Phytochemical Study of Euphorbia turcomanica Boiss.
Abstract
1. Introduction
2. Materials and Methods
2.1. General Experimental Procedures
2.2. Plant Materials
2.3. Extraction Procedure
2.4. Isolation of Terpenoids
3. Results
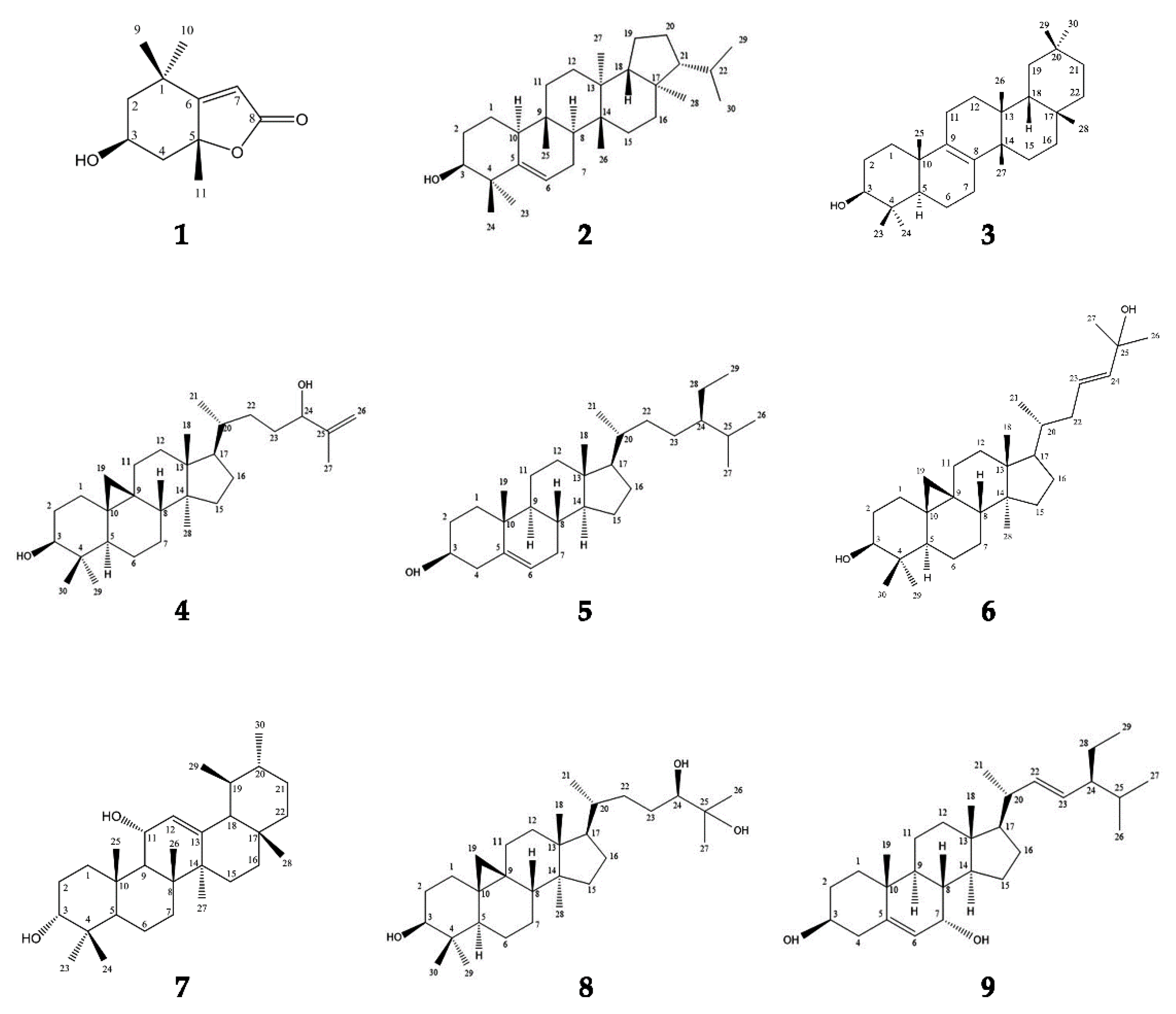
3.1. Spectral Data of Compounds 1–9
- Loliolide (Compound 1)
- 2.
