Understanding the Seasonal Effect of Metabolite Production in Terminalia catappa L. Leaves through a Concatenated MS- and NMR-Based Metabolomics Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Material
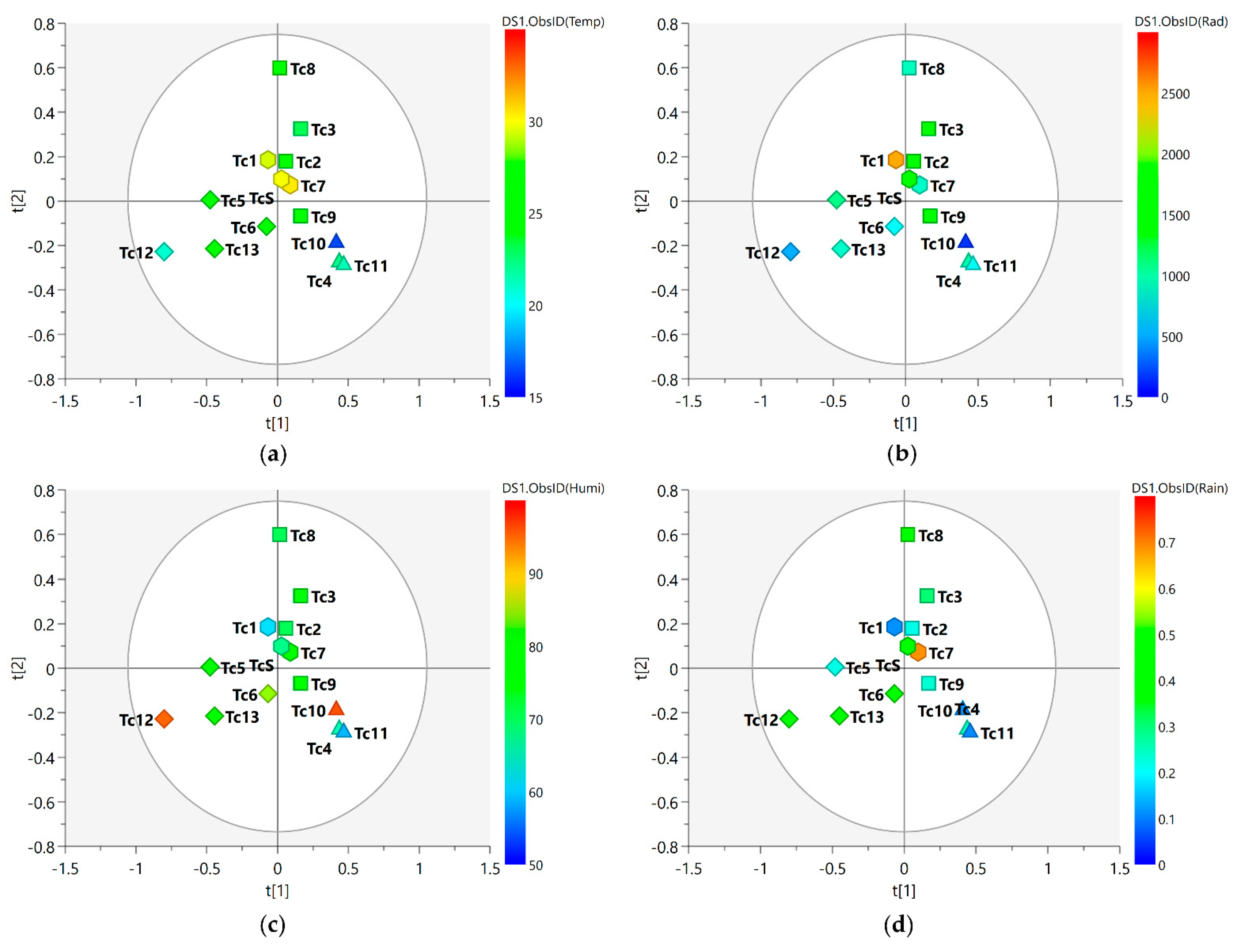
2.3. Climate Data
2.4. Ethanolic Extract and Samples Preparation
2.5. NMR Analysis
2.6. UHPLC-ESI-HRMS Analysis
2.7. Data Processing
2.8. Multivariate Data Analysis
3. Results
