Phytochemistry, Pharmacology and Molecular Mechanisms of Herbal Bioactive Compounds for Sickness Behaviour
Abstract
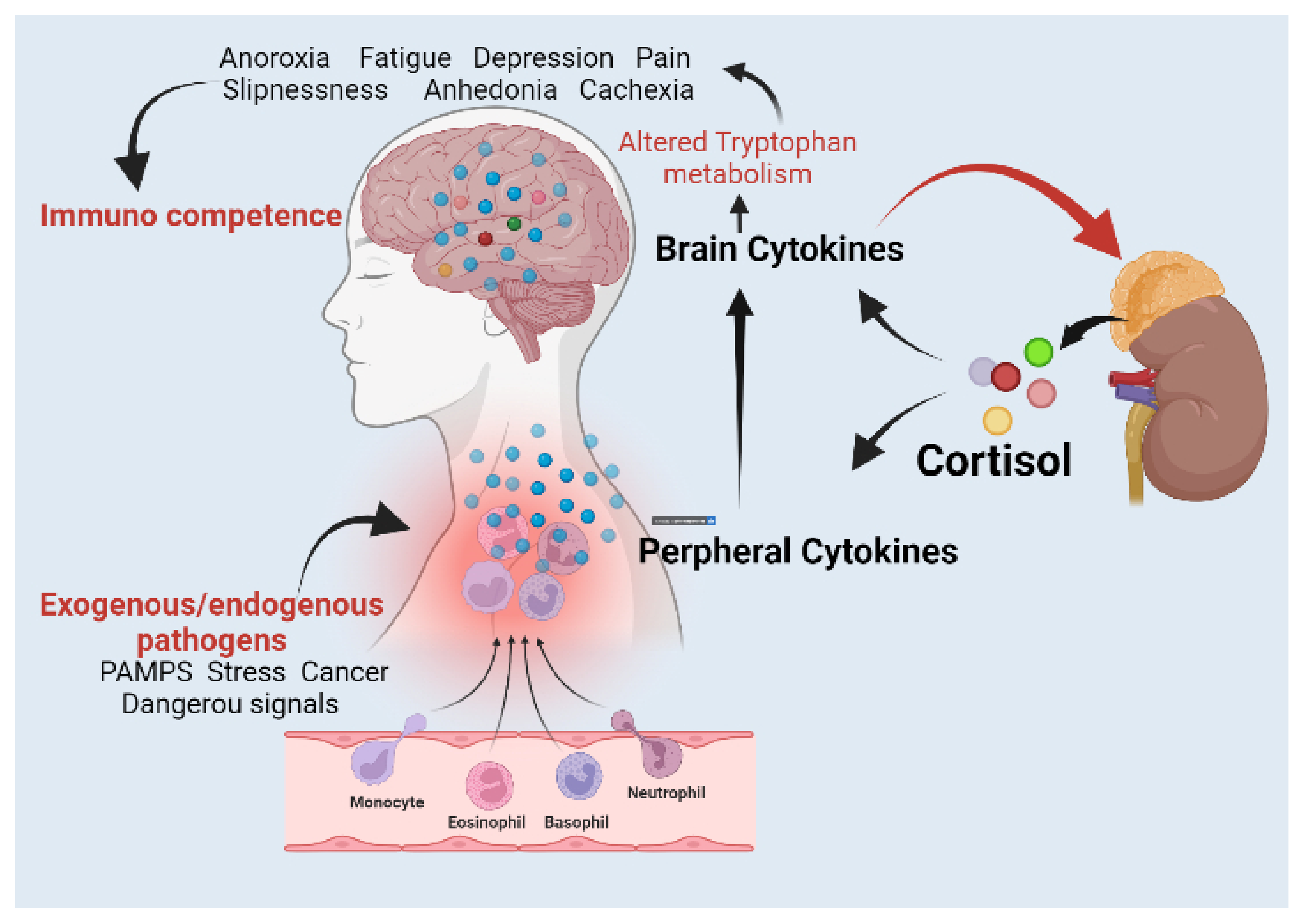
:1. Introduction
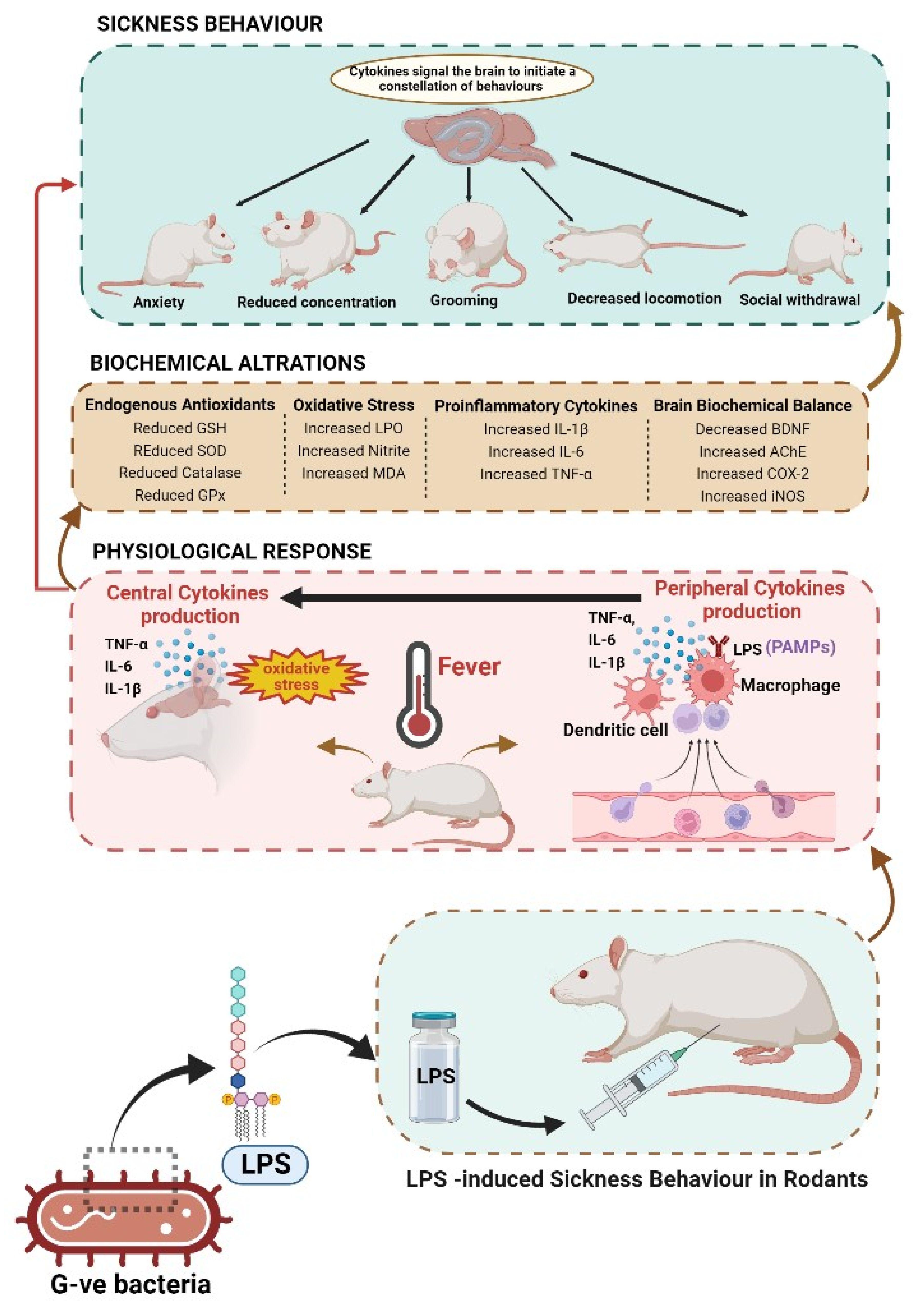
Lipopolysaccharide (LPS)-Induced Sickness Behaviour Model
2. Methods
3. Results
3.1. Selection of Articles
3.2. HBACs Conferring Protection in Mice
3.2.1. Ursolic Acid
3.2.2. Taraxasterol
3.2.3. Curcumin
3.2.4. Honokiol
3.2.5. Mangiferin
3.2.6. Esculetin
3.2.7. Caffeic Acid
3.2.8. Embelin
3.2.9. Gomisin N
3.2.10. Liquiritigenin
3.2.11. Paeonol
3.2.12. Trans-Astaxanthin
3.2.13. 2,3,4′,5-Tetrahydroxystilbene-2-O-β-D-glucoside
3.2.14. Ginsenoside Rg3
3.2.15. Lonchocarpine
3.2.16. Methyl Jasmonate
3.2.17. Proanthocyanidin
3.2.18. Gentiopicroside
3.2.19. Selanylimidazopyridine
3.2.20. 25-methoxyhispidol
3.2.21. Macranthol
3.2.22. Hesperidin
3.2.23. Resveratrol
3.2.24. Solidagenone
3.2.25. Diallyl Disulfide
3.2.26. Rosmarinic Acid
3.3. HBACs Conferring Protection in Rats
3.3.1. Paeoniflorin
3.3.2. Parthenolide
3.3.3. Quercetin
3.3.4. Thymoquinone
3.3.5. Gypenosides
3.3.6. Isovitexin
3.3.7. Carvacrol
3.3.8. Ellagic Acid
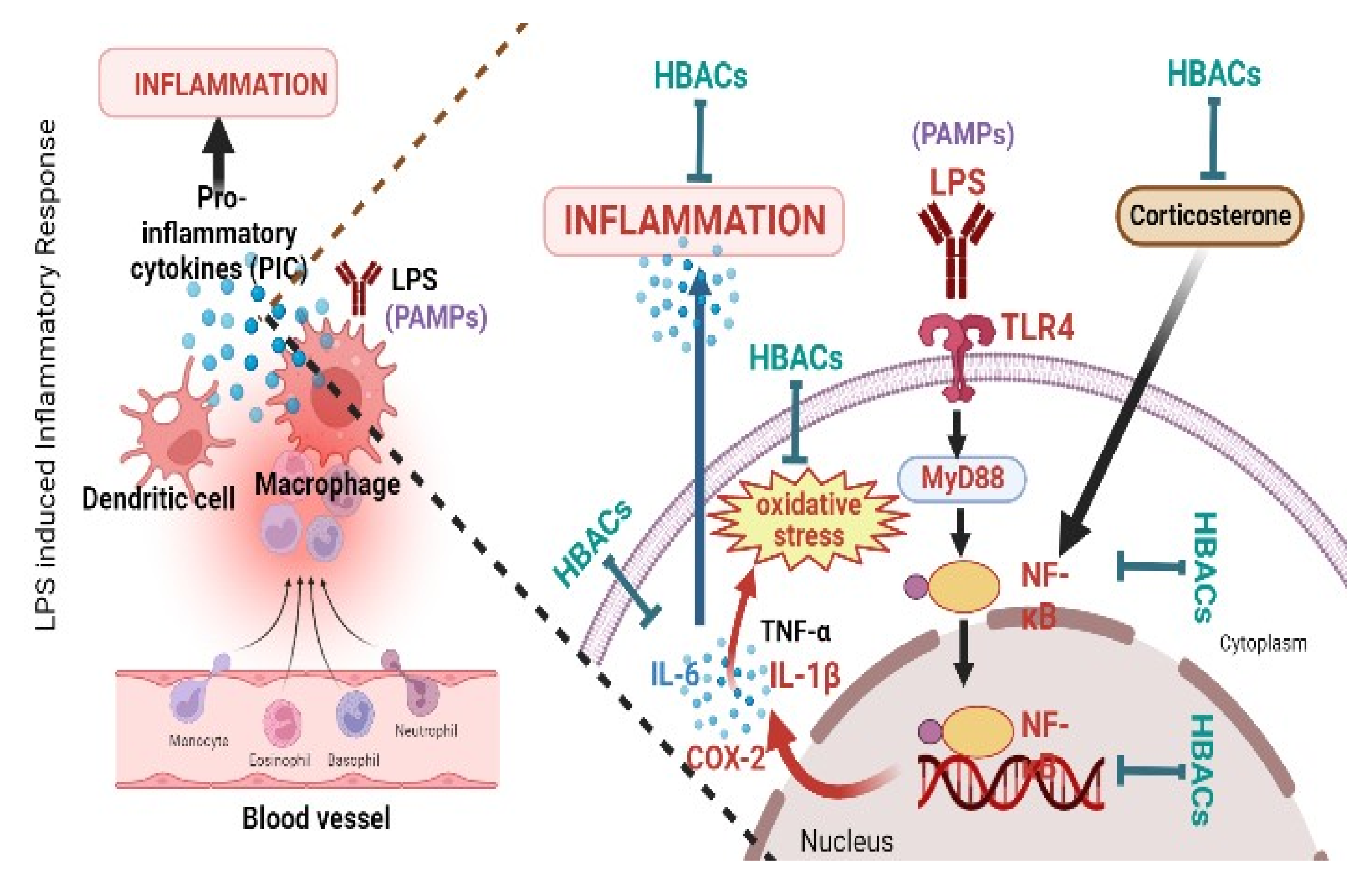
4. Mechanism of Action(s) of HBACs against LPS-Induced Sickness Behaviour
5. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alshehri, S.; Imam, S.S. Rosinidin Attenuates Lipopolysaccharide-Induced Memory Impairment in Rats: Possible Mechanisms of Action Include Antioxidant and Anti-Inflammatory Effects. Biomolecules 2021, 11, 1747. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, C.; Maclullich, A.M. At the extreme end of the psychoneuroimmunological spectrum: Delirium as a maladaptive sickness behaviour response. Brain Behav. Immun. 2013, 28, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maes, M.; Berk, M.; Goehler, L.; Song, C.; Anderson, G.; Gałecki, P.; Leonard, B. Depression and sickness behavior are Janus-faced responses to shared inflammatory pathways. BMC Med. 2012, 10, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilsterman, K.; Alonge, M.M.; Ernst, D.K.; Limber, C.; Treidel, L.A.; Bentley, G.E. Flexibility in an emergency life-history stage: Acute food deprivation prevents sickness behaviour but not the immune response. Proc. R. Soc. B Biol. Sci. 2020, 287, 20200842. [Google Scholar] [CrossRef] [PubMed]
- Prather, A.A. Sickness Behavior. In Encyclopedia of Behavioral Medicine; Gellman, M.D., Turner, J.R., Eds.; Springer New York: New York, NY, USA, 2013; pp. 1786–1788. [Google Scholar]
- Johnson, R.W. The concept of sickness behavior: A brief chronological account of four key discoveries. Vet Immunol Immunopathol 2002, 87, 443–450. [Google Scholar] [CrossRef]
- McCusker, R.H.; Kelley, K.W. Immune-neural connections: How the immune system’s response to infectious agents influences behavior. J. Exp. Biol. 2013, 216, 84–98. [Google Scholar] [CrossRef] [Green Version]
- Turner, M.D.; Nedjai, B.; Hurst, T.; Pennington, D.J. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2014, 1843, 2563–2582. [Google Scholar] [CrossRef] [Green Version]
- Eisenberger, N.I.; Moieni, M.; Inagaki, T.K.; Muscatell, K.A.; Irwin, M.R. In Sickness and in Health: The Co-Regulation of Inflammation and Social Behavior. Neuropsychopharmacology 2017, 42, 242–253. [Google Scholar] [CrossRef]
- Dantzer, R.; BluthÉ, R.-M.; Castanon, N.; Kelley, K.W.; Konsman, J.-P.; Laye, S.; Lestage, J.; Parnet, P. CHAPTER 14—Cytokines, Sickness Behavior, and Depression. In Psychoneuroimmunology, 4th ed.; Ader, R., Ed.; Academic Press: Burlington, NJ, USA, 2007; pp. 281–318. [Google Scholar]
- Shaikh, A.; Dhadde, S.B.; Durg, S.; Veerapur, V.P.; Badami, S.; Thippeswamy, B.S.; Patil, J.S. Effect of Embelin Against Lipopolysaccharide-induced Sickness Behaviour in Mice. Phytother. Res. PTR 2016, 30, 815–822. [Google Scholar] [CrossRef]
- Maldonado, R.F.; Sá-Correia, I.; Valvano, M.A. Lipopolysaccharide modification in Gram-negative bacteria during chronic infection. FEMS Microbiol. Rev. 2016, 40, 480–493. [Google Scholar] [CrossRef]
- Lasselin, J.; Schedlowski, M.; Karshikoff, B.; Engler, H.; Lekander, M.; Konsman, J.P. Comparison of bacterial lipopolysaccharide-induced sickness behavior in rodents and humans: Relevance for symptoms of anxiety and depression. Neurosci. Biobehav. Rev. 2020, 115, 15–24. [Google Scholar] [CrossRef]
- Sadraie, S.; Kiasalari, Z.; Razavian, M.; Azimi, S.; Sedighnejad, L.; Afshin-Majd, S.; Baluchnejadmojarad, T.; Roghani, M. Berberine ameliorates lipopolysaccharide-induced learning and memory deficit in the rat: Insights into underlying molecular mechanisms. Metab. Brain Dis. 2019, 34, 245–255. [Google Scholar] [CrossRef]
- Wang, M.; Li, H.; Wang, Y.; Hao, Y.; Huang, Y.; Wang, X.; Lu, Y.; Du, Y.; Fu, F.; Xin, W.; et al. Anti-Rheumatic Properties of Gentiopicroside Are Associated with Suppression of ROS-NF-κB-NLRP3 Axis in Fibroblast-Like Synoviocytes and NF-κB Pathway in Adjuvant-Induced Arthritis. Front. Pharm. 2020, 11, 515. [Google Scholar] [CrossRef]
- Shivasharan, B.D.; Nagakannan, P.; Thippeswamy, B.S.; Veerapur, V.P. Protective Effect of Calendula officinalis L. Flowers Against Monosodium Glutamate Induced Oxidative Stress and Excitotoxic Brain Damage in Rats. Indian J. Clin. Biochem. IJCB 2013, 28, 292–298. [Google Scholar] [CrossRef] [Green Version]
- Adebesin, A.; Adeoluwa, O.A.; Eduviere, A.T.; Umukoro, S. Methyl jasmonate attenuated lipopolysaccharide-induced depressive-like behaviour in mice. J. Psychiatr. Res. 2017, 94, 29–35. [Google Scholar] [CrossRef]
- Araki, R.; Hiraki, Y.; Nishida, S.; Inatomi, Y.; Yabe, T. Gomisin N ameliorates lipopolysaccharide-induced depressive-like behaviors by attenuating inflammation in the hypothalamic paraventricular nucleus and central nucleus of the amygdala in mice. J. Pharmacol. Sci. 2016, 132, 138–144. [Google Scholar] [CrossRef] [Green Version]
