Magnetic Resonance Imaging and Spectroscopy Methods to Study Hepatic Glucose Metabolism and Their Applications in the Healthy and Diabetic Liver
Abstract
:1. Introduction
1.1. Hepatic Glucose Metabolism
1.2. Regulation of Glucose Homeostasis and Distortions in Diabetes
1.3. Magnetic Resonance Imaging and Spectroscopy
2. MR Measurements of Liver Glucose and Glycogen Metabolism
2.1. 13C MRS
2.1.1. Measurement of Hepatic Glycogen with 13C MRS
2.1.2. Measurement of Hepatic Glucose with 13C MRS
2.2. Detecting Glucose and Glycogen in the Liver by Deuterium and Proton MRS/MRI
3. 13C MRS Investigations of Impairments of Hepatic Glucose and Glycogen Metabolism in Diabetes
3.1. Type 2 Diabetes
3.2. Type 1 Diabetes
3.3. Glycogen Storage Disease
4. Probing Gluconeogenesis and Glycolysis with Hyperpolarized 13C MR
4.1. Hyperpolarized 13C MRS
4.2. Use of Hyperpolarized [2-13C]Dihydroxyacetone to Simultaneously Assess Gluconeogenesis and Glycolysis
4.3. Use of Hyperpolarized [1-13C]Pyruvate to Assess Gluconeogenesis and PDH Flux
5. Limitations and Future Perspective
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Roden, M.; Petersen, K.F.; Shulman, G.I. Nuclear Magnetic Resonance Studies of Hepatic Glucose Metabolism in Humans. Recent Prog. Horm. Res. 2001, 56, 219–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, M.C.; Coate, K.C.; Winnick, J.J.; An, Z.; Cherrington, A.D. Regulation of Hepatic Glucose Uptake and Storage in Vivo. Adv. Nutr. 2012, 3, 286–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thorens, B. GLUT2, Glucose Sensing and Glucose Homeostasis. Diabetologia 2015, 58, 221–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rui, L. Energy Metabolism in the Liver. Compr. Physiol. 2014, 4, 177–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merritt, M.E.; Harrison, C.; Sherry, A.D.; Malloy, C.R.; Burgess, S.C. Flux through Hepatic Pyruvate Carboxylase and Phosphoenolpyruvate Carboxykinase Detected by Hyperpolarized 13C Magnetic Resonance. Proc. Natl. Acad. Sci. USA 2011, 108, 19084–19089. [Google Scholar] [CrossRef] [Green Version]
- Ordidge, R.J.; Connelly, A.; Lohman, J.A.B. Image-Selected in Vivo Spectroscopy (ISIS). A New Technique for Spatially Selective Nmr Spectroscopy. J. Magn. Reson. 1986, 66, 283–294. [Google Scholar] [CrossRef]
- Rothman, D.L.; Magnusson, I.; Katz, L.D.; Shulman, R.G.; Shulman, G.I. Quantitation of Hepatic Glycogenolysis and Gluconeogenesis in Fasting Humans with 13C NMR. Science 1991, 254, 573–576. [Google Scholar] [CrossRef]
- Hwang, J.H.; Perseghin, G.; Rothman, D.L.; Cline, G.W.; Magnusson, I.; Petersen, K.F.; Shulman, G.I. Impaired Net Hepatic Glycogen Synthesis in Insulin-Dependent Diabetic Subjects during Mixed Meal Ingestion: A 13C Nuclear Magnetic Resonance Spectroscopy Study. J. Clin. Investig. 1995, 95, 783–787. [Google Scholar] [CrossRef]
- Sillerud, L.O.; Shulman, R.G. Structure and Metabolism of Mammalian Liver Glycogen Monitored by Carbon-13 Nuclear Magnetic Resonance. Biochemistry 1983, 22, 1087–1094. [Google Scholar] [CrossRef]
- Stevens, A.N.; Richard, A.I.; Peter, G.M.; Griffiths, J.R. Detection of Glycogen in a Glycogen Storage Disease by 13C Nuclear Magnetic Resonance. FEBS Lett. 1982, 150, 489–493. [Google Scholar] [CrossRef]
