
- Dichtel, L.E.; Corey, K.E.; Misdraji, J.; Bredella, M.A.; Schorr, M.; Osganian, S.A.; Young, B.J.; Sung, J.C.; Miller, K.K. The Association Between IGF-1 Levels and the Histologic Severity of Nonalcoholic Fatty Liver Disease. Clin. Transl. Gastroenterol. 2017, 8, e217. [Google Scholar] [CrossRef] [PubMed]
- Dichtel, L.E.; Cordoba-Chacon, J.; Kineman, R.D. Growth Hormone and Insulin-Like Growth Factor 1 Regulation of Nonalcoholic Fatty Liver Disease. J. Clin. Endocrinol. Metab. 2022, 107, 1812–1824. [Google Scholar] [CrossRef]
- Osganian, S.A.; Subudhi, S.; Masia, R.; Drescher, H.K.; Bartsch, L.M.; Chicote, M.L.; Chung, R.T.; Gee, D.W.; Witkowski, E.R.; Bredella, M.A.; et al. Expression of IGF-1 receptor and GH receptor in hepatic tissue of patients with nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Growth Horm. IGF Res. 2022, 65, 101482. [Google Scholar] [CrossRef]
- Mauras, N.; O’Brien, K.O.; Welch, S.; Rini, A.; Helgeson, K.; Vieira, N.E.; Yergey, A.L. Insulin-like growth factor I and growth hormone (GH) treatment in GH-deficient humans: Differential effects on protein, glucose, lipid, and calcium metabolism. J. Clin. Endocrinol. Metab. 2000, 85, 1686–1694. [Google Scholar] [CrossRef]
- Lambert, J.E.; Ramos-Roman, M.A.; Browning, J.D.; Parks, E.J. Increased de novo lipogenesis is a distinct characteristic of individuals with nonalcoholic fatty liver disease. Gastroenterology 2014, 146, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Cordoba-Chacon, J.; Sarmento-Cabral, A.; Del Rio-Moreno, M.; Diaz-Ruiz, A.; Subbaiah, P.V.; Kineman, R.D. Adult-Onset Hepatocyte GH Resistance Promotes NASH in Male Mice, Without Severe Systemic Metabolic Dysfunction. Endocrinology 2018, 159, 3761–3774. