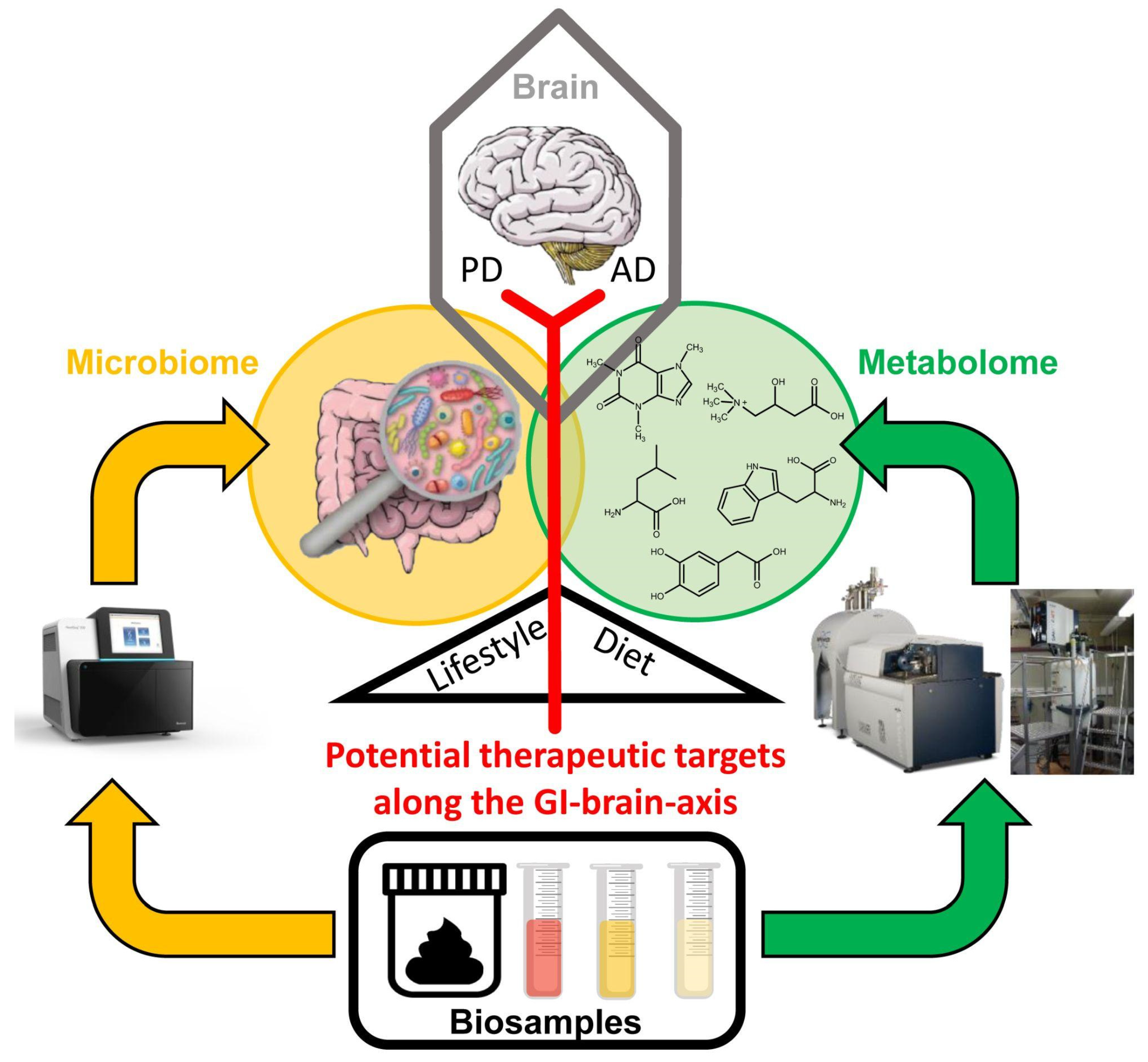
Microbiome and Metabolome Insights into the Role of the Gastrointestinal–Brain Axis in Parkinson’s and Alzheimer’s Disease: Unveiling Potential Therapeutic Targets
Abstract
:1. The Gastrointestinal–Brain Axis as a Potential Mediator of Microbiome Effects in Neurodegenerative Diseases
2. From the Microbiome to the Metabolome
2.1. Interrogating the Microbiome
2.2. Interrogating the Metabolome
3. Microbiome and Microbiome-Linked Metabolome Changes in Neurodegeneration
3.1. Microbiome Changes in Neurodegeneration
3.2. Metabolomic Changes in Neurodegeneration
| Publication | Study Question | Analytical Method | Sample Matrix | Additional Measurements | Study Population | Findings |
|---|---|---|---|---|---|---|
| (a) Parkinson’s disease | ||||||
| [97] | compare fecal and plasma levels of different SCFA subtypes in patients with PD and healthy controls | GC-MS and LC-MS/MS | feces and plasma | total fecal DNA | 96 PD patients and 85 controls | reduced fecal SCFAs and increased plasma SCFAs observed in patients with PD and correlated to the abundance of pro-inflammatory Clostridiales and Ruminococcus species and clinical severity of PD |
| [131] | characterize metabolite and lipoprotein profiles of newly diagnosed de novo drug-naïve PD patients | NMR | serum | - | 329 subjects including de novo drug-naïve PD patients, PD patients with advanced disease status, and healthy controls | metabolic differences between newly diagnosed de novo drug-naïve PD patients and healthy controls, which were more pronounced in male patients (particularly acetone and cholesterol); metabolic differences between de novo drug-naïve PD patients and advanced PD patients; metabolic differences between advanced PD patients and healthy controls |
| [132] | clinical relevance of microbiome and metabolome alterations in PD | NMR and LC-MS | feces | 16S-sequencing of fecal microbiota | 104 PD patients, 96 control subjects | increased abundance of Bacteroides fragilis, Lactobacillus acidophilus, unclassified Megasphaera and unclassified Gammaproteobacteria; greatest effect size for NMR-based metabolome; SCFAs, lipids, TMAO, ubiquinone and salicylate concentrations vary in PD patients; low SCFA levels correlate with poorer cognition and low BMI; low butyrate levels correlate with worse postural instability-gait disorder scores |
| [133] | Integration of longitudinal metabolomics data with constraint-based modeling of gut microbial communities | LC-MS | EDTA plasma | 16S-sequencing of fecal microbiota | 30 PD patients, 30 control subjects | combined omics-methods suggest correlation between sulfur co-metabolism and PD severity; dopaminergic medication affects lipidome; levels of taurine conjugated bile acids correlate with severity of motor symptoms; A. muciniphila and B. wadsworthia are predicted to alter sulfur metabolism |
| [134] | alterations in gut microbiota might be accompanied by altered concentrations of amino acids, leading to PD | LC-MS, GC-MS | feces | 16S-sequencing of fecal microbiota | PD patients and healthy controls | greater abundance of Alistipes, Rikenellaceae_RC9_gut_group, Bifidobacterium, Parabacteroides, while Faecalibacterium was decreased in PD feces specimens; fecal BCAAs and aromatic amino acids concentrations were significantly reduced in PD patients compared to controls |
| [135] | finding a cause-effect relationship between intestinal dysbiosis and PD | GC-MS | feces | 16S-sequencing of fecal microbiota | 64 PD patients, 51 control subjects | alteration of fecal metabolome regarding lipids, amino acids, vitamins, cadaverine, ethanolamine and hydroxy propionic acid; severe metabolomic alterations correlate with abundance of bacteria from the Lachnospiraceae family |
| [136] | identification of early biomarkers for PD | FT-ICR-MS | CSF | - | 31 patients, 95 control subjects | 243 metabolites were found to be affected in PD; 15 metabolites are predicted to be the main biological contributors; network analysis showed connection to Krebs-Cycle, possibly displaying mitochondrial dysfunction |
| [137] | Integrative metabolic modeling to identify roles of gut microbiota in host metabolism contributing to PD pathophysiology | LC-MS | serum | - | 31 early-stage L-DOPA-naïve PD male individuals, 28 matched controls | functional analysis reveals increased microbial capability to degrade mucin and host glycans in PD; personalized community-level metabolic modeling reveals microbial contribution to folate deficiency and hyperhomocysteinemia observed in patients with PD; decreased capacity to produce SCFAs by Bacteroides and Prevotella species observed |
| [111] | untargeted metabolomics approach to investigate metabolic changes associated with PD | LC-MS | plasma | - | 223 PD, 169 healthy controls, 68 neurological disease controls | significant reductions in fatty acids and caffeine metabolites, elevation of bile acids; metabolite PD panel with 4 biomarker candidates: FFA10:0, FFA12:0, indolelactic acid and phenylacetyl-glutamine |
| [78] | investigating sebum as potential diagnostic tool for PD; identify PD biomarkers in sebum | LC-MS | sebum | - | 80 drug-naïve PD, 138 medicated PD, 56 healthy controls | 10 metabolites present in samples of drug-naïve and treated PD patients associated with carnitine pathway and sphingolipid metabolism pathway |
| [71] | compare metabolomic profiles of whole blood obtained from treated PD patients, de-novo PD patients and controls, and study perturbations correlated with disease duration, disease stage and motor impairment | GC-MS | blood | - | 16 de-novo PD, 84 treated PD, 42 healthy controls | most prominent differences in butanoic acid and glutamic acid |
| [138] | identify distinct volatiles-associated signature of PD | GC-MS | sebum | - | 43 PD, 21 healthy controls | altered levels of perillic aldehyde, hippuric acid, eicosane, and octadecanal in PD specimens |
| [117] | characterization of metabolic patterns in PD plasma specimens | LC-MS | plasma | - | 28 PD, 18 healthy controls | 17 significantly altered metabolites associated with glycerol phospholipid metabolism, carnitine metabolism, bile acid biosynthesis and tyrosine biosynthesis |
| [72] | identify candidate metabolic biomarker(s) and pathomechanistic pathway(s) of PD | LC-MS | plasma | - | discovery cohort including 82 PD, 82 healthy controls; validation cohort including 118 PD, 22 Huntington’s Disease, 47 healthy controls | dopamine and putrescine/ornithine ratio upregulated in PD, octadecadienylcarnitine C18:2, asymmetric dimethylarginine, tryptophan, and kynurenine downregulated in PD |
| [113] | urinary metabolic profiling of idiopathic PD patients at three stages and normal control subjects | GC-MS, LC-MS | urine | - | 92 PD, 65 healthy controls | 18 differential metabolites associated with BCAA metabolism and steroid hormone biosynthesis |
| [93] | identify associations between intestinal microbiome, intestinal digestive function, and influence of systemic microbial metabolites on PD | LC-MS | feces, serum | - | 197 PD, 103 healthy controls | different intestinal microbiome composition in PD patients, with increased abundance of Akkermansia and Bifidobacterium and decreased abundance of Faecalibacterium and Lachnospiraceae; intestinal microbiome in PD patients had reduced capacity of carbohydrate fermentation and butyrate synthesis and showed increased proteolytic fermentation |
| (b) Alzheimer’s disease | ||||||
| [121] | investigate role of bile acid composition in AD | LC-MS | serum | - | 1464 subjects (370 cognitively normal, 284 early MCI, 505 late MCI, 305 AD patients) | in AD, cholic acid levels as a primary bile acid are significantly decreased and levels of the secondary bile acid deoxycholic acid are increased; levels of deoxycholic acid conjugated with taurine and glycine are also increased |
| [127] | investigating the metabolic output of gut microbiome dysbiosis in AD | LC-MS | feces | 16S-sequencing of fecal microbiota | 21 patients, 44 control subjects | in AD, 15 gut bacterial genera appear to be altered, 7 of those genera are associated with different series of metabolites; combination of bacterial genera Faecalibacterium and Pseudomonas, combined with 4 metabolites was able to discriminate between AD patients and controls |
| [120] | identify the relationship between microbiome-associated metabolites and dementia | LC, ion chromatography, GC-MS | feces | classification of fecal bacteria by T-RFLP | 82 control subjects, 25 patients | fecal ammonium and lactic acid were identified as markers for dementia |
| [128] | identify key microbial taxa that participate in the gut-brain axis | CE-FTMS | mouse brain | 16S-sequencing of fecal microbiota | 21 control subjects, 15 patients with MCI, 7 AD patients | Faecalibacterium prausnitzii was identified to participate in the gut-brain axis as its abundance decreased in patients with MCI, correlating with cognitive scores; oral treatment of GMO mice with Aβ-induced cognitive impairment with F. prausnitzii improved cognitive impairment and altered metabolic profile in brain tissue specimens |
