p-Coumaric Acid Nanoparticles Ameliorate Diabetic Nephropathy via Regulating mRNA Expression of KIM-1 and GLUT-2 in Streptozotocin-Induced Diabetic Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of p-Coumaric Acid-Loaded Nanoparticles
2.3. Animals
2.4. Induction of Diabetes Nephropathy
2.5. Experimental Design
2.6. Biochemical Assays
2.7. Histopathological Examination
2.8. Immunohistochemical Examination
2.9. RT-PCR
2.10. Statistical Analysis
3. Results
3.1. Body Weight
3.2. Kidney Weight and Hypertrophy
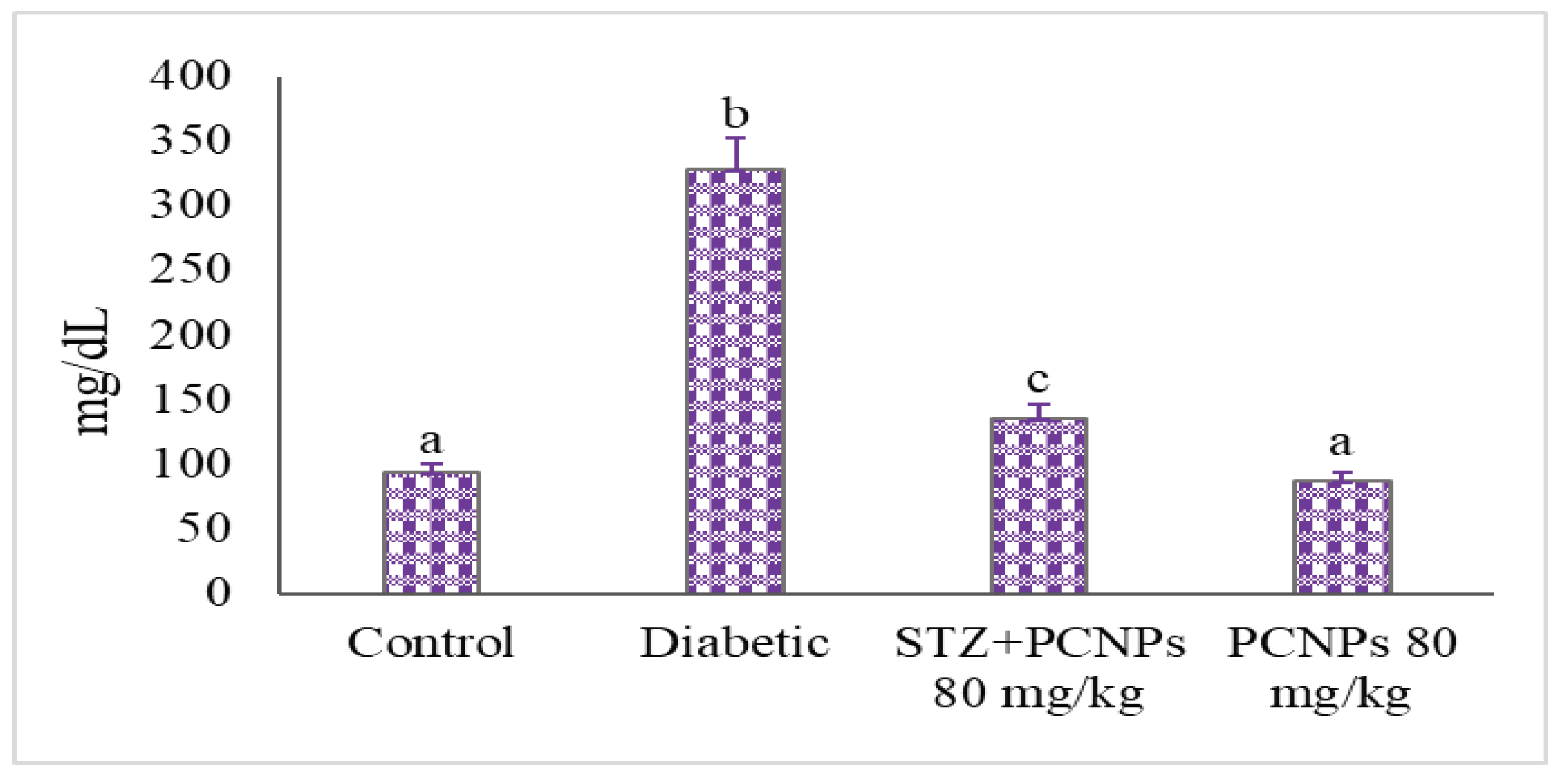
3.3. Blood Glucose and Insulin Level