3.1. Metabolite Profiling
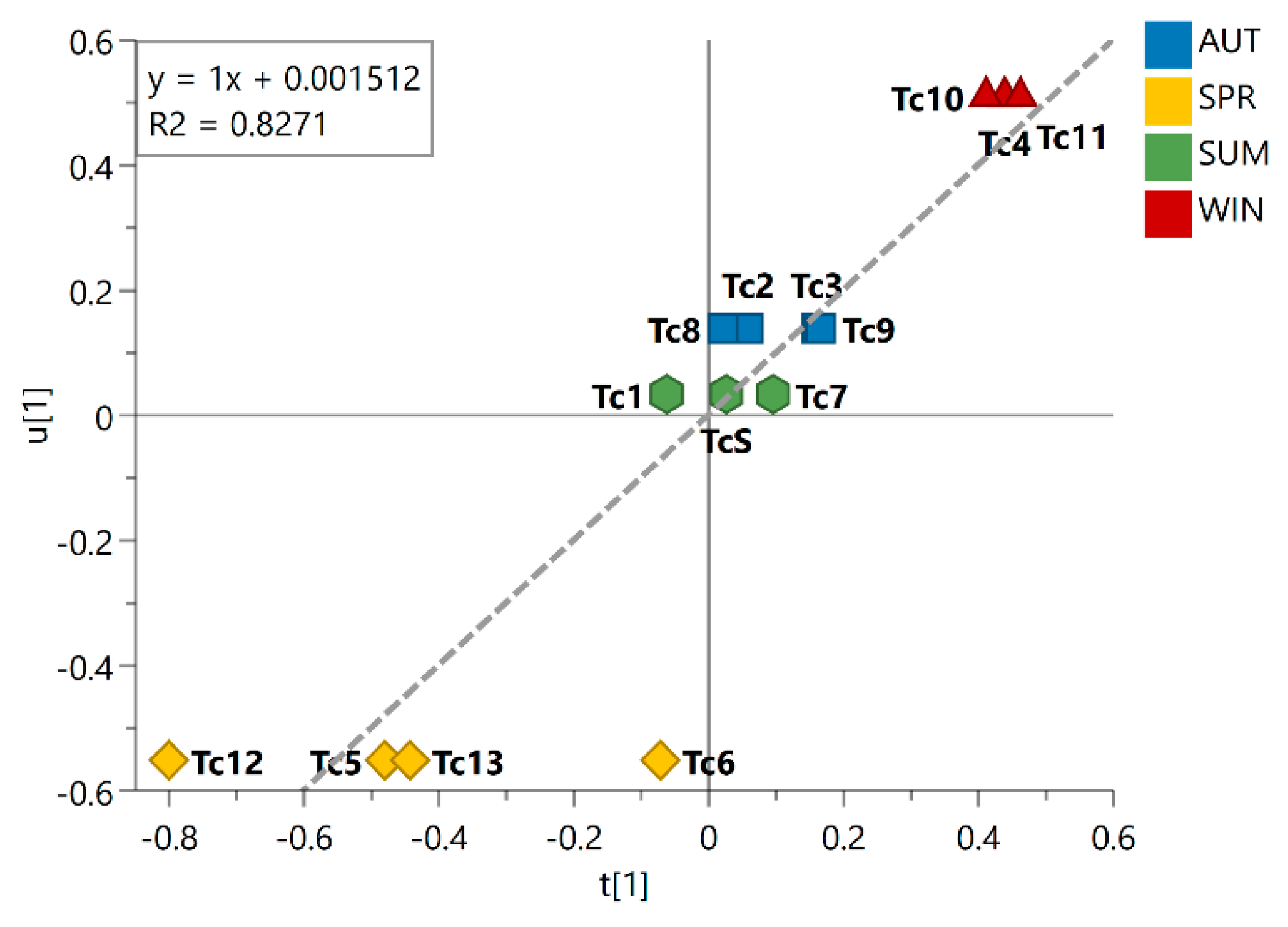
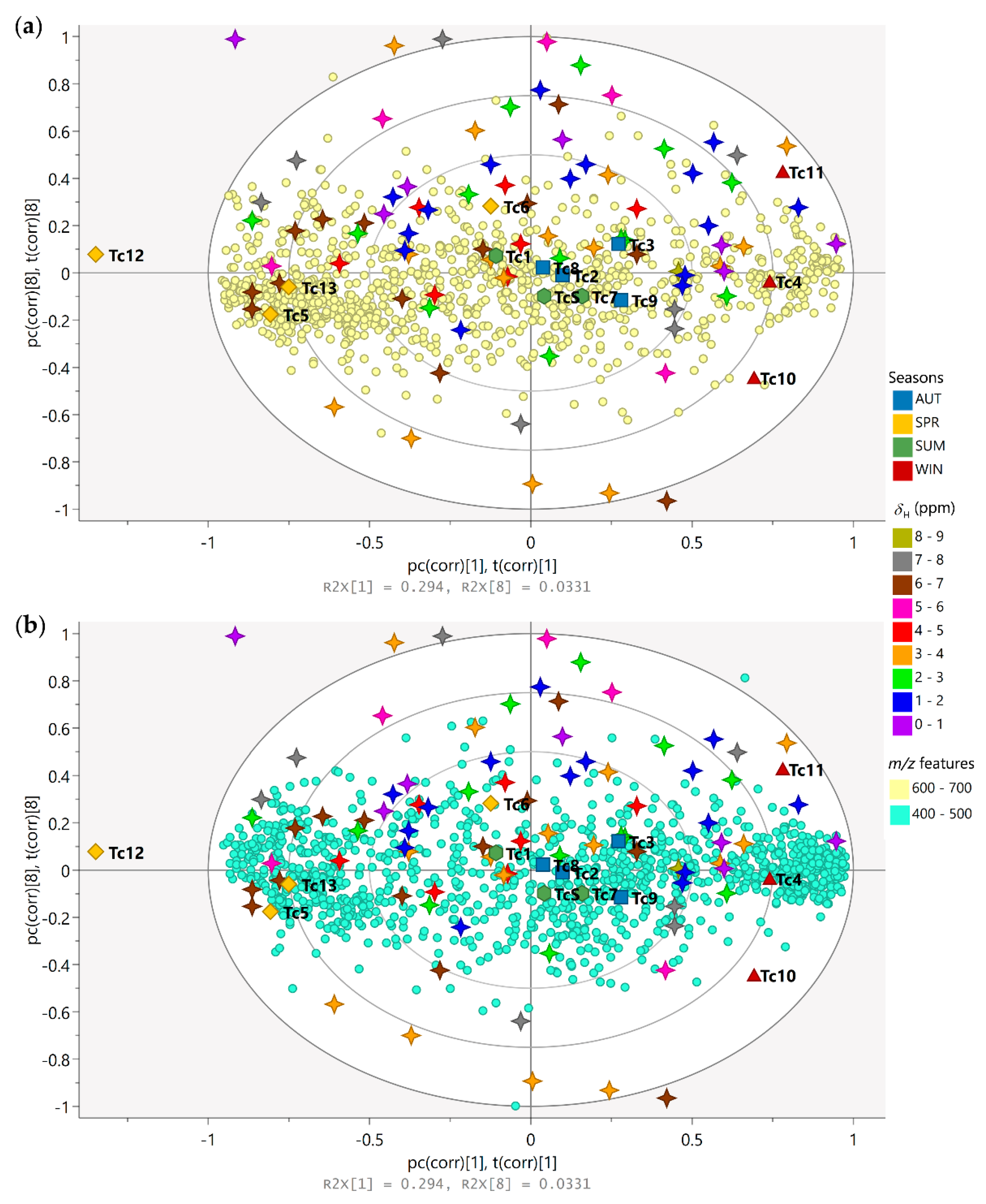
3.2. Seasonality Assessment from MS-NMR Fused Data
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Megadiverse Brazil: Giving Biodiversity an Online Boost. Available online: https://www.unep.org/news-and-stories/story/megadiverse-brazil-giving-biodiversity-online-boost (accessed on 8 December 2021).