| [123] | connecting bile acid profiles with standard biomarkers of AD progression | LC-MS | serum | imaging of brain atrophy with MRI, assessment of β-amyloid and tau deposits with PET | 305 control subjects, 98 subjective memory complaint patients, 284 early MCI patients, 505 late MCI patients, 305 AD patients | different bile acid profiles associated with Aβ1-42 in CSF and with p-Tau181 in CSF |
| [122] | metabolomic profiling of bile acids in serum and brain of AD patients | LC-MS | serum and brain tissue | metabolomic analysis of serum and brain samples were also performed in mice | 10 AD patients, 10 healthy subjects | serum levels of cholic acid in AD patients decreased; concentration of taurocholic acid reduced in brain tissue |
| [119] | identification of novel biomarkers for improved risk prediction in AD | LC-MS | serum and brain tissue (post mortem) | follow-up analysis of serum metabolome after 4.5 years | serum: 97 patients with MCI, 433 healthy subjects; brain (post mortem): 28 AD patients, 32 patients with MCI, 52 healthy subjects | peripheral and systemic metabolome appears to have minor overlaps; three serum acetylcarnitines identified as negative predictors for incident AD and cognitive decline; another 13 metabolites were found as predictors for longitudinal change in cognition |
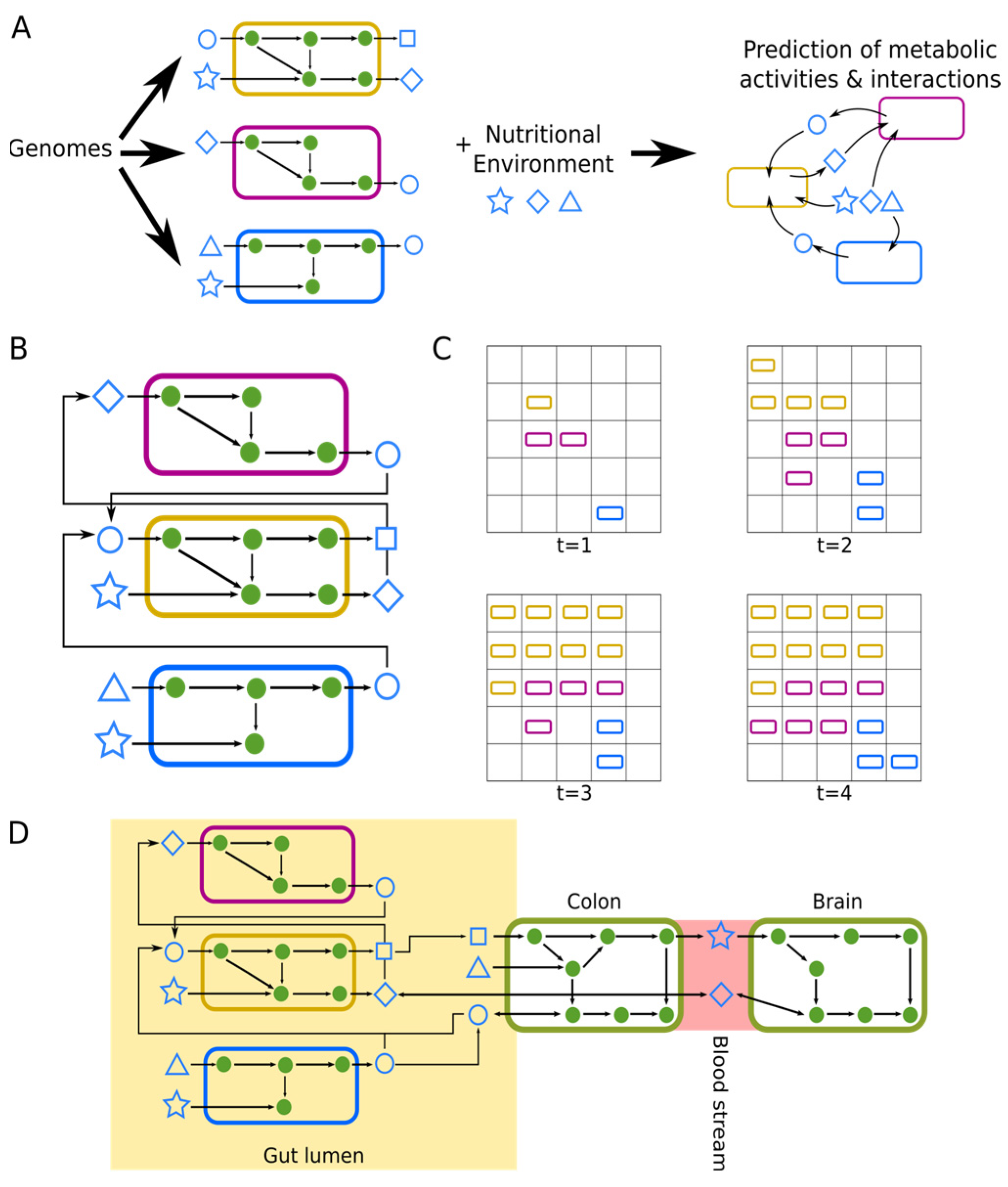
4. Metabolic Modeling of the Gut–Brain-Axis
4.1. Constraint-Based Metabolic Modelling
4.2. Microbial Community Modeling Yields Insights into Neurodegenerative Disease-Associated Changes in Microbiome Metabolic Activity
5. The Microbiome as Therapeutic Target in Neurodegenerative Diseases
5.1. Changes in Lifestyle: Diet and Exercise
5.2. Prebiotics and Probiotics
5.3. Antibiotics
5.4. Fecal Transplants
5.5. Medication
6. Summary and Future Research Perspectives
Funding
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| BCAA | branched chain amino acid |
| BMI | body-mass-index |
| CE-FTMS | capillary electrophoresis Fourier transform mass spectrometry |
| CSF | cerebrospinal fluid |
| FT-ICR-MS | Fourier transform ion cyclotron resonance mass spectrometry |
| GC-MS | gas chromatography mass spectrometry |
| LC-MS | liquid chromatography mass spectrometry |
| LC-MS/MS | liquid chromatography tandem mass spectrometry |
| MCI | mild cognitive impairment |
| MRI | magnetic resonance imaging |
| NMR | nuclear magnetic resonance |
| PD | Parkinson’s disease |
| PET | positron emission tomography |
| SCFA | short-chain fatty acid |
| TMAO | trimethylamine-N-oxide |
References
- Kivipelto, M.; Mangialasche, F.; Ngandu, T. Lifestyle Interventions to Prevent Cognitive Impairment, Dementia and Alzheimer Disease. Nat. Rev. Neurol. 2018, 14, 653–666. [Google Scholar] [CrossRef] [PubMed]
- Ascherio, A.; Schwarzschild, M.A. The Epidemiology of Parkinson’s Disease: Risk Factors and Prevention. Lancet Neurol. 2016, 15, 1257–1272. [Google Scholar] [CrossRef] [PubMed]
- Dorsey, E.R.; Sherer, T.; Okun, M.S.; Bloem, B.R. The Emerging Evidence of the Parkinson Pandemic. J. Park. Dis. 2018, 8, S3–S8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- GBD 2019 Dementia Forecasting Collaborators. Estimation of the Global Prevalence of Dementia in 2019 and Forecasted Prevalence in 2050: An Analysis for the Global Burden of Disease Study 2019. Lancet Public Health 2022, 7, e105–e125. [Google Scholar] [CrossRef] [PubMed]
- Berg, D.; Postuma, R.B.; Bloem, B.; Chan, P.; Dubois, B.; Gasser, T.; Goetz, C.G.; Halliday, G.M.; Hardy, J.; Lang, A.E.; et al. Time to Redefine PD? Introductory Statement of the MDS Task Force on the Definition of Parkinson’s Disease. Mov. Disord. 2014, 29, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.-E.; Lang, A.E. Parkinson Disease. Nat Rev. Dis. Primers 2017, 3, 17013. [Google Scholar] [CrossRef]
- Postuma, R.B.; Berg, D.; Stern, M.; Poewe, W.; Warren Olanow, C.; Oertel, W.; Obeso, J.; Marek, K.; Litvan, I.; Lang, A.E.; et al. MDS Clinical Diagnostic Criteria for Parkinson’s Disease. Mov. Disord. 2015, 30, 1591–1601. [Google Scholar] [CrossRef]
- Schrag, A.; Horsfall, L.; Walters, K.; Noyce, A.; Petersen, I. Prediagnostic Presentations of Parkinson’s Disease in Primary Care: A Case-Control Study. Lancet Neurol. 2015, 14, 57–64. [Google Scholar] [CrossRef] [Green Version]
- Fereshtehnejad, S.-M.; Yao, C.; Pelletier, A.; Montplaisir, J.Y.; Gagnon, J.-F.; Postuma, R.B. Evolution of Prodromal Parkinson’s Disease and Dementia with Lewy Bodies: A Prospective Study. Brain 2019, 142, 2051–2067. [Google Scholar] [CrossRef]
- Greenland, J.C.; Williams-Gray, C.H.; Barker, R.A. The Clinical Heterogeneity of Parkinson’s Disease and Its Therapeutic Implications. Eur. J. Neurosci. 2019, 49, 328–338. [Google Scholar] [CrossRef]
- Berg, D.; Borghammer, P.; Fereshtehnejad, S.-M.; Heinzel, S.; Horsager, J.; Schaeffer, E.; Postuma, R.B. Prodromal Parkinson Disease Subtypes—Key to Understanding Heterogeneity. Nat. Rev. Neurol. 2021, 17, 349–361. [Google Scholar] [CrossRef]
- Fereshtehnejad, S.-M.; Romenets, S.R.; Anang, J.B.M.; Latreille, V.; Gagnon, J.-F.; Postuma, R.B. New Clinical Subtypes of Parkinson Disease and Their Longitudinal Progression: A Prospective Cohort Comparison with Other Phenotypes. JAMA Neurol. 2015, 72, 863–873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horsager, J.; Andersen, K.B.; Knudsen, K.; Skjærbæk, C.; Fedorova, T.D.; Okkels, N.; Schaeffer, E.; Bonkat, S.K.; Geday, J.; Otto, M.; et al. Brain-First versus Body-First Parkinson’s Disease: A Multimodal Imaging Case-Control Study. Brain 2020, 143, 3077–3088. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a Risk Factor for Neurodegenerative Disease. Nat. Rev. Neurol. 2019, 15, 565–581. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Behl, T.; Bungau, S.; Kumar, A.; Uddin, M.S.; Mehta, V.; Zengin, G.; Mathew, B.; Shah, M.A.; Arora, S. Dysregulation of the Gut-Brain Axis, Dysbiosis and Influence of Numerous Factors on Gut Microbiota Associated Parkinson’s Disease. Curr. Neuropharmacol. 2021, 19, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Hanseeuw, B.J.; Betensky, R.A.; Jacobs, H.I.L.; Schultz, A.P.; Sepulcre, J.; Becker, J.A.; Cosio, D.M.O.; Farrell, M.; Quiroz, Y.T.; Mormino, E.C.; et al. Association of Amyloid and Tau with Cognition in Preclinical Alzheimer Disease: A Longitudinal Study. JAMA Neurol. 2019, 76, 915–924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, J.M.; Holtzman, D.M. Alzheimer Disease: An Update on Pathobiology and Treatment Strategies. Cell 2019, 179, 312–339. [Google Scholar] [CrossRef]
- Jellinger, K.A. Recent Update on the Heterogeneity of the Alzheimer’s Disease Spectrum. J. Neural Transm. 2022, 129, 1–24. [Google Scholar] [CrossRef]
- Dong, A.; Toledo, J.B.; Honnorat, N.; Doshi, J.; Varol, E.; Sotiras, A.; Wolk, D.; Trojanowski, J.Q.; Davatzikos, C.; Alzheimer’s Disease Neuroimaging Initiative. Heterogeneity of Neuroanatomical Patterns in Prodromal Alzheimer’s Disease: Links to Cognition, Progression and Biomarkers. Brain 2017, 140, 735–747. [Google Scholar] [CrossRef] [Green Version]
- Young, A.L.; Oxtoby, N.P.; Daga, P.; Cash, D.M.; Fox, N.C.; Ourselin, S.; Schott, J.M.; Alexander, D.C.; Alzheimer’s Disease Neuroimaging Initiative. A Data-Driven Model of Biomarker Changes in Sporadic Alzheimer’s Disease. Brain 2014, 137, 2564–2577. [Google Scholar] [CrossRef]
- Yang, Z.; Nasrallah, I.M.; Shou, H.; Wen, J.; Doshi, J.; Habes, M.; Erus, G.; Abdulkadir, A.; Resnick, S.M.; Albert, M.S.; et al. A Deep Learning Framework Identifies Dimensional Representations of Alzheimer’s Disease from Brain Structure. Nat. Commun. 2021, 12, 7065. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jiang, X.; Wang, Y.; Kim, Y. Applied Machine Learning in Alzheimer’s Disease Research: Omics, Imaging, and Clinical Data. Emerg. Top. Life Sci. 2021, 5, 765–777. [Google Scholar] [CrossRef] [PubMed]
- Kwak, K.; Giovanello, K.S.; Bozoki, A.; Styner, M.; Dayan, E.; Alzheimer’s Disease Neuroimaging Initiative. Subtyping of Mild Cognitive Impairment Using a Deep Learning Model Based on Brain Atrophy Patterns. Cell Rep. Med. 2021, 2, 100467. [Google Scholar] [CrossRef] [PubMed]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The Gut-Brain Axis: Interactions between Enteric Microbiota, Central and Enteric Nervous Systems. Ann. Gastroenterol. Hepatol. 2015, 28, 203–209. [Google Scholar]
- Rosario, D.; Boren, J.; Uhlen, M.; Proctor, G.; Aarsland, D.; Mardinoglu, A.; Shoaie, S. Systems Biology Approaches to Understand the Host-Microbiome Interactions in Neurodegenerative Diseases. Front. Neurosci. 2020, 14, 716. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut Microbiota in Human Metabolic Health and Disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- Morais, L.H.; Schreiber, H.L., 4th; Mazmanian, S.K. The Gut Microbiota-Brain Axis in Behaviour and Brain Disorders. Nat. Rev. Microbiol. 2021, 19, 241–255. [Google Scholar] [CrossRef]