- Gruetter, R.; Magnusson, I.; Rothman, D.L.; Avison, M.J.; Shulman, R.G.; Shulman, G.I. Validation of 13C NMR Measurements of Liver Glycogen in Vivo. Magn. Reson. Med. 1994, 31, 583–588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jue, T.; Lohman, J.A.B.; Ordidge, R.J.; Shulman, R.G. Natural Abundance 13C NMR Spectrum of Glycogen in Humans. Magn. Reson. Med. 1987, 5, 377–379. [Google Scholar] [CrossRef] [PubMed]
- Zang, L.H.; Laughlin, M.R.; Rothman, D.L.; Shulman, R.G. Carbon-13 NMR Relaxation Times of Hepatic Glycogen in Vitro and in Vivo. Biochemistry 1990, 29, 6815–6820. [Google Scholar] [CrossRef] [PubMed]
- Jue, T.; Rothman, D.L.; Tavitian, B.A.; Shulman, R.G. Natural-Abundance 13C NMR Study of Glycogen Repletion in Human Liver and Muscle. Proc. Natl. Acad. Sci. USA 1989, 86, 1439–1442. [Google Scholar] [CrossRef] [Green Version]
- Shalwitz, R.A.; Reo, N.V.; Becker, N.N.; Ackerman, J.J.H. Visibility of Mammalian Hepatic Glycogen to the NMR Experiment, in Vivo. Magn. Reson. Med. 1987, 5, 462–465. [Google Scholar] [CrossRef]
- Stephenson, M.C.; Leverton, E.; Khoo, E.Y.H.; Poucher, S.M.; Johansson, L.; Lockton, J.A.; Eriksson, J.W.; Mansell, P.; Morris, P.G.; Macdonald, I.A. Variability in Fasting Lipid and Glycogen Contents in Hepatic and Skeletal Muscle Tissue in Subjects with and without Type 2 Diabetes: A 1H and 13C MRS Study. NMR Biomed. 2013, 26, 1518–1526. [Google Scholar] [CrossRef]
- Buehler, T.; Bally, L.; Dokumaci, A.S.; Stettler, C.; Boesch, C. Methodological and Physiological Test-Retest Reliability of 13C-MRS Glycogen Measurements in Liver and in Skeletal Muscle of Patients with Type 1 Diabetes and Matched Healthy Controls. NMR Biomed. 2016, 29, 796–805. [Google Scholar] [CrossRef]
- Petersen, K.F.; Price, T.; Cline, G.W.; Rothman, D.L.; Shulman, G.I. Contribution of Net Hepatic Glycogenolysis to Glucose Production during the Early Postprandial Period. Am. J. Physiol. Metab. 1996, 270, E186–E191. [Google Scholar] [CrossRef]
- Iwayama, K.; Onishi, T.; Maruyama, K.; Takahashi, H. Diurnal Variation in the Glycogen Content of the Human Liver Using 13 C MRS. NMR Biomed. 2020, 33, e4289. [Google Scholar] [CrossRef]
- Kacerovsky, M.; Jones, J.; Schmid, A.I.; Barosa, C.; Lettner, A.; Kacerovsky-Bielesz, G.; Szendroedi, J.; Chmelik, M.; Nowotny, P.; Chandramouli, V.; et al. Postprandial and Fasting Hepatic Glucose Fluxes in Long-Standing Type 1 Diabetes. Diabetes 2011, 60, 1752–1758. [Google Scholar] [CrossRef]
- Bischof, M.G.; Krssak, M.; Krebs, M.; Bernroider, E.; Stingl, H.; Waldhäusl, W.; Roden, M. Effects of Short-Term Improvement of Insulin Treatment and Glycemia on Hepatic Glycogen Metabolism in Type 1 Diabetes. Diabetes 2001, 50, 392–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bischof, M.G.; Bernroider, E.; Krssak, M.; Krebs, M.; Stingl, H.; Nowotny, P.; Yu, C.; Shulman, G.I.; Waldhäusl, W.; Roden, M. Hepatic Glycogen Metabolism in Type 1 Diabetes after Long-Term near Normoglycemia. Diabetes 2002, 51, 49–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kishore, P.; Gabriely, I.; Cui, M.H.; Di Vito, J.; Gajavelli, S.; Hwang, J.H.; Shamoon, H. Role of Hepatic Glycogen Breakdown in Defective Counterregulation of Hypoglycemia in Intensively Treated Type 1 Diabetes. Diabetes 2006, 55, 659–666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petersen, K.F.; Cline, G.W.; Gerard, D.P.; Magnusson, I.; Rothman, D.L.; Shulman, G.I. Contribution of Net Hepatic Glycogen Synthesis to Disposal of an Oral Glucose Load in Humans. Metabolism. 2001, 50, 598–601. [Google Scholar] [CrossRef]