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.S.; Weiss, J.J.; Fourman, L.T.; Buckless, C.; Branch, K.L.; Lee, H.; Torriani, M.; Misra, M.; Stanley, T.L. Effect of recombinant human growth hormone on liver fat content in young adults with nonalcoholic fatty liver disease. Clin. Endocrinol. 2021, 94, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Hjelholt, A.; Høgild, M.; Bak, A.M.; Arlien-Søborg, M.C.; Bæk, A.; Jessen, N.; Richelsen, B.; Pedersen, S.B.; Møller, N.; Jørgensen, J.O.L. Growth Hormone and Obesity. Endocrinol. Metab. Clin. N. Am. 2020, 49, 239–250. [Google Scholar] [CrossRef]
- Hribal, M.L.; Procopio, T.; Petta, S.; Sciacqua, A.; Grimaudo, S.; Pipitone, R.M.; Perticone, F.; Sesti, G. Insulin-like growth factor-I, inflammatory proteins, and fibrosis in subjects with nonalcoholic fatty liver disease. J. Clin. Endocrinol. Metab. 2013, 98, E304–E308. [Google Scholar] [CrossRef]
- Attallah, H.; Friedlander, A.L.; Hoffman, A.R. Visceral obesity, impaired glucose tolerance, metabolic syndrome, and growth hormone therapy. Growth Horm. IGF Res. 2006, 16, S62–S67. [Google Scholar] [CrossRef]
- Bredella, M.A.; Gerweck, A.V.; Lin, E.; Landa, M.G.; Torriani, M.; Schoenfeld, D.A.; Hemphill, L.C.; Miller, K.K. Effects of GH on body composition and cardiovascular risk markers in young men with abdominal obesity. J. Clin. Endocrinol. Metab. 2013, 98, 3864–3872. [Google Scholar] [CrossRef]
| Variables | Mean (SD) or Median (25th–75th Centile) |
|---|---|
| Age, years | 12.9 (2.1) |
| Sex (M/F)% | 88/103 (46.2/53.8) |
| BMI, kg/sqm | 28.5 (4.7) |
| WC, cm (IQR) | 88.5 (81–99) |
| Uric acid, mg/dL | 5.2 (1.6) |
| ALT, UI/L (IQR) | 51 (31–78) |
| AST, UI/L (IQR) | 39 (27–54) |
| GGT, UI/L (IQR) | 21 (15–29) |
| Total-cholesterol, mg/dL (IQR) | 158 (132–179) |
| HDL cholesterol, mg/dL(IQR) | 43 (36–48) |
| Triglycerides, mg/dL (IQR) | 138 (73–151) |
