The Effect of 10 Crop Plants That Served as Hosts on the Primary Metabolic Profile of the Parasitic Plant Phelipanche aegyptiaca
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Sample Collection
2.2. Extraction of Primary Metabolites and Analysis Using Gas Chromatography-Mass Spectrometry (GC-MS)
2.3. Total Soluble Protein Content Determination
2.4. Total Phenolic Compound Content Determination
2.5. Statistical Analyses
3. Results
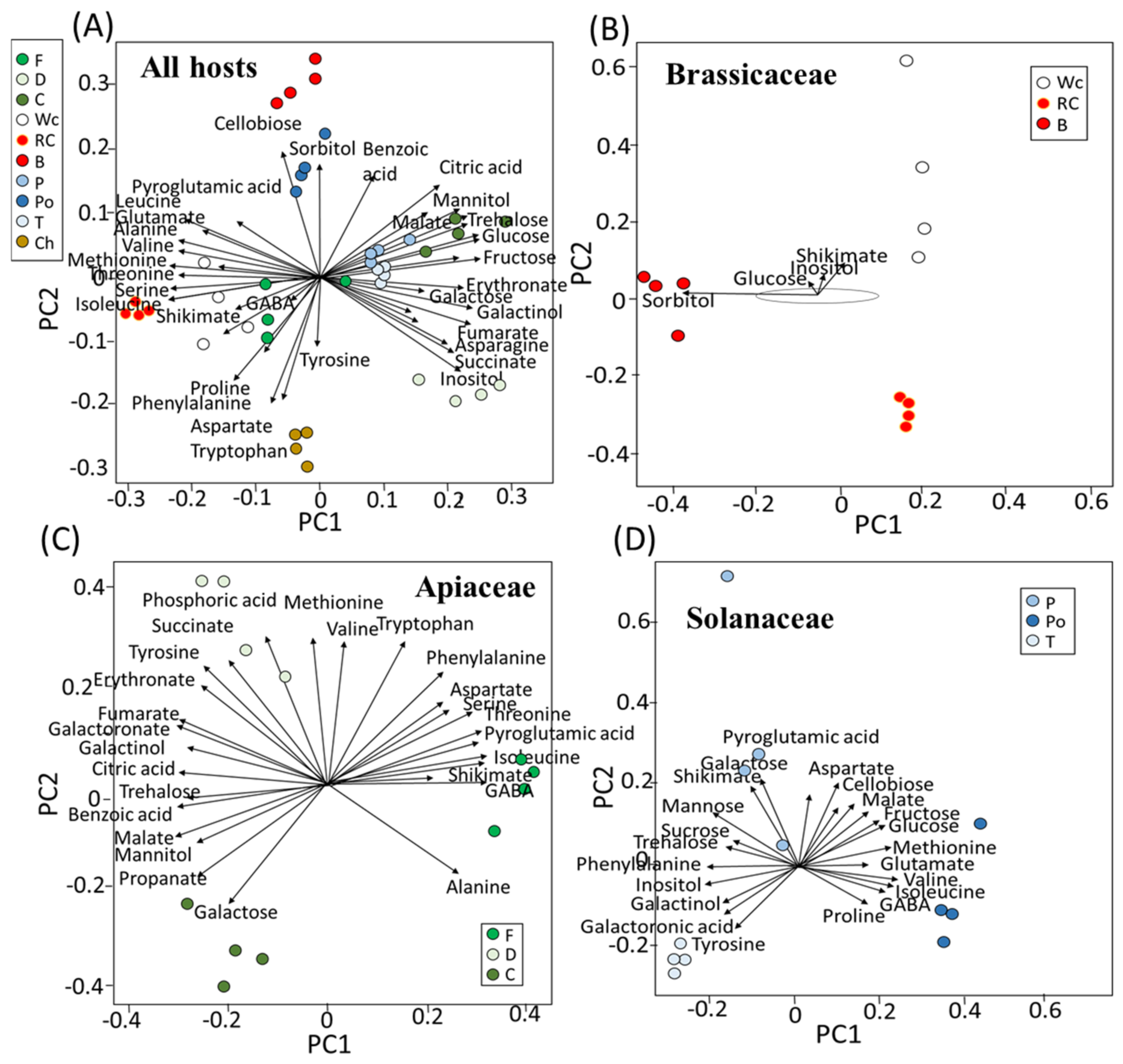
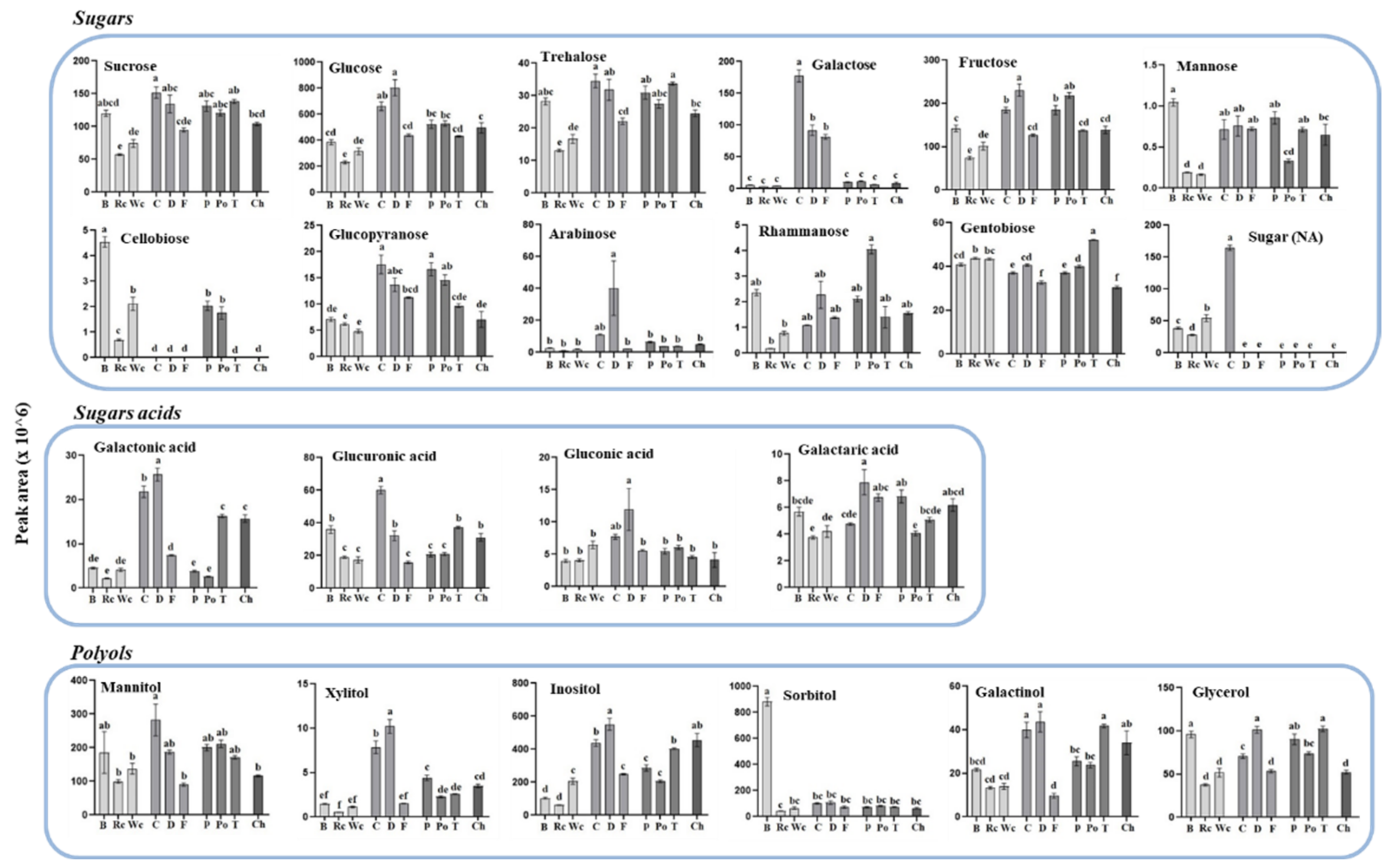
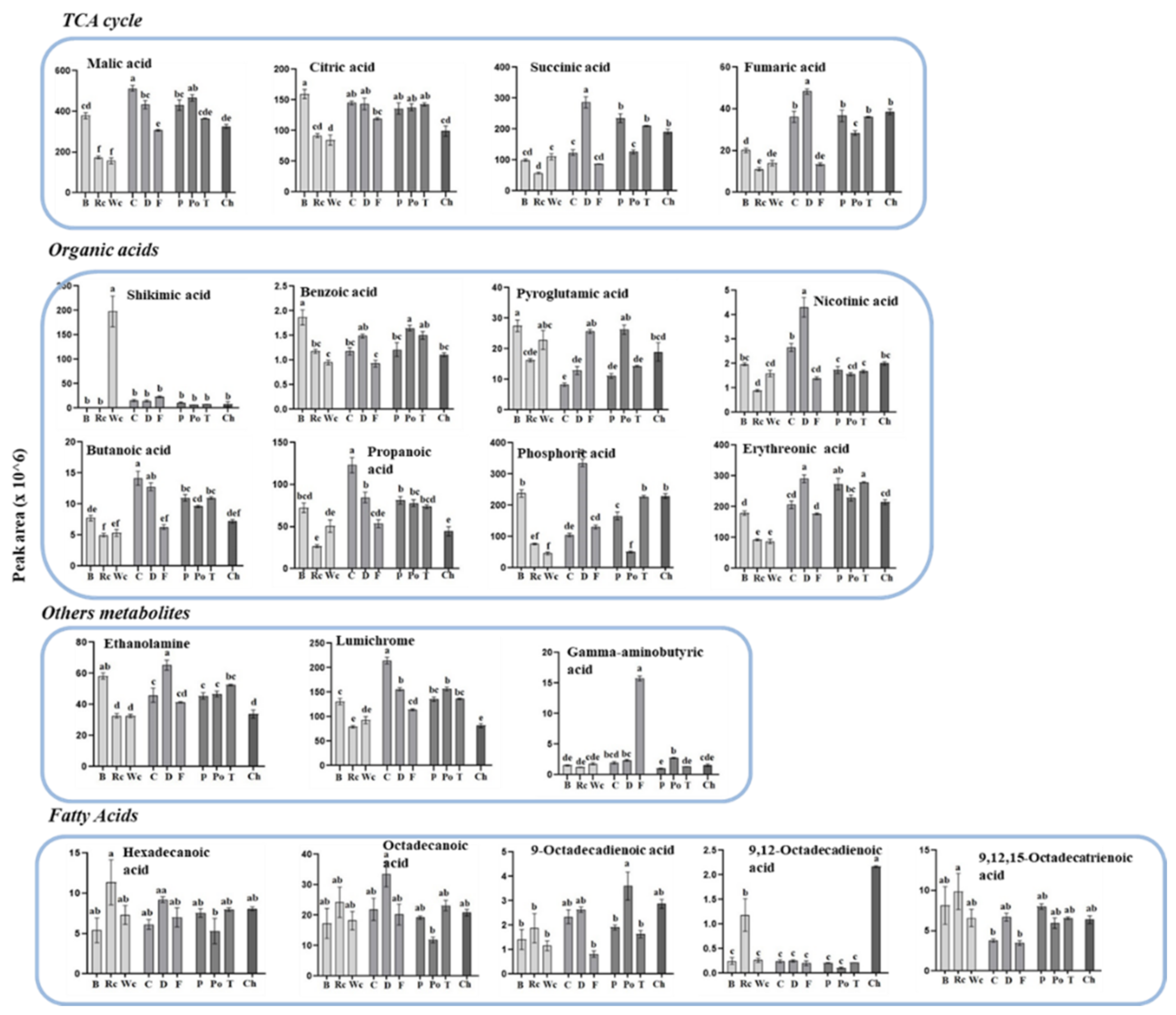
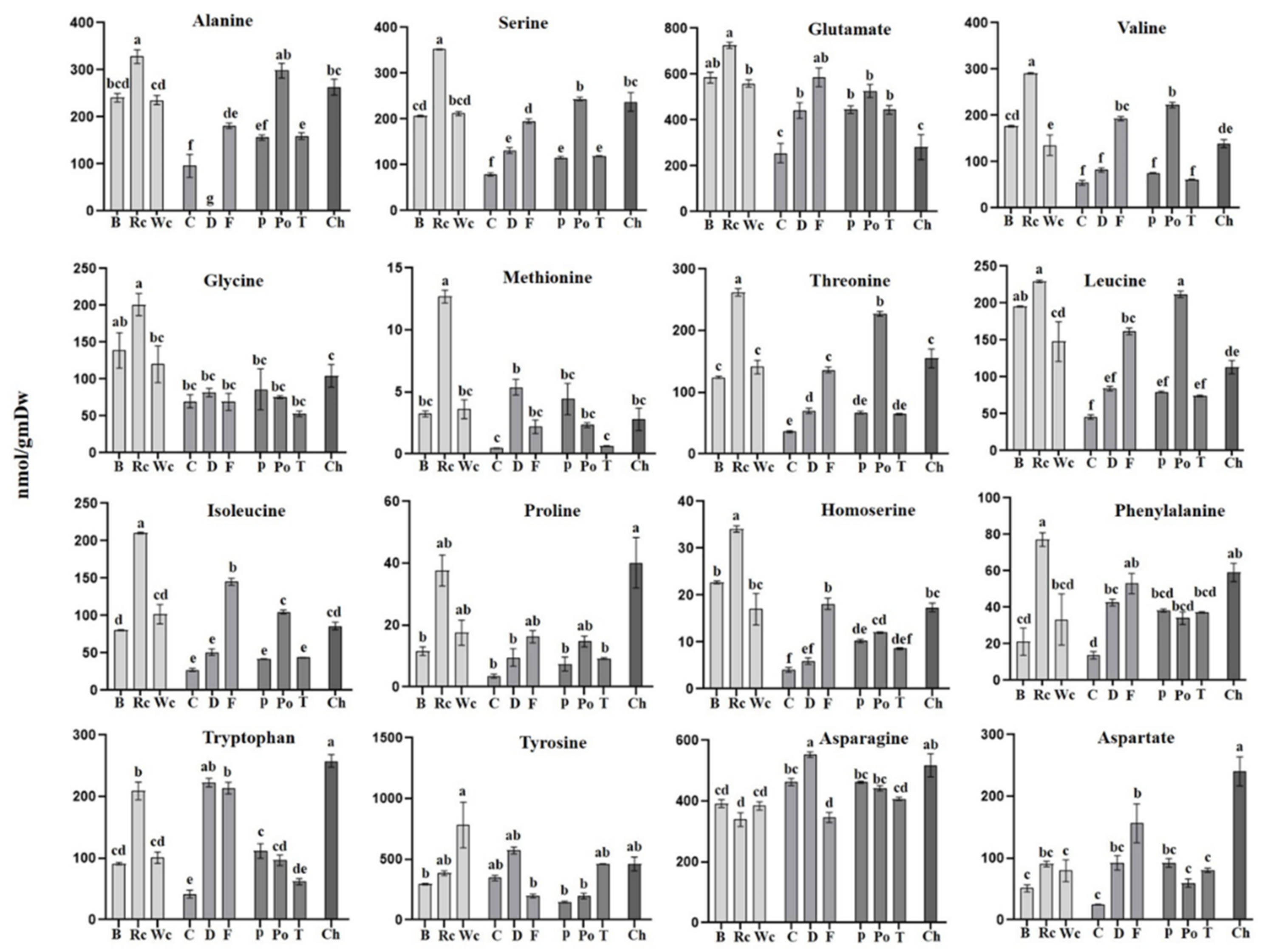
3.1. Primary Metabolic Profiling Analysis Using GC-MS Reveals a Differential Metabolic Accumulation in P. aegyptiaca with Respect to the Host Plants
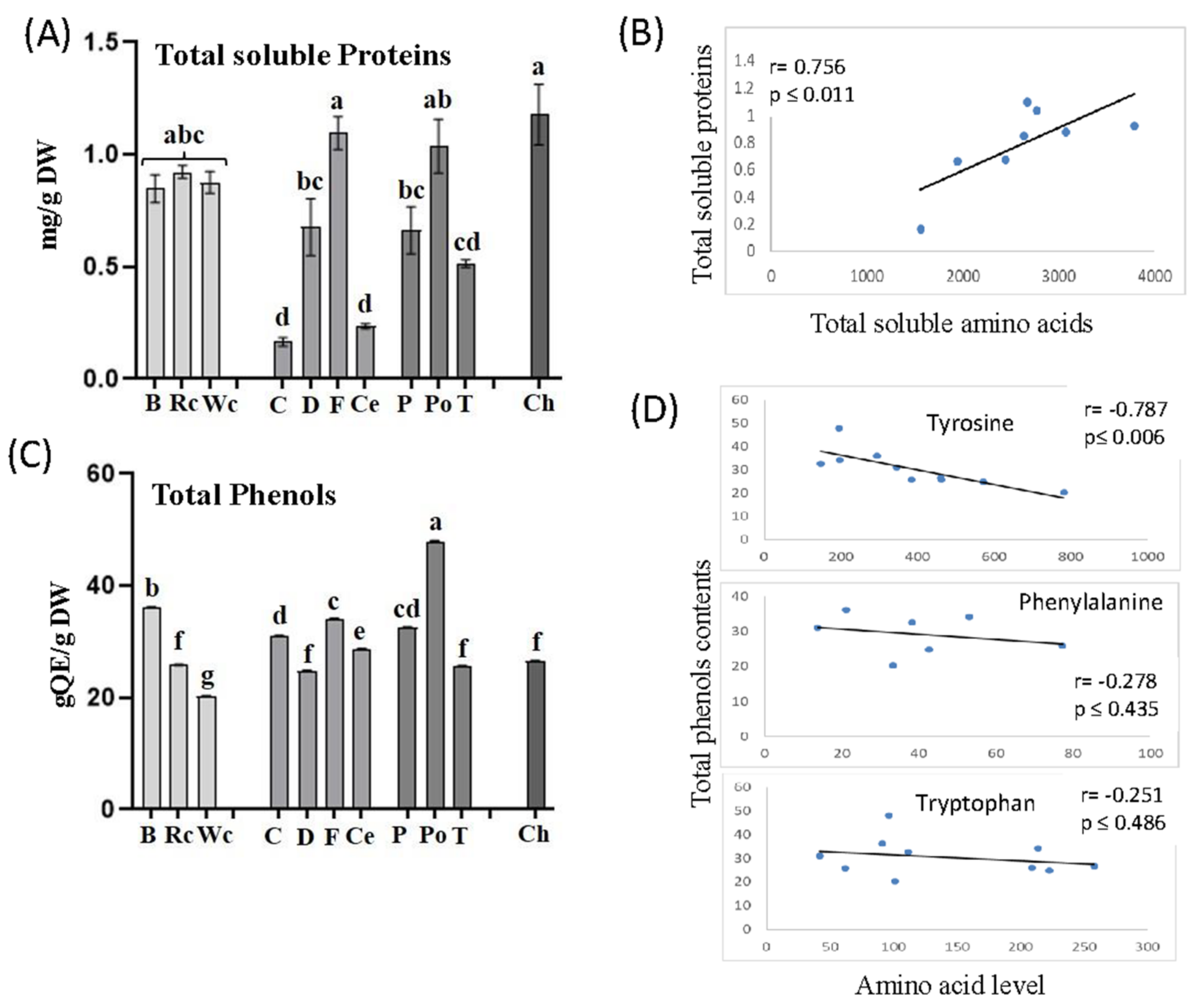
3.2. Determination of Total Soluble Proteins and Total Phenolic Compounds
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Joel, D.M. Direct Infection of Potato Tubers by the Root Parasite Orobanche aegyptiaca. Weed Res. 2007, 47, 276–279. [Google Scholar] [CrossRef]
- Yoshida, S.; Shirasu, K. Plants that Attack Plants: Molecular Elucidation of Plant Parasitism. Curr. Opin. Plant Biol. 2012, 15, 708–713. [Google Scholar] [CrossRef] [PubMed]
- Strelnikov, E.; Antonova, T.; Gorlova, L.; Trubina, V. The Environmentally Safe Method of Control of Broomrape (Orobanche cumana Wallr.) Parasitizing on Sunflower. BIO Web Conf. 2020, 21, 00039. [Google Scholar] [CrossRef]