- Valli, M.; Russo, H.M.; Bolzani, V.S. The Potential Contribution of the Natural Products from Brazilian Biodiversity to Bioeconomy. An. Acad. Bras. Cienc. 2018, 90, 763–778. [Google Scholar] [CrossRef] [PubMed]
- Dutra, R.C.; Campos, M.M.; Santos, A.R.S.; Calixto, J.B. Medicinal Plants in Brazil: Pharmacological Studies, Drug Discovery, Challenges and Perspectives. Pharmacol. Res. 2016, 112, 4–29. [Google Scholar] [CrossRef]
- da Silva Bolzani, V.; Valli, M.; Pivatto, M.; Viegas, C. Natural Products from Brazilian Biodiversity as a Source of New Models for Medicinal Chemistry. Pure Appl. Chem. 2012, 84, 1837–1846. [Google Scholar] [CrossRef]
- De Sedas, A.; Turner, B.L.; Winter, K.; Lopez, O.R. Salinity Responses of Inland and Coastal Neotropical Trees Species. Plant Ecol. 2020, 221, 695–708. [Google Scholar] [CrossRef]
- Panta, S.; Flowers, T.; Lane, P.; Doyle, R.; Haros, G.; Shabala, S. Halophyte Agriculture: Success Stories. Environ. Exp. Bot. 2014, 107, 71–83. [Google Scholar] [CrossRef]
- Ksouri, R.; Ksouri, W.M.; Jallali, I.; Debez, A.; Magné, C.; Hiroko, I.; Abdelly, C. Medicinal Halophytes: Potent Source of Health Promoting Biomolecules with Medical, Nutraceutical and Food Applications. Crit. Rev. Biotechnol. 2012, 32, 289–326. [Google Scholar] [CrossRef]
- Thomson, L.A.J.; Evans, B. Terminalia Catappa (Tropical Almond). In Traditional Trees of Pacific Islands: The Culture, Environment And Use; Elevitch, C.R., Ed.; Permanent Agriculture Resources: Holualoa, HI, USA, 2006; p. 816. ISBN 0970254458. [Google Scholar]
- Cock, I.E. The Medicinal Properties and Phytochemistry of Plants of the Genus Terminalia (Combretaceae). Inflammopharmacology 2015, 23, 203–229. [Google Scholar] [CrossRef]
- Pinheiro Silva, L.; Damacena de Angelis, C.; Bonamin, F.; Kushima, H.; José Mininel, F.; Campaner dos Santos, L.; Karina Delella, F.; Luis Felisbino, S.; Vilegas, W.; Regina Machado da Rocha, L.; et al. Terminalia catappa L.: A Medicinal Plant from the Caribbean Pharmacopeia with Anti-Helicobacter Pylori and Antiulcer Action in Experimental Rodent Models. J. Ethnopharmacol. 2015, 159, 285–295. [Google Scholar] [CrossRef]
- Mininel, F.J.; Leonardo Junior, C.S.; Espanha, L.G.; Resende, F.A.; Varanda, E.A.; Leite, C.Q.F.; Vilegas, W.; dos Santos, L.C. Characterization and Quantification of Compounds in the Hydroalcoholic Extract of the Leaves from Terminalia catappa Linn. (Combretaceae) and Their Mutagenic Activity. Evid. Based Complement. Altern. Med. 2014, 2014, 676902. [Google Scholar] [CrossRef]
- Terças, A.G.; de Souza Monteiro, A.; Moffa, E.B.; dos Santos, J.R.A.; de Sousa, E.M.; Pinto, A.R.B.; da Silva Costa, P.C.; Borges, A.C.R.; Torres, L.M.B.; Barros Filho, A.K.D.; et al. Phytochemical Characterization of Terminalia catappa Linn. Extracts and Their Antifungal Activities against Candida spp. Front. Microbiol. 2017, 8, 595. [Google Scholar] [CrossRef]
- Allyn, O.Q.; Kusumawati, E.; Nugroho, R.A. Antimicrobial Activity of Terminalia Catappa Brown Leaf Extracts against Staphylococcus aureus ATCC 25923 and Pseudomonas Aeruginosa ATCC 27853. F1000Research 2018, 7, 1406. [Google Scholar] [CrossRef] [PubMed]
- Mandloi, S.; Mishra, R.; Varma, R.; Varughese, B.; Tripathi, J. A Study on Phytochemical and Antifungal Activity of Leaf Extracts of Terminalia cattapa. Int. Pharma Bio Sci. 2013, 4, 85–93. [Google Scholar]
- Chyau, C.-C.; Ko, P.-T.; Mau, J.-L. Antioxidant Properties of Aqueous Extracts from Terminalia catappa Leaves. LWT Food Sci. Technol. 2006, 39, 1099–1108. [Google Scholar] [CrossRef]