- Hoyles, L.; Snelling, T.; Umlai, U.-K.; Nicholson, J.K.; Carding, S.R.; Glen, R.C.; McArthur, S. Microbiome-Host Systems Interactions: Protective Effects of Propionate upon the Blood-Brain Barrier. Microbiome 2018, 6, 55. [Google Scholar] [CrossRef] [Green Version]
- Topping, D.L.; Clifton, P.M. Short-Chain Fatty Acids and Human Colonic Function: Roles of Resistant Starch and Nonstarch Polysaccharides. Physiol. Rev. 2001, 81, 1031–1064. [Google Scholar] [CrossRef] [Green Version]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [Green Version]
- Roediger, W.E. The Starved Colon—Diminished Mucosal Nutrition, Diminished Absorption, and Colitis. Dis. Colon Rectum 1990, 33, 858–862. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, C.A.; Knight, R. Global Patterns in Bacterial Diversity. Proc. Natl. Acad. Sci. USA 2007, 104, 11436–11440. [Google Scholar] [CrossRef] [Green Version]
- Tyson, G.W.; Chapman, J.; Hugenholtz, P.; Allen, E.E.; Ram, R.J.; Richardson, P.M.; Solovyev, V.V.; Rubin, E.M.; Rokhsar, D.S.; Banfield, J.F. Community Structure and Metabolism through Reconstruction of Microbial Genomes from the Environment. Nature 2004, 428, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Zhernakova, A.; Kurilshikov, A.; Bonder, M.J.; Tigchelaar, E.F.; Schirmer, M.; Vatanen, T.; Mujagic, Z.; Vila, A.V.; Falony, G.; Vieira-Silva, S.; et al. Population-Based Metagenomics Analysis Reveals Markers for Gut Microbiome Composition and Diversity. Science 2016, 352, 565–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasolli, E.; Asnicar, F.; Manara, S.; Zolfo, M.; Karcher, N.; Armanini, F.; Beghini, F.; Manghi, P.; Tett, A.; Ghensi, P.; et al. Extensive Unexplored Human Microbiome Diversity Revealed by over 150,000 Genomes from Metagenomes Spanning Age, Geography, and Lifestyle. Cell 2019, 176, 649–662.e20. [Google Scholar] [CrossRef] [Green Version]
- Olm, M.R.; Crits-Christoph, A.; Bouma-Gregson, K.; Firek, B.A.; Morowitz, M.J.; Banfield, J.F. inStrain Profiles Population Microdiversity from Metagenomic Data and Sensitively Detects Shared Microbial Strains. Nat. Biotechnol. 2021, 39, 727–736. [Google Scholar] [CrossRef]
- Poyet, M.; Groussin, M.; Gibbons, S.M.; Avila-Pacheco, J.; Jiang, X.; Kearney, S.M.; Perrotta, A.R.; Berdy, B.; Zhao, S.; Lieberman, T.D.; et al. A Library of Human Gut Bacterial Isolates Paired with Longitudinal Multiomics Data Enables Mechanistic Microbiome Research. Nat. Med. 2019, 25, 1442–1452. [Google Scholar] [CrossRef]
- Almeida, A.; Nayfach, S.; Boland, M.; Strozzi, F.; Beracochea, M.; Shi, Z.J.; Pollard, K.S.; Sakharova, E.; Parks, D.H.; Hugenholtz, P.; et al. A Unified Catalog of 204,938 Reference Genomes from the Human Gut Microbiome. Nat. Biotechnol. 2021, 39, 105–114. [Google Scholar] [CrossRef]
- Gligorijević, V.; Renfrew, P.D.; Kosciolek, T.; Leman, J.K.; Berenberg, D.; Vatanen, T.; Chandler, C.; Taylor, B.C.; Fisk, I.M.; Vlamakis, H.; et al. Structure-Based Protein Function Prediction Using Graph Convolutional Networks. Nat. Commun. 2021, 12, 3168. [Google Scholar] [CrossRef]
- Gacesa, R.; Kurilshikov, A.; Vich Vila, A.; Sinha, T.; Klaassen, M.A.Y.; Bolte, L.A.; Andreu-Sánchez, S.; Chen, L.; Collij, V.; Hu, S.; et al. Environmental Factors Shaping the Gut Microbiome in a Dutch Population. Nature 2022, 604, 732–739. [Google Scholar] [CrossRef]
- Morton, J.T.; Aksenov, A.A.; Nothias, L.F.; Foulds, J.R.; Quinn, R.A.; Badri, M.H.; Swenson, T.L.; Van Goethem, M.W.; Northen, T.R.; Vazquez-Baeza, Y.; et al. Learning Representations of Microbe-Metabolite Interactions. Nat. Methods 2019, 16, 1306–1314. [Google Scholar] [CrossRef] [PubMed]
- Duvallet, C.; Gibbons, S.M.; Gurry, T.; Irizarry, R.A.; Alm, E.J. Meta-Analysis of Gut Microbiome Studies Identifies Disease-Specific and Shared Responses. Nat. Commun. 2017, 8, 1784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDonald, D.; Hyde, E.; Debelius, J.W.; Morton, J.T.; Gonzalez, A.; Ackermann, G.; Aksenov, A.A.; Behsaz, B.; Brennan, C.; Chen, Y.; et al. American Gut: An Open Platform for Citizen Science Microbiome Research. mSystems 2018, 3, e00031-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Y.; Wu, W.; Zheng, H.-M.; Li, P.; McDonald, D.; Sheng, H.-F.; Chen, M.-X.; Chen, Z.-H.; Ji, G.-Y.; Zheng, Z.-D.-X.; et al. Regional Variation Limits Applications of Healthy Gut Microbiome Reference Ranges and Disease Models. Nat. Med. 2018, 24, 1532–1535. [Google Scholar] [CrossRef]
- Groussin, M.; Poyet, M.; Sistiaga, A.; Kearney, S.M.; Moniz, K.; Noel, M.; Hooker, J.; Gibbons, S.M.; Segurel, L.; Froment, A.; et al. Elevated Rates of Horizontal Gene Transfer in the Industrialized Human Microbiome. Cell 2021, 184, 2053–2067.e18. [Google Scholar] [CrossRef]
- Costea, P.I.; Zeller, G.; Sunagawa, S.; Pelletier, E.; Alberti, A.; Levenez, F.; Tramontano, M.; Driessen, M.; Hercog, R.; Jung, F.-E.; et al. Towards Standards for Human Fecal Sample Processing in Metagenomic Studies. Nat. Biotechnol. 2017, 35, 1069–1076. [Google Scholar] [CrossRef] [Green Version]
- Sinha, R.; The Microbiome Quality Control Project Consortium; Abu-Ali, G.; Vogtmann, E.; Fodor, A.A.; Ren, B.; Amir, A.; Schwager, E.; Crabtree, J.; Ma, S.; et al. Assessment of Variation in Microbial Community Amplicon Sequencing by the Microbiome Quality Control (MBQC) Project Consortium. Nat. Biotechnol. 2017, 35, 1077–1086. [Google Scholar] [CrossRef] [Green Version]
- Thorsen, J.; Brejnrod, A.; Mortensen, M.; Rasmussen, M.A.; Stokholm, J.; Al-Soud, W.A.; Sørensen, S.; Bisgaard, H.; Waage, J. Large-Scale Benchmarking Reveals False Discoveries and Count Transformation Sensitivity in 16S rRNA Gene Amplicon Data Analysis Methods Used in Microbiome Studies. Microbiome 2016, 4, 62. [Google Scholar] [CrossRef] [Green Version]
- Mirzayi, C.; Renson, A.; Genomic Standards Consortium; Massive Analysis and Quality Control Society; Zohra, F.; Elsafoury, S.; Geistlinger, L.; Kasselman, L.J.; Eckenrode, K.; van de Wijgert, J.; et al. Reporting Guidelines for Human Microbiome Research: The STORMS Checklist. Nat. Med. 2021, 27, 1885–1892. [Google Scholar] [CrossRef]
- Seo, D.-O.; Holtzman, D.M. Gut Microbiota: From the Forgotten Organ to a Potential Key Player in the Pathology of Alzheimer’s Disease. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 1232–1241. [Google Scholar] [CrossRef] [Green Version]
- Friedland, R.P.; Chapman, M.R. The Role of Microbial Amyloid in Neurodegeneration. PLoS Pathog. 2017, 13, e1006654. [Google Scholar] [CrossRef] [PubMed]
- Glassner, K.L.; Abraham, B.P.; Quigley, E.M.M. The Microbiome and Inflammatory Bowel Disease. J. Allergy Clin. Immunol. 2020, 145, 16–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romano, S.; Savva, G.M.; Bedarf, J.R.; Charles, I.G.; Hildebrand, F.; Narbad, A. Meta-Analysis of the Parkinson’s Disease Gut Microbiome Suggests Alterations Linked to Intestinal Inflammation. NPJ Park. Dis. 2021, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Li, G.; Huang, P.; Liu, Z.; Zhao, B. The Gut Microbiota and Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 58, 1–15. [Google Scholar] [CrossRef]
- Smith, P.; Willemsen, D.; Popkes, M.; Metge, F.; Gandiwa, E.; Reichard, M.; Valenzano, D.R. Regulation of Life Span by the Gut Microbiota in the Short-Lived African Turquoise Killifish. eLife 2017, 6, e27014. [Google Scholar] [CrossRef]
- Walter, J.; Armet, A.M.; Finlay, B.B.; Shanahan, F. Establishing or Exaggerating Causality for the Gut Microbiome: Lessons from Human Microbiota-Associated Rodents. Cell 2020, 180, 221–232. [Google Scholar] [CrossRef]
- Zacharias, H.U.; Altenbuchinger, M.; Gronwald, W. Statistical Analysis of NMR Metabolic Fingerprints: Established Methods and Recent Advances. Metabolites 2018, 8, 47. [Google Scholar] [CrossRef] [Green Version]
- Bauermeister, A.; Mannochio-Russo, H.; Costa-Lotufo, L.V.; Jarmusch, A.K.; Dorrestein, P.C. Mass Spectrometry-Based Metabolomics in Microbiome Investigations. Nat. Rev. Microbiol. 2022, 20, 143–160. [Google Scholar] [CrossRef]
- Keppler, E.A.H.; Jenkins, C.L.; Davis, T.J.; Bean, H.D. Advances in the Application of Comprehensive Two-Dimensional Gas Chromatography in Metabolomics. Trends Anal. Chem. 2018, 109, 275–286. [Google Scholar] [CrossRef]
- Papadimitropoulos, M.-E.P.; Vasilopoulou, C.G.; Maga-Nteve, C.; Klapa, M.I. Untargeted GC-MS Metabolomics. Methods Mol. Biol. 2018, 1738, 133–147. [Google Scholar]
- Herderich, M.; Richling, E.; Roscher, R.; Schneider, C.; Schwab, W.; Humpf, H.-U.; Schreier, P. Application of Atmospheric Pressure Ionization HPLC-MS-MS for the Analysis of Natural Products. Chromatographia 1997, 45, 127–132. [Google Scholar] [CrossRef]