- Abbes, Z.; Kharrat, M.; Delavault, P.; Chaïbi, W.; Simier, P. Osmoregulation and Nutritional Relationships between Orobanche foetida and Faba Bean. Plant Signal. Behav. 2009, 4, 336–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hibberd, J.M.; Jeschke, W.D. Solute Flux into Parasitic Plants. J. Exp. Bot. 2001, 52, 2043–2049. [Google Scholar] [CrossRef] [Green Version]
- Péron, T.; Candat, A.; Montiel, G.; Veronesi, C.; Macherel, D.; Delavault, P.; Simier, P. New Insights into Phloem Unloading and Expression of Sucrose Transporters in Vegetative Sinks of the Parasitic Plant Phelipanche ramosa L. (Pomel). Front. Plant Sci. 2017, 7, 2048. [Google Scholar] [CrossRef] [Green Version]
- Flores-Sánchez, I.J.; Garza-Ortiz, A. Is there a secondary/specialized metabolism in the genus Cuscuta and which is the role of the host plant? Phytochem. Rev. 2019, 18, 1299–1335. [Google Scholar] [CrossRef]
- Smith, J.D.; Mescher, M.C.; De Moraes, C.M. Implications of Bioactive Solute Transfer from Hosts to Parasitic plants. Curr. Opin. Plant Biol. 2013, 16, 464–472. [Google Scholar] [CrossRef]
- Clermont, K.; Wang, Y.; Liu, S.; Yang, Z.; dePamphilis, C.W.; Yoder, J.I. Comparative metabolomics of early development of the parasitic plants Phelipanche aegyptiaca and Triphysaria versicolor. Metabolites 2019, 9, 114. [Google Scholar] [CrossRef] [Green Version]