- Bargi, R.; Asgharzadeh, F.; Beheshti, F.; Hosseini, M.; Sadeghnia, H.R.; Khazaei, M. The effects of thymoquinone on hippocampal cytokine level, brain oxidative stress status and memory deficits induced by lipopolysaccharide in rats. Cytokine 2017, 96, 173–184. [Google Scholar] [CrossRef]
- Chen, Z.; Huang, C.; He, H.; Ding, W. 2, 3, 5, 4′-Tetrahydroxystilbene-2-O-β-D-glucoside prevention of lipopolysaccharide-induced depressive-like behaviors in mice involves neuroinflammation and oxido-nitrosative stress inhibition. Behav. Pharmacol. 2017, 28, 365–374. [Google Scholar] [CrossRef]
- Deng, Y.-t.; Zhao, M.-g.; Xu, T.-j.; Jin, H.; Li, X.-h. Gentiopicroside abrogates lipopolysaccharide-induced depressive-like behavior in mice through tryptophan-degrading pathway. Metab. Brain Dis. 2018, 33, 1413–1420. [Google Scholar] [CrossRef]
- Domingues, M.; Casaril, A.M.; Birmann, P.T.; Lourenço, D.d.A.; Vieira, B.; Begnini, K.; Lenardão, E.J.; Collares, T.; Seixas, F.K.; Savegnago, L. Selanylimidazopyridine Prevents Lipopolysaccharide-Induced Depressive-Like Behavior in Mice by Targeting Neurotrophins and Inflammatory/Oxidative Mediators. Front. Neurosci. 2018, 12, 486. [Google Scholar] [CrossRef] [Green Version]
- Dornelles, G.L.; de Oliveira, J.S.; de Almeida, E.J.R.; Mello, C.B.E.; e Rodrigues, B.R.; da Silva, C.B.; Petry, L.d.S.; Pillat, M.M.; Palma, T.V.; de Andrade, C.M. Ellagic Acid Inhibits Neuroinflammation and Cognitive Impairment Induced by Lipopolysaccharides. Neurochem. Res. 2020, 45, 2456–2473. [Google Scholar] [CrossRef] [PubMed]
- Sulakhiya, K.; Kumar, P.; Gurjar, S.S.; Barua, C.C.; Hazarika, N.K. Beneficial effect of honokiol on lipopolysaccharide induced anxiety-like behavior and liver damage in mice. Pharmacol. Biochem. Behav. 2015, 132, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Jangra, A.; Lukhi, M.M.; Sulakhiya, K.; Baruah, C.C.; Lahkar, M. Protective effect of mangiferin against lipopolysaccharide-induced depressive and anxiety-like behaviour in mice. Eur. J. Pharmacol. 2014, 740, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Jangra, A.; Kwatra, M.; Singh, T.; Pant, R.; Kushwah, P.; Sharma, Y.; Saroha, B.; Datusalia, A.K.; Bezbaruah, B.K. Piperine Augments the Protective Effect of Curcumin Against Lipopolysaccharide-Induced Neurobehavioral and Neurochemical Deficits in Mice. Inflammation 2016, 39, 1025–1038. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.-H.; Park, J.-S.; Kim, D.-H.; Kang, J.L.; Kim, H.-S. Anti-inflammatory mechanism of lonchocarpine in LPS- or poly(I:C)-induced neuroinflammation. Pharmacol. Res. 2017, 119, 431–442. [Google Scholar] [CrossRef]
- Jiang, X.; Chen, L.; Shen, L.; Chen, Z.; Xu, L.; Zhang, J.; Yu, X. Trans-astaxanthin attenuates lipopolysaccharide-induced neuroinflammation and depressive-like behavior in mice. Brain Res. 2016, 1649, 30–37. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, J.; Lin, Q.; Mao, K.; Tian, F.; Jing, C.; Wang, C.; Ding, L.; Pang, C. Proanthocyanidin prevents lipopolysaccharide-induced depressive-like behavior in mice via neuroinflammatory pathway. Brain Res. Bull. 2017, 135, 40–46. [Google Scholar] [CrossRef]
- Kang, A.; Xie, T.; Zhu, D.; Shan, J.; Di, L.; Zheng, X. Suppressive Effect of Ginsenoside Rg3 against Lipopolysaccharide-Induced Depression-Like Behavior and Neuroinflammation in Mice. J. Agric. Food Chem. 2017, 65, 6861–6869. [Google Scholar] [CrossRef]
- Kim, I.D.; Ha, B.J. The effects of paeoniflorin on LPS-induced liver inflammatory reactions. Arch. Pharmacal Res. 2010, 33, 959–966. [Google Scholar] [CrossRef]
- Kwatra, M.; Ahmed, S.; Gawali, B.; Panda, S.R.; Naidu, V.G.M. Hesperidin alleviates chronic restraint stress and lipopolysaccharide-induced Hippocampus and Frontal cortex damage in mice: Role of TLR4/NF-κB, p38 MAPK/JNK, Nrf2/ARE signaling. Neurochem. Int. 2020, 140, 104835. [Google Scholar] [CrossRef]
- Lee, B.; Yeom, M.; Shim, I.; Lee, H.; Hahm, D.-h. Inhibitory effect of carvacrol on lipopolysaccharide-induced memory impairment in rats. KJPP 2019, 24, 27–37. [Google Scholar] [CrossRef] [Green Version]
- Liao, Y.R.; Lin, J.Y. Quercetin intraperitoneal administration ameliorates lipopolysaccharide-induced systemic inflammation in mice. Life Sci. 2015, 137, 89–97. [Google Scholar] [CrossRef]
- Liu, B.; Huang, B.; Hu, G.; He, D.; Li, Y.; Ran, X.; Du, J.; Fu, S.; Liu, D. Isovitexin-Mediated Regulation of Microglial Polarization in Lipopolysaccharide-Induced Neuroinflammation via Activation of the CaMKKβ/AMPK-PGC-1α Signaling Axis. Front. Immunol. 2019, 10, 2650. [Google Scholar] [CrossRef]
- Locateli, G.; de Oliveira Alves, B.; Miorando, D.; Ernetti, J.; Alievi, K.; Zilli, G.A.L.; Serpa, P.Z.; Vecchia, C.A.D.; Mota da Silva, L.; Müller, L.G.; et al. Antidepressant-like effects of solidagenone on mice with bacterial lipopolysaccharide (LPS)-induced depression. Behav. Brain Res. 2020, 395, 112863. [Google Scholar] [CrossRef]
- Basu Mallik, S.; Mudgal, J.; Nampoothiri, M.; Hall, S.; Dukie, S.A.; Grant, G.; Rao, C.M.; Arora, D. Caffeic acid attenuates lipopolysaccharide-induced sickness behaviour and neuroinflammation in mice. Neurosci. Lett. 2016, 632, 218–223. [Google Scholar] [CrossRef]
- Thingore, C.; Kshirsagar, V.; Juvekar, A. Amelioration of oxidative stress and neuroinflammation in lipopolysaccharide-induced memory impairment using Rosmarinic acid in mice. Metab. Brain Dis. 2021, 36, 299–313. [Google Scholar] [CrossRef]
- Rummel, C.; Gerstberger, R.; Roth, J.; Hübschle, T. Parthenolide attenuates LPS-induced fever, circulating cytokines and markers of brain inflammation in rats. Cytokine 2011, 56, 739–748. [Google Scholar] [CrossRef]