- Taylor, R.; Magnusson, I.; Rothman, D.L.; Cline, G.W.; Caumo, A.; Cobelli, C.; Shulman, G.I. Direct Assessment of Liver Glycogen Storage by 13C Nuclear Magnetic Resonance Spectroscopy and Regulation of Glucose Homeostasis after a Mixed Meal in Normal Subjects. J. Clin. Investig. 1996, 97, 126–132. [Google Scholar] [CrossRef]
- Moriarty, K.T.; Mclntyre, D.G.O.; Bingham, K.; Coxon, R.; Glover, P.M.; Greenhaff, P.L.; Macdonald, I.A.; Bachelard, H.S.; Morris, P.G. Glycogen Resynthesis in Liver and Muscle after Exercise: Measurement of the Rate of Resynthesis By13C Magnetic Resonance Spectroscopy. Magma Magn. Reson. Mater. Phys. Biol. Med. 1994, 2, 429–432. [Google Scholar] [CrossRef]
- Casey, A.; Mann, R.; Banister, K.; Fox, J.; Morris, P.G.; Macdonald, I.A.; Greenhaff, P.L. Effect of Carbohydrate Ingestion on Glycogen Resynthesis in Human Liver and Skeletal Muscle, Measured by 13C MRS. Am. J. Physiol. Endocrinol. Metab. 2000, 278, E65–E75. [Google Scholar] [CrossRef] [Green Version]
- Shulman, G.I.; Rothman, D.L.; Chung, Y.; Rossetti, L.; Petit, W.A.; Barrett, E.J.; Shulman, R.G. 13C NMR Studies of Glycogen Turnover in the Perfused Rat Liver. J. Biol. Chem. 1988, 263, 5027–5029. [Google Scholar] [CrossRef]
- Beckmann, N.; Fried, R.; Turkalj, I.; Seelig, J.; Keller, U.; Stalder, G. Noninvasive Observation of Hepatic Glycogen Formation in Man by 13C MRS after Oral and Intravenous Glucose Administration. Magn. Reson. Med. 1993, 29, 583–590. [Google Scholar] [CrossRef]
- Künnecke, B.; Küstermann, E.; Seelig, J. Simultaneous in Vivo Monitoring of Hepatic Glucose and Glucose-6-Phosphate by 13C-NMR Spectroscopy. Magn. Reson. Med. 2000, 44, 556–562. [Google Scholar] [CrossRef]
- Tomiyasu, M.; Obata, T.; Nishi, Y.; Nakamoto, H.; Nonaka, H.; Takayama, Y.; Autio, J.; Ikehira, H.; Kanno, I. Monitoring of Liver Glycogen Synthesis in Diabetic Patients Using Carbon-13 MR Spectroscopy. Eur. J. Radiol. 2010, 73, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Stender, S.; Zaha, V.G.; Malloy, C.R.; Sudderth, J.; DeBerardinis, R.J.; Park, J.M. Assessment of Rapid Hepatic Glycogen Synthesis in Humans Using Dynamic 13C Magnetic Resonance Spectroscopy. Hepatol. Commun. 2020, 4, 425–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosman, K.J.R.; Taylor, P.D.P. Isotopic Compositions of the Elements 1997 (Technical Report). Pure Appl. Chem. 1998, 70, 217–235. [Google Scholar] [CrossRef]
- Chen, H.; De Feyter, H.M.; Brown, P.B.; Rothman, D.L.; Cai, S.; de Graaf, R.A. Comparison of Direct 13C and Indirect 1H-[13C] MR Detection Methods for the Study of Dynamic Metabolic Turnover in the Human Brain. J. Magn. Reson. 2017, 283, 33–44. [Google Scholar] [CrossRef] [PubMed]
- De Feyter, H.M.; de Graaf, R.A. Deuterium Metabolic Imaging—Back to the Future. J. Magn. Reson. 2021, 326, 106932. [Google Scholar] [CrossRef] [PubMed]