- Nickrent, D.L.; Musselman, L. Introduction to Parasitic Flowering Plants. Plant Health Instr. 2004, 13, 25–31. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Jiang, L.; Li, Z.; Zhang, M. Metabolomics Analysis Provides New Insights into the Molecular Mechanisms of Parasitic Plant Dodder Elongation in vitro. Front. Plant Sci. 2022, 13, 921245. [Google Scholar] [CrossRef]
- Hacham, Y.; Hershenhorn, J.; Dor, E.; Amir, R. Primary Metabolic Profiling of Egyptian Broomrape (Phelipanche aegyptiaca) Compared to its Host Tomato Roots. J. Plant Physiol. 2016, 205, 11–19. [Google Scholar] [CrossRef]
- Nandula, V.K.; Foster, J.G.; Foy, C.L. Impact of Egyptian Broomrape (Orobanche aegyptiaca (Pers.) Parasitism on Amino Acid Composition of Carrot (Daucus carota L.). J. Agric. Food Chem. 2000, 48, 3930–3934. [Google Scholar] [CrossRef]
- Wakabayashi, T.; Joseph, B.; Yasumoto, S.; Akashi, T.; Aoki, T.; Harada, K.; Muranaka, S.; Bamba, T.; Fukusaki, E.; Takeuchi, Y.; et al. Planteose as a Storage Carbohydrate Required for Early Stage of Germination of Orobanche minor and its Metabolism as a Possible Target for Selective Control. J. Exp. Bot. 2015, 66, 3085–3097. [Google Scholar] [CrossRef] [Green Version]
- Dor, E.; Galili, S.; Smirnov, E.; Hacham, Y.; Amir, R.; Hershenhorn, J. The Effects of Herbicides Targeting Aromatic and Branched Chain Amino Acid biosynthesis support the Presence of Functional Pathways in Broomrape. Front. Plant Sci. 2017, 8, 707. [Google Scholar] [CrossRef] [Green Version]