- Sah, S.P.; Tirkey, N.; Kuhad, A.; Chopra, K. Effect of quercetin on lipopolysaccharide induced-sickness behavior and oxidative stress in rats. Indian J. Pharmacol. 2011, 43, 192–196. [Google Scholar] [CrossRef] [Green Version]
- Shal, B.; Khan, A.; Naveed, M.; Ali, H.; Seo, E.K.; Choi, H.; Khan, S. Neuroprotective effect of 25-Methoxyhispidol A against CCl(4)-induced behavioral alterations by targeting VEGF/BDNF and caspase-3 in mice. Life Sci. 2020, 253, 117684. [Google Scholar] [CrossRef]
- Sorrenti, V.; Contarini, G.; Sut, S.; Dall’Acqua, S.; Confortin, F.; Pagetta, A.; Giusti, P.; Zusso, M. Curcumin Prevents Acute Neuroinflammation and Long-Term Memory Impairment Induced by Systemic Lipopolysaccharide in Mice. Front. Pharm. 2018, 9, 183. [Google Scholar] [CrossRef]
- Su, Q.; Tao, W.; Huang, H.; Du, Y.; Chu, X.; Chen, G. Protective effect of liquiritigenin on depressive-like behavior in mice after lipopolysaccharide administration. Psychiatry Res. 2016, 240, 131–136. [Google Scholar] [CrossRef]
- Sulakhiya, K.; Kumar, P.; Jangra, A.; Dwivedi, S.; Hazarika, N.K.; Baruah, C.C.; Lahkar, M. Honokiol abrogates lipopolysaccharide-induced depressive like behavior by impeding neuroinflammation and oxido-nitrosative stress in mice. Eur. J. Pharmacol. 2014, 744, 124–131. [Google Scholar] [CrossRef]
- Tao, W.; Wang, H.; Su, Q.; Chen, Y.; Xue, W.; Xia, B.; Duan, J.; Chen, G. Paeonol attenuates lipopolysaccharide-induced depressive-like behavior in mice. Psychiatry Res. 2016, 238, 116–121. [Google Scholar] [CrossRef]
- Tian, Q.; Fan, X.; Ma, J.; Han, Y.; Li, D.; Jiang, S.; Zhang, F.; Guang, H.; Shan, X.; Chen, R.; et al. Resveratrol ameliorates lipopolysaccharide-induced anxiety-like behavior by attenuating YAP-mediated neuro-inflammation and promoting hippocampal autophagy in mice. Toxicol. Appl. Pharmacol. 2020, 408, 115261. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Lu, J.; Wu, D.-m.; Zheng, Z.-h.; Zheng, Y.-L.; Wang, X.-h.; Ruan, J.; Sun, X.; Shan, Q.; Zhang, Z.-f. Ursolic acid attenuates lipopolysaccharide-induced cognitive deficits in mouse brain through suppressing p38/NF-κB mediated inflammatory pathways. Neurobiol. Learn. Mem. 2011, 96, 156–165. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Q.; Yuan, L.; Wang, S.; Liu, L.; Yang, X.; Li, G.; Liu, D. The effects of curcumin on depressive-like behavior in mice after lipopolysaccharide administration. Behav. Brain Res. 2014, 274, 282–290. [Google Scholar] [CrossRef]
- Wei, X.; Ma, Y.; Li, F.; He, H.; Huang, H.; Huang, C.; Chen, Z.; Chen, D.; Chen, J.; Yuan, X. Acute Diallyl Disulfide Administration Prevents and Reveres Lipopolysaccharide-Induced Depression-Like Behaviors in Mice via Regulating Neuroinflammation and Oxido-Nitrosative Stress. Inflammation 2021, 44, 1381–1395. [Google Scholar] [CrossRef]
- Weng, L.; Dong, S.; Wang, S.; Yi, L.; Geng, D. Macranthol attenuates lipopolysaccharide-induced depressive-like behaviors by inhibiting neuroinflammation in prefrontal cortex. Physiol. Behav. 2019, 204, 33–40. [Google Scholar] [CrossRef]
- Zhang, X.; Xiong, H.; Li, H.; Cheng, Y. Protective effect of taraxasterol against LPS-induced endotoxic shock by modulating inflammatory responses in mice. Immunopharmacol. Immunotoxicol. 2014, 36, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-x.; Li, E.; Yan, J.-p.; Fu, W.; Shen, P.; Tian, S.-W.; You, Y. Apelin attenuates depressive-like behavior and neuroinflammation in rats co-treated with chronic stress and lipopolysaccharide. Neuropeptides 2019, 77, 101959. [Google Scholar] [CrossRef]
- Zhu, L.; Nang, C.; Luo, F.; Pan, H.; Zhang, K.; Liu, J.; Zhou, R.; Gao, J.; Chang, X.; He, H.; et al. Esculetin attenuates lipopolysaccharide (LPS)-induced neuroinflammatory processes and depressive-like behavior in mice. Physiol. Behav. 2016, 163, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.Y.; Lee, S.R.; Heo, J.-W.; No, M.-H.; Rhee, B.D.; Ko, K.S.; Kwak, H.-B.; Han, J. Ursolic acid in health and disease. Korean J. Physiol. Pharm. 2018, 22, 235–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wirngo, F.E.; Lambert, M.N.; Jeppesen, P.B. The Physiological Effects of Dandelion (Taraxacum Officinale) in Type 2 Diabetes. Rev. Diabet. Stud. 2016, 13, 113–131. [Google Scholar] [CrossRef] [Green Version]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef] [Green Version]
- Rinwa, P.; Kumar, A. Piperine potentiates the protective effects of curcumin against chronic unpredictable stress-induced cognitive impairment and oxidative damage in mice. Brain Res. 2012, 1488, 38–50. [Google Scholar] [CrossRef]
- Singh, S.; Jamwal, S.; Kumar, P. Piperine Enhances the Protective Effect of Curcumin Against 3-NP Induced Neurotoxicity: Possible Neurotransmitters Modulation Mechanism. Neurochem. Res. 2015, 40, 1758–1766. [Google Scholar] [CrossRef]
- Woodbury, A.; Yu, S.P.; Wei, L.; Garcia, P. Neuro-Modulating Effects of Honokiol: A Review. Front. Neurol. 2013, 4, 130. [Google Scholar] [CrossRef] [Green Version]
- Imran, M.; Arshad, M.S.; Butt, M.S.; Kwon, J.-H.; Arshad, M.U.; Sultan, M.T. Mangiferin: A natural miracle bioactive compound against lifestyle related disorders. Lipids Health Dis. 2017, 16, 84. [Google Scholar] [CrossRef]
- Liang, C.; Ju, W.; Pei, S.; Tang, Y.; Xiao, Y. Pharmacological Activities and Synthesis of Esculetin and Its Derivatives: A Mini-Review. Molecules 2017, 22, 387. [Google Scholar] [CrossRef] [Green Version]
- Sulakhiya, K.; Keshavlal, G.P.; Bezbaruah, B.B.; Dwivedi, S.; Gurjar, S.S.; Munde, N.; Jangra, A.; Lahkar, M.; Gogoi, R. Lipopolysaccharide induced anxiety- and depressive-like behaviour in mice are prevented by chronic pre-treatment of esculetin. Neurosci. Lett. 2016, 611, 106–111. [Google Scholar] [CrossRef]