| Glucose, mg/dL (IQR) | 82.9 (76–91) |
| Insulin, μUI/ML (IQR) | 17.6 (10–28.3) |
| HOMA-IR | 3.7 (3.4) |
| DBP, mmHg (IQR) | 68 (60–74) |
| SBP, mmHg (IQR) | 110 (101–118) |
| GH, ng/mL (IQR) | 7.8 (5.8–9) |
| IGF1, ng/mL (IQR) | 266 (198–345) |
| IGFBP-3, µg/mL (IQR) | 2.34 (1.5–3.2) |
| TNF-a (IQR), pg/ml | 51 (7.5–77.3) |
| IL-6 (IQR), pg/mL | 29 (19–33.5) |
| Tanner’s Stage I–II–III (Approximatively 12–15 Years) | Tanner’s Stage IV–V (Approximatively > 15 Years) | Tanner’s Stage: I–II–III vs. IV–V p Values | |||||
|---|---|---|---|---|---|---|---|
| All (151) | F (85) | M (66) | All (40) | F (18) | M (22) | ||
| Age, years | 13.5 (1.33) | 12.9 (1.3) | 12.6(1.3) | 15.9 (1.1) | 15.9 (1.3) | 16 (0.9) | 0.0001 |
| BMI, kg/sqm | 27.1 (4.2) | 27 (24–29) | 27.1 (24–29.5) | 29.8 (5.6) | 30 (25–33) | 28.8 (25–31) | 0.01 |
| WC, cm (IQR) | 84 (78–92) | 83 (78–92) | 83 (77–90.5) | 88 (78–97) | 87 (76–96) | 88 (81–97) | 0.04 |
| Uric acid, mg/dL | 5.2 (1.6) | 5.4 (1.6) | 5 (1.5) | 5.7 (1.6) | 5.3 (1.9) | 6 (1.2) | 0.14 |
| ALT, UI/L (IQR) | 59.7 (32–75.5) | 63 (33–78) | 55 (31–73) | 73 (28–96) | 85 (32–130) | 65 (28–78) | 0.08 |
| AST, UI/L (IQR) | 43 (27–52.5) | 44 (30–56) | 41.5 (26–49) | 45 (25–58) | 57 (24–67) | 39.5 (25–44.7) | 0.65 |
| GGT, UI/L (IQR) | 23.6 (15–27) | 25 (15–30) | 22 (14–25) | 32 (14–39) | 39 (31–18) | 26 (13–28) | 0.01 |
| Total-cholesterol, mg/dL (IQR) | 158 (132–178) | 165 (145–189) | 149 (123–169) | 161 (131–180) | 173 (131–216) | 151 (132–165) | 0.66 |
| HDL, mg/dL (IQR) | 46 (38–48.5) | 44.6 (39–49) | 47 (36–48) | 45 (36–48) | 46 (36–53) | 45 (35–44) | 0.82 |
| Triglycerides, mg/dL (IQR) | 104 (69–117) | 109 (97–122) | 99 (59–110) | 126 (89–168) | 125 (90–170) | 127 (89–163) | 0.05 |
| Glucose, mg/dL (IQR) | 83.6 (76–90) | 83 (76–89) | 84 (77–93) | 80 (76–86) | 85 (77–92) | 78 (71–83) | 0.13 |