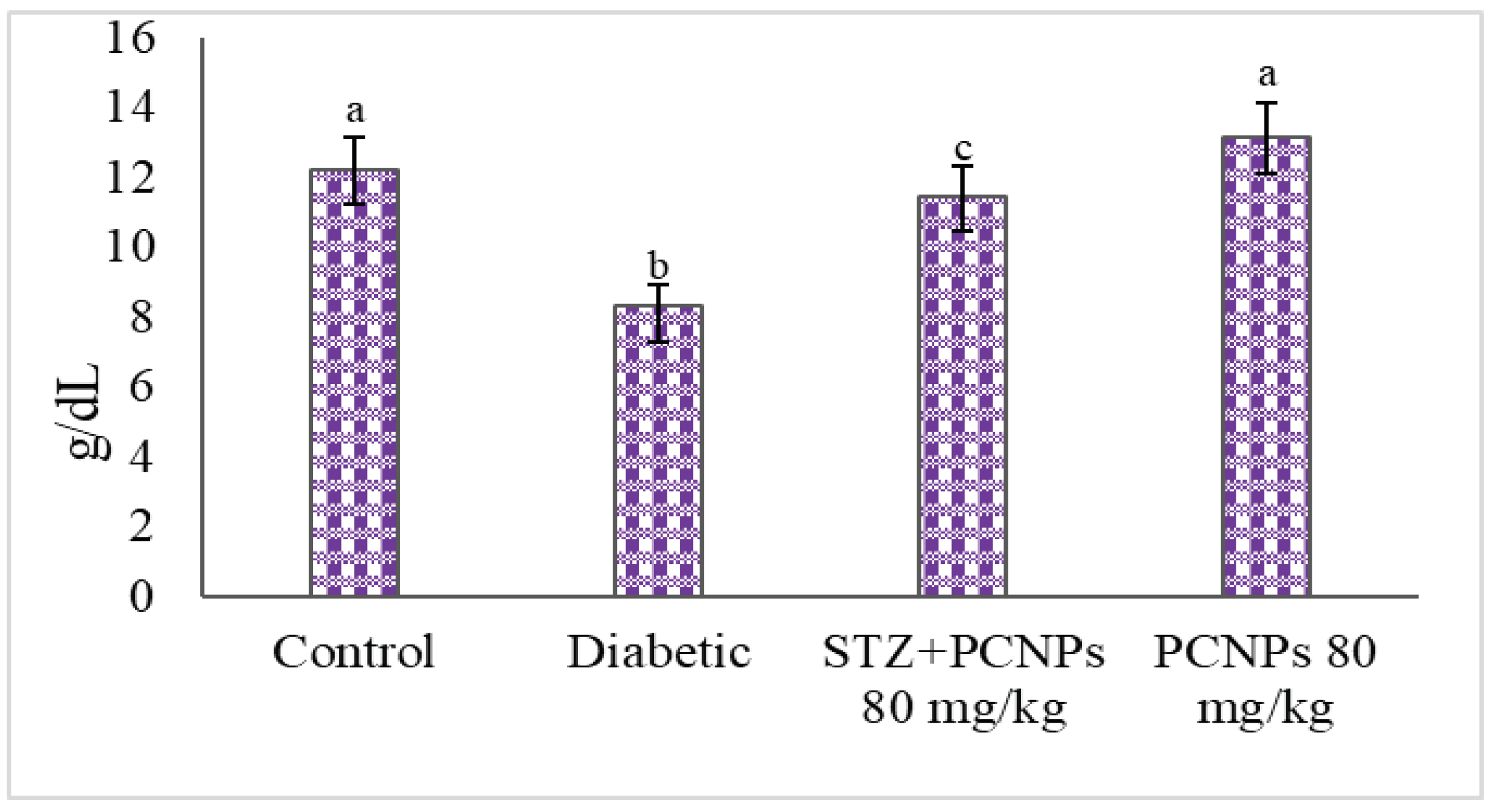
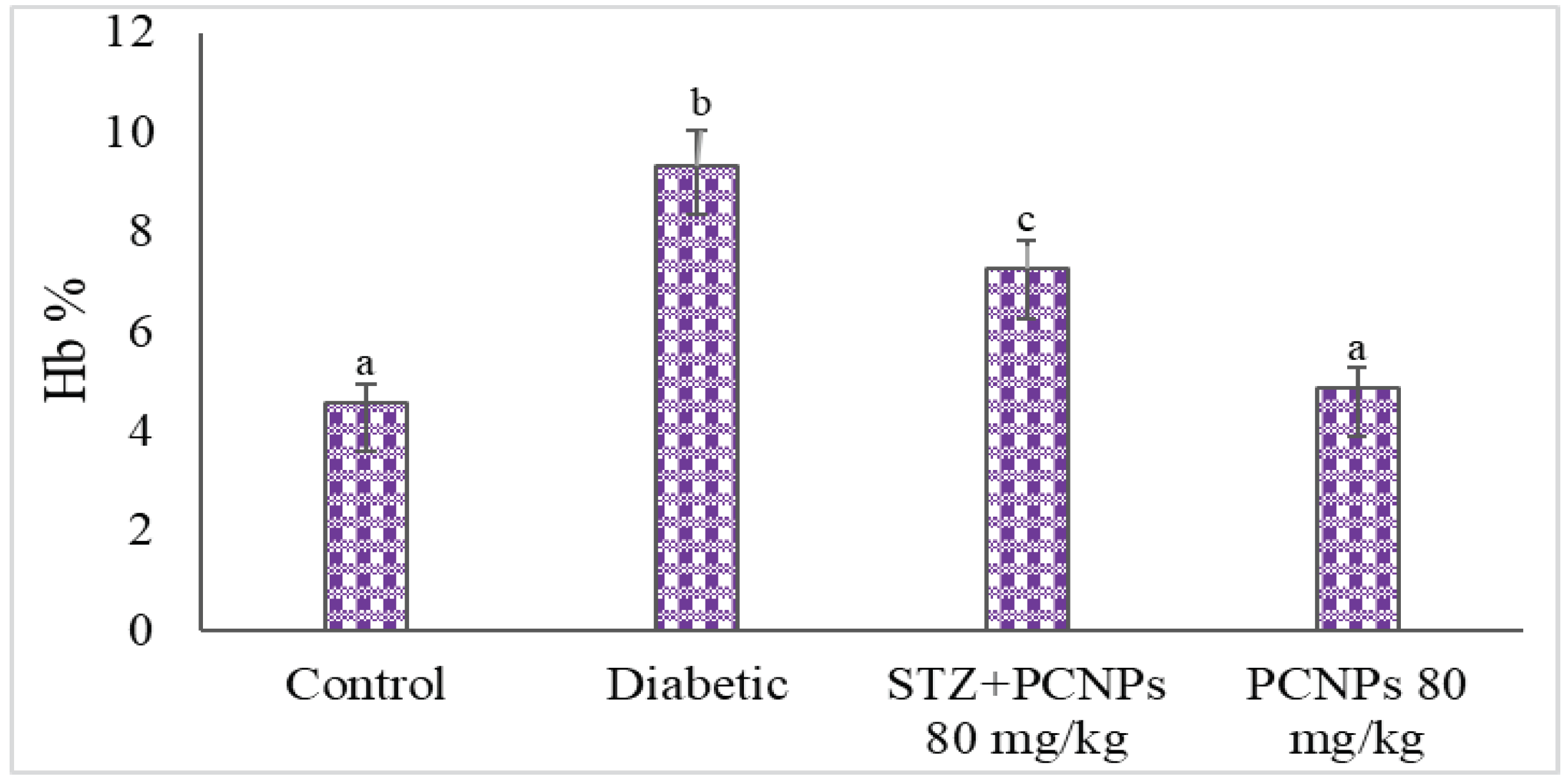
3.4. Hb and HbA1c Level
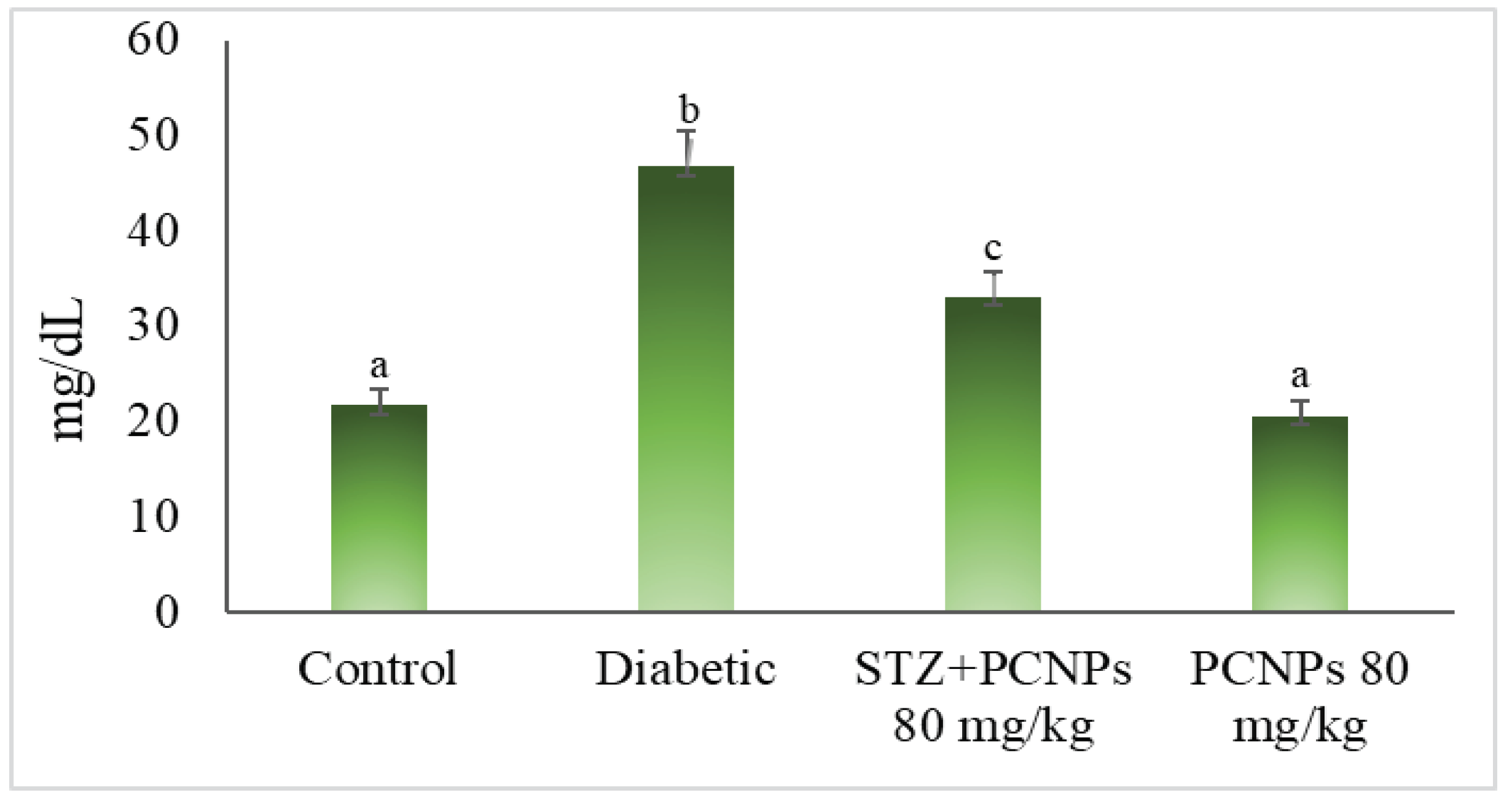