- Kinoshita, S.; Inoue, Y.; Nakama, S.; Ichiba, T.; Aniya, Y. Antioxidant and Hepatoprotective Actions of Medicinal Herb, Terminalia catappa L. from Okinawa Island and Its Tannin Corilagin. Phytomedicine 2007, 14, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.M.; Xu, L.Z.; Gao, J.; Wang, Y.; Tang, X.H.; Zhao, X.N.; Zhang, Z.X. Phytochemical and Antiinflammatory Studies on Terminalia catappa. Fitoterapia 2004, 75, 253–260. [Google Scholar] [CrossRef]
- Ohara, R.; Périco, L.L.; Rodrigues, V.P.; Bueno, G.; Zanatta, A.C.; Campaner dos Santos, L.; Vilegas, W.; Constatino, F.B.; Justulin, L.A.; Hiruma-Lima, C.A. Terminalia catappa L. Infusion Accelerates the Healing Process of Gastric Ischemia-Reperfusion Injury in Rats. J. Ethnopharmacol. 2020, 256, 112793. [Google Scholar] [CrossRef]
- Mansoor Ahmed, S.; Swamy, V.B.; Gopkumar Dhanapal, P.R.; Chandrashekara, V. Anti-Diabetic Activity of Terminalia catappa Linn. Leaf Extracts in Alloxan-Induced Diabetic Rats. Iran. J. Pharmacol. Ther. 2005, 4, 36–39. [Google Scholar]
- Iheagwam, F.N.; Iheagwam, O.T.; Onuoha, M.K.; Ogunlana, O.O.; Chinedu, S.N. Terminalia catappa Aqueous Leaf Extract Reverses Insulin Resistance, Improves Glucose Transport and Activates PI3K/AKT Signalling in High Fat/Streptozotocin-Induced Diabetic Rats. Sci. Rep. 2022, 12, 10711. [Google Scholar] [CrossRef]
- Chung, H.-H.; Hsieh, M.-J.; Hsieh, Y.-S.; Chen, P.-N.; Ko, C.-P.; Yu, N.-Y.; Lin, C.-W.; Yang, S.-F. The Inhibitory Effects of Terminalia Catappa L. Extract on the Migration and Invasion of Human Glioblastoma Multiforme Cells. Pharmaceuticals 2021, 14, 1183. [Google Scholar] [CrossRef]
- Yeh, C.-B.; Hsieh, M.-J.; Hsieh, Y.-S.; Chien, M.-H.; Lin, P.-Y.; Chiou, H.-L.; Yang, S.-F. Terminalia catappa Exerts Antimetastatic Effects on Hepatocellular Carcinoma through Transcriptional Inhibition of Matrix Metalloproteinase-9 by Modulating NF- κ B and AP-1 Activity. Evidence-Based Complement. Altern. Med. 2012, 2012, 595292. [Google Scholar] [CrossRef]
- Mudi, S.; Muhammad, A. Antimalaria Activity of Ethanolic Extracts of Leaves of Terminalia catappa L. Combretaceae (Indian Almond). Bayero J. Pure Appl. Sci. 2010, 2, 14–18. [Google Scholar] [CrossRef]
- Ngemenya, M.N.; Abwenzoh, G.N.; Zofou, D.; Kang, T.R.; Mbah, J.A. Antiplasmodial Activity against Resistant Strains, Toxicity and Effect on Mouse Liver Enzymes of Extracts of Terminalia Species Found in Southwest Cameroon. J. Herbmed Pharmacol. 2020, 10, 132–138. [Google Scholar] [CrossRef]
- Ratnasooriya, W.D.; Dharmasiri, M.G.; Rajapakse, R.A.S.; De Silva, M.S.; Jayawardena, S.P.M.; Fernando, P.U.D.; De Silva, W.N.; Nawela, A.J.M.D.N.B.; Warusawithana, R.P.Y.T.; Jayakody, J.R.C.; et al. Tender Leaf Extract of Terminalia catappa Antinociceptive Activity in Rats. Pharm. Biol. 2002, 40, 60–66. [Google Scholar] [CrossRef]
- Venkatalakshmi, P.; Vadivel, V.; Brindha, P. Identification of Flavonoids in Different Parts of Terminalia catappa L. Using LC-ESI-MS/MS and Investigation of Their Anticancer Effect in EAC Cell Line Model. J. Pharm. Sci. Res. 2016, 8, 176–183. [Google Scholar]
- Lin, T.-C.; Hsu, F.-L. Tannin and Related Compounds from Terminalia catappa and Terminalia parviflora. J. Chinese Chem. Soc. 1999, 46, 613–618. [Google Scholar] [CrossRef]
- Lin, Y.-L.; Kuo, Y.-H.; Shiao, M.-S.; Chen, C.-C.; Ou, J.-C. Flavonoid Glycosides from Terminalia catappa L. J. Chin. Chem. Soc. 2000, 47, 253–256. [Google Scholar] [CrossRef]
- Ghorbanpour, M.; Varma, A. (Eds.) Medicinal Plants and Environmental Challenges; Springer International Publishing: Cham, Switzerland, 2017; ISBN 978-3-319-68716-2. [Google Scholar]
- Länger, R.; Stöger, E.; Kubelka, W.; Helliwell, K. Quality Standards for Herbal Drugs and Herbal Drug Preparations—Appropriate or Improvements Necessary? Planta Med. 2018, 84, 350–360. [Google Scholar] [CrossRef]