- Want, E.J.; Nordström, A.; Morita, H.; Siuzdak, G. From Exogenous to Endogenous: The Inevitable Imprint of Mass Spectrometry in Metabolomics. J. Proteome Res. 2007, 6, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Pasikanti, K.K.; Ho, P.C.; Chan, E.C.Y. Gas Chromatography/mass Spectrometry in Metabolic Profiling of Biological Fluids. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2008, 871, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, B.; Holmes, E.; Heude, C.; Tolson, R.F.; Harvey, N.; Lodge, S.L.; Chetwynd, A.J.; Cannet, C.; Fang, F.; Pearce, J.T.M.; et al. Quantitative Lipoprotein Subclass and Low Molecular Weight Metabolite Analysis in Human Serum and Plasma by H NMR Spectroscopy in a Multilaboratory Trial. Anal. Chem. 2018, 90, 11962–11971. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Huhman, D.V.; Sumner, L.W. Mass Spectrometry Strategies in Metabolomics. J. Biol. Chem. 2011, 286, 25435–25442. [Google Scholar] [CrossRef] [Green Version]
- Erve, J.C.L.; Demaio, W.; Talaat, R.E. Rapid Metabolite Identification with Sub Parts-per-Million Mass Accuracy from Biological Matrices by Direct Infusion Nanoelectrospray Ionization after Clean-up on a ZipTip and LTQ/Orbitrap Mass Spectrometry. Rapid Commun. Mass Spectrom. 2008, 22, 3015–3026. [Google Scholar] [CrossRef]
- Beckonert, O.; Keun, H.C.; Ebbels, T.M.D.; Bundy, J.; Holmes, E.; Lindon, J.C.; Nicholson, J.K. Metabolic Profiling, Metabolomic and Metabonomic Procedures for NMR Spectroscopy of Urine, Plasma, Serum and Tissue Extracts. Nat. Protoc. 2007, 2, 2692–2703. [Google Scholar] [CrossRef]
- Zacharias, H.; Hochrein, J.; Klein, M.; Samol, C.; Oefner, P.; Gronwald, W. Current Experimental, Bioinformatic and Statistical Methods Used in NMR Based Metabolomics. Curr. Metab. 2013, 1, 253–268. [Google Scholar] [CrossRef]
- Schultheiss, U.T.; Kosch, R.; Kotsis, F.; Altenbuchinger, M.; Zacharias, H.U. Chronic Kidney Disease Cohort Studies: A Guide to Metabolome Analyses. Metabolites 2021, 11, 460. [Google Scholar] [CrossRef]
- Trezzi, J.-P.; Galozzi, S.; Jaeger, C.; Barkovits, K.; Brockmann, K.; Maetzler, W.; Berg, D.; Marcus, K.; Betsou, F.; Hiller, K.; et al. Distinct Metabolomic Signature in Cerebrospinal Fluid in Early Parkinson’s Disease. Mov. Disord. 2017, 32, 1401–1408. [Google Scholar] [CrossRef]
- Troisi, J.; Landolfi, A.; Vitale, C.; Longo, K.; Cozzolino, A.; Squillante, M.; Savanelli, M.C.; Barone, P.; Amboni, M. A Metabolomic Signature of Treated and Drug-Naïve Patients with Parkinson’s Disease: A Pilot Study. Metabolomics 2019, 15, 90. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.-H.; Cheng, M.-L.; Tang, H.-Y.; Huang, C.-Y.; Wu, Y.-R.; Chen, C.-M. Alternations of Metabolic Profile and Kynurenine Metabolism in the Plasma of Parkinson’s Disease. Mol. Neurobiol. 2018, 55, 6319–6328. [Google Scholar] [CrossRef] [PubMed]
- Michell, A.W.; Mosedale, D.; Grainger, D.J.; Barker, R.A. Metabolomic Analysis of Urine and Serum in Parkinson’s Disease. Metabolomics 2008, 4, 191–201. [Google Scholar] [CrossRef]
- Li, X.; Fan, X.; Yang, H.; Liu, Y. Review of Metabolomics-Based Biomarker Research for Parkinson’s Disease. Mol. Neurobiol. 2022, 59, 1041–1057. [Google Scholar] [CrossRef] [PubMed]
- Unger, M.M.; Spiegel, J.; Dillmann, K.-U.; Grundmann, D.; Philippeit, H.; Bürmann, J.; Faßbender, K.; Schwiertz, A.; Schäfer, K.-H. Short Chain Fatty Acids and Gut Microbiota Differ between Patients with Parkinson’s Disease and Age-Matched Controls. Park. Relat. Disord. 2016, 32, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Le, W. Recent Advances and Perspectives of Metabolomics-Based Investigations in Parkinson’s Disease. Mol. Neurodegener. 2019, 14, 3. [Google Scholar] [CrossRef] [Green Version]
- Kumari, S.; Goyal, V.; Kumaran, S.S.; Dwivedi, S.N.; Srivastava, A.; Jagannathan, N.R. Quantitative Metabolomics of Saliva Using Proton NMR Spectroscopy in Patients with Parkinson’s Disease and Healthy Controls. Neurol. Sci. 2020, 41, 1201–1210. [Google Scholar] [CrossRef]
- Sinclair, E.; Trivedi, D.K.; Sarkar, D.; Walton-Doyle, C.; Milne, J.; Kunath, T.; Rijs, A.M.; de Bie, R.M.A.; Goodacre, R.; Silverdale, M.; et al. Metabolomics of Sebum Reveals Lipid Dysregulation in Parkinson’s Disease. Nat. Commun. 2021, 12, 1592. [Google Scholar] [CrossRef]
- Bouatra, S.; Aziat, F.; Mandal, R.; Guo, A.C.; Wilson, M.R.; Knox, C.; Bjorndahl, T.C.; Krishnamurthy, R.; Saleem, F.; Liu, P.; et al. The Human Urine Metabolome. PLoS ONE 2013, 8, e73076. [Google Scholar] [CrossRef] [Green Version]
- Bogdanov, M.; Matson, W.R.; Wang, L.; Matson, T.; Saunders-Pullman, R.; Bressman, S.S.; Flint Beal, M. Metabolomic Profiling to Develop Blood Biomarkers for Parkinson’s Disease. Brain 2008, 131, 389–396. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Ni, Y.; Su, M.; Li, H.; Dong, F.; Chen, W.; Wei, R.; Zhang, L.; Guiraud, S.P.; Martin, F.-P.; et al. High Throughput and Quantitative Measurement of Microbial Metabolome by Gas Chromatography/Mass Spectrometry Using Automated Alkyl Chloroformate Derivatization. Anal. Chem. 2017, 89, 5565–5577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, A.H.; Lim, S.Y.; Lang, A.E. The Microbiome-Gut-Brain Axis in Parkinson Disease—From Basic Research to the Clinic. Nat. Rev. Neurol. 2022, 18, 476–495. [Google Scholar] [CrossRef] [PubMed]
- Sampson, T.R.; Debelius, J.W.; Thron, T.; Janssen, S.; Shastri, G.G.; Ilhan, Z.E.; Challis, C.; Schretter, C.E.; Rocha, S.; Gradinaru, V.; et al. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell 2016, 167, 1469–1480.e12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plassais, J.; Gbikpi-Benissan, G.; Figarol, M.; Scheperjans, F.; Gorochov, G.; Derkinderen, P.; Cervino, A.C.L. Gut Microbiome Alpha-Diversity Is Not a Marker of Parkinson’s Disease and Multiple Sclerosis. Brain Commun. 2021, 3, fcab113. [Google Scholar] [CrossRef]
- Aggarwal, V.; Sunder, S.; Verma, S.R. Disease-Associated Dysbiosis and Potential Therapeutic Role of Akkermansia Muciniphila, a Mucus Degrading Bacteria of Gut Microbiome. Folia Microbiol. 2022, 67, 811–824. [Google Scholar] [CrossRef]
- Toh, T.S.; Chong, C.W.; Lim, S.-Y.; Bowman, J.; Cirstea, M.; Lin, C.-H.; Chen, C.-C.; Appel-Cresswell, S.; Finlay, B.B.; Tan, A.H. Gut Microbiome in Parkinson’s Disease: New Insights from Meta-Analysis. Park. Relat. Disord. 2022, 94, 1–9. [Google Scholar] [CrossRef]
- Heintz-Buschart, A.; Pandey, U.; Wicke, T.; Sixel-Döring, F.; Janzen, A.; Sittig-Wiegand, E.; Trenkwalder, C.; Oertel, W.H.; Mollenhauer, B.; Wilmes, P. The Nasal and Gut Microbiome in Parkinson’s Disease and Idiopathic Rapid Eye Movement Sleep Behavior Disorder. Mov. Disord. 2018, 33, 88–98. [Google Scholar] [CrossRef] [Green Version]
- Heinzel, S.; Aho, V.T.E.; Suenkel, U.; von Thaler, A.-K.; Schulte, C.; Deuschle, C.; Paulin, L.; Hantunen, S.; Brockmann, K.; Eschweiler, G.W.; et al. Gut Microbiome Signatures of Risk and Prodromal Markers of Parkinson Disease. Ann. Neurol. 2021, 90, E1–E12. [Google Scholar] [CrossRef]
- Aho, V.T.E.; Pereira, P.A.B.; Voutilainen, S.; Paulin, L.; Pekkonen, E.; Auvinen, P.; Scheperjans, F. Gut Microbiota in Parkinson’s Disease: Temporal Stability and Relations to Disease Progression. EBioMedicine 2019, 44, 691–707. [Google Scholar] [CrossRef] [Green Version]
- Lubomski, M.; Xu, X.; Holmes, A.J.; Muller, S.; Yang, J.Y.H.; Davis, R.L.; Sue, C.M. The Gut Microbiome in Parkinson’s Disease: A Longitudinal Study of the Impacts on Disease Progression and the Use of Device-Assisted Therapies. Front. Aging Neurosci. 2022, 14, 875261. [Google Scholar] [CrossRef]
- Qian, Y.; Yang, X.; Xu, S.; Huang, P.; Li, B.; Du, J.; He, Y.; Su, B.; Xu, L.-M.; Wang, L.; et al. Gut Metagenomics-Derived Genes as Potential Biomarkers of Parkinson’s Disease. Brain 2020, 143, 2474–2489. [Google Scholar] [CrossRef]
- Mao, L.; Zhang, Y.; Tian, J.; Sang, M.; Zhang, G.; Zhou, Y.; Wang, P. Cross-Sectional Study on the Gut Microbiome of Parkinson’s Disease Patients in Central China. Front. Microbiol. 2021, 12, 728479. [Google Scholar] [CrossRef]
- Cirstea, M.S.; Yu, A.C.; Golz, E.; Sundvick, K.; Kliger, D.; Radisavljevic, N.; Foulger, L.H.; Mackenzie, M.; Huan, T.; Finlay, B.B.; et al. Microbiota Composition and Metabolism Are Associated with Gut Function in Parkinson’s Disease. Mov. Disord. 2020, 35, 1208–1217. [Google Scholar] [CrossRef]
- Aho, V.T.E.; Houser, M.C.; Pereira, P.A.B.; Chang, J.; Rudi, K.; Paulin, L.; Hertzberg, V.; Auvinen, P.; Tansey, M.G.; Scheperjans, F. Relationships of Gut Microbiota, Short-Chain Fatty Acids, Inflammation, and the Gut Barrier in Parkinson’s Disease. Mol. Neurodegener. 2021, 16, 6. [Google Scholar] [CrossRef]
- Shin, C.; Lim, Y.; Lim, H.; Ahn, T.-B. Plasma Short-Chain Fatty Acids in Patients with Parkinson’s Disease. Mov. Disord. 2020, 35, 1021–1027. [Google Scholar] [CrossRef]
- He, X.; Qian, Y.; Xu, S.; Zhang, Y.; Mo, C.; Guo, W.; Yang, X.; Xiao, Q. Plasma Short-Chain Fatty Acids Differences in Multiple System Atrophy from Parkinson’s Disease. J. Park. Dis. 2021, 11, 1167–1176. [Google Scholar] [CrossRef]
- Chen, S.-J.; Chen, C.-C.; Liao, H.-Y.; Lin, Y.-T.; Wu, Y.-W.; Liou, J.-M.; Wu, M.-S.; Kuo, C.-H.; Lin, C.-H. Association of Fecal and Plasma Levels of Short-Chain Fatty Acids with Gut Microbiota and Clinical Severity in Patients with Parkinson Disease. Neurology 2022, 98, e848–e858. [Google Scholar] [CrossRef] [PubMed]