- Simiarenol: 3β-hydroxy-E:B-friedo-hop-5-ene (Compound 2)
- 3.
- Isomultiflorenol (Compound 3)
- 4.
- Cycloart-25-ene-3β,24-diol (Compound 4)
- 5.
- β-sitosterol: Stigmast-5-en-3β-ol (Compound 5)
- 6.
- Cycloart-23-ene-3β,25-diol (Compound 6)
- 7.
- 3α, 11α-dihydroxyurs-12-ene (Compound 7)
- 8.
- 3β, 24β, 25-trihydroxycycloartane (Compound 8)
- 9.
- 7α-hydroxystigmasterol (Compound 9)
3.2. Structure Identification of Compounds 1–9
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vasas, A.; Hohmann, J. Euphorbia diterpenes: Isolation, structure, biological activity, and synthesis (2008–2012). Chem. Rev. 2014, 114, 8579–8612. [Google Scholar] [CrossRef] [PubMed]
- Jassbi, A.R. Chemistry and biological activity of secondary metabolites in Euphorbia from Iran. Phytochemistry 2006, 67, 1977–1984. [Google Scholar] [CrossRef] [PubMed]
- Pahlevani, A.H.; Liede-Schumann, S.; Akhani, H. Diversity, distribution, endemism and conservation status of Euphorbia (Euphorbiaceae) in SW Asia and adjacent countries. Plant Syst. Evol. 2020, 306, 80. [Google Scholar] [CrossRef]
- Pahlevani, A.H.; Riina, R. A synopsis of Euphorbia subgen. Chamaesyce (Euphorbiaceae) in Iran. Ann. Bot. Fenn. 2011, 48, 304–316. [Google Scholar] [CrossRef]
- Masyita, A.; Sari, R.M.; Astuti, A.D.; Yasir, B.; Rumata, N.R.; Emran, T.B.; Nainu, F.; Simal-Gandara, J. Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives. Food Chem. X 2022, 753, 100217. [Google Scholar] [CrossRef]
- Shi, Q.-W.; Su, X.-H.; Kiyota, H. Chemical and pharmacological research of the plants in genus Euphorbia. Chem. Rev. 2008, 108, 4295–4327. [Google Scholar] [CrossRef]
- Kemboi, D.; Siwe-Noundou, X.; Krause, R.W.; Langat, M.K.; Tembu, V.J. Euphorbia Diterpenes: An Update of Isolation, Structure, Pharmacological Activities and Structure–Activity Relationship. Molecules 2021, 26, 5055. [Google Scholar] [CrossRef]
- Mitu, S.A.; Stewart, P.; Tran, T.D.; Reddell, P.W.; Cummins, S.F.; Ogbourne, S.M. Identification of Gene Biomarkers for Tigilanol Tiglate Content in Fontainea picrosperma. Molecules 2022, 27, 3980. [Google Scholar] [CrossRef]
- Kemboi, D.; Peter, X.; Langat, M.; Tembu, J. A review of the ethnomedicinal uses, biological activities, and triterpenoids of Euphorbia species. Molecules 2020, 25, 4019. [Google Scholar] [CrossRef]
- Saleem, H.; Ahmad, I.; Gill, M.S.A. Phytochemical screening and diuretic activity of Euphorbia granulata. Bangladesh J. Pharm. 2015, 10, 584–587. [Google Scholar] [CrossRef]
- Dey, P.M.; Harborne, J.B. Plant biochemistry; Elsevier: Amsterdam, The Netherlands, 1997. [Google Scholar]
- Zare, H.; Noori, A.; Yusefzadi, M.; Banaee, M. Acute toxicity of Euphorbia turcomanica on Aphanius dispar. Int. J. Aquat. Biol. 2015, 3, 346–351. [Google Scholar]
- Zolfaghari, B.; Yazdiniapour, Z.; Ghanadian, M.; Lanzotti, V. Cyclomyrsinane and premyrsinane diterpenes from Euphorbia sogdiana Popov. Tetrahedron 2016, 72, 5394–5401. [Google Scholar] [CrossRef]
- Sukor, S.; Zahari, Z.; Rahim, N.; Yusoff, J.; Salim, F. Chemical Constituents and Antiproliferative Activity of Eleusine indica (L.) Gaertn. Sains Malays. 2022, 51, 873–882. [Google Scholar] [CrossRef]