- Klein-Junior, L.C.; de Souza, M.R.; Viaene, J.; Bresolin, T.M.B.; de Gasper, A.L.; Henriques, A.T.; Heyden, Y. Vander Quality Control of Herbal Medicines: From Traditional Techniques to State-of-the-Art Approaches. Planta Med. 2021, 87, 964–988. [Google Scholar] [CrossRef]
- Bueno, P.C.P.; Lopes, N.P. Metabolomics to Characterize Adaptive and Signaling Responses in Legume Crops under Abiotic Stresses. ACS Omega 2020, 5, 1752–1763. [Google Scholar] [CrossRef]
- Fraga-Corral, M.; Carpena, M.; Garcia-Oliveira, P.; Pereira, A.G.; Prieto, M.A.; Simal-Gandara, J. Analytical Metabolomics and Applications in Health, Environmental and Food Science. Crit. Rev. Anal. Chem. 2022, 52, 712–734. [Google Scholar] [CrossRef]
- Utpott, M.; Rodrigues, E.; de Oliveira Rios, A.; Mercali, G.D.; Flôres, S.H. Metabolomics: An Analytical Technique for Food Processing Evaluation. Food Chem. 2022, 366, 130685. [Google Scholar] [CrossRef]
- Marshall, D.D.; Powers, R. Beyond the Paradigm: Combining Mass Spectrometry and Nuclear Magnetic Resonance for Metabolomics. Prog. Nucl. Magn. Reson. Spectrosc. 2017, 100, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Zacharias, H.; Altenbuchinger, M.; Gronwald, W. Statistical Analysis of NMR Metabolic Fingerprints: Established Methods and Recent Advances. Metabolites 2018, 8, 47. [Google Scholar] [CrossRef]
- Zanatta, A.C.; Vilegas, W.; Edrada-Ebel, R. UHPLC-(ESI)-HRMS and NMR-Based Metabolomics Approach to Access the Seasonality of Byrsonima intermedia and Serjania marginata From Brazilian Cerrado Flora Diversity. Front. Chem. 2021, 9, 534. [Google Scholar] [CrossRef]
- Sampaio, B.L.; Edrada-Ebel, R.; Da Costa, F.B. Effect of the Environment on the Secondary Metabolic Profile of Tithonia diversifolia: A Model for Environmental Metabolomics of Plants. Sci. Rep. 2016, 6, 29265. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.M.A.; Filho, E.G.A.; Rodrigues, T.H.S.; Louredo, F.J.C.; Zocolo, G.J.; Canuto, K.M.; Mikich, S.B.; Liebsch, D.; De Almeida, A.; De Brito, E.S. Metabolomic Profiling of Phloem Sap from Different Pine Species and Implications on Black Capuchin. J. Chem. Ecol. 2022, 48, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Borges, R.M.; Resende, J.V.M.; Pinto, A.P.; Garrido, B.C. Exploring Correlations between MS and NMR for Compound Identification Using Essential Oils: A Pilot Study. Phytochem. Anal. 2022, 33, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Macintyre, L.; Zhang, T.; Viegelmann, C.; Juarez Martinez, I.; Cheng, C.; Dowdells, C.; Ramadan Abdelmohsen, U.; Gernert, C.; Hentschel, U.; Edrada-Ebel, R. Metabolomic Tools for Secondary Metabolite Discovery from Marine Microbial Symbionts. Mar. Drugs 2014, 12, 3416–3448. [Google Scholar] [CrossRef] [PubMed]
- Dudzik, D.; Barbas-Bernardos, C.; García, A.; Barbas, C. Quality Assurance Procedures for Mass Spectrometry Untargeted Metabolomics. a Review. J. Pharm. Biomed. Anal. 2018, 147, 149–173. [Google Scholar] [CrossRef]
- Pluskal, T.; Castillo, S.; Villar-Briones, A.; Orešič, M. MZmine 2: Modular Framework for Processing, Visualizing, and Analyzing Mass Spectrometry-Based Molecular Profile Data. BMC Bioinform. 2010, 11, 395. [Google Scholar] [CrossRef]
- Katajamaa, M.; Miettinen, J.; Oreš Ič, M. Data and Text Mining MZmine: Toolbox for Processing and Visualization of Mass Spectrometry Based Molecular Profile Data. Bioinformatics 2006, 22, 634–636. [Google Scholar] [CrossRef] [PubMed]
- Pluskal, T.; Uehara, T.; Yanagida, M. Highly Accurate Chemical Formula Prediction Tool Utilizing High-Resolution Mass Spectra, MS/MS Fragmentation, Heuristic Rules, and Isotope Pattern Matching. Anal. Chem. 2012, 84, 4396–4403. [Google Scholar] [CrossRef] [PubMed]
- Dictionary of Natural Products 30.1 Chemical Search. Available online: https://dnp.chemnetbase.com/faces/chemical/ChemicalSearch.xhtml (accessed on 3 December 2021).