- Shilo, T.; Rubin, B.; Plakhine, D.; Gal, S.; Amir, R.; Hacham, Y.; Wolf, S.; Eizenberg, H. Secondary Effects of Glyphosate Action in Phelipanche aegyptiaca: Inhibition of solute transport from the host plant to the parasite. Front. Plant Sci. 2017, 8, 255. [Google Scholar] [CrossRef] [Green Version]
- Emran, S.; Nawade, B.; Yahyaa, M.; Abu Nassar, J.; Tholl, D.; Eizenberg, H.; Ibdah, M. Broomrape Infestation in Carrot (Daucus carota): Changes in Carotenoid Gene Expression and Carotenoid Accumulation in the Parasitic Weed Phelipanche aegyptiaca and its host. Sci. Rep. 2020, 10, 324. [Google Scholar] [CrossRef] [Green Version]
- Kumar, K.; Amir, R. The Effect of a Host on the Primary Metabolic Profiling of Cuscuta campestris’ Main Organs, Haustoria, Stem and Flower. Plants 2021, 10, 2098. [Google Scholar] [CrossRef]
- Nativ, N.; Hacham, Y.; Hershenhorn, J.; Dor, E.; Amir, R. Metabolic Investigation of Phelipanche aegyptiaca Reveals Significant Changes during Developmental Stages and in Its Different Organs. Front. Plant Sci. 2017, 8, 491. [Google Scholar] [CrossRef] [Green Version]
- Cohen, H.; Israeli, H.; Matityahu, I.; Amir, R. Seed-Specific Expression of a Feedback-Insensitive form of CYSTATHIONINE-γ-SYNTHASE in Arabidopsis Stimulates Metabolic and Transcriptomic Responses Associated with Desiccation Stress. Plant Physiol. 2014, 166, 1575–1592. [Google Scholar] [CrossRef]