- Espíndola, K.M.M.; Ferreira, R.G.; Narvaez, L.E.M.; Silva Rosario, A.C.R.; da Silva, A.H.M.; Silva, A.G.B.; Vieira, A.P.O.; Monteiro, M.C. Chemical and Pharmacological Aspects of Caffeic Acid and Its Activity in Hepatocarcinoma. Front. Oncol. 2019, 9, 541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhadde, S.B.; Nagakannan, P.; Roopesh, M.; Anand Kumar, S.R.; Thippeswamy, B.S.; Veerapur, V.P.; Badami, S. Effect of embelin against 3-nitropropionic acid-induced Huntington’s disease in rats. Biomed. Pharmacother. 2016, 77, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Thippeswamy, B.S.; Nagakannan, P.; Shivasharan, B.D.; Mahendran, S.; Veerapur, V.P.; Badami, S. Protective effect of embelin from Embelia ribes Burm. against transient global ischemia-induced brain damage in rats. Neurotox. Res. 2011, 20, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Szopa, A.; Ekiert, R.; Ekiert, H. Current knowledge of Schisandra chinensis (Turcz.) Baill. (Chinese magnolia vine) as a medicinal plant species: A review on the bioactive components, pharmacological properties, analytical and biotechnological studies. Phytochem. Rev. 2017, 16, 195–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramalingam, M.; Kim, H.; Lee, Y.; Lee, Y.-I. Phytochemical and Pharmacological Role of Liquiritigenin and Isoliquiritigenin From Radix Glycyrrhizae in Human Health and Disease Models. Front. Aging Neurosci. 2018, 10, 348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Himaya, S.W.A.; Ryu, B.; Qian, Z.-J.; Kim, S.-K. Paeonol from Hippocampus kuda Bleeler suppressed the neuro-inflammatory responses in vitro via NF-κB and MAPK signaling pathways. Toxicol. Vitr. 2012, 26, 878–887. [Google Scholar] [CrossRef]
- Jiang, X.; Zhu, K.; Xu, Q.; Wang, G.; Zhang, J.; Cao, R.; Ye, J.; Yu, X. The antidepressant-like effect of trans-astaxanthin involves the serotonergic system. Oncotarget 2017, 8, 25552–25563. [Google Scholar] [CrossRef] [Green Version]
- Xu, S.; Liu, J.; Shi, J.; Wang, Z.; Ji, L. 2,3,4′,5-tetrahydroxystilbene-2-O-β-D-glucoside exacerbates acetaminophen-induced hepatotoxicity by inducing hepatic expression of CYP2E1, CYP3A4 and CYP1A2. Sci. Rep. 2017, 7, 16511. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.-H. Pharmacological and medical applications of Panax ginseng and ginsenosides: A review for use in cardiovascular diseases. J. Ginseng Res. 2018, 42, 264–269. [Google Scholar] [CrossRef]
- Jeong, Y.-H.; Park, J.-S.; Kim, D.-H.; Kim, H.-S. Lonchocarpine Increases Nrf2/ARE-Mediated Antioxidant Enzyme Expression by Modulating AMPK and MAPK Signaling in Brain Astrocytes. Biomol. Ther. 2016, 24, 581–588. [Google Scholar] [CrossRef]
- Arun, M.; Satish, S.; Anima, P. Phytopharmacological Profile of Jasminum grandiflorum Linn. (Oleaceae). Chin. J. Integr. Med. 2016, 22, 311–320. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Iahtisham Ul, H.; Patel, S.; Pan, X.; Naz, S.; Sanches Silva, A.; Saeed, F.; Rasul Suleria, H.A. Proanthocyanidins: A comprehensive review. Biomed. Pharmacother. 2019, 116, 108999. [Google Scholar] [CrossRef]
- Yang, L.; Xian, D.; Xiong, X.; Lai, R.; Song, J.; Zhong, J. Proanthocyanidins against Oxidative Stress: From Molecular Mechanisms to Clinical Applications. BioMed. Res. Int. 2018, 2018, 8584136. [Google Scholar] [CrossRef] [Green Version]
- Domingues, M.; Casaril, A.M.; Smaniotto, T.Â.; Birmann, P.T.; Lourenço, D.d.A.; Bampi, S.R.; Vieira, B.; Lenardão, E.J.; Savegnago, L. Selanylimidazopyridine abolishes inflammation- and stress-induced depressive-like behaviors by modulating the oxido-nitrosative system. Eur. J. Pharmacol. 2022, 914, 174570. [Google Scholar] [CrossRef]
- Chung, H.J.; Park, E.J.; Pyee, Y.; Hua Xu, G.; Lee, S.H.; Kim, Y.S.; Lee, S.K. 25-Methoxyhispidol A, a novel triterpenoid of Poncirus trifoliata, inhibits cell growth via the modulation of EGFR/c-Src signaling pathway in MDA-MB-231 human breast cancer cells. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2011, 49, 2942–2946. [Google Scholar] [CrossRef]
- Sy, L.-K.; Saunders, R.M.K.; Brown, G.D. Phytochemistry of Illicium dunnianum and the systematic position of the illiciaceae. Phytochemistry 1997, 44, 1099–1108. [Google Scholar] [CrossRef]
- Li, J.; Geng, D.; Xu, J.; Weng, L.J.; Liu, Q.; Yi, L.T. Antidepressant-like effect of macranthol isolated from Illicium dunnianum tutch in mice. Eur. J. Pharmacol. 2013, 707, 112–119. [Google Scholar] [CrossRef]
- Luo, L.; Liu, X.-L.; Li, J.; Mu, R.-H.; Liu, Q.; Yi, L.-T.; Geng, D. Macranthol promotes hippocampal neuronal proliferation in mice via BDNF-TrkB-PI3K/Akt signaling pathway. Eur. J. Pharmacol. 2015, 762, 357–363. [Google Scholar] [CrossRef]
- Zanwar, A.A.; Badole, S.L.; Shende, P.S.; Hegde, M.V.; Bodhankar, S.L. Chapter 76—Cardiovascular Effects of Hesperidin: A Flavanone Glycoside. In Polyphenols in Human Health and Disease; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 989–992. [Google Scholar]
- Andrade, S.; Ramalho, M.J.; Pereira, M.d.C.; Loureiro, J.A. Resveratrol Brain Delivery for Neurological Disorders Prevention and Treatment. Front. Pharm. 2018, 9, 1261. [Google Scholar] [CrossRef] [Green Version]
- Kuršvietienė, L.; Stanevičienė, I.; Mongirdienė, A.; Bernatonienė, J. Multiplicity of effects and health benefits of resveratrol. Medicina 2016, 52, 148–155. [Google Scholar] [CrossRef]
- Vasconcelos, J.F.; Santos, I.P.; de Oliveira, T.B.; Kelly, A.M.; do Reis, B.P.Z.C.; Orge, I.D.; Meira, C.S.; Valverde, S.S.; Soares, M.B.P. The protective effect of solidagenone from Solidago chilensis Meyen in a mouse model of airway inflammation. Basic Clin. Pharmacol. Toxicol. 2022, 130, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Bortoleti, B.T.d.S.; Gonçalves, M.D.; Tomiotto-Pellissier, F.; Contato, V.M.; Silva, T.F.; de Matos, R.L.N.; Detoni, M.B.; Rodrigues, A.C.J.; Carloto, A.C.; Lazarin, D.B.; et al. Solidagenone acts on promastigotes of L. amazonensis by inducing apoptosis-like processes on intracellular amastigotes by IL-12p70/ROS/NO pathway activation. Phytomedicine 2021, 85, 153536. [Google Scholar] [CrossRef]