| Insulin, μUI/ML (IQR) | 17 (10–24) | 19 (10–25.5) | 16 (10–21) | 18(10–24) | 22 (11–29) | 15 (10–19) | 0.71 |
| HOMA-IR (IQR) | 3.7 (2–4.9) | 3.9 (2.2–4.9) | 3.4 (2–5) | 3.8 (2–4.5) | 4.9 (2.8–6.9) | 3.1 (1.8–4) | 0.81 |
| DBP, mmHg (IQR) | 67 (60–74) | 67 (59–79) | 67.5 (61–73) | 66 (60–73) | 70 (68–75) | 63 (58–68) | 0.49 |
| SBP, mmHg (IQR) | 110 (101–118) | 110.5 (102–118) | 111 (102–115) | 111.5 (103–120) | 110 (105–118) | 112 (102–120) | 0.62 |
| TNF-a | 40 (7–47) | 41 (7–22.5) | 36.5(12–59) | 55.2 (43–89) | 44.5 (30–49) | 41 (33–78) | 0.06 |
| IL-6 | 26 (18–31.2) | 24 (19–29.5) | 27 (18.5–32) | 36.5 (25–50) | 25 (21–35) | 39 (26–49) | 0.13 |
| GH, ng/mL (IQR) | 10.2 (8–11) | 10.5(3.2) | 10 (8–11) | 10 (8–11) | 8.1 (7.5–11) | 10.4 (2–11.3) | 0.67 |
| IGF1, ng/mL(IQR) | 272 (198–345) | 267 (198–305) | 279 (208–347) | 268 (198–345) | 233 (164–305) | 395 (125–205) | 0.81 |
| IGFBP-3, µg/mL (IQR) | 2.4 (1.5–3.2) | 2.4 (1.5–3.2) | 2.4 (1.5–3.2) | 2.2 (1.5–2.8) | 2.2 (1.4–2.6) | 2.3 (1.6–2.9) | 0.5 |
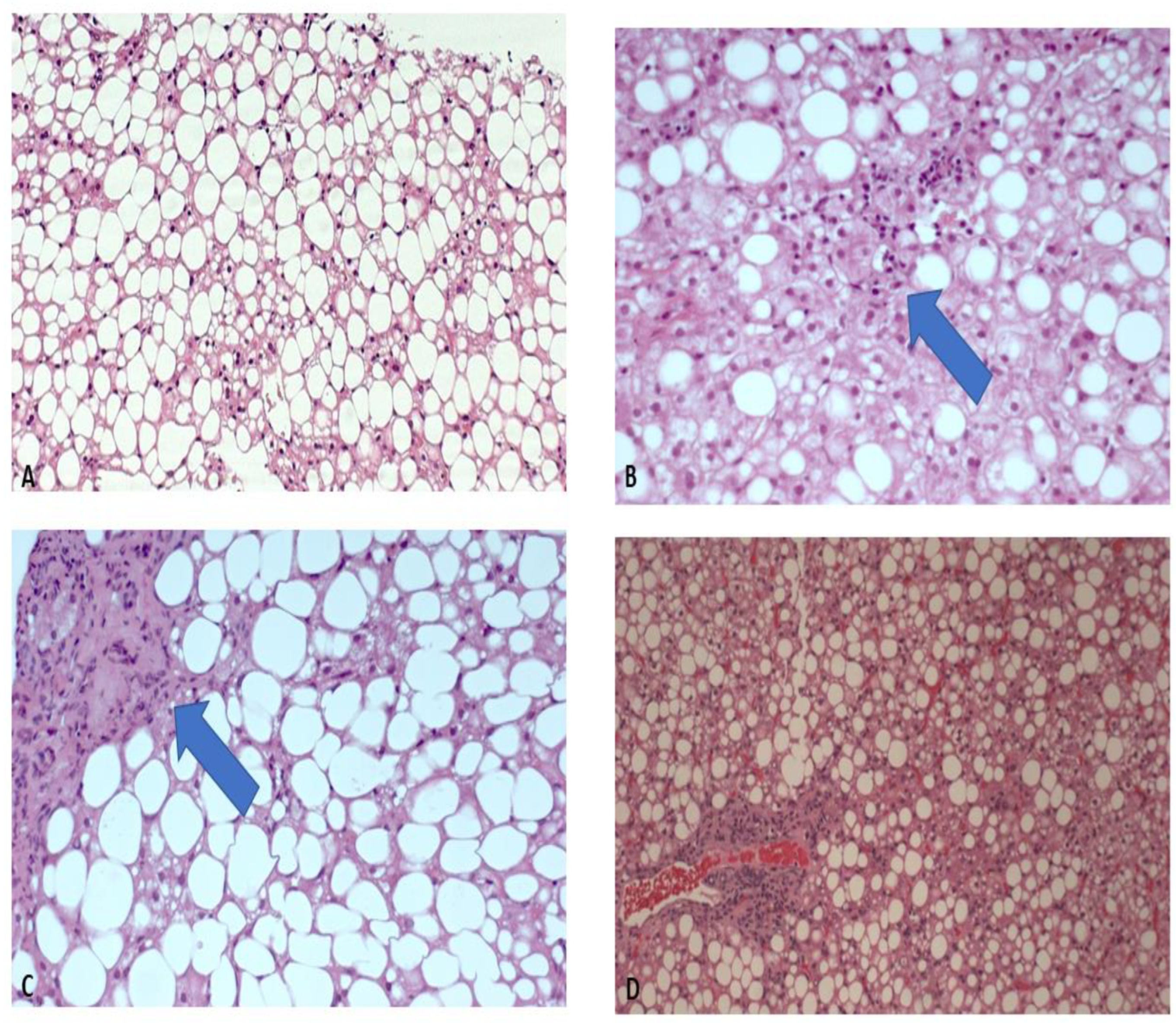
| Histologic Characteristic | Number of Patients | Percentage |
|---|---|---|
| Steatosis | ||
| 0 | 16 | 8.4% |
| 1 | 64 | 33.7% |
| 2 | 72 | 37.9% |
| 3 | 38 | 20% |
| Portal inflammation | ||
| 0 | 19 | 10% |
| 1 | 139 | 73.2% |
| 2 | 32 | 16.8% |
| Lobular inflammation | ||
| 0 | 73 | 38.6% |
| 1 | 100 | 52.9% |
| 2 | 16 | 8.5% |
| Ballooning | ||
| 0 | 99 | 52.1% |
| 1 | 62 | 32.6% |
| 2 | 29 | 15.3% |
| Fibrosis | ||
| 0 | 57 | 30% |
| 1 | 108 | 56.8% |
| 2 | 18 | 9.5% |
| 3 | 7 | 3.7% |
| NAS | ||
| 0 | 13 | 6.8% |
| 1 | 11 | 5.8% |
| 2 | 43 | 22.6% |
| 3 | 34 | 17.9% |
| 4 | 39 | 20.5% |
| 5 | 23 | 12.6% |
| 6 | 24 | 12.6% |
| 7 | 3 | 1.6% |
| Non-Steatohepatitis (N 102) | Steatohepatitis (N 89) | p | |