- Yuan, Z.; Zheng, X.; Zhao, Y.; Liu, Y.; Zhou, S.; Wei, C.; Hu, Y.; Shao, H. Phytotoxic compounds isolated from leaves of the invasive weed Xanthium spinosum. Molecules 2018, 23, 2840. [Google Scholar] [CrossRef]
- Le, D.K.; Hoang, M.H. Triterpenoids isolated from Helicteres hirsuta. J. Tech. Educ. 2020, 33, 12–16. [Google Scholar]
- Amin, E.; Moawad, A.; Hassan, H. Biologically-guided isolation of leishmanicidal secondary metabolites from Euphorbia peplus L. Saudi Pharm. J. 2017, 25, 236–240. [Google Scholar] [CrossRef]
- Carréu, J.P.M. Bioactive Terpenoids from Euphorbia Pubescens: Isolation and Derivatization. Master’s Thesis, University of Lisbon, Lisbon, Portugal, 2020. [Google Scholar]
- Ayatollahi, A.M.; Ghanadian, M.; Afsharypuor, S.; Mesaik, M.A.; Abdalla, O.M.; Shahlaei, M.; Farzandi, G.; Mostafavi, H. Cycloartanes from Euphorbia aellenii Rech. f. and their Antiproliferative Activity. Iran J. Pharm Res. 2011, 10, 105. [Google Scholar]
- Takahashi, S.; Satoh, H.; Hongo, Y.; Koshino, H. Structural Revision of Terpenoids with a (3 Z)-2-Methyl-3-penten-2-ol Moiety by the Synthesis of (23 E)-and (23 Z)-Cycloart-23-ene-3β, 25-diols. J. Org. Chem. Res. 2007, 72, 4578–4581. [Google Scholar] [CrossRef]
- Rauter, A.P.; Filipe, M.M.; Prata, C.; Noronha, J.P.; Sampayo, M.A.; Justino, J.; Bermejo, J. A new dihydroxysterol from the marine phytoplankton Diacronema sp. Fitoterapia 2005, 76, 433–438. [Google Scholar] [CrossRef]
- Ododo, M.M.; Choudhury, M.K.; Dekebo, A.H. Structure elucidation of β-sitosterol with antibacterial activity from the root bark of Malva parviflora. Springerplus 2016, 5, 1210. [Google Scholar] [CrossRef]
- Ghannadian, M.; Akhavan, A.; Abdalla, O.; Ayatollahi, A.; Mohammadi-Kamalabadi, M.; Ghazanfari, H. Triterpenes from Euphorbia spinidens with immunomodulatory activity. Res. Pharm. Sci. 2013, 8, 205. [Google Scholar]
- Khan, M.T.H.; Khan, S.B.; Ather, A. Tyrosinase inhibitory cycloartane type triterpenoids from the methanol extract of the whole plant of Amberboa ramosa Jafri and their structure–activity relationship. Bioorg. Med. Chem. 2006, 14, 938–943. [Google Scholar] [CrossRef] [PubMed]
- Hajhashemi, V.; Ghanadian, M.; Palizaban, A.; Mahnam, K.; Eshaghi, H.; Gheisari, B.; Sadeghi-Aliabadi, H. Cycloarta-23-ene-3beta, 25-diol a pentacyclic steroid from Euphorbia spinidens, as COX inhibitor with molecular docking, and in vivo study of its analgesic and anti-inflammatory activities in male swiss mice and wistar rats. Prostaglandins Other Lipid Mediat. 2020, 150, 106473. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.; Braga, P.A.; Macedo, M.L.; Silva, M.; Ferreira, A.G.; Fernandes, J.B.; Vieira, P.C. Phytochemistry of Trattinnickia burserifolia, T. rhoifolia, and Dacryodes hopkinsii: Chemosystematic implications. J. Braz. Chem. Soc. 2004, 15, 385–394. [Google Scholar] [CrossRef]
- Ajithabai, M.; Sreedevi, S.; Jayakumar, G.; Nair, M.S.; Nair, D.P.; SP, S.R. Phytochemical Analysis and Radical Scavenging Activity of the Extracts of Costus picatus Linn and Coccinia indica W and A, two Ethnic Medicinal Plants used in the Treatment of Diabetes mellitus. Free Radic. Antioxid. 2011, 1, 77–83. [Google Scholar] [CrossRef]