- Bayan, L.; Koulivand, P.H.; Gorji, A. Garlic: A review of potential therapeutic effects. Avicenna J. Phytomed. 2014, 4, 1–14. [Google Scholar] [PubMed]
- Lu, J.; He, H.; Huang, C.; Chen, Z. Effect of Diallyl Disulfide on Lipopolysaccharide-Induced Depression-Like Behavior in Mice. 2019. Available online: https://www.researchsquare.com/article/rs-10369/v1 (accessed on 9 November 2022). [CrossRef]
- De Oliveira, J.R.; Camargo, S.E.A.; de Oliveira, L.D. Rosmarinus officinalis L. (rosemary) as therapeutic and prophylactic agent. J. Biomed. Sci. 2019, 26, 5. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Liang, X.; Wang, S.; Lee, P.; Zhang, Y. The Underlying Mechanism of Paeonia lactiflora Pall. in Parkinson’s Disease Based on a Network Pharmacology Approach. Front. Pharm. 2020, 11, 581984. [Google Scholar] [CrossRef]
- Tizard, I. Sickness behavior, its mechanisms and significance. Anim. Health Res. Rev. 2008, 9, 87–99. [Google Scholar] [CrossRef]
- Pareek, A.; Suthar, M.; Rathore, G.S.; Bansal, V. Feverfew (Tanacetum parthenium L.): A systematic review. Pharm. Rev 2011, 5, 103–110. [Google Scholar] [CrossRef] [Green Version]
- Goyal, S.N.; Prajapati, C.P.; Gore, P.R.; Patil, C.R.; Mahajan, U.B.; Sharma, C.; Talla, S.P.; Ojha, S.K. Therapeutic Potential and Pharmaceutical Development of Thymoquinone: A Multitargeted Molecule of Natural Origin. Front. Pharm. 2017, 8, 656. [Google Scholar] [CrossRef]
- Khader, M.; Eckl, P.M. Thymoquinone: An emerging natural drug with a wide range of medical applications. Iran. J. Basic Med. Sci. 2014, 17, 950–957. [Google Scholar]
- Li, Y.; Lin, W.; Huang, J.; Xie, Y.; Ma, W. Anti-cancer effects of Gynostemma pentaphyllum (Thunb.) Makino (Jiaogulan). Chin. Med. 2016, 11, 43. [Google Scholar] [CrossRef]
- Shin, K.S.; Zhao, T.T.; Park, K.H.; Park, H.J.; Hwang, B.Y.; Lee, C.K.; Lee, M.K. Gypenosides attenuate the development of L-DOPA-induced dyskinesia in 6-hydroxydopamine-lesioned rat model of Parkinson’s disease. BMC Neurosci. 2015, 16, 23. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.; Shim, I.; Lee, H. Gypenosides Attenuate Lipopolysaccharide-Induced Neuroinflammation and Memory Impairment in Rats. Evid. Based Complement. Altern. Med. 2018, 2018, 4183670. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Qi, Z.; Wang, W.; Wang, L.; Cao, F.; Zhao, L.; Fang, X. Isovitexin Inhibits Ginkgolic Acids-Induced Inflammation Through Downregulating SHP2 Activation. Front. Pharm. 2021, 12, 630320. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Varoni, E.M.; Iriti, M.; Martorell, M.; Setzer, W.N.; Del Mar Contreras, M.; Salehi, B.; Soltani-Nejad, A.; Rajabi, S.; Tajbakhsh, M.; et al. Carvacrol and human health: A comprehensive review. Phytother. Res. PTR 2018, 32, 1675–1687. [Google Scholar] [CrossRef]
- Javed, H.; Meeran, M.F.N.; Jha, N.K.; Ojha, S. Carvacrol, a Plant Metabolite Targeting Viral Protease (Mpro) and ACE2 in Host Cells Can Be a Possible Candidate for COVID-19. Front. Plant Sci. 2021, 11, 2237. [Google Scholar] [CrossRef]
- Baliga, M.S.; Shivashankara, A.R.; Venkatesh, S.; Bhat, H.P.; Palatty, P.L.; Bhandari, G.; Rao, S. Chapter 7—Phytochemicals in the Prevention of Ethanol-Induced Hepatotoxicity: A Revisit. In Dietary Interventions in Liver Disease; Watson, R.R., Preedy, V.R., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 79–89. [Google Scholar]
- Miranda, M.; Morici, J.F.; Zanoni, M.B.; Bekinschtein, P. Brain-Derived Neurotrophic Factor: A Key Molecule for Memory in the Healthy and the Pathological Brain. Front. Cell. Neurosci. 2019, 13, 363. [Google Scholar] [CrossRef]
- Bathina, S.; Das, U.N. Brain-derived neurotrophic factor and its clinical implications. Arch. Med. Sci. AMS 2015, 11, 1164–1178. [Google Scholar] [CrossRef]


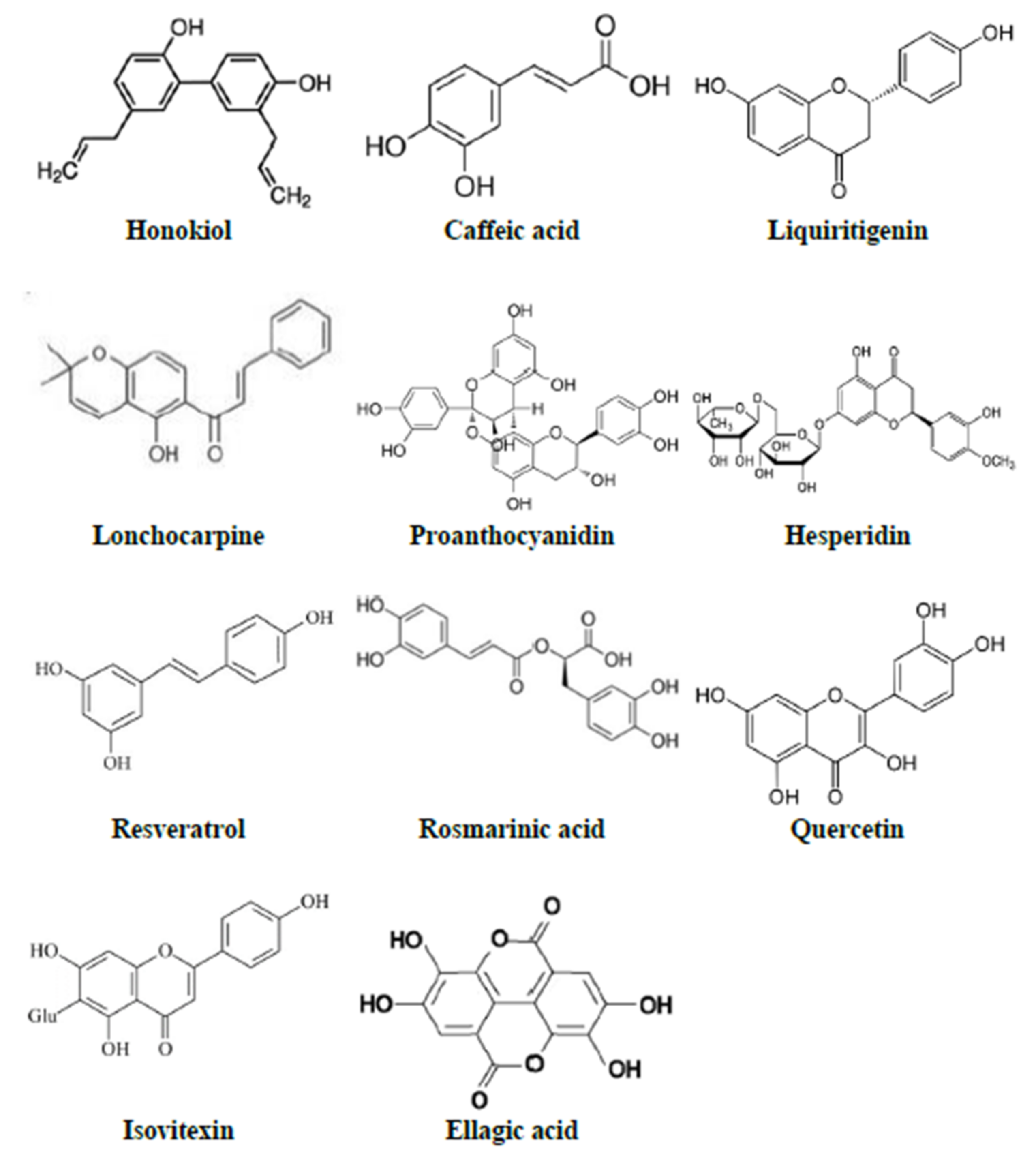
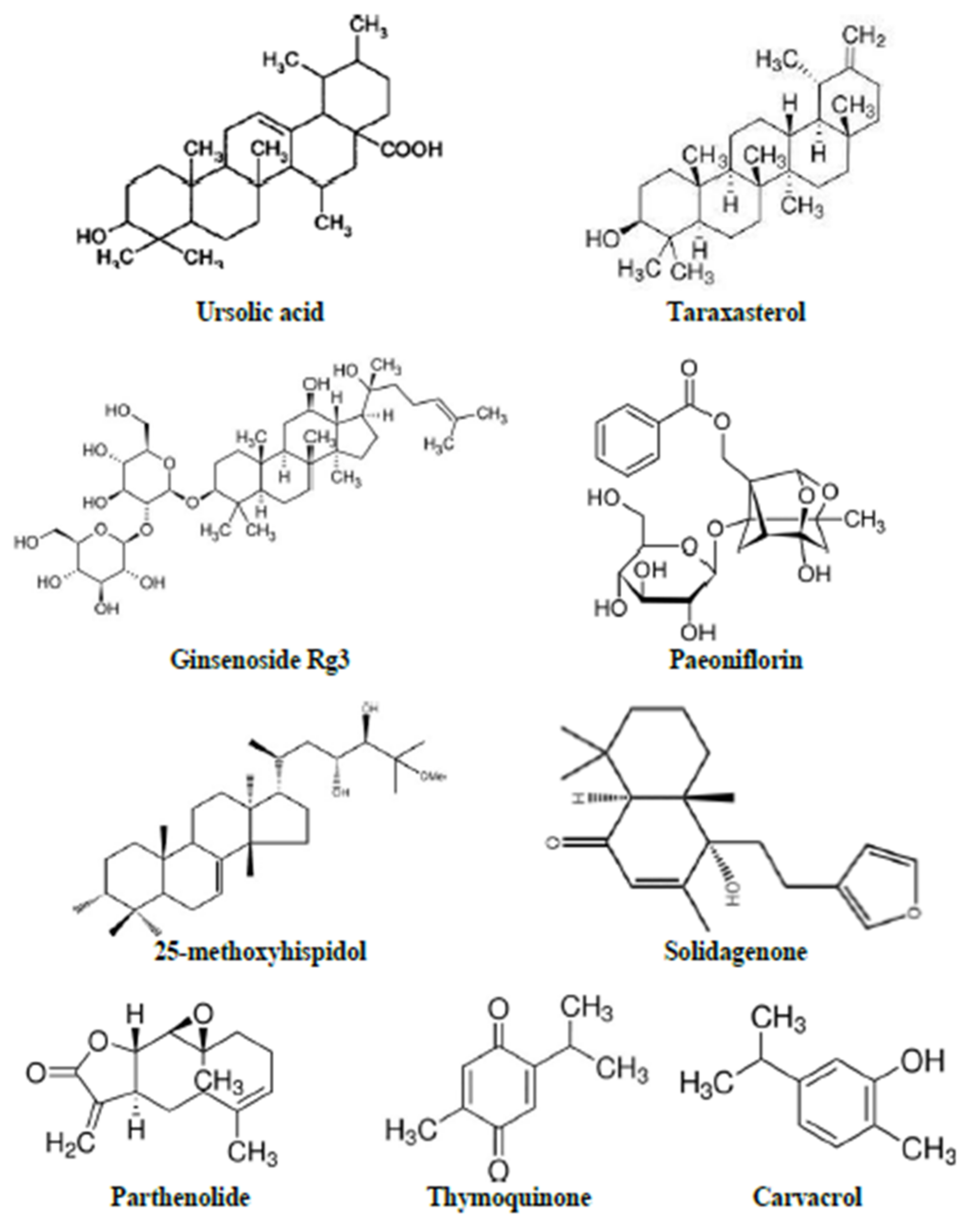
| Phytoconstituent | Isolated from | Reported Activities | Animal/Dose | Dose of LPS | Parameters Evaluated | Reference |
|---|---|---|---|---|---|---|
| Methyl Jasmonate | Jasmonium grandiflorum | Antinociceptive, anti-amnesic, and adaptogenic properties. | Male Swiss mice/5, 10, 20 mg/kg/7 days | 830 mg/kg (i.p.) | Behavioural Sucrose preference test Tail suspension test and Forced swim test Locomotor activity Biochemical Estimation Corticosterone Glutathione Malondialdehyde Super oxide dismutase TNF-α | [17] |
| Gomisin N | Schisandra chinensis (Turcz.) | Antioxidant and protective effects against tissue injury of heart, liver, kidney, and brain. | Mice | 500 mg/kg (i.p.) | Behavioural Object exploration test Forced swim test Locomotor activity Biochemical Estimation Griess assay c-Fos immunohistochemistry Quantitative real-time PCR MTS assay | [18] |
| Thymoquinone (Found in seeds of Nigella sativa L) | Purchased from Sigma-Aldrich | Anti-inflammatory and neuroprotective effects. | Male Wistar rats/2, 5, and 10 mg/kg (i.p.) | 1 mg/kg/day (i.p.) for two weeks | Behavioural Morris water maze test Passive avoidance Biochemical Estimation IL-6 TNF-α MDA Thiol Superoxide dismutase Catalase Nitric oxide | [19] |
| 2,3,5,4′-Tetrahydroxystilbene-2-O-β-D-glucoside (TSG) (Found in Polygonum multiflorum Thunb.) | Purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China) | Antioxidative, free radical scavenging, and antiplatelet activities. | Male ICR mice, 30 and 60 mg/kg (i.p.) | 0.83 mg/kg (i.p.) | Behavioural Tail suspension test Forced swim test Open field test Biochemical Estimation IL-1β IL-6 TNF-α MDA GSH level BDNF Nitrite level | [20] |