- Ben Nasr, C.; Ayed, N.; Metche, M. Quantitative Determination of the Polyphenolic Content of Pomegranate peel. Z. Lebensm.-Unters. Forsch. 1996, 203, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Sinelnikov, I.V.; Han, B.; Wishart, D.S. MetaboAnalyst 3.0-making Metabolomics more Meaningful. Nucleic Acids Res. 2015, 43, W251–W257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rhodes, D.; Handa, S.; Bressan, R.A. Metabolic Changes Associated with Adaptation of Plant Cells to Water Stress. Plant Physiol. 1986, 82, 890–903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jander, G.; Joshi, V. Recent Progress in Deciphering the Biosynthesis of Aspartate-Derived Amino Acids in Plants. Mol. Plant 2010, 3, 54–65. [Google Scholar] [CrossRef]
- Ahmad, A.; Tandon, S.; Xuan, T.D.; Nooreen, Z. A Review on Phytoconstituents and Biological activities of Cuscuta species. Biomed. Pharmacother. 2017, 92, 772–795. [Google Scholar] [CrossRef]
- Al-Gburi, B.K.H.; Al-Sahaf, F.H.; Al-fadhal, F.A.; Del Monte, J.P. Detection of Phytochemical Compounds and Pigments in Seeds and Shoots of Cuscuta campestris parasitizing on eggplant. Physiol. Mol. Biol. Plants 2019, 25, 253–261. [Google Scholar] [CrossRef]
- Kaiser, B.; Vogg, G.; Fürst, U.B.; Albert, M. Parasitic Plants of the Genus Cuscuta and their Interaction with Susceptible and Resistant Host Plants. Front. Plant Sci. 2015, 6, 45. [Google Scholar] [CrossRef] [Green Version]