- De Feyter, H.M.; Behar, K.L.; Corbin, Z.A.; Fulbright, R.K.; Brown, P.B.; McIntyre, S.; Nixon, T.W.; Rothman, D.L.; De Graaf, R.A. Deuterium Metabolic Imaging (DMI) for MRI-Based 3D Mapping of Metabolism in Vivo. Sci. Adv. 2018, 4, eaat7314. [Google Scholar] [CrossRef] [Green Version]
- Lu, M.; Zhu, X.H.; Zhang, Y.; Mateescu, G.; Chen, W. Quantitative Assessment of Brain Glucose Metabolic Rates Using in Vivo Deuterium Magnetic Resonance Spectroscopy. J. Cereb. Blood Flow Metab. 2017, 37, 3518–3530. [Google Scholar] [CrossRef]
- De Feyter, H.M.; Thomas, M.A.; Behar, K.L.; de Graaf, R.A. NMR Visibility of Deuterium-Labeled Liver Glycogen in Vivo. Magn. Reson. Med. 2021, 86, 62–68. [Google Scholar] [CrossRef]
- Chen, W.; Avison, M.J.; Bloch, G.; Shulman, R.G. Proton NMR Observation of Glycogen in Vivo. Magn. Reson. Med. 1994, 31, 576–579. [Google Scholar] [CrossRef]
- Ouwerkerk, R.; Pettigrew, R.I.; Gharib, A.M. Liver Metabolite Concentrations Measured with 1H MR Spectroscopy. Radiology 2012, 265, 565–575. [Google Scholar] [CrossRef]
- Inai, K.; Noriki, S.; Kinoshita, K.; Nishijima, A.; Sakai, T.; Kimura, H.; Naiki, H. Feasibility of Liver Weight Estimation by Postmortem Computed Tomography Images: An Autopsy Study. Pathol. Int. 2014, 64, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Weis, J.; Kullberg, J.; Ahlström, H. Multiple Breath-Hold Proton Spectroscopy of Human Liver at 3T: Relaxation Times and Concentrations of Glycogen, Choline, and Lipids. J. Magn. Reson. Imaging 2018, 47, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Van Zijl, P.C.M.; Jones, C.K.; Ren, J.; Malloy, C.R.; Sherry, A.D. MRI Detection of Glycogen in Vivo by Using Chemical Exchange Saturation Transfer Imaging (GlycoCEST). Proc. Natl. Acad. Sci. USA 2007, 104, 4359–4364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; van Zijl, P.C.M.; Xu, X.; Xu, J.; Li, Y.; Chen, L.; Yadav, N.N. Magnetic Resonance Imaging of Glycogen Using Its Magnetic Coupling with Water. Proc. Natl. Acad. Sci. USA 2020, 117, 3144–3149. [Google Scholar] [CrossRef]
- Magnusson, I.; Rothman, D.L.; Katz, L.D.; Shulman, R.G.; Shulman, G.I. Increased Rate of Gluconeogenesis in Type II Diabetes Mellitus a 13C Nuclear Magnetic Resonance Study. J. Clin. Investig. 1992, 90, 1323–1327. [Google Scholar] [CrossRef]
- Krssak, M.; Brehm, A.; Bernroider, E.; Anderwald, C.; Nowotny, P.; Dalla Man, C.; Cobelli, C.; Cline, G.W.; Shulman, G.I.; Waldhäusl, W.; et al. Alterations in Postprandial Hepatic Glycogen Metabolism in Type 2 Diabetes. Diabetes 2004, 53, 3048–3056. [Google Scholar] [CrossRef] [Green Version]
- Velho, G.; Petersen, K.F.; Perseghin, G.; Hwang, J.H.; Rothman, D.L.; Pueyo, M.E.; Cline, G.W.; Froguel, P.; Shulman, G.I. Impaired Hepatic Glycogen Synthesis in Glucokinase-Deficient (MODY-2) Subjects. J. Clin. Investig. 1996, 98, 1755–1761. [Google Scholar] [CrossRef] [Green Version]
- Bailey, C.J.; Turner, R.C. Metformin. N. Engl. J. Med. 1996, 334, 574–579. [Google Scholar] [CrossRef]