- Johnsson, L.; Andersson, R.E.; Dutta, P.C. Side-chain autoxidation of stigmasterol and analysis of a mixture of phytosterol oxidation products by chromatographic and spectroscopic methods. JAOCS J. Am. Oil Chem. Soc. 2003, 80, 777–783. [Google Scholar] [CrossRef]
- Tasyriq, M.; Najmuldeen, I.A.; In, L.L.; Mohamad, K.; Awang, K.; Hasima, N. 7α-Hydroxy-β-sitosterol from Chisocheton tomentosus induces apoptosis via dysregulation of cellular Bax/Bcl-2 ratio and cell cycle arrest by downregulating ERK1/2 activation. Evid. Based Complement. Altern. Med. 2012, 2012, 765316. [Google Scholar] [CrossRef]
- Aliomrani, M.; Jafarian, A.; Zolfaghari, B. Phytochemical screening and cytotoxic evaluation of Euphorbia turcomanica on Hela and HT-29 tumor cell lines. Adv. Biomed. Res. 2017, 6, 68. [Google Scholar]
- Abdel-Monem, A.R.; Abdel-Sattar, E.; Harraz, F.M.; Petereit, F. Chemical Investigation of Euphorbia schimperi C. Presl. Rec. Nat. Prod. 2008, 2, 39–45. [Google Scholar]
- Li, P.; Feng, Z.X.; Ye, D.; Huan, W.; Da Gang, W.; Dong, L.X. Chemical constituents from the whole plant of Euphorbia altotibetic. Helv. Chim. Acta 2003, 86, 2525–2532. [Google Scholar] [CrossRef]
- Madureira, A.; Ascenso, J.; Valdeira, L.; Duarte, A.; Frade, J.; Freitas, G.; Ferreira, M. Evaluation of the antiviral and antimicrobial activities of triterpenes isolated from Euphorbia segetalis. Nat. Prod. Res. 2003, 17, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, T.; Akihisa, T.; Tokuda, H.; Ukiya, M.; Watanabe, K.; Nishino, H. Cancer chemopreventive effects of cycloartane-type and related triterpenoids in in vitro and in vivo models. J. Nat. Prod. 2007, 70, 918–922. [Google Scholar] [CrossRef] [PubMed]
- Badole, S.L.; Zanwar, A.A.; Khopade, A.N.; Bodhankar, S.L. In vitro antioxidant and antimicrobial activity cycloart–23–ene–3β,-25–diol (B2) isolated from Pongamia pinnata (L. Pierre). Asian Pac. J. Trop. Med. 2011, 4, 910–916. [Google Scholar] [CrossRef] [PubMed]
- Badole, S.L.; Mahamuni, S.P.; Bagul, P.P.; Khose, R.D.; Joshi, A.C.; Ghule, A.E.; Bodhankar, S.L.; Raut, C.G.; Khedkar, V.M.; Coutinho, E.C. Cycloart-23-ene-3β, 25-diol stimulates GLP-1 (7–36) amide secretion in streptozotocin–nicotinamide induced diabetic Sprague Dawley rats: A mechanistic approach. Eur. J. Pharmacol. 2013, 698, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Jassbi, A.R.; Zamanizadehnajari, S.; Tahara, S. Chemical constituents of Euphorbia marschalliana Boiss. Z Nat. C J. Biosci 2004, 59, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Shamsabadipour, S.; Zarei, S.M.; Ghanadian, M.; Ayatollahi, S.A.; Rahimnejad, M.R.; Saeedi, H.; Aghaei, M. A new taraxastane triterpene from Euphorbia denticulata with cytotoxic activity against prostate cancer cells. Iran. J. Pharm. Res. 2018, 17, 336. [Google Scholar]
- Tanaka, R.; Matsunaga, S. Fernane and multiflorane triterpene ketols from Euphorbia supina. Phytochemistry 1991, 30, 4093–4097. [Google Scholar] [CrossRef]
- Li, J.; Chen, Y. Anticancer activity of isomultiflorenol against human cervical cancer cells due to G2/M cell cycle arrest, autophagy and mitochondrial mediated apoptosis. Trop. J. Pharm. Res. 2020, 19, 1423–1428. [Google Scholar] [CrossRef]