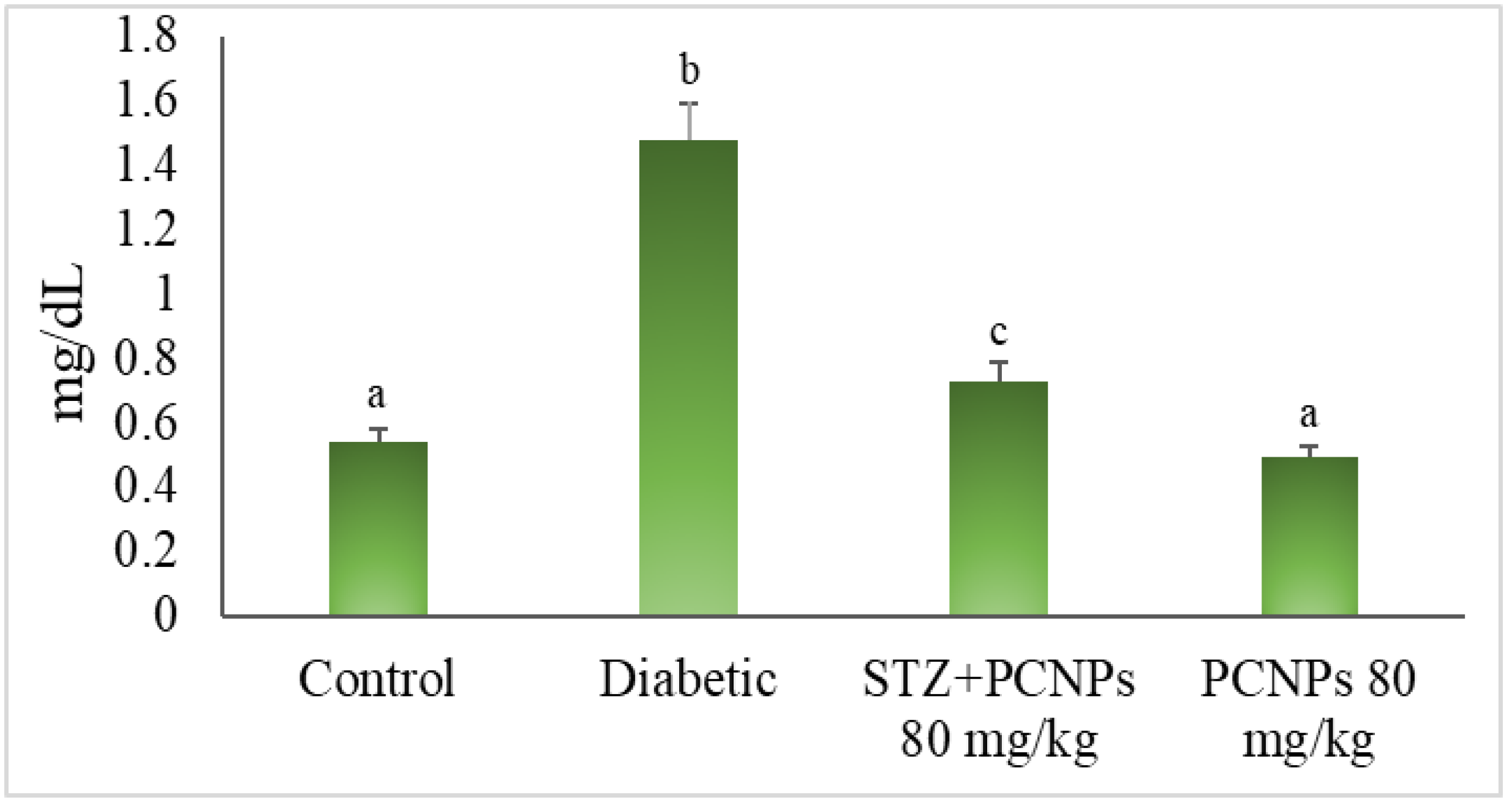
3.5. Blood Urea and Serum Creatinine
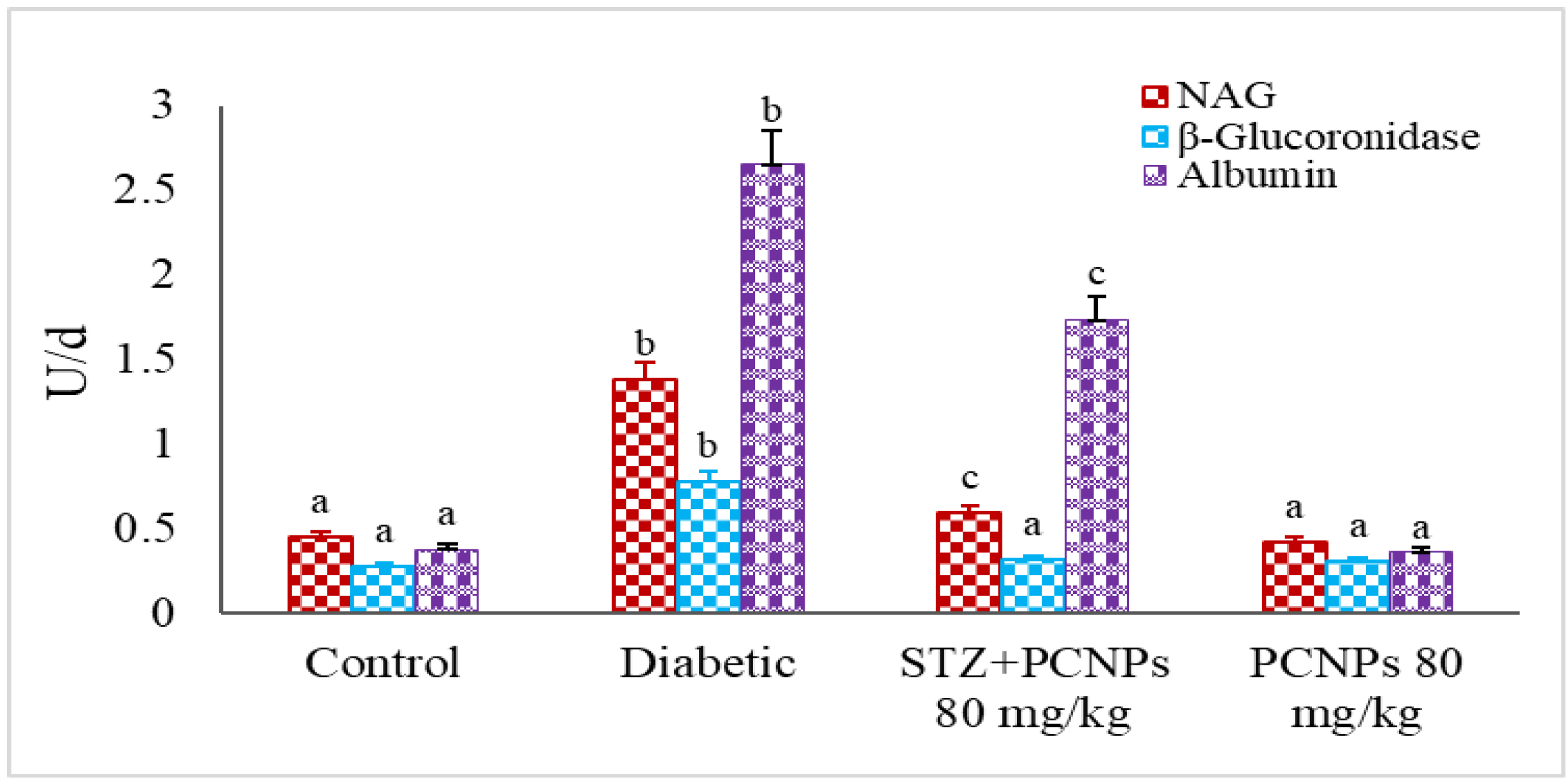
3.6. Urinary NAG, β-Glucuronidase