| Gentiopicroside (Gent) (Found in Gentiana rigescens) | Purchased from Spring & Autumn Biologic Engineering Co., Ltd. (Nanjing, China) | Anti-inflammatory activity. | 8–10 week old male BALB/C mice, 50 mg/kg (i.p.) once a day | 0.5 mg/kg (i.p.) | Behavioural Forced swimming test Tail suspension test Open field test Biochemical Estimation IL-1β TNF-α Protein expression of NMDA receptors (Western blot) | [21] |
| 3-((4-methoxyphenyl) selanyl)-2 phenylimidazo [1,2-a] pyridine (Selanylimidazopyridin) | Synthesised by the Laboratory of Clean Organic Synthesis (LASOL-UFPel) | Anti-inflammatory, antioxidant, antidepressant, antineuroinflammatory, and antioxidant. | Male Swiss mice, (MPI; 20 and 50 mg/kg, intragastrically) | 0.83 mg/kg (i.p.) | Behavioural Open field test Forced swimming test Biochemical Estimation Lipid peroxidation Reactive oxygen species (ROS) BDNF TBARS level Gene expression | [22] |
| Ellagic acid (Found in strawberries, raspberries, blackberries, cherries, and walnuts) | Purchased from Sigma-Aldrich | Antioxidant, anti-Alzheimer’s, and anti-Parkinson’s activities. | Male Wistar rats, 100 mg/kg intragastric gavage | 250 μg/kg (i.p.) | Behavioural Open field test Object recognition test Biochemical Estimation Lipid peroxidation Reactive oxygen species (ROS) Protein carbonylation T-SHs level GSH level Acetylcholinesterase activity TBARS measurement Protein carbonyl level | [23] |
| Esculetin (Found in Artemisia eriopoda, Euphorbia decipiens) | Purchased from Sigma-Aldrich | Antioxidant, anti-inflammatory, antiproliferative, and antidepressant and cognitive enhancer. | Mice 25 and 50 mg/kg (p.o.) | 0.83 mg/kg (i.p.) | Behavioural Elevated plus maze Open field test Forced swim test Tail suspension test Biochemical Estimation Cytokines MDA level GSH level CORT IL-1β IL-6 TNF-α Oxidative stress | [24] |
| Mangiferin (Found in Mangifera indica) | Purchased from Sigma-Aldrich | Antioxidant, anti-inflammatory, and immunomodulatory activities. | Mice, 20 and 40 mg/kg (p.o.) | 0.83 mg/kg (i.p.) | Behavioural Elevated plus maze Light–dark box Open field test Sucrose preference Biochemical Estimation IL-1β SOD Catalase MDA level GSH level Nitrite assay TNF-α BDNF | [25] |
| Curcumin and piperine | Purchased from Sigma-Aldrich | Antioxidant, anti-inflammatory, hepato- and nephroprotective activity, and antimicrobial and neuroprotective properties. | Male Swiss albino mice; curcumin alone—100, 200, and 400 mg/kg (p.o.); curcumin and piperine—20 mg/kg (p.o.) | 0.83 mg/kg (i.p.) | Behavioural Elevated plus Maze Light–dark box test Open field test Sucrose preference test Tail suspension test Forced swimming test Biochemical Estimation IL-1β TBARS level CORT level MDA level Nitrite assay TNF-α BDNF | [26] |
| Lonchocarpine | Isolated from Abrus precatorius | Anti-inflammatory, anti-edematogenic, antibacterial, gastroprotective, and cytoprotective effects. | Male ICR mice (10–11 weeks), 50 mg/kg (i.p.) | 5 mg/kg (i.p.) | Biochemical Estimation IL-6 IL-10 Nitrite assay TNF-α ROS level Western blot RT-PCR Immunohistochemistry Transient transfection and luciferase assay Co-immunoprecipitation assay | [27] |
| Trans-astaxanthin | Purchased from Sigma-Aldrich, USA | Anti-inflammatory and antioxidative activities. | Male ICR mice (4–6 weeks, 20–22 g); 20, 40, and 80 mg/kg (p.o.) | 0.83 mg/kg (i.p.) | Behavioural Tail suspension test Forced swimming test Locomotor activity Biochemical Estimation IL-1β IL-6 iNOS, nNOS, eNOS level COX-2 level NF-κB p65 level Nitrite assay TNF-α RT-PCR | [28] |
| Proanthocyanidin (Found in algae, yeast, salmon, trout, krill, shrimpm and crayfish) | Purchased from Tianjin Jianfeng Natural Product R & D Co., Ltd. (Tianjin, China) | Anti-inflammatory and antioxidative activities. | Six-week-old male ICR mice (20–22 g), 80 mg/kg (p.o.) | 0.83 mg/kg (i.p.) | Behavioural Forced swimming test Locomotor activity Marble-burying test Elevated plus maze test Biochemical Estimation IL-1β IL-6 iNOS level COX-2 level NF-κB p65 level TNF-α RT-PCR | [29] |
| Ginsenoside Rg3 (Found in Panax ginseng) | Purchased from the College of Chemistry, Jilin University 83 (Changchun, China). | Antioxidative, anti-inflammatory, and immunomodulatory effects. | Male ICR mice (8 weeks old), 20 and 40 mg/kg intragastric administration | 0.83 mg/kg (i.p.) | Behavioural Forced swimming test Tail suspension test Open field test Biochemical Estimation IL-6 IL-1β IDO mRNA NF-κB level TNF-α RT-PCR Western blot | [30] |
| Paeoniflorin (Found in Paeonia, Paeonia tenuifolia) | Provided by Wako Pure Chemical Industries, Ltd. (Osaka, Japan) | Anti-inflammatory, anti-allergic, immunoregulatory, analgesic, neuromuscular blocking, cognition enhancement, and steroid protein-binding inhibition. | Female Sprague Dawley rats; 2.5, 5, and 10 mg/kg (i.p.) at a dose of 1.5 mL/kg | 1.5 mL/kg (i.p.) | Biochemical Estimation Triglyceride (TG) Total cholesterol (TC) Total lipid (TL) High-density lipoproteins (HDLs) Malondialdehyde (MDA) | [31] |
| Hesperidin (Commonly found in citrus fruits) | Procured from Sigma, Aldrich USA | Anti-inflammatory, anti-apoptotic, and antioxidant activities. | Male Balb/c mice (8–10 weeks), 100 mg/kg (p.o.) | 0.83 mg/kg (i.p.) | Behavioural Elevated plus Maze Light–dark box test Open field test Sucrose preference test Tail suspension test Forced swimming test Biochemical Estimation IL-1β IL-10 MDA level Nitrite level GSH level SOD and CAT activity Total protein CORT TNF-α BDNF RTPCR Western Blot | [32] |
| Carvacrol (Found in Origanum vulgare, Thymus vulgaris, and Lepidium flavum) | Purchased from Sigma-Aldrich Company (Sigma- Aldrich Co., St. Louis, MO, USA) | Antioxidative, anti-inflammatory, and anti-apoptotic effects. | Male Sprague–Dawley (SD) rat, (25, 50, and 100 mg/kg) | 2 μL/1 min (total 5 min) intracerebroventricularly | Behavioural Object recognition task Morris water maze test Open field test Biochemical Estimation IL-1β Il-6 TNF-α COX-2 level NF-κB level iNOS level TLR4 level BDNF RTPCR | [33] |