|---|---|---|---|
| Age, years | 12.9 (1.6) | 13.1 (1.8) | 0.40 |
| Sex, (F/M) | 57/45 | 46/43 | 0.79 |
| BMI, kg/sqm | 26.8 (4.2) | 28.3 (4.4) | 0.01 |
| WC, cm (IQR) | 85.9 (72–95) | 90 (80–99) | 0.02 |
| Uric acid, mg/dL | 5.4 (1.6) | 6 (1.4) | 0.01 |
| ALT, UI/L (IQR) | 49 (24–66) | 75 (39–90) | 0.001 |
| AST, UI/L (IQR) | 37 (25–45) | 50 (33–62) | 0.001 |
| GGT, UI/L (IQR) | 21 (13–24) | 30 (19–38) | 0.04 |
| Total-cholesterol, mg/dL (IQR) | 158 (93–175) | 161 (78–183) | 0.22 |
| HDL, mg/dL (IQR) | 44 (37–49) | 47 (35–48) | 0.70 |
| Triglycerides, mg/dL (IQR) | 100 (73–138) | 127 (74–146) | 0.04 |
| Glucose, mg/dL (IQR) | 80 (75–86) | 89 (76–96) | 0.03 |
| Insulin, μUI/ML (IQR) | 17 (10–24) | 24 (18–32) | 0.02 |
| HOMA-IR (IQR) | 3.5 (2.2–4.5) | 4.6 (3.2–6.2) | 0.001 |
| DBP, mmHg (IQR) | 65 (58–72) | 68 (61–75) | 0.08 |
| SBP, mmHg (IQR) | 110 (101–118) | 114 (103–121) | 0.31 |
| TNF-a, pg/mL | 44 (7.5–44) | 72 (12–72) | 0.02 |
| IL-6, pg/mL | 25 (22–35.5) | 35 (15–39) | 0.048 |
| GH, ng/mL (IQR) | 7.5 (6–8.2) | 5.4 (4–6.8) | 0.001 |
| IGF1, ng/mL(IQR) | 284 (208–345) | 245 (150–310) | 0.01 |
| IGFBP-3, µg/mL (IQR) | 2.6 (1.6–3.4) | 2 (1.4–2.8) | 0.02 |
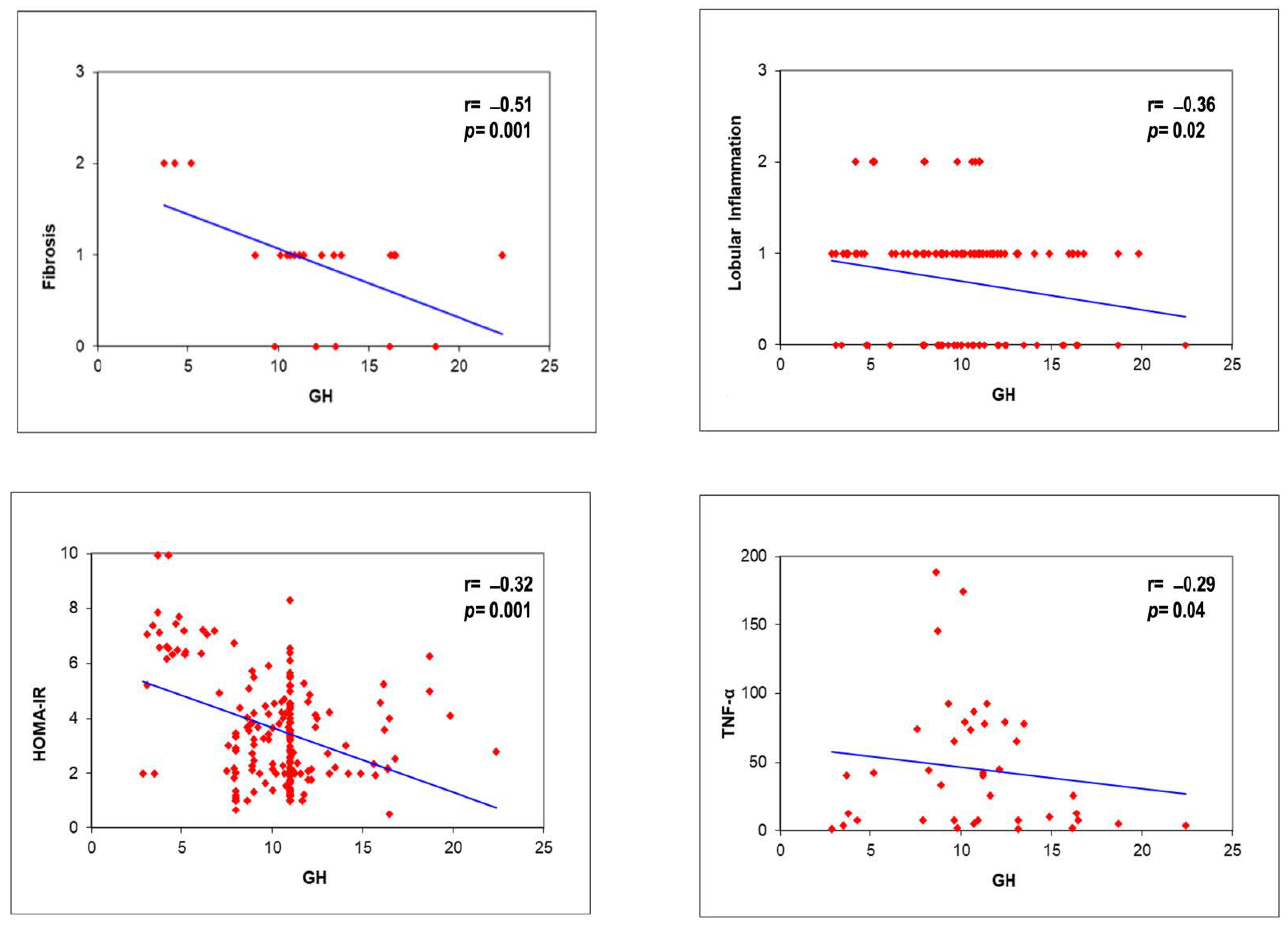
| Logistic Regression Analysis * | Standardized Beta Coefficients | SE | p Value |
|---|---|---|---|
| GH, ng/mL | |||
| Total Cholesterol | 0.13 | 0.04 | 0.003 |
| HDL-Cholesterol | −0.17 | 0.06 | 0.008 |
| Triglycerides | −0.01 | 0.02 | 0.37 |
| Glucose | 1.7 | 0.02 | 0.002 |
| Insulin | 0.03 | 0.04 | 0.45 |
| HOMA-IR | −0.33 | 0.2 | 0.09 |
| Fibrosis | −2.3 | 0.31 | 0.001 |
| Portal Inflammation | 0.56 | 0.51 | 0.41 |
| Lobular Inflammation | −0.36 | 0.34 | 0.28 |
| TNF-a | −1.2 | 0.12 | 0.04 |
| IGF1, ng/mL | |||
| Total Cholesterol | 0.41 | 1.3 | 0.75 |