- Bairamian, D.; Sha, S.; Rolhion, N.; Sokol, H.; Dorothée, G.; Lemere, C.A.; Krantic, S. Microbiota in Neuroinflammation and Synaptic Dysfunction: A Focus on Alzheimer’s Disease. Mol. Neurodegener. 2022, 17, 1–23. [Google Scholar] [CrossRef]
- Mariat, D.; Firmesse, O.; Levenez, F.; Guimarăes, V.D.; Sokol, H.; Doré, J.; Corthier, G.; Furet, J.-P. The Firmicutes/Bacteroidetes Ratio of the Human Microbiota Changes with Age. BMC Microbiol. 2009, 9, 123. [Google Scholar] [CrossRef] [PubMed]
- Vogt, N.M.; Kerby, R.L.; Dill-McFarland, K.A.; Harding, S.J.; Merluzzi, A.P.; Johnson, S.C.; Carlsson, C.M.; Asthana, S.; Zetterberg, H.; Blennow, K.; et al. Gut Microbiome Alterations in Alzheimer’s Disease. Sci. Rep. 2017, 7, 13537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shukla, P.K.; Delotterie, D.F.; Xiao, J.; Pierre, J.F.; Rao, R.; McDonald, M.P.; Khan, M.M. Alterations in the Gut-Microbial-Inflammasome-Brain Axis in a Mouse Model of Alzheimer’s Disease. Cells 2021, 10, 779. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.-L.; Li, W.-W.; Wang, J.; Xu, Y.-L.; Sun, H.-L.; Tian, D.-Y.; Wang, Y.-J.; Yao, X.-Q. Gut Microbiota Alteration and Its Time Course in a Tauopathy Mouse Model. J. Alzheimer’s Dis. 2019, 70, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Erny, D.; Hrabě de Angelis, A.L.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T.; et al. Host Microbiota Constantly Control Maturation and Function of Microglia in the CNS. Nat. Neurosci. 2015, 18, 965–977. [Google Scholar] [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marizzoni, M.; Cattaneo, A.; Mirabelli, P.; Festari, C.; Lopizzo, N.; Nicolosi, V.; Mombelli, E.; Mazzelli, M.; Luongo, D.; Naviglio, D.; et al. Short-Chain Fatty Acids and Lipopolysaccharide as Mediators Between Gut Dysbiosis and Amyloid Pathology in Alzheimer’s Disease. J. Alzheimer’s Dis. 2020, 78, 683–697. [Google Scholar] [CrossRef] [PubMed]
- Cuervo-Zanatta, D.; Syeda, T.; Sánchez-Valle, V.; Irene-Fierro, M.; Torres-Aguilar, P.; Torres-Ramos, M.A.; Shibayama-Salas, M.; Silva-Olivares, A.; Noriega, L.G.; Torres, N.; et al. Dietary Fiber Modulates the Release of Gut Bacterial Products Preventing Cognitive Decline in an Alzheimer’s Mouse Model. Cell Mol. Neurobiol. 2022, 1–24. [Google Scholar] [CrossRef]
- Shi, Y.; Holtzman, D.M. Interplay between Innate Immunity and Alzheimer Disease: APOE and TREM2 in the Spotlight. Nat. Rev. Immunol. 2018, 18, 759–772. [Google Scholar] [CrossRef]
- Tran, T.T.T.; Corsini, S.; Kellingray, L.; Hegarty, C.; Le Gall, G.; Narbad, A.; Müller, M.; Tejera, N.; O’Toole, P.W.; Minihane, A.-M.; et al. Genotype Influences the Gut Microbiome Structure and Function in Humans and Mice: Relevance for Alzheimer’s Disease Pathophysiology. FASEB J. 2019, 33, 8221–8231. [Google Scholar] [CrossRef] [Green Version]
- Friedland, R.P.; McMillan, J.; Kurlawala, Z. What Are the Molecular Mechanisms by Which Functional Bacterial Amyloids Influence Amyloid Beta Deposition and Neuroinflammation in Neurodegenerative Disorders? Int. J. Mol. Sci. 2020, 21, 1652. [Google Scholar] [CrossRef] [Green Version]
- Holmqvist, S.; Chutna, O.; Bousset, L.; Aldrin-Kirk, P.; Li, W.; Björklund, T.; Wang, Z.-Y.; Roybon, L.; Melki, R.; Li, J.-Y. Direct Evidence of Parkinson Pathology Spread from the Gastrointestinal Tract to the Brain in Rats. Acta Neuropathol. 2014, 128, 805–820. [Google Scholar] [CrossRef] [Green Version]
- Shao, Y.; Li, T.; Liu, Z.; Wang, X.; Xu, X.; Li, S.; Xu, G.; Le, W. Comprehensive Metabolic Profiling of Parkinson’s Disease by Liquid Chromatography-Mass Spectrometry. Mol. Neurodegener. 2021, 16, 4. [Google Scholar] [CrossRef] [PubMed]
- Hatano, T.; Saiki, S.; Okuzumi, A.; Mohney, R.P.; Hattori, N. Identification of Novel Biomarkers for Parkinson’s Disease by Metabolomic Technologies. J. Neurol. Neurosurg. Psychiatry 2016, 87, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Luan, H.; Liu, L.-F.; Tang, Z.; Zhang, M.; Chua, K.-K.; Song, J.-X.; Mok, V.C.T.; Li, M.; Cai, Z. Comprehensive Urinary Metabolomic Profiling and Identification of Potential Noninvasive Marker for Idiopathic Parkinson’s Disease. Sci. Rep. 2015, 5, 13888. [Google Scholar] [CrossRef] [Green Version]
- Olsson, E.; Byberg, L.; Höijer, J.; Kilander, L.; Larsson, S.C. Milk and Fermented Milk Intake and Parkinson’s Disease: Cohort Study. Nutrients 2020, 12, 2763. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Ju, C.; Jiang, H.; Zhang, D. Dairy Foods Intake and Risk of Parkinson’s Disease: A Dose-Response Meta-Analysis of Prospective Cohort Studies. Eur. J. Epidemiol. 2014, 29, 613–619. [Google Scholar] [CrossRef]
- Tosukhowong, P.; Boonla, C.; Dissayabutra, T.; Kaewwilai, L.; Muensri, S.; Chotipanich, C.; Joutsa, J.; Rinne, J.; Bhidayasiri, R. Biochemical and Clinical Effects of Whey Protein Supplementation in Parkinson’s Disease: A Pilot Study. J. Neurol. Sci. 2016, 367, 162–170. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, C.; Zhao, N.; Li, W.; Yang, Z.; Liu, X.; Le, W.; Zhang, X. Potential Biomarkers of Parkinson’s Disease Revealed by Plasma Metabolic Profiling. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2018, 1081–1082, 101–108. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, S.; Tan, J.; Gao, Y.; Yan, H.; Liu, Y.; Yi, S.; Xiao, Z.; Wu, H. Tryptophan in the Diet Ameliorates Motor Deficits in a Rotenone-Induced Rat Parkinson’s Disease Model via Activating the Aromatic Hydrocarbon Receptor Pathway. Brain Behav. 2021, 11, e2226. [Google Scholar] [CrossRef]
- Huo, Z.; Yu, L.; Yang, J.; Zhu, Y.; Bennett, D.A.; Zhao, J. Brain and Blood Metabolome for Alzheimer’s Dementia: Findings from a Targeted Metabolomics Analysis. Neurobiol. Aging 2020, 86, 123–133. [Google Scholar] [CrossRef]
- Saji, N.; Murotani, K.; Hisada, T.; Kunihiro, T.; Tsuduki, T.; Sugimoto, T.; Kimura, A.; Niida, S.; Toba, K.; Sakurai, T. Relationship between Dementia and Gut Microbiome-Associated Metabolites: A Cross-Sectional Study in Japan. Sci. Rep. 2020, 10, 8088. [Google Scholar] [CrossRef]
- MahmoudianDehkordi, S.; Arnold, M.; Nho, K.; Ahmad, S.; Jia, W.; Xie, G.; Louie, G.; Kueider-Paisley, A.; Moseley, M.A.; Thompson, J.W.; et al. Altered Bile Acid Profile Associates with Cognitive Impairment in Alzheimer’s Disease-An Emerging Role for Gut Microbiome. Alzheimer’s Dement. 2019, 15, 76–92. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Elliott, C.T.; McGuinness, B.; Passmore, P.; Kehoe, P.G.; Hölscher, C.; McClean, P.L.; Graham, S.F.; Green, B.D. Metabolomic Profiling of Bile Acids in Clinical and Experimental Samples of Alzheimer’s Disease. Metabolites 2017, 7, 28. [Google Scholar] [CrossRef] [PubMed]
- Nho, K.; Kueider-Paisley, A.; MahmoudianDehkordi, S.; Arnold, M.; Risacher, S.L.; Louie, G.; Blach, C.; Baillie, R.; Han, X.; Kastenmüller, G.; et al. Altered Bile Acid Profile in Mild Cognitive Impairment and Alzheimer’s Disease: Relationship to Neuroimaging and CSF Biomarkers. Alzheimer’s Dement. 2019, 15, 232–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Copple, B.L.; Li, T. Pharmacology of Bile Acid Receptors: Evolution of Bile Acids from Simple Detergents to Complex Signaling Molecules. Pharmacol. Res. 2016, 104, 9–21. [Google Scholar] [CrossRef] [Green Version]
- Mertens, K.L.; Kalsbeek, A.; Soeters, M.R.; Eggink, H.M. Bile Acid Signaling Pathways from the Enterohepatic Circulation to the Central Nervous System. Front. Neurosci. 2017, 11, 617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quinn, M.; McMillin, M.; Galindo, C.; Frampton, G.; Pae, H.Y.; DeMorrow, S. Bile Acids Permeabilize the Blood Brain Barrier after Bile Duct Ligation in Rats via Rac1-Dependent Mechanisms. Dig. Liver Dis. 2014, 46, 527–534. [Google Scholar] [CrossRef] [Green Version]
- Xi, J.; Ding, D.; Zhu, H.; Wang, R.; Su, F.; Wu, W.; Xiao, Z.; Liang, X.; Zhao, Q.; Hong, Z.; et al. Disturbed Microbial Ecology in Alzheimer’s Disease: Evidence from the Gut Microbiota and Fecal Metabolome. BMC Microbiol. 2021, 21, 226. [Google Scholar] [CrossRef] [PubMed]
- Ueda, A.; Shinkai, S.; Shiroma, H.; Taniguchi, Y.; Tsuchida, S.; Kariya, T.; Kawahara, T.; Kobayashi, Y.; Kohda, N.; Ushida, K.; et al. Identification of Faecalibacterium prausnitzii Strains for Gut Microbiome-Based Intervention in Alzheimer’s-Type Dementia. Cell Rep. Med. 2021, 2, 100398. [Google Scholar] [CrossRef]
- Lenoir, M.; Martín, R.; Torres-Maravilla, E.; Chadi, S.; González-Dávila, P.; Sokol, H.; Langella, P.; Chain, F.; Bermúdez-Humarán, L.G. Butyrate Mediates Anti-Inflammatory Effects of Faecalibacterium Prausnitzii in Intestinal Epithelial Cells through Dact3. Gut Microbes 2020, 12, 1–16. [Google Scholar] [CrossRef]
- Jia, L.; Yang, J.; Zhu, M.; Pang, Y.; Wang, Q.; Wei, Q.; Li, Y.; Li, T.; Li, F.; Wang, Q.; et al. A Metabolite Panel That Differentiates Alzheimer’s Disease from Other Dementia Types. Alzheimer’s Dement. 2022, 18, 1345–1356. [Google Scholar] [CrossRef]