| Quercetin (Found mostly in onions, grapes, berries, cherries, broccoli, and citrus fruits) | Purchased from Sigma-Aldrich Co., Steinheim, Switzerland | Anti-inflammatory, antiproliferative, and anti-atherosclerotic effects. | Female BALB/c inbred mice (7 weeks old), 0.06 or 0.15 μmol/mouse | 8 and 16 mg/kg BW (i.p.) | Biochemical Estimation IL-6 IL-1β IL-17 IL-10 TNF-α | [34] |
| Isovitexin | Purchased from Sigma- Aldrich, St. Louis, MO, USA | Anti-inflammatory, antioxidant, and anxiolytic activities. | Male C57BL/6 mice (8–10 weeks and 20–25 g), 10 mg/kg (i.p.) | 0.33 mg/kg (i.p.) | Behavioural Open field test Sickness behaviour Biochemical Estimation IL-6 RTPCR IL-1β IL-17 IL-10 TNF-α COX-2 iNOS Western blot | [35] |
| Solidagenone | Solidago chilensis Meyen | Anti-inflammatory, hypoglycaemic, analgesic, and hypolipidemic activities. | Male Swiss mice (25–35 g); 1, 10, or 100 mg/kg (p.o) | 600 μg/kg (i.p.) | Behavioural Open field test Sickness behaviour Tail suspension test Biochemical Estimation MPO IL-6 TNF-α GSH level LOOH level SOD activity CAT activity GST activity | [36] |
| Diallyl disulfide | Purchased from Sigma | Anti-inflammatory and antioxidant activities. | Male C57BL6/J mice (6–8 weeks), 40 or 80 mg/kg (i.p.) | 100 μg/kg (i.p.) | Behavioural Tail suspension test Forced swim test Open field test Biochemical Estimation IL-6 IL-1β TNF-α Nitric oxide level GSH level MDA level | [35] |
| Caffeic acid | Purchased from Sigma-Aldrich Co., LLC (St. Louis, MO, USA) | Antioxidant, antitumour, antinociceptive, antidementiam, and anti-inflammatory activities. | Male Swiss albino mice (8–10 weeks, 20–30 g), 30 mg/kg (p.o.) | 1.5 mg/kg (i.p.) | Behavioural Open field test Forced swim test Tail suspension test Biochemical Estimation IL-6 TNF-α GSH level MDA level | [37] |
| Rosmarinic acid | Obtained from Sigma Aldrich Co. (St. Louis, MO, USA) | Anti-inflammatory, hepatoprotection, and renoprotection activities. | Adult Swiss albino mice, 0.5 mg/kg and 1 mg/kg (i.p.) | 0.25 mg/kg (i.p.) | Behavioural Morris water maze Y maze Tail suspension test Biochemical Estimation SOD IL-6 TNF-α Caspase-3 C-Jun GSH level MDA level AChE activity TBARS assay | [38] |
| Parthenolide | Purchased from Sigma Chemicals, Deisenhofen, Germany | Anti-inflammatory and immunomodulatory effects. | Male Wistar rat, 1 mg/kg (i.p.) | 100 μg/kg (i.p.) | Behavioural Morris water maze Y maze Tail suspension test Biochemical Estimation IL-6 TNF-α COX-2 level NF-κB/NF-IL6 pathway RTPCR PGC1a Trib1 | [39] |
| Quercetin | Purchased from Sigma, St. Louis, MO, USA | Anti-inflammatory, antioxidant, antiallergic, antiapoptotic, nephro-, gastro-, angio-, cardio-, and chondroprotective properties. | Wistar albino rats, 2 and 25 mg/kg, (i.p.) | LPS, 1 mg/kg (i.p.) | Behavioural Elevated plus maze Open field test Biochemical Estimation IL-6 IL-1β TNF-α TBARS GSH | [40] |
| Embelin | Embelia ribes Burm | Anti-inflammatory, neuroprotective, anxiolytic, antitumour, analgesic, and anticonvulsant activities. | Adult male Swiss albino mice, 10 and 20 mg/kg (p.o.) | 400 μg/kg (i.p.) | Behavioural Open field test Plus maze Light–dark box Forced swim test Social behaviour assessment Sucrose preference test Biochemical Estimation GSH level MDA level | [11] |
| 25-methoxyhispidol | Poncirus trifoliate | Antibacterial, anti-inflammatory, and anticancer activities. | Male albino mice (3–4 weeks of age); 1, 5, and 10 mg/kg (i.p.) | 0.83 mg/kg (i.p.) | Behavioural Elevated plus maze test Forced swim test Light dark box test Tail suspension test Open field test Biochemical Estimation IL-6 IL-1β TNF-α GSH level GST level ALT AST | [41] |
| Curcumin | Purchased from Sigma-Aldrich, Milan, Italy | Anti-inflammatory, antitumour, antioxidative, anti-amyloidogenic, metal-chelating, and cardiovascular protective effects. | 3 month old male C57BL/6 mice, 50 mg/kg (p.o.) | 5 mg/kg (i.p.) | Behavioural Open field test Novel object recognition test Biochemical Estimation IL-6 IL-1β TNF-α NLRP3 inflammasome COX-2 RTPCR | [42] |
| Liquiritigenin | Purchased from National Institutes for Food and Drug Control (Beijing, China) | Anti-inflammatory and neuroprotective activities. | ICR mice, 7.5 and 15 mg/kg intragastric | 0.5 mg/kg (s.c.) | Behavioural Tail suspension test Forced swimming test Biochemical Estimation IL-6 TNF-α BDNF B(TrkB) Western blot RTPCR | [43] |
| Honokiol | Purchased from Sigma-Aldrich, St. Louis, MO, USA | Antioxidant, anti-inflammatory, anxiolytic, antidepressant, and neuroprotective activities. | Adult male Swiss albino mice, (22–30 g), 2.5 and5 mg/kg (i.p.) | 0.83 mg/kg (i.p.) | Behavioural Tail suspension test Forced swim test Biochemical Estimation IL-6 IL-1β TNF-α BDNF CORT level TBARS level Nitrite level | [44] |
| Honokiol | Purchased from Sigma-Aldrich, St. Louis, MO, USA | Antiarrhythmic, anti-inflammatory, antithrombocytic, anti-angiogenesis, antitumour, anxiolytic, and antioxidative activities. | Adult male Swiss albino mice (22–30 g), 2.5 and 5 mg/kg (i.p.) | 0.83 mg/kg (i.p.) | Behavioural Elevated plus maze test Open field test Biochemical Estimation IL-6 IL-10 IL-1β TNF-α BDNF AST level ALT level TBARS level GSH level | [24] |
| Paeonol | Provided by the National Institutes for Food and Drug Control (Beijing, China) | Anti-inflammatory, antioxidant, antiatherosclerosis, antidiabetic, antimutagenic agent, and antineuroinflammatory activities. | Male ICR mice, 10 and 20 mg/kg (i.p.) | 0.5 mg/kg (i.p.) | Behavioural Forced swimming test Open field test Tail suspension test Biochemical Estimation 5-HT level NE level IL-6 TNF-α Western blot BDNF and NF-κB TrkB | [45] |