- Hundal, R.S.; Krssak, M.; Dufour, S.; Laurent, D.; Lebon, V.; Chandramouli, V.; Inzucchi, S.E.; Schumann, W.C.; Petersen, K.F.; Landau, B.R.; et al. Mechanism by Which Metformin Reduces Glucose Production in Type 2 Diabetes. Diabetes 2000, 49, 2063–2069. [Google Scholar] [CrossRef] [Green Version]
- Davies, M.J.; D’Alessio, D.A.; Fradkin, J.; Kernan, W.N.; Mathieu, C.; Mingrone, G.; Rossing, P.; Tsapas, A.; Wexler, D.J.; Buse, J.B. Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018, 41, 2669–2701. [Google Scholar] [CrossRef]
- Ferrannini, E.; Muscelli, E.; Frascerra, S.; Baldi, S.; Mari, A.; Heise, T.; Broedl, U.C.; Woerle, H.J. Metabolic Response to Sodium-Glucose Cotransporter 2 Inhibition in Type 2 Diabetic Patients. J. Clin. Investig. 2014, 124, 499–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolf, P.; Fellinger, P.; Pfleger, L.; Beiglböck, H.; Krumpolec, P.; Barbieri, C.; Gastaldelli, A.; Harreiter, J.; Metz, M.; Scherer, T.; et al. Gluconeogenesis, But Not Glycogenolysis, Contributes to the Increase in Endogenous Glucose Production by SGLT-2 Inhibition. Diabetes Care 2021, 44, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, M.A.; Eisenbarth, G.S.; Michels, A.W. Type 1 Diabetes. Lancet 2014, 383, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matyka, K.; Dixon, R.M.; Mohn, A.; Rajagopalan, B.; Shmueli, E.; Styles, P.; Dunger, D.B. Daytime Liver Glycogen Accumulation, Measured by 13C Magnetic Resonance Spectroscopy, in Young Children with Type 1 Diabetes Mellitus. Diabet. Med. 2001, 18, 659–662. [Google Scholar] [CrossRef] [PubMed]
- Castle, J.R.; El Youssef, J.; Bakhtiani, P.A.; Cai, Y.; Stobbe, J.M.; Branigan, D.; Ramsey, K.; Jacobs, P.; Reddy, R.; Woods, M.; et al. Effect of Repeated Glucagon Doses on Hepatic Glycogen in Type 1 Diabetes: Implications for a Bihormonal Closed-Loop System. Diabetes Care 2015, 38, 2115–2119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roser, W.; Beckmann, N.; Wiesmann, U.; Seelig, J. Absolute Quantification of the Hepatic Glycogen Content in a Patient with Glycogen Storage Disease by 13C Magnetic Resonance Spectroscopy. Magn. Reson. Imaging 1996, 14, 1217–1220. [Google Scholar] [CrossRef]
- Ardenkjær-Larsen, J.H.; Fridlund, B.; Gram, A.; Hansson, G.; Hansson, L.; Lerche, M.H.; Servin, R.; Thaning, M.; Golman, K. Increase in Signal-to-Noise Ratio of >10,000 Times in Liquid-State NMR. Proc. Natl. Acad. Sci. USA 2003, 100, 10158–10163. [Google Scholar] [CrossRef] [Green Version]
- Golman, K.; Ardenkjær-Larsen, J.H.; Petersson, J.S.; Månsson, S.; Leunbach, I. Molecular Imaging with Endogenous Substances. Proc. Natl. Acad. Sci. USA 2003, 100, 10435–10439. [Google Scholar] [CrossRef] [Green Version]
- Golman, K.; In ’t Zandt, R.; Thaning, M. Real-Time Metabolic Imaging. Proc. Natl. Acad. Sci. USA 2006, 103, 11270–11275. [Google Scholar] [CrossRef] [Green Version]
- Moreno, K.X.; Satapati, S.; DeBerardinis, R.J.; Burgess, S.C.; Malloy, C.R.; Merritt, M.E. Real-Time Detection of Hepatic Gluconeogenic and Glycogenolytic States Using Hyperpolarized [2-13C]Dihydroxyacetone. J. Biol. Chem. 2014, 289, 35859–35867. [Google Scholar] [CrossRef]
- Ragavan, M.; McLeod, M.A.; Giacalone, A.G.; Merritt, M.E. Hyperpolarized Dihydroxyacetone Is a Sensitive Probe of Hepatic Gluconeogenic State. Metabolites 2021, 11, 441. [Google Scholar] [CrossRef] [PubMed]