and Microalbumin Level
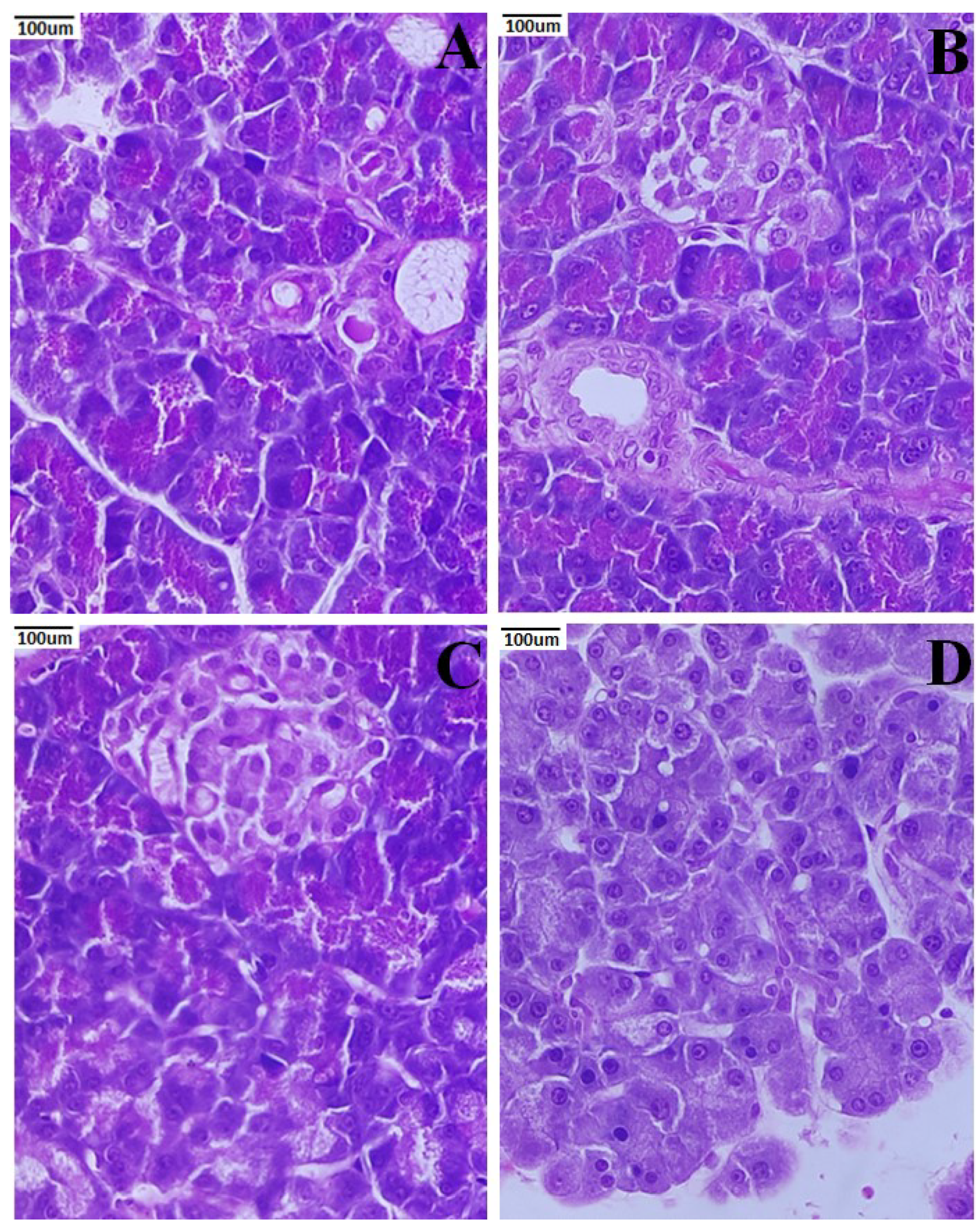
3.7. Histopathology of Pancreas
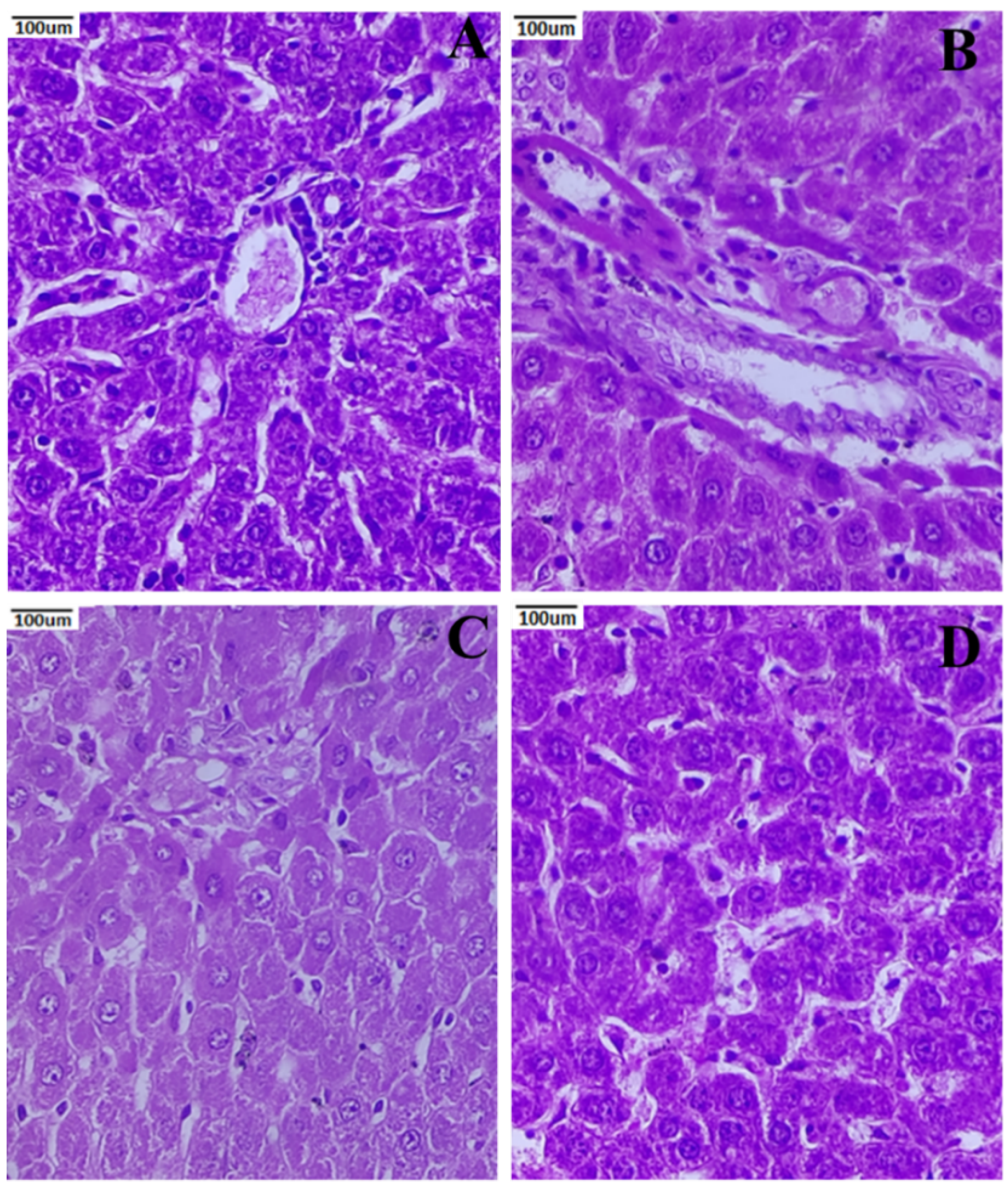