- Hemmers, H.; Gülz, P.-G.; Marner, F.-J.; Wray, V. Pentacyclic triterpenoids in epicuticular waxes from Euphorbia lathyris L., Euphorbiaceae. Z. Für Nat. C 1989, 44, 193–201. [Google Scholar] [CrossRef]
- Gülz, P.-G.; Bodden, J.; Müller, E.; Marner, F.-J. Epicuticular wax of Euphorbia aphylla brouss. ex. willd., Euphorbiaceae. Z. Für Nat. C 1988, 43, 19–23. [Google Scholar] [CrossRef]
- Sultana, A.; Hossain, M.J.; Kuddus, M.R.; Rashid, M.A.; Zahan, M.S.; Mitra, S.; Roy, A.; Alam, S.; Sarker, M.M.R.; Naina Mohamed, I. Ethnobotanical Uses, Phytochemistry, toxicology, and pharmacological properties of Euphorbia neriifolia Linn. against infectious diseases: A comprehensive review. Molecules 2022, 27, 4374. [Google Scholar] [CrossRef] [PubMed]
- Akande, R.; Fouche, G.; Famuyide, I.; Makhubu, F.; Nkadimeng, S.; Aro, A.; Kayoka-Kabongo, P.; McGaw, L. Anthelmintic and antimycobacterial activity of fractions and compounds isolated from Cissampelos mucronata. J. Ethnopharmacol. 2022, 292, 115130. [Google Scholar] [CrossRef] [PubMed]
- Azemi, A.K.; Nordin, M.L.; Hambali, K.A.; Noralidin, N.A.; Mokhtar, S.S.; Rasool, A.H.G. Phytochemical Contents and Pharmacological Potential of Parkia speciosa Hassk. for Diabetic Vasculopathy: A Review. Antioxidants 2022, 11, 431. [Google Scholar] [CrossRef]
- Karim, S.; Akhter, M.H.; Burzangi, A.S.; Alkreathy, H.; Alharthy, B.; Kotta, S.; Md, S.; Rashid, M.A.; Afzal, O.; Altamimi, A.S. Phytosterol-Loaded Surface-Tailored Bioactive-Polymer Nanoparticles for Cancer Treatment: Optimization, In Vitro Cell Viability, Antioxidant Activity, and Stability Studies. Gels 2022, 8, 219. [Google Scholar] [CrossRef]
- Wang, K.N.; Hu, Y.; Han, L.L.; Zhao, S.S.; Song, C.; Sun, S.W.; Lv, H.Y.; Jiang, N.N.; Xv, L.Z.; Zhao, Z.W. Salvia chinensis Benth Inhibits Triple-Negative Breast Cancer Progression by Inducing the DNA Damage Pathway. Front. Oncol. 2022, 12, 882784. [Google Scholar] [CrossRef] [PubMed]
- Elhady, S.S.; Ibrahim, E.A.; Goda, M.S.; Nafie, M.S.; Samir, H.; Diri, R.M.; Alahdal, A.M.; Thomford, A.K.; El Gindy, A.; Hadad, G.M. GC-MS/MS Quantification of EGFR Inhibitors, β-Sitosterol, Betulinic Acid,(+) Eriodictyol,(+) Epipinoresinol, and Secoisolariciresinol, in Crude Extract and Ethyl Acetate Fraction of Thonningia sanguinea. Molecules 2022, 27, 4109. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.-Y.; Lee, C.-F.; Chou, W.-T.; Hwang, J.-J.; Tyan, Y.-S.; Chuang, H.-Y. Liposomal β-Sitosterol Suppresses Metastasis of CT26/luc Colon Carcinoma via Inhibition of MMP-9 and Evoke of Immune System. Pharmaceutics 2022, 14, 1214. [Google Scholar] [CrossRef]
- Vasanth, K.; Minakshi, G.C.; Velu, K.; Priya, T.; Kumar, R.M.; Kaliappan, I.; Dubey, G.P. Anti-adipogenic β-sitosterol and lupeol from Moringa oleifera suppress adipocyte differentiation through regulation of cell cycle progression. J. Food Biochem. 2022, 46, e14170. [Google Scholar] [CrossRef]
- Witkowska, A.M.; Waśkiewicz, A.; Zujko, M.E.; Cicha-Mikołajczyk, A.; Mirończuk-Chodakowska, I.; Drygas, W. Dietary plant sterols and phytosterol-enriched margarines and their relationship with cardiovascular disease among polish men and women: The WOBASZ II cross-sectional study. Nutrients 2022, 14, 2665. [Google Scholar] [CrossRef]