- Marco-Rius, I.; Wright, A.J.; Hu, D.; Savic, D.; Miller, J.J.; Timm, K.N.; Tyler, D.; Brindle, K.M.; Comment, A. Probing Hepatic Metabolism of [2-13C]Dihydroxyacetone in Vivo with 1H-Decoupled Hyperpolarized 13C-MR. Magn. Reson. Mater. Phys. Biol. Med. 2021, 34, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Chen, A.P.; Zierhut, M.L.; Bok, R.; Yen, Y.F.; Schroeder, M.A.; Hurd, R.E.; Nelson, S.J.; Kurhanewicz, J.; Vigneron, D.B. In Vivo Carbon-13 Dynamic MRS and MRSI of Normal and Fasted Rat Liver with Hyperpolarized 13C-Pyruvate. Mol. Imaging Biol. 2009, 11, 399–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faarkrog Høyer, K.; Laustsen, C.; Ringgaard, S.; Qi, H.; Mariager, C.Ø.; Nielsen, T.S.; Sundekilde, U.K.; Treebak, J.T.; Jessen, N.; Stødkilde-Jørgensen, H. Assessment of Mouse Liver [1-13C]Pyruvate Metabolism by Dynamic Hyperpolarized MRS. J. Endocrinol. 2019, 242, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.M. Carbon-13 NMR Study of the Effects of Fasting and Diabetes on the Metabolism of Pyruvate in the Tricarboxylic Acid Cycle and of the Utilization of Pyruvate and Ethanol in Lipogenesis in Perfused Rat Liver. Biochemistry 1987, 26, 581–589. [Google Scholar] [CrossRef]
- Jin, E.S.; Moreno, K.X.; Wang, J.X.; Fidelino, L.; Merritt, M.E.; Sherry, A.D.; Malloy, C.R. Metabolism of Hyperpolarized [1-13C]Pyruvate through Alternate Pathways in Rat Liver. NMR Biomed. 2016, 29, 466–474. [Google Scholar] [CrossRef]
- Lee, P.; Leong, W.; Tan, T.; Lim, M.; Han, W.; Radda, G.K. In Vivo Hyperpolarized Carbon-13 Magnetic Resonance Spectroscopy Reveals Increased Pyruvate Carboxylase Flux in an Insulin-Resistant Mouse Model. Hepatology 2013, 57, 515–524. [Google Scholar] [CrossRef]
- Leon, B.M. Diabetes and Cardiovascular Disease: Epidemiology, Biological Mechanisms, Treatment Recommendations and Future Research. World J. Diabetes 2015, 6, 1246. [Google Scholar] [CrossRef]
- Le Page, L.M.; Ball, D.R.; Ball, V.; Dodd, M.S.; Miller, J.J.; Heather, L.C.; Tyler, D.J. Simultaneous in Vivo Assessment of Cardiac and Hepatic Metabolism in the Diabetic Rat Using Hyperpolarized MRS. NMR Biomed. 2016, 29, 1759–1767. [Google Scholar] [CrossRef]
- Meyer, C.; Stumvoll, M.; Nadkarni, V.; Dostou, J.; Mitrakou, A.; Gerich, J. Abnormal Renal and Hepatic Glucose Metabolism in Type 2 Diabetes Mellitus. J. Clin. Investig. 1998, 102, 619–624. [Google Scholar] [CrossRef]
- von Morze, C.; Chang, G.Y.; Larson, P.E.Z.; Shang, H.; Allu, P.K.R.; Bok, R.A.; Crane, J.C.; Olson, M.P.; Tan, C.T.; Marco-Rius, I.; et al. Detection of Localized Changes in the Metabolism of Hyperpolarized Gluconeogenic Precursors 13C-Lactate and 13C-Pyruvate in Kidney and Liver. Magn. Reson. Med. 2017, 77, 1429–1437. [Google Scholar] [CrossRef] [Green Version]