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Schrimpe-Rutledge, A.C.; Codreanu, S.G.; Sherrod, S.D.; McLean, J.A. Untargeted Metabolomics Strategies—Challenges and Emerging Directions. J. Am. Soc. Mass Spectrom. 2016, 27, 1897. [Google Scholar] [CrossRef]
- Ebbels, T.M.D.; Lindon, J.C.; Coen, M. Processing and Modeling of Nuclear Magnetic Resonance (NMR) Metabolic Profiles. Methods Mol. Biol. 2011, 708, 365–388. [Google Scholar] [CrossRef]
- Nothias, L.-F.; Petras, D.; Schmid, R.; Dührkop, K.; Rainer, J.; Sarvepalli, A.; Protsyuk, I.; Ernst, M.; Tsugawa, H.; Fleischauer, M.; et al. Feature-Based Molecular Networking in the GNPS Analysis Environment. Nat. Methods 2020, 17, 905–908. [Google Scholar] [CrossRef] [PubMed]
- Stuart, K.A.; Welsh, K.; Walker, M.C.; Edrada-Ebel, R. Metabolomic Tools Used in Marine Natural Product Drug Discovery. Expert Opin. Drug Discov. 2020, 15, 499–522. [Google Scholar] [CrossRef]
- Zeng, Q.; Chen, J.; Zhan, C.; Lin, Y.; Chen, Z. Fully Exploiting the Power of 2D NMR J-Resolved Spectroscopy. Anal. Chem. 2020, 92, 6893–6899. [Google Scholar] [CrossRef]
- Chang, Z.; Zhang, Q.; Liang, W.; Zhou, K.; Jian, P.; She, G.; Zhang, L. A Comprehensive Review of the Structure Elucidation of Tannins from Terminalia Linn. Evidence-Based Complement. Altern. Med. 2019, 2019, 8623909. [Google Scholar] [CrossRef]
- Yoshida, T.; Amakura, Y.; Yoshimura, M. Structural Features and Biological Properties of Ellagitannins in Some Plant Families of the Order Myrtales. Int. J. Mol. Sci. 2010, 11, 79–106. [Google Scholar] [CrossRef]
- Aqil, F.; Munagala, R.; Vadhanam, M.V.; Kausar, H.; Jeyabalan, J.; Schultz, D.J.; Gupta, R.C. Anti-Proliferative Activity and Protection against Oxidative DNA Damage by Punicalagin Isolated from Pomegranate Husk. Food Res. Int. 2012, 49, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.L.; Kang, K.S. Protection Effect of Punicalagin Isolated from Pomegranate on Inflammation and Ethanol-Induced Gastric Mucosal Injury. Bull. Korean Chem. Soc. 2016, 37, 1778–1782. [Google Scholar] [CrossRef]
- Reddy, M.; Gupta, S.; Jacob, M.; Khan, S.; Ferreira, D. Antioxidant, Antimalarial and Antimicrobial Activities of Tannin-Rich Fractions, Ellagitannins and Phenolic Acids from Punica granatum L. Planta Med. 2007, 73, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, C.C.; da Costa Santos, S.; de Souza Lino, R.; Bara, M.T.F.; Chaibub, B.A.; de Melo Reis, P.R.; Chaves, D.A.; da Silva, A.J.R.; Silva, L.S.; de Melo e Silva, D.; et al. Chemopreventive Effect and Angiogenic Activity of Punicalagin Isolated from Leaves of Lafoensia pacari A. St.-Hil. Toxicol. Appl. Pharmacol. 2016, 310, 1–8. [Google Scholar] [CrossRef]
- Luo, J.; Long, Y.; Ren, G.; Zhang, Y.; Chen, J.; Huang, R.; Yang, L. Punicalagin Reversed the Hepatic Injury of Tetrachloromethane by Antioxidation and Enhancement of Autophagy. J. Med. Food 2019, 22, 1271–1279. [Google Scholar] [CrossRef]
- Wang, W.; Ali, Z.; Shen, Y.; Li, X.-C.; Khan, I.A. Ursane Triterpenoids from the Bark of Terminalia arjuna. Fitoterapia 2010, 81, 480–484. [Google Scholar] [CrossRef]