- de Oliveira, L.S.; de Araújo, M.F.; Braz-Filho, R.; Vieira, I.J.C. Dois Novos Diterpenos do Tipo Labdano e outros Compostos de Conchocarpus cyrtanthus (Rutaceae). Rev. Virtual Quim. 2016, 8, 87–96. [Google Scholar] [CrossRef]
- Silva, J.; Alves, C.; Martins, A.; Susano, P.; Simões, M.; Guedes, M.; Rehfeldt, S.; Pinteus, S.; Gaspar, H.; Rodrigues, A. Loliolide, a new therapeutic option for neurological diseases? In vitro neuroprotective and anti-inflammatory activities of a monoterpenoid lactone isolated from Codium tomentosum. Int. J. Mol. Sci. 2021, 22, 1888. [Google Scholar] [CrossRef] [PubMed]
- Radman, S.; Čižmek, L.; Babić, S.; Cikoš, A.M.; Čož-Rakovac, R.; Jokić, S.; Jerković, I. Bioprospecting of less-polar fractions of Ericaria crinita and Ericaria amentacea: Developmental Toxicity and antioxidant activity. Mar. Drugs 2022, 20, 57. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Wang, G.C.; Khan, G.J.; Su, X.H.; Guo, S.L.; Niu, Y.M.; Cao, W.G.; Wang, W.T.; Zhai, K.F. Identification and characterization of potential antioxidant components in Isodon amethystoides (Benth.) Hara tea leaves by UPLC-LTQ-Orbitrap-MS. FCT 2021, 148, 111961. [Google Scholar] [CrossRef] [PubMed]
- Chy, M.N.U.; Adnan, M.; Chowdhury, M.R.; Pagano, E.; Kamal, A.M.; Oh, K.K.; Cho, D.H.; Capasso, R. Central and peripheral pain intervention by Ophiorrhiza rugosa leaves: Potential underlying mechanisms and insight into the role of pain modulators. J. Ethnopharmacol. 2021, 276, 114182. [Google Scholar]
- Fernando, I.P.S.; Dias, M.K.H.M.; Madusanka, D.M.D.; Kim, H.-S.; Han, E.-J.; Kim, M.-J.; Seo, M.-J.; Ahn, G. Effects of (–)-Loliolide against Fine Dust Preconditioned Keratinocyte Media-Induced Dermal Fibroblast Inflammation. Antioxidants 2021, 10, 675. [Google Scholar] [CrossRef]
- Lee, D.; Kim, K.H.; Jang, T.S.; Kang, K.S. Identification of bioactive compounds from mulberry enhancing glucose-stimulated insulin secretion. Bioorganic Med. Chem. Lett. 2021, 43, 128096. [Google Scholar] [CrossRef]
- Sinan, K.I.; Chiavaroli, A.; Orlando, G.; Bene, K.; Zengin, G.; Cziáky, Z.; Jekő, J.; Fawzi Mahomoodally, M.; Picot-Allain, M.C.N.; Menghini, L. Evaluation of pharmacological and phytochemical profiles of Piptadeniastrum africanum (Hook. f.) brenan stem bark extracts. Biomolecules 2020, 10, 516. [Google Scholar] [CrossRef]
- Jeyasri, R.; Muthuramalingam, P.; Suba, V.; Ramesh, M.; Chen, J.-T. Bacopa monnieri and their bioactive compounds inferred multi-target treatment strategy for neurological diseases: A cheminformatics and system pharmacology approach. Biomolecules 2020, 10, 536. [Google Scholar] [CrossRef]
- Swantara, M.D.; Rita, W.S.; Dira, M.A.; Agustina, K.K. Cervical anticancer activities of Annona squamosa Linn. leaf isolate. Vet. World 2022, 15, 124. [Google Scholar] [CrossRef]