- Von Morze, C.; Allu, P.K.R.; Chang, G.Y.; Marco-Rius, I.; Milshteyn, E.; Wang, Z.J.; Ohliger, M.A.; Gleason, C.E.; Kurhanewicz, J.; Vigneron, D.B.; et al. Non-Invasive Detection of Divergent Metabolic Signals in Insulin Deficiency vs. Insulin Resistance in Vivo. Sci. Rep. 2018, 8, 2088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.J.; Ohliger, M.A.; Larson, P.E.Z.; Gordon, J.W.; Bok, R.A.; Slater, J.; Villanueva-Meyer, J.E.; Hess, C.P.; Kurhanewicz, J.; Vigneron, D.B. Hyperpolarized 13C MRI: State of the Art and Future Directions. Radiology 2019, 291, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.M.; Chen, H.Y.; Gordon, J.W.; Wang, Z.J.; Bok, R.; Hashoian, R.; Kim, Y.; Liu, X.; Nickles, T.; Cheung, K.; et al. Whole-Abdomen Metabolic Imaging of Healthy Volunteers Using Hyperpolarized [1-13C]Pyruvate MRI. J. Magn. Reson. Imaging 2022, 56, 1792–1806. [Google Scholar] [CrossRef] [PubMed]
- de Graaf, R.A.; Hendriks, A.D.; Klomp, D.W.J.; Kumaragamage, C.; Welting, D.; Arteaga de Castro, C.S.; Brown, P.B.; McIntyre, S.; Nixon, T.W.; Prompers, J.J.; et al. On the Magnetic Field Dependence of Deuterium Metabolic Imaging. NMR Biomed. 2020, 33, e4235. [Google Scholar] [CrossRef]
- Ladd, M.E.; Bachert, P.; Meyerspeer, M.; Moser, E.; Nagel, A.M.; Norris, D.G.; Schmitter, S.; Speck, O.; Straub, S.; Zaiss, M. Pros and Cons of Ultra-High-Field MRI/MRS for Human Application. Prog. Nucl. Magn. Reson. Spectrosc. 2018, 109, 1–50. [Google Scholar] [CrossRef]
- Poli, S.; Emara, A.F.; Ballabani, E.; Buser, A.; Schiavon, M.; Herzig, D.; Man, C.D.; Tappy, L.; Kreis, R.; Bally, L. Real-Time Observation of Postprandial Hepatic Glucose Metabolism with Interleaved 2H Metabolic Imaging and 13C-MRS at 7 T. In Proceedings of the 2022 Joint Annual Meeting ISMRM-ESMRMB & ISMRT 31st Annual Meeting, London, UK, 7–12 May 2022; p. 628. [Google Scholar]
- Gursan, A.; Hendriks, A.D.; Welting, D.; Klomp, D.W.J.; Prompers, J.J. Monitoring Glucose Uptake and Metabolism in Human Kidney with Dynamic 3D DMI at 7 T. In Proceedings of the 2022 Joint Annual Meeting ISMRM-ESMRMB & ISMRT 31st Annual Meeting, London, UK, 7–12 May 2022; p. 128. [Google Scholar]
- Gursan, A.; Hendriks, A.D.; Welting, D.; Klomp, D.W.J.; Prompers, J.J. Measurement of Gastric Emptying with Dynamic 3D DMI Using a Deuterium Body Array at 7 T. In Proceedings of the 2022 Joint Annual Meeting ISMRM-ESMRMB & ISMRT 31st Annual Meeting, London, UK, 7–12 May 2022; p. 2542. [Google Scholar]
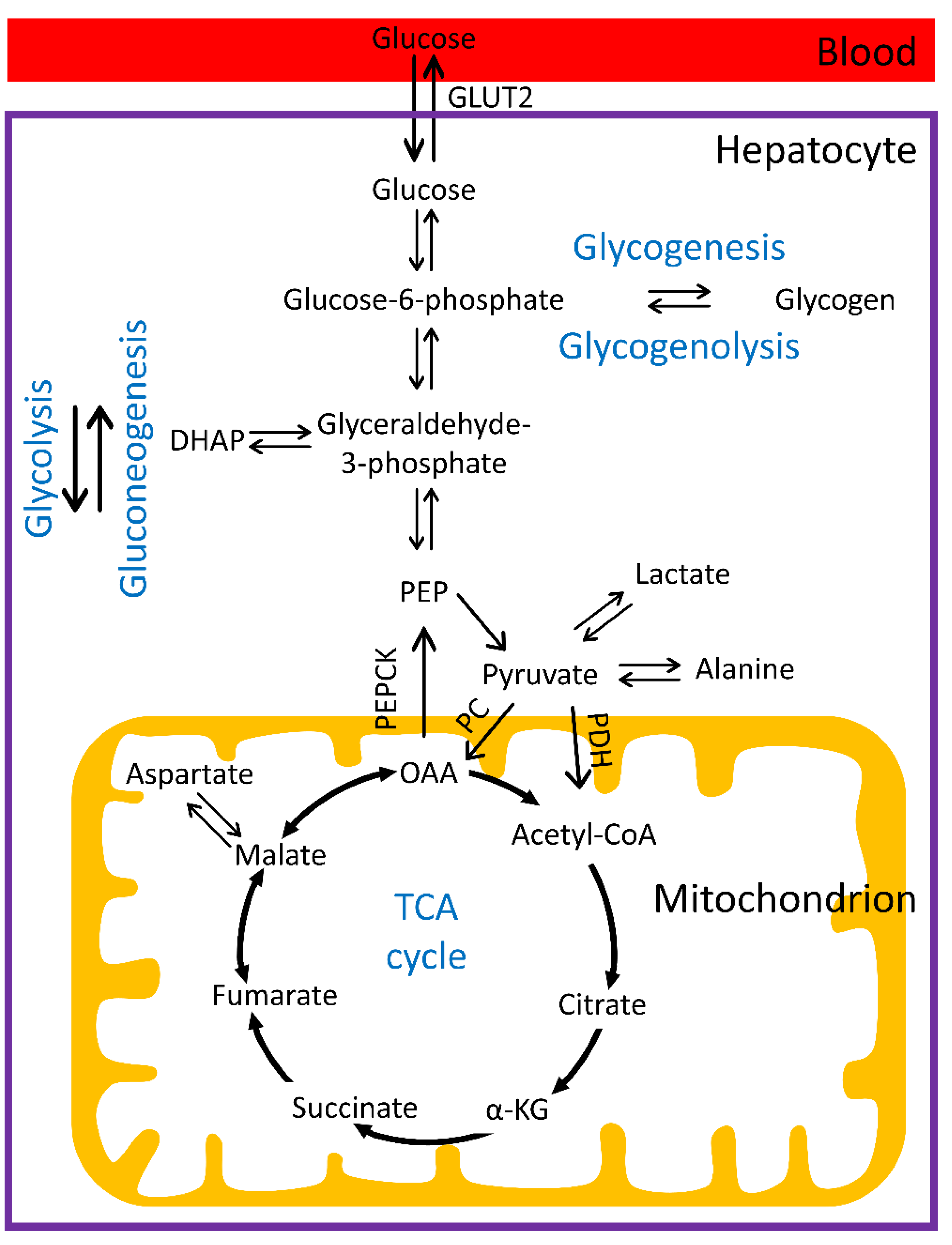
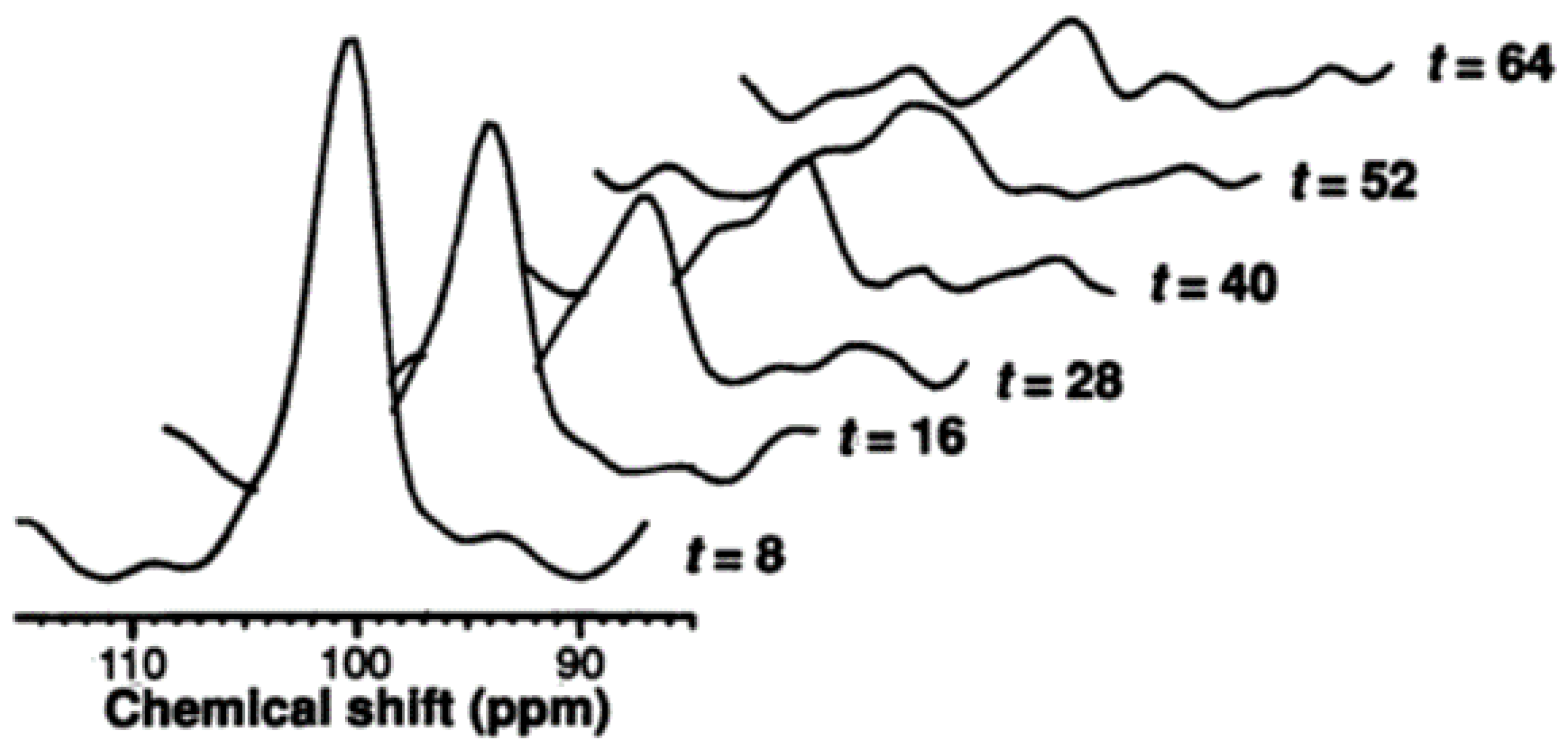
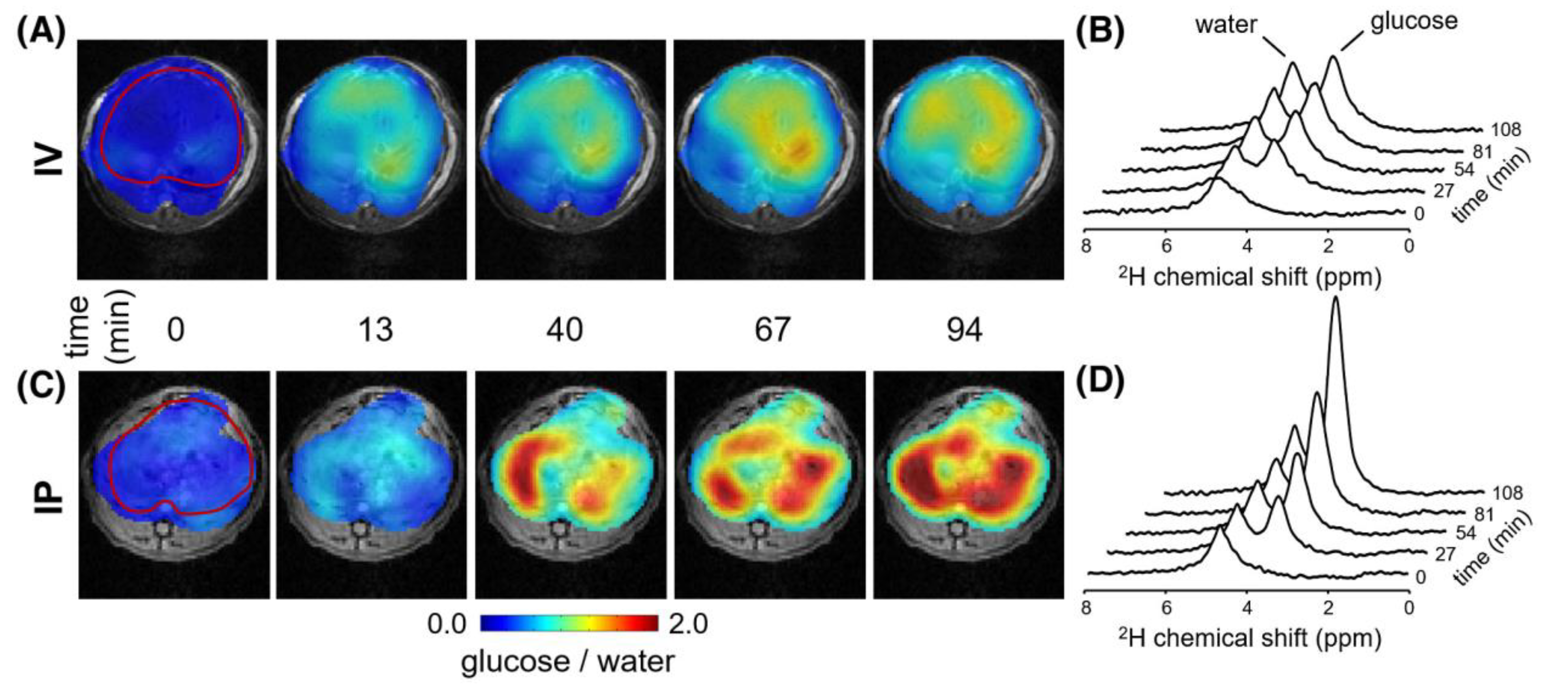

| Technique | Measurement | Metabolic Process in the Liver | Insights | References |
|---|---|---|---|---|
| 13C MRS | Liver glycogen levels after meal/glucose intake | Glycogenesis | In healthy subjects, about 17% of an oral glucose load is stored as glycogen in the liver and net hepatic glycogenesis continues up to 4 h after meal intake. | [24,25] |
| Hepatic glycogenesis is reduced in T2D, MODY, and (adult) T1D patients. | [8,21,31,45,46,47] | |||
| (Long-term intensive) insulin treatment restores (or even normalizes) glycogenesis in T1D patients. | [20,21,22] | |||
| Liver glycogen levels during fasting | Glycogenolysis | In healthy subjects, the contribution of hepatic glycogenolysis to whole-body glucose production is ~45% during the first period of fasting, and it gradually declines thereafter. | [7,18] | |
| Hepatic glycogenolysis is reduced in T2D and T1D patients. | [21,45,46,49,52] | |||
| Metformin tends to increase glycogenolysis in T2D patients, but the SGLT-2 inhibitor dapagliflozin has no effect on glycogenolysis. | [49,52] | |||