- Huang, H.; Ullah, F.; Zhou, D.-X.; Yi, M.; Zhao, Y. Mechanisms of ROS Regulation of Plant Development and Stress Responses. Front. Plant Sci. 2019, 10, 800. [Google Scholar] [CrossRef]
- Jajic, I.; Sarna, T.; Strzalka, K. Senescence, Stress, and Reactive Oxygen Species. Plants 2015, 4, 393–411. [Google Scholar] [CrossRef]
- Sun, T.; Zou, L.; Zhang, L.; Zhang, J.; Wang, X. Methyl Jasmonate Induces Triterpenoid Biosynthesis in Inonotus baumii. Biotechnol. Biotechnol. Equip. 2017, 31, 312–317. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, J.-W.; Lu, S.-H.; Zhang, H.; Liu, F.; Fu, B.; Zhao, M.-Q.; Liu, H. Transcriptome Divergence between Developmental Senescence and Premature Senescence in Nicotiana tabacum L. Sci. Rep. 2020, 10, 20556. [Google Scholar] [CrossRef]
- Misra, R.C.; Maiti, P.; Chanotiya, C.S.; Shanker, K.; Ghosh, S. Methyl Jasmonate-Elicited Transcriptional Responses and Pentacyclic Triterpene Biosynthesis in Sweet Basil. Plant Physiol. 2014, 164, 1028–1044. [Google Scholar] [CrossRef] [PubMed]
- González-Coloma, A.; López-Balboa, C.; Santana, O.; Reina, M.; Fraga, B.M. Triterpene-Based Plant Defenses. Phytochem. Rev. 2011, 10, 245–260. [Google Scholar] [CrossRef]
- Kortbeek, R.W.J.; van der Gragt, M.; Bleeker, P.M. Endogenous Plant Metabolites against Insects. Eur. J. Plant Pathol. 2019, 154, 67–90. [Google Scholar] [CrossRef]
- Harborne, J.B. (Ed.) The Flavonoids: Advances in Research Since 1986; Chapman & Hall: London, UK, 1994. [Google Scholar]
- Agrawal, P.K. NMR Spectroscopy in the Structural Elucidation of Oligosaccharides and Glycosides. Phytochemistry 1992, 31, 3307–3330. [Google Scholar] [CrossRef]
- Karageorgou, P.; Manetas, Y. The Importance of Being Red When Young: Anthocyanins and the Protection of Young Leaves of Quercus coccifera from Insect Herbivory and Excess Light. Tree Physiol. 2006, 26, 613–621. [Google Scholar] [CrossRef]
- Jiang, N.; Doseff, A.; Grotewold, E. Flavones: From Biosynthesis to Health Benefits. Plants 2016, 5, 27. [Google Scholar] [CrossRef]
- Almeida, T.; Pinto, G.; Correia, B.; Gonçalves, S.; Meijón, M.; Escandón, M. In-Depth Analysis of the Quercus suber Metabolome under Drought Stress and Recovery Reveals Potential Key Metabolic Players. Plant Sci. 2020, 299, 110606. [Google Scholar] [CrossRef]
- Ji, H.G.; Lee, Y.R.; Lee, M.S.; Hwang, K.H.; Park, C.Y.; Kim, E.H.; Park, J.S.; Hong, Y.S. Diverse Metabolite Variations in Tea (Camellia sinensis L.) Leaves Grown under Various Shade Conditions Revisited: A Metabolomics Study. J. Agric. Food Chem. 2018, 66, 1889–1897. [Google Scholar] [CrossRef]
- Robson, T.M.; Klem, K.; Urban, O.; Jansen, M.A.K. Re-Interpreting Plant Morphological Responses to UV-B Radiation. Plant. Cell Environ. 2015, 38, 856–866. [Google Scholar] [CrossRef]
- Halliwell, B. Reactive Species and Antioxidants. Redox Biology Is a Fundamental Theme of Aerobic Life. Plant Physiol. 2006, 141, 312–322. [Google Scholar] [CrossRef] [PubMed]

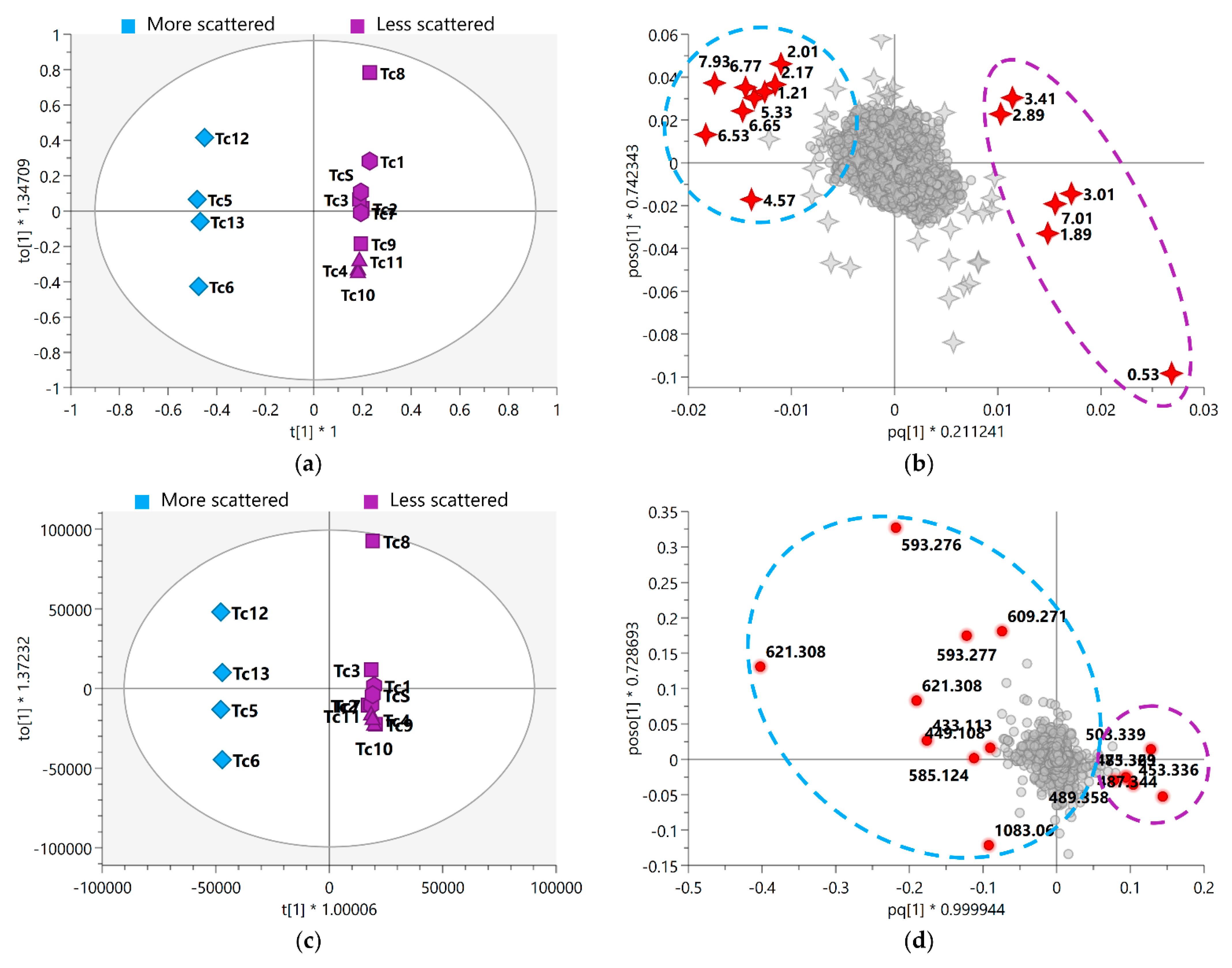

| ID | δH (ppm) 1 | δH, Mult. (J in Hz) | Type of Proton | Annotation of Metabolite Class or Compound | p-Value | FDR 2 |
|---|---|---|---|---|---|---|
| 50 | 6.53–6.57 | 6.55, s | Aromatic, Ar-H | Flavones | 0.00357 | 0.005 |