- Westwood, J.H.; dePamphilis, C.W.; Das, M.; Fernández-Aparicio, M.; Honaas, L.A.; Timko, M.P.; Wafula, E.K.; Wickett, N.J.; Yoder, J.I. The Parasitic Plant Genome Project: New Tools for Understanding the Biology of Orobanche and Striga. Weed Sci. 2012, 60, 295–306. [Google Scholar] [CrossRef]
- Fernández-Aparicio, M.; Reboud, X.; Gibot-Leclerc, S. Broomrape Weeds. Underground Mechanisms of Parasitism and Associated Strategies for their Control: A review. Front. Plant Sci. 2016, 7, 135. [Google Scholar] [CrossRef] [Green Version]
- Delavault, P.; Simier, P.; Thoiron, S.; Véronési, C.; Fer, A.; Thalouarn, P. Isolation of Mannose 6-phosphate Reductase cDNA, Changes in Enzyme Activity and Mannitol Content in Broomrape (Orobanche ramosa) Parasitic on Tomato Roots. Physiol. Plant. 2002, 115, 48–55. [Google Scholar] [CrossRef]
- Furuhashi, T.; Nakamura, T.; Iwase, K. Analysis of Metabolites in Stem Parasitic Plant Interactions: Interaction of Cuscuta–Momordica versus Cassytha–Ipomoea. Plants 2016, 5, 421. [Google Scholar] [CrossRef]
- Fer, A.; Russo, N.; Simier, P.; Arnaud, M.C.; Thalouarn, P. Physiological Changes in a Root Hemiparasitic Angiosperm, Thesium humile (Santalaceae), before and after Attachment to the Host Plant (Triticum vulgare). J. Plant Physiol. 1994, 143, 704–710. [Google Scholar] [CrossRef]
- Stoop, J.M.H.; Williamson, J.D.; Phar, D.R. Mannitol Metabolism in Plants: A Method for Coping with Stress. Trends Plant Sci. 1996, 1, 139–144. [Google Scholar] [CrossRef]