| (Long-term intensive) insulin treatment restores (or even normalizes) glycogenolysis in T1D patients. | [21,22] | |||
| Liver glycogen levels during fasting, in combination with plasma measurements of whole body glucose production | Gluconeogenesis | In healthy subjects, the contribution of hepatic gluconeogenesis to whole-body glucose production during early fasting is more than 50% and remains relatively constant with longer fasting. | [7,18] | |
| Hepatic gluconeogenesis is elevated in T2D, MODY, and T1D patients. | [8,45,46,47,49,52] | |||
| Metformin reduces gluconeogenesis in T2D patients, but the SGLT-2 inhibitor dapagliflozin has no effect on gluconeogenesis in T2D patients, while it increases gluconeogenesis in healthy subjects. | [49,52] | |||
| Long-term intensive insulin treatment normalizes gluconeogenesis in T1D patients. | [20,22] | |||
| 13C MRS/DMI | Liver glucose levels after [1-13C]glucose/[6,6′-2H2]glucose administration, either orally/IP or IV | Glucose uptake | Hepatic glucose uptake is higher after oral/IP glucose administration compared to IV infusion. | [29,38] |
| Hyperpolarized 13C MRS | 13C labeling of hexoses relative to 3-carbon intermediates after infusion of [2-13C]DHA | Gluconeogenesis (vs. glycolysis) | Hepatic gluconeogenesis is elevated under fasting conditions and in a T2D animal model. | [60,61] |
| 13C labeling of [1-13C]alanine and [1-13C]lactate after infusion of [1-13C]pyruvate | Gluconeogenesis | Hepatic gluconeogenesis is elevated under fasting conditions and in T2D and T1D animal models. | [5,63,64,69,71,72] | |
| 13C labeling of [1-13C]malate and [1-13C]aspartate after infusion of [1-13C]pyruvate | PC flux (gluconeogenesis) | Hepatic PC flux is elevated in a T2D animal model and can be reduced with metformin. | [67] | |
| 13C labeling of [1-13C]bicarbonate after infusion of [1-13C]pyruvate | PC and/or PDH flux | Under fasting conditions, PC flux is dominant in the liver, but PDH flux is dominant in fed conditions. | [5,64,66] | |
| Hepatic PDH flux is decreased in a T2D animal model. | [69] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gursan, A.; Prompers, J.J. Magnetic Resonance Imaging and Spectroscopy Methods to Study Hepatic Glucose Metabolism and Their Applications in the Healthy and Diabetic Liver. Metabolites 2022, 12, 1223. https://doi.org/10.3390/metabo12121223
Gursan A, Prompers JJ. Magnetic Resonance Imaging and Spectroscopy Methods to Study Hepatic Glucose Metabolism and Their Applications in the Healthy and Diabetic Liver. Metabolites. 2022; 12(12):1223. https://doi.org/10.3390/metabo12121223
Chicago/Turabian StyleGursan, Ayhan, and Jeanine J. Prompers. 2022. "Magnetic Resonance Imaging and Spectroscopy Methods to Study Hepatic Glucose Metabolism and Their Applications in the Healthy and Diabetic Liver" Metabolites 12, no. 12: 1223. https://doi.org/10.3390/metabo12121223
APA StyleGursan, A., & Prompers, J. J. (2022). Magnetic Resonance Imaging and Spectroscopy Methods to Study Hepatic Glucose Metabolism and Their Applications in the Healthy and Diabetic Liver. Metabolites, 12(12), 1223. https://doi.org/10.3390/metabo12121223