| Resveratrol | Purchased from Sigma-Aldrich (St. Louis, MO, USA) | Anti-inflammatory, antioxidant, and anti-anxiety activities. | Adult male C57BL/6J mice, 50 mg/kg (i.p.) | 1 mg/kg (i.p.) | Behavioural Elevated plus maze test Open field test Morris water maze Biochemical Estimation IL-6 IL-1β IL-2 COX-2 iNOS NF-κB Western blot qRT-PCR | [46] |
| Ursolic acid | Purchased from Sigma-Aldrich (St. Louis, MO, USA) | Antioxidant, antitumour, and anti-inflammatory activities. | Male C57BL/6 mice, 10 mg/kg (p.o.) | 10 or 20 mg/kg (i.p.) | Behavioural Step through passive avoidance test Open field test Morris water maze Biochemical Estimation IL-6 IL-1β COX-2 iNOS TNF-α NF-κB MAPK pathway Akt pathway | [47] |
| Curcumin | Purchased from Sigma–Aldrich | Anti-inflammatory, antioxidant, anticarcinogenic, and neuroprotective activities. | Adult Kun-Ming mice (male), 50 mg/kg (i.p.) | 0.83 mg/kg (i.p.) | Behavioural Forced swimming test Tail suspension test Sucrose preference test Locomotor activity Biochemical Estimation IL-1β TNF-α COX-2 iNOS NF-κB Western blot RT-PCR NF-κB | [48] |
| Diallyl disulfide | Purchased from Sigma | Antimicrobial and anti-inflammatory activities. | Male C57BL6/J mice (6–8 weeks), 40 or 80 mg/kg (i.p.) | 100 μg/kg (i.p.) | Behavioural Open field test Tail suspension test Forced swim test Biochemical Estimation IL-1β TNF-α Nitric oxide (NO) levels MDA level GSH level | [49] |
| Macranthol | Illicium dunnianum Tutch | Neuroprotective activities. | Male ICR mice, 20 mg/kg (p.o.) | 0.83 mg/kg (i.p.) | Behavioural Sucrose preference test Forced swimming test Biochemical Estimation IL-1β IL-6 TNF-α qPCR iba1 | [50] |
| Taraxasterol | Obtained from Chengdu Fenruisi BioTechnology Co. (Chengdu, China) | Antirheumatic, anti-inflammatory, and antimastopathy activities. | Male Kunming mice; 2.5, 5, and 10 mg/kg, intragastric | 32 mg/kg (i.p.) | Behavioural Morris water maze test Passive avoidance Biochemical Estimation IL-6 IL-1β TNF-α IFN- γ MDA NO PGE2 | [51] |
| Apelin | Purchased from Sigma-Aldrich Co. (St. Louis, MO, USA) | Antineuroinflammatory effects. | Male Wistar rats (200–220 g), 2 μg/kg (i.c.v.) | 2 μg/kg (i.c.v.) | Behavioural Forced swimming test Sucrose preference test Passive avoidance Biochemical Estimation IL-1β TNF-α NF-κB p-IKKβ Western blot | [52] |
| Esculetin | Purchased from the National Institutes for Food and Drug Control (Beijing, China) | Antioxidant, anti-inflammatory, and hepatoprotective activities. | Male ICR mice (18–22 g), 20 and 40 mg/kg, intragastric administration | 0.83 mg/kg (i.p.) | Behavioural Forced swimming test Tail suspension test Open field test Biochemical Estimation IL-6 IL-1β TNF-α COX-2 iNOS NF-κB BDNF p-TrkB Western blot RT-PCR NF-κB | [53] |
| HABC | Suppression of Oxidative Stress | Attenuation of Cytokine Levels | Suppression of Nitrosative Stress | COX-2 Inhibition | Suppression of Corticosterone | Restoration of BDNF Levels |
|---|---|---|---|---|---|---|
| Caffeic acid | Yes | Yes | - | - | - | - |
| Carvacrol | - | - | - | - | - | Yes |
| Curcumin | - | Yes | Yes | Yes | - | - |
| Diallyl disulfide | Yes | Yes | - | - | - | - |
| Ellagic Acid | Yes | - | - | - | - | - |
| Embelin | Yes | - | - | - | - | - |
| Esculetin | Yes | - | - | - | Yes | - |
| Gentiopicroside | - | Yes | - | - | - | - |
| Ginsenoside Rg3 | - | Yes | - | - | - | - |
| Gomisin N | - | - | - | - | - | - |
| Gypenosides | - | - | - | - | - | Yes |
| Hesperidin | - | - | - | - | - | - |
| Honokiol | Yes | Yes | Yes | - | - | Yes |
| Isovitexin | - | - | - | - | - | - |
| Liquiritigenin | - | Yes | - | - | - | Yes |
| Lonchocarpine | - | Yes | - | - | - | - |
| Macranthol | - | Yes | - | - | - | - |
| Mangiferin | Yes | - | - | - | - | Yes |
| Methyl jasmonate | Yes | Yes | - | - | Yes | - |
| Paeoniflorin | Yes | - | - | - | - | - |
| Paeonol | - | Yes | - | - | - | Yes |
| Parthenolide | Yes | Yes | - | - | - | - |
| Curcumin + Piperine | Yes | - | Yes | - | - | - |
| Proanthocyanidin | - | Yes | - | Yes | - | - |
| Quercetin | Yes | Yes | - | - | - | - |
| Resveratrol | - | Yes | - | - | - | - |
| Rosmarinic acid | Yes | Yes | - | - | - | - |
| Selanylimidazopyridine | Yes | - | - | - | - | Yes |
| Solidagenone | Yes | - | - | - | - | - |
| Taraxasterol | - | Yes | - | - | - | - |
| Thymoquinone | Yes | Yes | - | - | - | - |
| Trans-astaxanthin | - | Yes | - | - | - | - |
| Ursolic acid | - | Yes | - | Yes | - | - |
| 2, 3, 5, 4’-Tetrahydroxystilbene-2-O-β-D-glucoside | Yes | - | Yes | - | - | - |
| 25-Methoxy hispidol | Yes | Yes | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alotaibi, G.H.S.; Shivanandappa, T.B.; Chinnadhurai, M.; Reddy Dachani, S.; Dabeer Ahmad, M.; Abdullah Aldaajanii, K. Phytochemistry, Pharmacology and Molecular Mechanisms of Herbal Bioactive Compounds for Sickness Behaviour. Metabolites 2022, 12, 1215. https://doi.org/10.3390/metabo12121215
Alotaibi GHS, Shivanandappa TB, Chinnadhurai M, Reddy Dachani S, Dabeer Ahmad M, Abdullah Aldaajanii K. Phytochemistry, Pharmacology and Molecular Mechanisms of Herbal Bioactive Compounds for Sickness Behaviour. Metabolites. 2022; 12(12):1215. https://doi.org/10.3390/metabo12121215
Chicago/Turabian StyleAlotaibi, Ghallab Hamoud Sinhat, Thippeswamy Boreddy Shivanandappa, Maheswari Chinnadhurai, Sudharshan Reddy Dachani, Mahmad Dabeer Ahmad, and Khalid Abdullah Aldaajanii. 2022. "Phytochemistry, Pharmacology and Molecular Mechanisms of Herbal Bioactive Compounds for Sickness Behaviour" Metabolites 12, no. 12: 1215. https://doi.org/10.3390/metabo12121215
APA StyleAlotaibi, G. H. S., Shivanandappa, T. B., Chinnadhurai, M., Reddy Dachani, S., Dabeer Ahmad, M., & Abdullah Aldaajanii, K. (2022). Phytochemistry, Pharmacology and Molecular Mechanisms of Herbal Bioactive Compounds for Sickness Behaviour. Metabolites, 12(12), 1215. https://doi.org/10.3390/metabo12121215