- Gangadhar, K.N.; Rodrigues, M.J.; Pereira, H.; Gaspar, H.; Malcata, F.X.; Barreira, L.; Varela, J. Anti-hepatocellular carcinoma (HepG2) activities of monoterpene hydroxy lactones isolated from the marine microalga Tisochrysis lutea. Mar. Drugs 2020, 18, 567. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Rahman, A.A.; Elsayed, K.N.; Abd El-Mageed, H.; Mohamed, H.S.; Ahmed, S.A. Cytotoxic activity, molecular docking, pharmacokinetic properties and quantum mechanics calculations of the brown macroalga Cystoseira trinodis compounds. J. Biomol. Struct. Dyn 2021, 39, 3855–3873. [Google Scholar] [CrossRef] [PubMed]
- Hamed, A.N.; Abouelela, M.E.; El Zowalaty, A.E.; Badr, M.M.; Abdelkader, M.S. Chemical constituents from Carica papaya Linn. leaves as potential cytotoxic, EGFR wt and aromatase (CYP19A) inhibitors; a study supported by molecular docking. RSC Adv. 2022, 12, 9154–9162. [Google Scholar] [CrossRef] [PubMed]
- El-Mekkawy, S.; Hassan, A.Z.; Abdelhafez, M.A.; Mahmoud, K.; Mahrous, K.F.; Meselhy, M.R.; Sendker, J.; Abdel-Sattar, E. Cytotoxicity, genotoxicity, and gene expression changes induced by methanolic extract of Moringa stenopetala leaf with LC-qTOF-MS metabolic profile. Toxicon 2021, 203, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Elasbali, A.M.; Al-Soud, W.A.; Al-Oanzi, Z.H.; Qanash, H.; Alharbi, B.; Binsaleh, N.K.; Alreshidi, M.; Patel, M.; Adnan, M. Cytotoxic Activity, Cell Cycle Inhibition, and Apoptosis-Inducing Potential of Athyrium hohenackerianum (Lady Fern) with Its Phytochemical Profiling. Evid.-Based Complement. Altern. Med. 2022, 2022. [Google Scholar] [CrossRef]
- Stojakowska, A.; Galanty, A.; Malarz, J.; Michalik, M. Major terpenoids from Telekia speciosa flowers and their cytotoxic activity in vitro. Nat. Prod. Res. 2019, 33, 1804–1808. [Google Scholar] [CrossRef]
- Tanaka, R.; Matsunaga, S. Loliolide and olean-12-en-3β, 9α, 11α-triol from Euphorbia supina. Phytochemistry 1989, 28, 1699–1702. [Google Scholar] [CrossRef]
- Tao, Y.; Tian, Y.; Xu, W.; Guo, Q.; Shi, J. Terpenoids from Euphorbia micractina. Acta Pharm. Sin. 2016, 51, 411–419. [Google Scholar]
- Hlengwa, S.S. Isolation and Characterisation of Bioactive Compounds from Antidesma Venosum E. Mey. ex Tul. and Euphorbia cooperi NE Br. ex A. Berger. Master’s Thesis, University of KwaZulu-Natal, Pietermaritzburg, South Africa, 2018. [Google Scholar]
- Rozimamat, R.; Kehrimen, N.; Aisa, H.A. New compound from Euphorbia alatavica Boiss. Nat. Prod. Res. 2019, 33, 380–385. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Motinia, N.; Ghannadian, M.; Zolfaghari, B.; Yazdiniapour, Z. Phytochemical Study of Euphorbia turcomanica Boiss. Metabolites 2022, 12, 1200. https://doi.org/10.3390/metabo12121200
Motinia N, Ghannadian M, Zolfaghari B, Yazdiniapour Z. Phytochemical Study of Euphorbia turcomanica Boiss. Metabolites. 2022; 12(12):1200. https://doi.org/10.3390/metabo12121200
Chicago/Turabian StyleMotinia, Newsha, Mustafa Ghannadian, Behzad Zolfaghari, and Zeinab Yazdiniapour. 2022. "Phytochemical Study of Euphorbia turcomanica Boiss." Metabolites 12, no. 12: 1200. https://doi.org/10.3390/metabo12121200
APA StyleMotinia, N., Ghannadian, M., Zolfaghari, B., & Yazdiniapour, Z. (2022). Phytochemical Study of Euphorbia turcomanica Boiss. Metabolites, 12(12), 1200. https://doi.org/10.3390/metabo12121200