| HDL-Cholesterol | 0.68 | 1.97 | 0.72 |
| Triglycerides | −0.07 | 0.35 | 0.83 |
| Glucose | −0.69 | 0.71 | 0.32 |
| Insulin | 3.8 | 1.5 | 0.01 |
| HOMA-IR | −2.7 | 1.7 | 0.001 |
| Fibrosis | −2.8 | 1.1 | 0.001 |
| Portal Inflammation | −1.3 | 1.7 | 0.09 |
| Lobular Inflammation | −1.0 | 1.1 | 0.42 |
| TNF-a | −0.78 | 0.34 | 0.25 |
| IGFBP-3, µg/mL | |||
| Total Cholesterol | 0.01 | 0.01 | 0.46 |
| HDL-Cholesterol | −0.02 | 0.03 | 0.21 |
| Triglycerides | 0.01 | 0.04 | 0.69 |
| Glucose | −0.01 | 0.07 | 0.16 |
| Insulin | −0.02 | 0.016 | 0.94 |
| HOMA-IR | −0.09 | 0.07 | 0.20 |
| Fibrosis | −0.02 | 0.04 | 0.45 |
| Portal Inflammation | 0.41 | 0.54 | 0.35 |
| Lobular Inflammation | −0.01 | 0.9 | 0.74 |
| TNF-a | −0.11 | 0.02 | 0.85 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mosca, A.; Della Volpe, L.; Alisi, A.; Panera, N.; Maggiore, G.; Vania, A. The Role of the GH/IGF1 Axis on the Development of MAFLD in Pediatric Patients with Obesity. Metabolites 2022, 12, 1221. https://doi.org/10.3390/metabo12121221
Mosca A, Della Volpe L, Alisi A, Panera N, Maggiore G, Vania A. The Role of the GH/IGF1 Axis on the Development of MAFLD in Pediatric Patients with Obesity. Metabolites. 2022; 12(12):1221. https://doi.org/10.3390/metabo12121221
Chicago/Turabian StyleMosca, Antonella, Luca Della Volpe, Anna Alisi, Nadia Panera, Giuseppe Maggiore, and Andrea Vania. 2022. "The Role of the GH/IGF1 Axis on the Development of MAFLD in Pediatric Patients with Obesity" Metabolites 12, no. 12: 1221. https://doi.org/10.3390/metabo12121221
APA StyleMosca, A., Della Volpe, L., Alisi, A., Panera, N., Maggiore, G., & Vania, A. (2022). The Role of the GH/IGF1 Axis on the Development of MAFLD in Pediatric Patients with Obesity. Metabolites, 12(12), 1221. https://doi.org/10.3390/metabo12121221