- Meoni, G.; Tenori, L.; Schade, S.; Licari, C.; Pirazzini, C.; Bacalini, M.G.; Garagnani, P.; Turano, P.; PROPAG-AGEING Consortium; Trenkwalder, C.; et al. Metabolite and Lipoprotein Profiles Reveal Sex-Related Oxidative Stress Imbalance in de Novo Drug-Naive Parkinson’s Disease Patients. NPJ Park. Dis. 2022, 8, 14. [Google Scholar] [CrossRef]
- Tan, A.H.; Chong, C.W.; Lim, S.-Y.; Yap, I.K.S.; Teh, C.S.J.; Loke, M.F.; Song, S.-L.; Tan, J.Y.; Ang, B.H.; Tan, Y.Q.; et al. Gut Microbial Ecosystem in Parkinson Disease: New Clinicobiological Insights from Multi-Omics. Ann. Neurol. 2021, 89, 546–559. [Google Scholar] [CrossRef] [PubMed]
- Hertel, J.; Harms, A.C.; Heinken, A.; Baldini, F.; Thinnes, C.C.; Glaab, E.; Vasco, D.A.; Pietzner, M.; Stewart, I.D.; Wareham, N.J.; et al. Integrated Analyses of Microbiome and Longitudinal Metabolome Data Reveal Microbial-Host Interactions on Sulfur Metabolism in Parkinson’s Disease. Cell Rep. 2019, 29, 1767–1777.e8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, Z.; Yang, F.; Cao, J.; Ding, W.; Yan, S.; Shi, W.; Wen, S.; Yao, L. Alterations of Gut Microbiota and Metabolome with Parkinson’s Disease. Microb. Pathog. 2021, 160, 105187. [Google Scholar] [CrossRef] [PubMed]
- Vascellari, S.; Palmas, V.; Melis, M.; Pisanu, S.; Cusano, R.; Uva, P.; Perra, D.; Madau, V.; Sarchioto, M.; Oppo, V.; et al. Gut Microbiota and Metabolome Alterations Associated with Parkinson’s Disease. mSystems 2020, 5, e00561-20. [Google Scholar] [CrossRef]
- Willkommen, D.; Lucio, M.; Moritz, F.; Forcisi, S.; Kanawati, B.; Smirnov, K.S.; Schroeter, M.; Sigaroudi, A.; Schmitt-Kopplin, P.; Michalke, B. Metabolomic Investigations in Cerebrospinal Fluid of Parkinson’s Disease. PLoS ONE 2018, 13, e0208752. [Google Scholar] [CrossRef] [Green Version]
- Rosario, D.; Bidkhori, G.; Lee, S.; Bedarf, J.; Hildebrand, F.; Le Chatelier, E.; Uhlen, M.; Ehrlich, S.D.; Proctor, G.; Wüllner, U.; et al. Systematic Analysis of Gut Microbiome Reveals the Role of Bacterial Folate and Homocysteine Metabolism in Parkinson’s Disease. Cell Rep. 2021, 34, 108807. [Google Scholar] [CrossRef]
- Trivedi, D.K.; Sinclair, E.; Xu, Y.; Sarkar, D.; Walton-Doyle, C.; Liscio, C.; Banks, P.; Milne, J.; Silverdale, M.; Kunath, T.; et al. Discovery of Volatile Biomarkers of Parkinson’s Disease from Sebum. ACS Cent. Sci. 2019, 5, 599–606. [Google Scholar] [CrossRef] [Green Version]
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef] [Green Version]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A Human Gut Microbial Gene Catalogue Established by Metagenomic Sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef] [Green Version]
- Fischbach, M.A. Microbiome: Focus on Causation and Mechanism. Cell 2018, 174, 785–790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koh, A.; Bäckhed, F. From Association to Causality: The Role of the Gut Microbiota and Its Functional Products on Host Metabolism. Mol. Cell 2020, 78, 584–596. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Ji, B.; Zengler, K.; Nielsen, J. Modelling Approaches for Studying the Microbiome. Nat. Microbiol. 2019, 4, 1253–1267. [Google Scholar] [CrossRef] [PubMed]
- Heinken, A.; Basile, A.; Hertel, J.; Thinnes, C.; Thiele, I. Genome-Scale Metabolic Modeling of the Human Microbiome in the Era of Personalized Medicine. Annu. Rev. Microbiol. 2021, 75, 199–222. [Google Scholar] [CrossRef]
- Blazier, A.S.; Papin, J.A. Integration of Expression Data in Genome-Scale Metabolic Network Reconstructions. Front. Physiol. 2012, 3, 299. [Google Scholar] [CrossRef] [Green Version]
- Hertel, J.; Heinken, A.; Martinelli, F.; Thiele, I. Integration of Constraint-Based Modeling with Fecal Metabolomics Reveals Large Deleterious Effects of Spp. on Community Butyrate Production. Gut Microbes 2021, 13, 1–23. [Google Scholar] [CrossRef]
- Cruz, F.; Faria, J.P.; Rocha, M.; Rocha, I.; Dias, O. A Review of Methods for the Reconstruction and Analysis of Integrated Genome-Scale Models of Metabolism and Regulation. Biochem. Soc. Trans. 2020, 48, 1889–1903. [Google Scholar] [CrossRef]
- Baldini, F.; Heinken, A.; Heirendt, L.; Magnusdottir, S.; Fleming, R.M.T.; Thiele, I. The Microbiome Modeling Toolbox: From Microbial Interactions to Personalized Microbial Communities. Bioinformatics 2019, 35, 2332–2334. [Google Scholar] [CrossRef] [Green Version]
- Bauer, E.; Zimmermann, J.; Baldini, F.; Thiele, I.; Kaleta, C. BacArena: Individual-Based Metabolic Modeling of Heterogeneous Microbes in Complex Communities. PLoS Comput. Biol. 2017, 13, e1005544. [Google Scholar] [CrossRef] [Green Version]
- Thiele, I.; Sahoo, S.; Heinken, A.; Hertel, J.; Heirendt, L.; Aurich, M.K.; Fleming, R.M. Personalized Whole-Body Models Integrate Metabolism, Physiology, and the Gut Microbiome. Mol. Syst. Biol. 2020, 16, e8982. [Google Scholar] [CrossRef]
- Heinken, A.; Hertel, J.; Thiele, I. Metabolic Modelling Reveals Broad Changes in Gut Microbial Metabolism in Inflammatory Bowel Disease Patients with Dysbiosis. NPJ Syst. Biol. Appl. 2021, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Aden, K.; Rehman, A.; Waschina, S.; Pan, W.-H.; Walker, A.; Lucio, M.; Nunez, A.M.; Bharti, R.; Zimmerman, J.; Bethge, J.; et al. Metabolic Functions of Gut Microbes Associate with Efficacy of Tumor Necrosis Factor Antagonists in Patients with Inflammatory Bowel Diseases. Gastroenterology 2019, 157, 1279–1292.e11. [Google Scholar] [CrossRef]
- Pryor, R.; Norvaisas, P.; Marinos, G.; Best, L.; Thingholm, L.B.; Quintaneiro, L.M.; De Haes, W.; Esser, D.; Waschina, S.; Lujan, C.; et al. Host-Microbe-Drug-Nutrient Screen Identifies Bacterial Effectors of Metformin Therapy. Cell 2019, 178, 1299–1312.e29. [Google Scholar] [CrossRef] [Green Version]
- Baldini, F.; Hertel, J.; Sandt, E.; Thinnes, C.C.; Neuberger-Castillo, L.; Pavelka, L.; Betsou, F.; Krüger, R.; Thiele, I.; NCER-PD Consortium. Parkinson’s Disease-Associated Alterations of the Gut Microbiome Predict Disease-Relevant Changes in Metabolic Functions. BMC Biol. 2020, 18, 62. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.; Schmitt-Kopplin, P. The Role of Fecal Sulfur Metabolome in Inflammatory Bowel Diseases. Int. J. Med. Microbiol. 2021, 311, 151513. [Google Scholar] [CrossRef] [PubMed]
- Matheus, F.C.; Aguiar, A.S., Jr.; Castro, A.A.; Villarinho, J.G.; Ferreira, J.; Figueiredo, C.P.; Walz, R.; Santos, A.R.S.; Tasca, C.I.; Prediger, R.D.S. Neuroprotective Effects of Agmatine in Mice Infused with a Single Intranasal Administration of 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine (MPTP). Behav. Brain Res. 2012, 235, 263–272. [Google Scholar] [CrossRef]
- Song, J.; Hur, B.E.; Bokara, K.K.; Yang, W.; Cho, H.J.; Park, K.A.; Lee, W.T.; Lee, K.M.; Lee, J.E. Agmatine Improves Cognitive Dysfunction and Prevents Cell Death in a Streptozotocin-Induced Alzheimer Rat Model. Yonsei Med. J. 2014, 55, 689–699. [Google Scholar] [CrossRef] [Green Version]
- Campbell, J.M.; Stephenson, M.D.; de Courten, B.; Chapman, I.; Bellman, S.M.; Aromataris, E. Metformin Use Associated with Reduced Risk of Dementia in Patients with Diabetes: A Systematic Review and Meta-Analysis. J. Alzheimer’s Dis. 2018, 65, 1225–1236. [Google Scholar] [CrossRef] [Green Version]
- Patil, S.P.; Jain, P.D.; Ghumatkar, P.J.; Tambe, R.; Sathaye, S. Neuroprotective Effect of Metformin in MPTP-Induced Parkinson’s Disease in Mice. Neuroscience 2014, 277, 747–754. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet Rapidly and Reproducibly Alters the Human Gut Microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [Green Version]
- Cassani, E.; Barichella, M.; Ferri, V.; Pinelli, G.; Iorio, L.; Bolliri, C.; Caronni, S.; Faierman, S.A.; Mottolese, A.; Pusani, C.; et al. Dietary Habits in Parkinson’s Disease: Adherence to Mediterranean Diet. Park. Relat. Disord. 2017, 42, 40–46. [Google Scholar] [CrossRef]
- Ghosh, T.S.; Rampelli, S.; Jeffery, I.B.; Santoro, A.; Neto, M.; Capri, M.; Giampieri, E.; Jennings, A.; Candela, M.; Turroni, S.; et al. Mediterranean Diet Intervention Alters the Gut Microbiome in Older People Reducing Frailty and Improving Health Status: The NU-AGE 1-Year Dietary Intervention across Five European Countries. Gut 2020, 69, 1218–1228. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly-Y, M.; Glickman, J.N.; Garrett, W.S. The Microbial Metabolites, Short-Chain Fatty Acids, Regulate Colonic Treg Cell Homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kujawska, M.; Jodynis-Liebert, J. Polyphenols in Parkinson’s Disease: A Systematic Review of In Vivo Studies. Nutrients 2018, 10, 642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, L.; Zhao, D.; Ono, K.; Ruan, K.; Mogno, I.; Tsuji, M.; Carry, E.; Brathwaite, J.; Sims, S.; Frolinger, T.; et al. Heterogeneity in Gut Microbiota Drive Polyphenol Metabolism That Influences α-Synuclein Misfolding and Toxicity. J. Nutr. Biochem. 2019, 64, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Mischley, L.K.; Lau, R.C.; Bennett, R.D. Role of Diet and Nutritional Supplements in Parkinson’s Disease Progression. Oxid. Med. Cell. Longev. 2017, 2017, 6405278. [Google Scholar] [CrossRef] [Green Version]