- Bäumel, P.; Czygan, F.C.; Proksch, P.; Jeschke, W.D.; Witte, L. Uptake and Transport of Quinolizidine Alkaloids in Cuscuta Reflex a Parasitizing on Lupinusc angustifolius. Z. Naturforsch. Sect. C J. Biosci. 1993, 48, 436–443. [Google Scholar] [CrossRef]
- Bäumel, P.; Jeschke, W.D.; Räth, N.; Czygan, F.C.; Proksch, P. Modelling of Quinolizidine Alkaloid Net Flows in Lupinus albus and between L. albus and the Parasite Cuscuta reflexa: New Insights into the Site of Quinolizidine Alkaloid Synthesis. J. Exp. Bot. 1995, 46, 1721–1730. [Google Scholar] [CrossRef]
- Furuhashi, T.; Fragner, L.; Furuhashi, K.; Valledor, L.; Sun, X.; Weckwerth, W. Metabolite Changes with Induction of Cuscuta haustorium and Translocation from Host Plants. J. Plant Interact. 2012, 7, 84–93. [Google Scholar] [CrossRef]
- Anis, E.; Mustafa, G.; Ullah, N.; Malik, A. Phytochemical Studies on Cuscuta reflexa. Pak. J. Sci. Ind. Res. 1999, 42, 170–172. [Google Scholar]
- Bais, N.; Kakkar, A. Phytochemical Analysis of Methanolic Extract of Cuscuta reflexa Grown on Cassia fistula and Ficus benghalensis by GC-MS. Int. J. Pharm. Sci. Rev. Res. 2014, 25, 33–36. [Google Scholar]
- Tanruean, K.; Kaewnarin, K.; Suwannarach, N.; Lumyong, S. Comparative Evaluation of Phytochemicals, and Antidiabetic and Antioxidant Activities of Cuscuta reflexa Grown on Different Hosts in Northern Thailand. Nat. Prod. Commun. 2017, 12, 51–54. [Google Scholar] [CrossRef]
- Wiese, A.J. Comparative Analyses of Primary Carbon Metabolism in Parasitic Plant Species in Faculty of Agricultural Sciences; Stellenbosch University: Stellenbosch, South Africa, 2013; pp. 1–106. [Google Scholar]