| 15 | 7.93–7.97 | 7.94, d (8.8) | Aromatic, Ar-H | Flavones | 0.00358 | 0.010 |
| 159 | 2.17–2.21 | 2.19, t (7.4) | Alkyl (methylene), -CH2 | NA | 0.01110 | 0.015 |
| 200 | 0.53–0.57 | 0.55, s | Alkyl (methyl), -CH3 | Triterpenes | 0.01240 | 0.020 |
| 138 | 3.01–3.05 | 3.04, m | Alkyl (methine), -CH-OH | Triterpenes | 0.01270 | 0.025 |
| 99 | 4.57–4.61 | 4.59, d (10.0) | Anomeric proton, -CH-OH | Saccharide unit | 0.01940 | 0.030 |
| 80 | 5.33–5.37 | 5.34, m | Alkyl (methine), -CH-OH | Saccharide unit | 0.02010 | 0.035 |
| 166 | 1.89–1.93 | 1.92, s | Alkyl (methine), -CH | Triterpenes | 0.03480 | 0.040 |
| 47 | 6.65–6.69 | 6.67, s | Aromatic, Ar-H | Flavones/Galloyl group | 0.04150 | 0.045 |
| 44 | 6.77–6.81 | 6.79, s | Aromatic, Ar-H | Flavones/Galloyl group | 0.04400 | 0.050 |
| MZmine ID 1 | Rt (min) | Adduct (m/z) | Chemical Formula | Annotation of Metabolite Class or Compound | p-Value | FDR 2 |
|---|---|---|---|---|---|---|
| P2185 | 8.42 | 585.1239 [M+H]+ | C28H24O14 | Apigenin-6-C-(-O-galloyl)-hexose | 0.00005 | 0.006 |
| P4607 | 7.67 | 433.1129 [M+H]+ | C21H20O10 | Apigenin-6-C-hexose (isovitexin) | 0.00047 | 0.011 |
| P291 | 22.92 | 473.3629 [M+H]+ | C30H48O4 | Hydroxyursolic acid | 0.00581 | 0.017 |
| N4 | 17.41 | 487.3441 [M-H]− | C30H48O5 | Trihydroxyurs-12-en-28-oic acid (Asiatic acid) | 0.00661 | 0.022 |
| N3 | 15.67 | 503.3393 [M-H]− | C30H48O6 | Tetrahydroxyolean-12-en-28-oic acid | 0.00896 | 0.028 |
| P304 | 6.74 | 449.1077 [M+H]+ | C21H20O11 | Luteolin-6-C-hexose (isoorientin) | 0.00898 | 0.033 |
| N9 | 16.50 | 485.3286 [M-H]− | C30H46O5 | Trihydroxyursadien-28-oic acid | 0.00976 | 0.039 |
| P288 | 17.41 | 489.3575 [M+H]+ | C30H48O5 | Trihydroxyurs-12-en-28-oic acid (Asiatic acid) | 0.01360 | 0.044 |
| P285 | 17.42 | 453.3362 [M+H]+ | C30H44O3 | 3-oxo-urs-12,18-dien-28-oic acid | 0.01500 | 0.050 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zanatta, A.C.; Vieira, N.C.; Dantas-Medeiros, R.; Vilegas, W.; Edrada-Ebel, R. Understanding the Seasonal Effect of Metabolite Production in Terminalia catappa L. Leaves through a Concatenated MS- and NMR-Based Metabolomics Approach. Metabolites 2023, 13, 349. https://doi.org/10.3390/metabo13030349
Zanatta AC, Vieira NC, Dantas-Medeiros R, Vilegas W, Edrada-Ebel R. Understanding the Seasonal Effect of Metabolite Production in Terminalia catappa L. Leaves through a Concatenated MS- and NMR-Based Metabolomics Approach. Metabolites. 2023; 13(3):349. https://doi.org/10.3390/metabo13030349
Chicago/Turabian StyleZanatta, Ana C., Natália Carolina Vieira, Renato Dantas-Medeiros, Wagner Vilegas, and RuAngelie Edrada-Ebel. 2023. "Understanding the Seasonal Effect of Metabolite Production in Terminalia catappa L. Leaves through a Concatenated MS- and NMR-Based Metabolomics Approach" Metabolites 13, no. 3: 349. https://doi.org/10.3390/metabo13030349
APA StyleZanatta, A. C., Vieira, N. C., Dantas-Medeiros, R., Vilegas, W., & Edrada-Ebel, R. (2023). Understanding the Seasonal Effect of Metabolite Production in Terminalia catappa L. Leaves through a Concatenated MS- and NMR-Based Metabolomics Approach. Metabolites, 13(3), 349. https://doi.org/10.3390/metabo13030349