- Magnusson, K.R.; Hauck, L.; Jeffrey, B.M.; Elias, V.; Humphrey, A.; Nath, R.; Perrone, A.; Bermudez, L.E. Relationships between Diet-Related Changes in the Gut Microbiome and Cognitive Flexibility. Neuroscience 2015, 300, 128–140. [Google Scholar] [CrossRef]
- Olson, C.A.; Vuong, H.E.; Yano, J.M.; Liang, Q.Y.; Nusbaum, D.J.; Hsiao, E.Y. The Gut Microbiota Mediates the Anti-Seizure Effects of the Ketogenic Diet. Cell 2018, 173, 1728–1741.e13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boulos, M.I.; Jairam, T.; Kendzerska, T.; Im, J.; Mekhael, A.; Murray, B.J. Normal Polysomnography Parameters in Healthy Adults: A Systematic Review and Meta-Analysis. Lancet Respir. Med. 2019, 7, 533–543. [Google Scholar] [CrossRef]
- Yassine, H.N.; Samieri, C.; Livingston, G.; Glass, K.; Wagner, M.; Tangney, C.; Plassman, B.L.; Ikram, M.A.; Voigt, R.M.; Gu, Y.; et al. Nutrition State of Science and Dementia Prevention: Recommendations of the Nutrition for Dementia Prevention Working Group. Lancet Healthy Longev. 2022, 3, e501–e512. [Google Scholar] [CrossRef]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal Microbiota Metabolism of L-Carnitine, a Nutrient in Red Meat, Promotes Atherosclerosis. Nat. Med. 2013, 19, 576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirsch, M.A.; Iyer, S.S.; Sanjak, M. Exercise-Induced Neuroplasticity in Human Parkinson’s Disease: What Is the Evidence Telling Us? Park. Relat. Disord. 2016, 22 (Suppl. 1), S78–S81. [Google Scholar] [CrossRef] [PubMed]
- Svensson, M.; Lexell, J.; Deierborg, T. Effects of Physical Exercise on Neuroinflammation, Neuroplasticity, Neurodegeneration, and Behavior: What We Can Learn From Animal Models in Clinical Settings. Neurorehabil. Neural Repair 2015, 29, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.M.; Berg Miller, M.E.; Pence, B.D.; Whitlock, K.; Nehra, V.; Gaskins, H.R.; White, B.A.; Fryer, J.D.; Woods, J.A. Voluntary and Forced Exercise Differentially Alters the Gut Microbiome in C57BL/6J Mice. J. Appl. Physiol. 2015, 118, 1059–1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petriz, B.A.; Castro, A.P.; Almeida, J.A.; Gomes, C.P.; Fernandes, G.R.; Kruger, R.H.; Pereira, R.W.; Franco, O.L. Exercise Induction of Gut Microbiota Modifications in Obese, Non-Obese and Hypertensive Rats. BMC Genom. 2014, 15, 511. [Google Scholar] [CrossRef] [Green Version]
- Clarke, S.F.; Murphy, E.F.; O’Sullivan, O.; Lucey, A.J.; Humphreys, M.; Hogan, A.; Hayes, P.; O’Reilly, M.; Jeffery, I.B.; Wood-Martin, R.; et al. Exercise and Associated Dietary Extremes Impact on Gut Microbial Diversity. Gut 2014, 63, 1913–1920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inamori, T. Experimental and clinical study of endolymphatic hydrops by means of electrocochleogram. Nihon Jibiinkoka Gakkai Kaiho 1984, 87, 325–339. [Google Scholar] [CrossRef]
- Burokas, A.; Arboleya, S.; Moloney, R.D.; Peterson, V.L.; Murphy, K.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Targeting the Microbiota-Gut-Brain Axis: Prebiotics Have Anxiolytic and Antidepressant-like Effects and Reverse the Impact of Chronic Stress in Mice. Biol. Psychiatry 2017, 82, 472–487. [Google Scholar] [CrossRef]
- Azpiroz, F.; Dubray, C.; Bernalier-Donadille, A.; Cardot, J.-M.; Accarino, A.; Serra, J.; Wagner, A.; Respondek, F.; Dapoigny, M. Effects of scFOS on the Composition of Fecal Microbiota and Anxiety in Patients with Irritable Bowel Syndrome: A Randomized, Double Blind, Placebo Controlled Study. Neurogastroenterol. Motil. 2017, 29, e12911. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Ho, L.; Faith, J.; Ono, K.; Janle, E.M.; Lachcik, P.J.; Cooper, B.R.; Jannasch, A.H.; D’Arcy, B.R.; Williams, B.A.; et al. Role of Intestinal Microbiota in the Generation of Polyphenol-Derived Phenolic Acid Mediated Attenuation of Alzheimer’s Disease β-Amyloid Oligomerization. Mol. Nutr. Food Res. 2015, 59, 1025–1040. [Google Scholar] [CrossRef] [Green Version]
- Barichella, M.; Pacchetti, C.; Bolliri, C.; Cassani, E.; Iorio, L.; Pusani, C.; Pinelli, G.; Privitera, G.; Cesari, I.; Faierman, S.A.; et al. Probiotics and Prebiotic Fiber for Constipation Associated with Parkinson Disease: An RCT. Neurology 2016, 87, 1274–1280. [Google Scholar] [CrossRef] [PubMed]
- Becker, A.; Schmartz, G.P.; Gröger, L.; Grammes, N.; Galata, V.; Philippeit, H.; Weiland, J.; Ludwig, N.; Meese, E.; Tierling, S.; et al. Effects of Resistant Starch on Symptoms, Fecal Markers, and Gut Microbiota in Parkinson’s Disease—The RESISTA-PD Trial. Genom. Proteom. Bioinform. 2022, 20, 274–287. [Google Scholar] [CrossRef] [PubMed]
- Davari, S.; Talaei, S.A.; Alaei, H.; Salami, M. Probiotics Treatment Improves Diabetes-Induced Impairment of Synaptic Activity and Cognitive Function: Behavioral and Electrophysiological Proofs for Microbiome-Gut-Brain Axis. Neuroscience 2013, 240, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.V.; Bested, A.C.; Beaulne, T.M.; Katzman, M.A.; Iorio, C.; Berardi, J.M.; Logan, A.C. A Randomized, Double-Blind, Placebo-Controlled Pilot Study of a Probiotic in Emotional Symptoms of Chronic Fatigue Syndrome. Gut Pathog. 2009, 1, 6. [Google Scholar] [CrossRef] [Green Version]
- Akbari, E.; Asemi, Z.; Daneshvar Kakhaki, R.; Bahmani, F.; Kouchaki, E.; Tamtaji, O.R.; Hamidi, G.A.; Salami, M. Effect of Probiotic Supplementation on Cognitive Function and Metabolic Status in Alzheimer’s Disease: A Randomized, Double-Blind and Controlled Trial. Front. Aging Neurosci. 2016, 8, 256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamtaji, O.R.; Taghizadeh, M.; Daneshvar Kakhaki, R.; Kouchaki, E.; Bahmani, F.; Borzabadi, S.; Oryan, S.; Mafi, A.; Asemi, Z. Clinical and Metabolic Response to Probiotic Administration in People with Parkinson’s Disease: A Randomized, Double-Blind, Placebo-Controlled Trial. Clin. Nutr. 2019, 38, 1031–1035. [Google Scholar] [CrossRef]
- Avis, T.; Wilson, F.X.; Khan, N.; Mason, C.S.; Powell, D.J. Targeted Microbiome-Sparing Antibiotics. Drug Discov. Today 2021, 26, 2198–2203. [Google Scholar] [CrossRef]
- Park, E.M.; Chelvanambi, M.; Bhutiani, N.; Kroemer, G.; Zitvogel, L.; Wargo, J.A. Targeting the Gut and Tumor Microbiota in Cancer. Nat. Med. 2022, 28, 690–703. [Google Scholar] [CrossRef]
- Mertsalmi, T.H.; Pekkonen, E.; Scheperjans, F. Antibiotic Exposure and Risk of Parkinson’s Disease in Finland: A Nationwide Case-Control Study. Mov. Disord. 2020, 35, 431–442. [Google Scholar] [CrossRef]
- Angelucci, F.; Cechova, K.; Amlerova, J.; Hort, J. Antibiotics, Gut Microbiota, and Alzheimer’s Disease. J. Neuroinflamm. 2019, 16, 108. [Google Scholar] [CrossRef] [Green Version]
- Itzhaki, R.F. Herpes Simplex Virus Type 1 and Alzheimer’s Disease: Possible Mechanisms and Signposts. FASEB J. 2017, 31, 3216–3226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, F.K.L.; To, K.F.; Wu, J.C.Y.; Yung, M.Y.; Leung, W.K.; Kwok, T.; Hui, Y.; Chan, H.L.Y.; Chan, C.S.Y.; Hui, E.; et al. Eradication of Helicobacter Pylori and Risk of Peptic Ulcers in Patients Starting Long-Term Treatment with Non-Steroidal Anti-Inflammatory Drugs: A Randomised Trial. Lancet 2002, 359, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Vickers, R.J.; Tillotson, G.S.; Nathan, R.; Hazan, S.; Pullman, J.; Lucasti, C.; Deck, K.; Yacyshyn, B.; Maliakkal, B.; Pesant, Y.; et al. Efficacy and Safety of Ridinilazole Compared with Vancomycin for the Treatment of Clostridium Difficile Infection: A Phase 2, Randomised, Double-Blind, Active-Controlled, Non-Inferiority Study. Lancet Infect. Dis. 2017, 17, 735–744. [Google Scholar] [CrossRef] [Green Version]
- Mishra, Y.K.; Adelung, R. ZnO Tetrapod Materials for Functional Applications. Mater. Today 2018, 21, 631–651. [Google Scholar] [CrossRef]
- Antoine, T.E.; Hadigal, S.R.; Yakoub, A.M.; Mishra, Y.K.; Bhattacharya, P.; Haddad, C.; Valyi-Nagy, T.; Adelung, R.; Prabhakar, B.S.; Shukla, D. Intravaginal Zinc Oxide Tetrapod Nanoparticles as Novel Immunoprotective Agents against Genital Herpes. J. Immunol. 2016, 196, 4566–4575. [Google Scholar] [CrossRef] [Green Version]
- Siebert, L.; Luna-Cerón, E.; García-Rivera, L.E.; Oh, J.; Jang, J.; Rosas-Gómez, D.A.; Pérez-Gómez, M.D.; Maschkowitz, G.; Fickenscher, H.; Oceguera-Cuevas, D.; et al. Light-controlled Growth Factors Release on Tetrapodal ZnO-incorporated 3D-printed Hydrogels for Developing Smart Wound Scaffold. Adv. Funct. Mater. 2021, 31, 2007555. [Google Scholar] [CrossRef]
- Nasajpour, A.; Mandla, S.; Shree, S.; Mostafavi, E.; Sharifi, R.; Khalilpour, A.; Saghazadeh, S.; Hassan, S.; Mitchell, M.J.; Leijten, J.; et al. Nanostructured Fibrous Membranes with Rose Spike-Like Architecture. Nano Lett. 2017, 17, 6235–6240. [Google Scholar] [CrossRef] [Green Version]
- Nasajpour, A.; Samandari, M.; Patil, C.D.; Abolhassani, R.; Suryawanshi, R.K.; Adelung, R.; Rubahn, H.-G.; Khademhosseini, A.; Mishra, Y.K.; Shukla, D.; et al. Nanoengineered Antiviral Fibrous Arrays with Rose-Thorn-Inspired Architectures. ACS Mater. Lett. 2021, 3, 1566–1571. [Google Scholar] [CrossRef]