| Hosts | Short Name | Locations | Date |
|---|---|---|---|
| Potato | Po | South Hula Valley | 26 December 2018 |
| Tomato | T | Beit She’an Valley | 12 June 2019 |
| Paprika pepper | P | Beit She’an Valley | 12 June 2019 |
| Broccoli | B | Jezreel Valley | 11 January 2019 |
| White cabbage | Wc | Jezreel Valley | 11 January 2019 |
| Red cabbage | Rc | Jezreel Valley | 11 January 2019 |
| Fennel | F | Jezreel Valley | 11 January 2019 |
| Dill | D | Beit She’an Valley | 21 May 2019 |
| Carrot | C | North Hula Valley | 14 April 2019 |
| Chickpea | CH | Beit She’an Valley | 21 May 2019 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, K.; Hacham, Y.; Amir, R. The Effect of 10 Crop Plants That Served as Hosts on the Primary Metabolic Profile of the Parasitic Plant Phelipanche aegyptiaca. Metabolites 2022, 12, 1195. https://doi.org/10.3390/metabo12121195
Kumar K, Hacham Y, Amir R. The Effect of 10 Crop Plants That Served as Hosts on the Primary Metabolic Profile of the Parasitic Plant Phelipanche aegyptiaca. Metabolites. 2022; 12(12):1195. https://doi.org/10.3390/metabo12121195
Chicago/Turabian StyleKumar, Krishna, Yael Hacham, and Rachel Amir. 2022. "The Effect of 10 Crop Plants That Served as Hosts on the Primary Metabolic Profile of the Parasitic Plant Phelipanche aegyptiaca" Metabolites 12, no. 12: 1195. https://doi.org/10.3390/metabo12121195