- Gough, E.; Shaikh, H.; Manges, A.R. Systematic Review of Intestinal Microbiota Transplantation (fecal Bacteriotherapy) for Recurrent Clostridium Difficile Infection. Clin. Infect. Dis. 2011, 53, 994–1002. [Google Scholar] [CrossRef] [Green Version]
- Van Nood, E.; Vrieze, A.; Nieuwdorp, M.; Fuentes, S.; Zoetendal, E.G.; de Vos, W.M.; Visser, C.E.; Kuijper, E.J.; Bartelsman, J.F.W.M.; Tijssen, J.G.P.; et al. Duodenal Infusion of Donor Feces for Recurrent Clostridium Difficile. N. Engl. J. Med. 2013, 368, 407–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fehily, S.R.; Basnayake, C.; Wright, E.K.; Kamm, M.A. Fecal Microbiota Transplantation Therapy in Crohn’s Disease: Systematic Review. J. Gastroenterol. Hepatol. 2021, 36, 2672–2686. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Deng, Y.; Yang, K.; Chen, J.; He, Q.; Chen, H. Safety and Efficacy of Fecal Microbiota Transplantation for Autoimmune Diseases and Autoinflammatory Diseases: A Systematic Review and Meta-Analysis. Front. Immunol. 2022, 13, 944387. [Google Scholar] [CrossRef]
- Tian, H.; Ge, X.; Nie, Y.; Yang, L.; Ding, C.; McFarland, L.V.; Zhang, X.; Chen, Q.; Gong, J.; Li, N. Fecal Microbiota Transplantation in Patients with Slow-Transit Constipation: A Randomized, Clinical Trial. PLoS ONE 2017, 12, e0171308. [Google Scholar] [CrossRef] [Green Version]
- Bercik, P.; Denou, E.; Collins, J.; Jackson, W.; Lu, J.; Jury, J.; Deng, Y.; Blennerhassett, P.; Macri, J.; McCoy, K.D.; et al. The Intestinal Microbiota Affect Central Levels of Brain-Derived Neurotropic Factor and Behavior in Mice. Gastroenterology 2011, 141, 599–609.e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dimitriu, P.A.; Boyce, G.; Samarakoon, A.; Hartmann, M.; Johnson, P.; Mohn, W.W. Temporal Stability of the Mouse Gut Microbiota in Relation to Innate and Adaptive Immunity. Environ. Microbiol. Rep. 2013, 5, 200–210. [Google Scholar] [CrossRef] [PubMed]
- De Palma, G.; Lynch, M.D.J.; Lu, J.; Dang, V.T.; Deng, Y.; Jury, J.; Umeh, G.; Miranda, P.M.; Pigrau Pastor, M.; Sidani, S.; et al. Transplantation of Fecal Microbiota from Patients with Irritable Bowel Syndrome Alters Gut Function and Behavior in Recipient Mice. Sci. Transl. Med. 2017, 9, eaaf6397. [Google Scholar] [CrossRef]
- Zhao, Z.; Ning, J.; Bao, X.-Q.; Shang, M.; Ma, J.; Li, G.; Zhang, D. Fecal Microbiota Transplantation Protects Rotenone-Induced Parkinson’s Disease Mice via Suppressing Inflammation Mediated by the Lipopolysaccharide-TLR4 Signaling Pathway through the Microbiota-Gut-Brain Axis. Microbiome 2021, 9, 226. [Google Scholar] [CrossRef]
- Sun, M.-F.; Zhu, Y.-L.; Zhou, Z.-L.; Jia, X.-B.; Xu, Y.-D.; Yang, Q.; Cui, C.; Shen, Y.-Q. Neuroprotective Effects of Fecal Microbiota Transplantation on MPTP-Induced Parkinson’s Disease Mice: Gut Microbiota, Glial Reaction and TLR4/TNF-α Signaling Pathway. Brain Behav. Immun. 2018, 70, 48–60. [Google Scholar] [CrossRef]
- Sun, J.; Xu, J.; Ling, Y.; Wang, F.; Gong, T.; Yang, C.; Ye, S.; Ye, K.; Wei, D.; Song, Z.; et al. Fecal Microbiota Transplantation Alleviated Alzheimer’s Disease-like Pathogenesis in APP/PS1 Transgenic Mice. Transl. Psychiatry 2019, 9, 189. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.-S.; Kim, Y.; Choi, H.; Kim, W.; Park, S.; Lee, D.; Kim, D.K.; Kim, H.J.; Choi, H.; Hyun, D.-W.; et al. Transfer of a Healthy Microbiota Reduces Amyloid and Tau Pathology in an Alzheimer’s Disease Animal Model. Gut 2020, 69, 283–294. [Google Scholar] [CrossRef]
- Kuai, X.-Y.; Yao, X.-H.; Xu, L.-J.; Zhou, Y.-Q.; Zhang, L.-P.; Liu, Y.; Pei, S.-F.; Zhou, C.-L. Evaluation of Fecal Microbiota Transplantation in Parkinson’s Disease Patients with Constipation. Microb. Cell Fact. 2021, 20, 98. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Xu, H.; Luo, Q.; He, J.; Li, M.; Chen, H.; Tang, W.; Nie, Y.; Zhou, Y. Fecal Microbiota Transplantation to Treat Parkinson’s Disease with Constipation: A Case Report. Medicine 2019, 98, e16163. [Google Scholar] [CrossRef]
- Segal, A.; Zlotnik, Y.; Moyal-Atias, K.; Abuhasira, R.; Ifergane, G. Fecal Microbiota Transplant as a Potential Treatment for Parkinson’s Disease—A Case Series. Clin. Neurol. Neurosurg. 2021, 207, 106791. [Google Scholar] [CrossRef]
- Fecal Microbiota Transplantation for Parkinson’s Disease. Available online: https://clinicaltrials.gov/ct2/show/NCT03808389 (accessed on 23 November 2022).
- Hazan, S. Rapid Improvement in Alzheimer’s Disease Symptoms Following Fecal Microbiota Transplantation: A Case Report. J. Int. Med. Res. 2020, 48, 300060520925930. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-H.; Lee, J.H.; Shin, J.; Kim, J.-S.; Cha, B.; Lee, S.; Kwon, K.S.; Shin, Y.W.; Choi, S.H. Cognitive Function Improvement after Fecal Microbiota Transplantation in Alzheimer’s Dementia Patient: A Case Report. Curr. Med. Res. Opin. 2021, 37, 1739–1744. [Google Scholar] [CrossRef] [PubMed]
- Oral Fecal Microbiota Transplant Feasibility Study in Alzheimer’s Disease—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03998423 (accessed on 23 November 2022).
- Van Laar, T.; Boertien, J.M.; Herranz, A.H. Faecal Transplantation, Pro- and Prebiotics in Parkinson’s Disease; Hope or Hype? J. Park. Dis. 2019, 9, S371–S379. [Google Scholar] [CrossRef] [Green Version]
- Antunes, L.C.M.; Han, J.; Ferreira, R.B.R.; Lolić, P.; Borchers, C.H.; Finlay, B.B. Effect of Antibiotic Treatment on the Intestinal Metabolome. Antimicrob. Agents Chemother. 2011, 55, 1494–1503. [Google Scholar] [CrossRef] [Green Version]
- Imhann, F.; Bonder, M.J.; Vich Vila, A.; Fu, J.; Mujagic, Z.; Vork, L.; Tigchelaar, E.F.; Jankipersadsing, S.A.; Cenit, M.C.; Harmsen, H.J.M.; et al. Proton Pump Inhibitors Affect the Gut Microbiome. Gut 2016, 65, 740–748. [Google Scholar] [CrossRef] [Green Version]
- Bodrug, S.E.; Warner, B.J.; Bath, M.L.; Lindeman, G.J.; Harris, A.W.; Adams, J.M. Cyclin D1 Transgene Impedes Lymphocyte Maturation and Collaborates in Lymphomagenesis with the Myc Gene. EMBO J. 1994, 13, 2124–2130. [Google Scholar] [CrossRef]
- Van Kessel, S.P.; Frye, A.K.; El-Gendy, A.O.; Castejon, M.; Keshavarzian, A.; van Dijk, G.; El Aidy, S. Gut Bacterial Tyrosine Decarboxylases Restrict Levels of Levodopa in the Treatment of Parkinson’s Disease. Nat. Commun. 2019, 10, 310. [Google Scholar] [CrossRef] [Green Version]
- Jangi, S.; Gandhi, R.; Cox, L.M.; Li, N.; von Glehn, F.; Yan, R.; Patel, B.; Mazzola, M.A.; Liu, S.; Glanz, B.L.; et al. Alterations of the Human Gut Microbiome in Multiple Sclerosis. Nat. Commun. 2016, 7, 12015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levi, I.; Gurevich, M.; Perlman, G.; Magalashvili, D.; Menascu, S.; Bar, N.; Godneva, A.; Zahavi, L.; Chermon, D.; Kosower, N.; et al. Potential Role of Indolelactate and Butyrate in Multiple Sclerosis Revealed by Integrated Microbiome-Metabolome Analysis. Cell Rep. Med. 2021, 2, 100246. [Google Scholar] [CrossRef] [PubMed]
- Kandpal, M.; Indari, O.; Baral, B.; Jakhmola, S.; Tiwari, D.; Bhandari, V.; Pandey, R.K.; Bala, K.; Sonawane, A.; Jha, H.C. Dysbiosis of Gut Microbiota from the Perspective of the Gut-Brain Axis: Role in the Provocation of Neurological Disorders. Metabolites 2022, 12, 64. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zacharias, H.U.; Kaleta, C.; Cossais, F.; Schaeffer, E.; Berndt, H.; Best, L.; Dost, T.; Glüsing, S.; Groussin, M.; Poyet, M.; et al. Microbiome and Metabolome Insights into the Role of the Gastrointestinal–Brain Axis in Parkinson’s and Alzheimer’s Disease: Unveiling Potential Therapeutic Targets. Metabolites 2022, 12, 1222. https://doi.org/10.3390/metabo12121222
Zacharias HU, Kaleta C, Cossais F, Schaeffer E, Berndt H, Best L, Dost T, Glüsing S, Groussin M, Poyet M, et al. Microbiome and Metabolome Insights into the Role of the Gastrointestinal–Brain Axis in Parkinson’s and Alzheimer’s Disease: Unveiling Potential Therapeutic Targets. Metabolites. 2022; 12(12):1222. https://doi.org/10.3390/metabo12121222
Chicago/Turabian StyleZacharias, Helena U., Christoph Kaleta, François Cossais, Eva Schaeffer, Henry Berndt, Lena Best, Thomas Dost, Svea Glüsing, Mathieu Groussin, Mathilde Poyet, and et al. 2022. "Microbiome and Metabolome Insights into the Role of the Gastrointestinal–Brain Axis in Parkinson’s and Alzheimer’s Disease: Unveiling Potential Therapeutic Targets" Metabolites 12, no. 12: 1222. https://doi.org/10.3390/metabo12121222
APA StyleZacharias, H. U., Kaleta, C., Cossais, F., Schaeffer, E., Berndt, H., Best, L., Dost, T., Glüsing, S., Groussin, M., Poyet, M., Heinzel, S., Bang, C., Siebert, L., Demetrowitsch, T., Leypoldt, F., Adelung, R., Bartsch, T., Bosy-Westphal, A., Schwarz, K., & Berg, D. (2022). Microbiome and Metabolome Insights into the Role of the Gastrointestinal–Brain Axis in Parkinson’s and Alzheimer’s Disease: Unveiling Potential Therapeutic Targets. Metabolites, 12(12), 1222. https://doi.org/10.3390/metabo12121222