3.8. Histopathology of the Liver
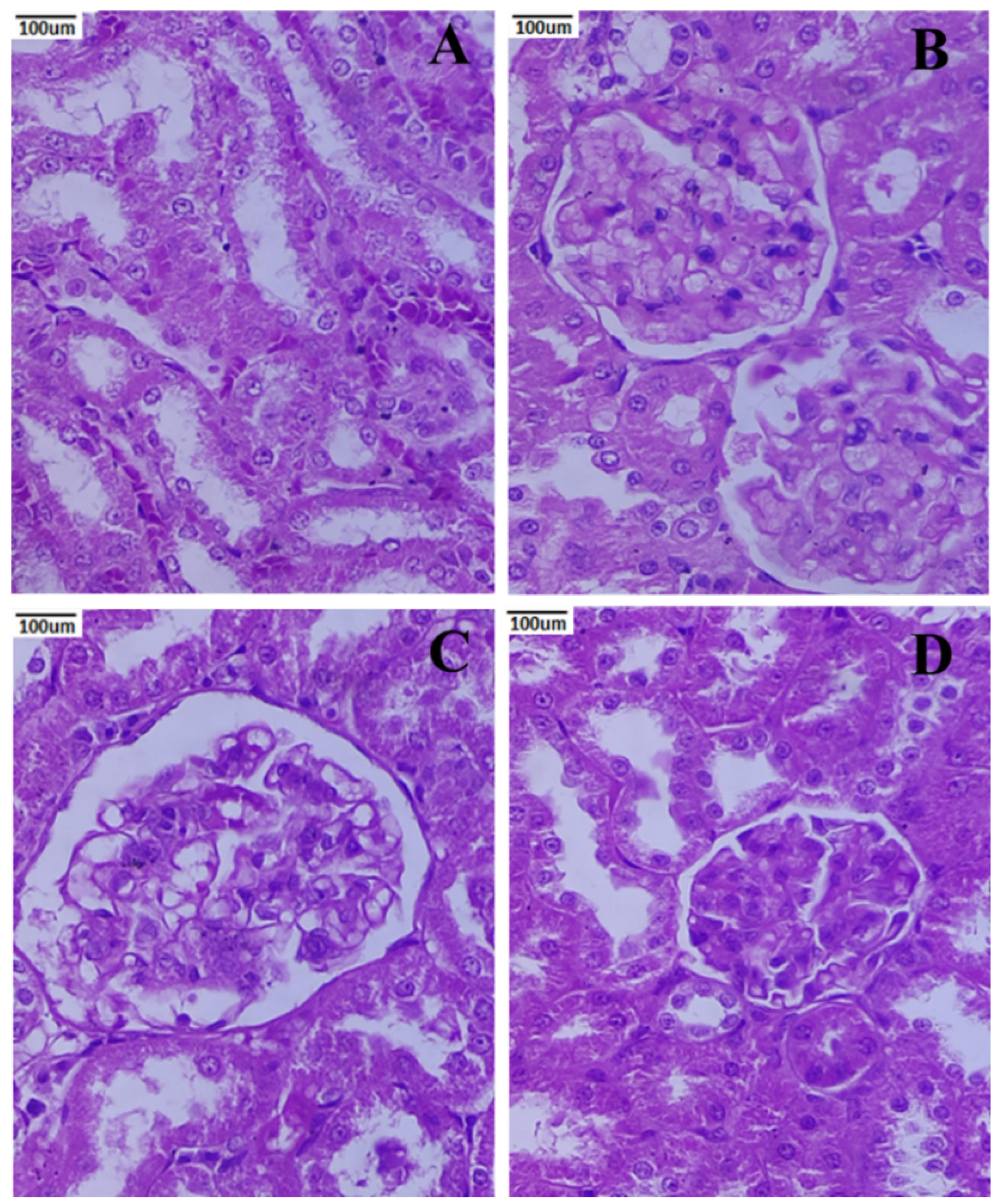
3.9. Histopathology of the Kidney
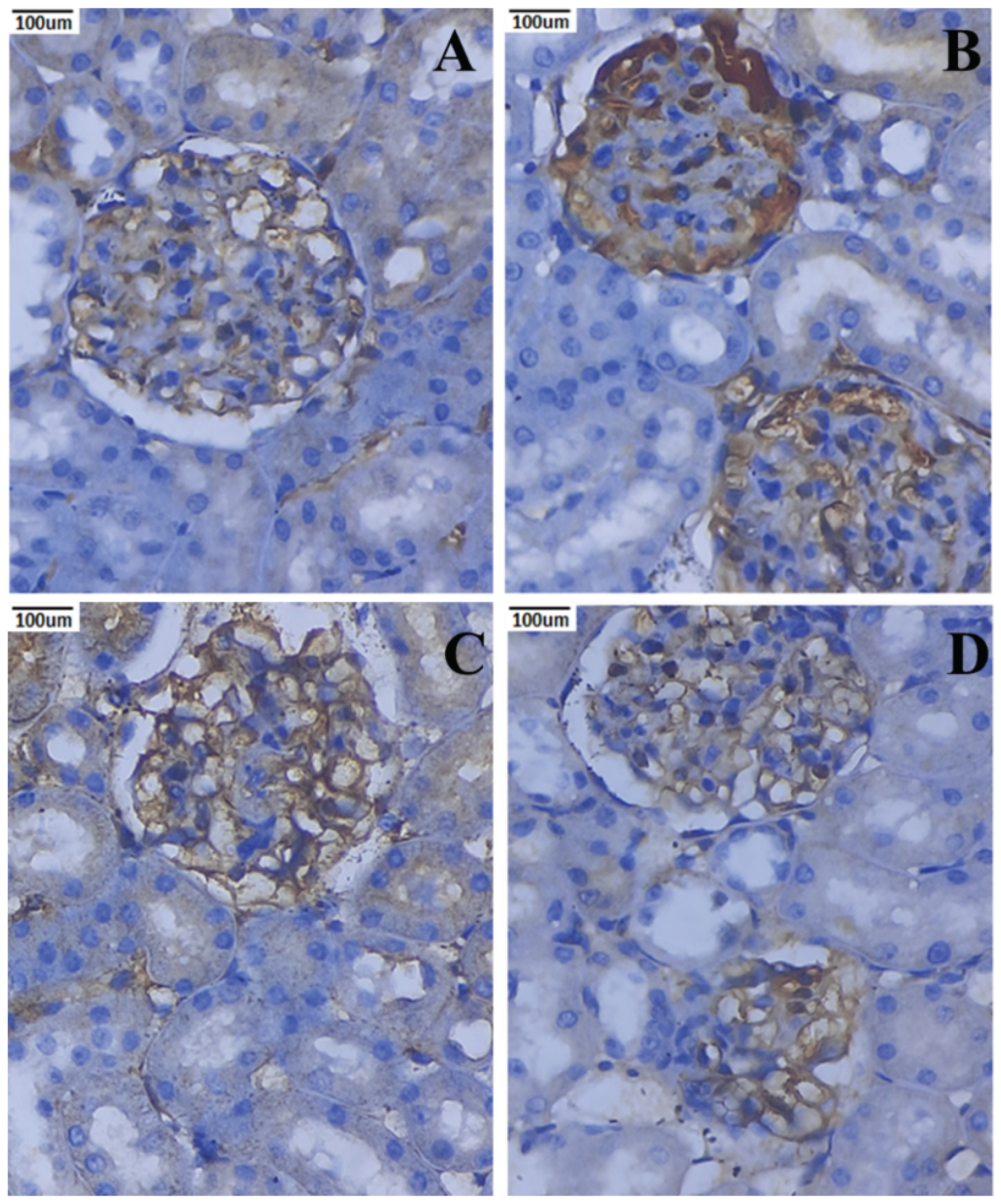
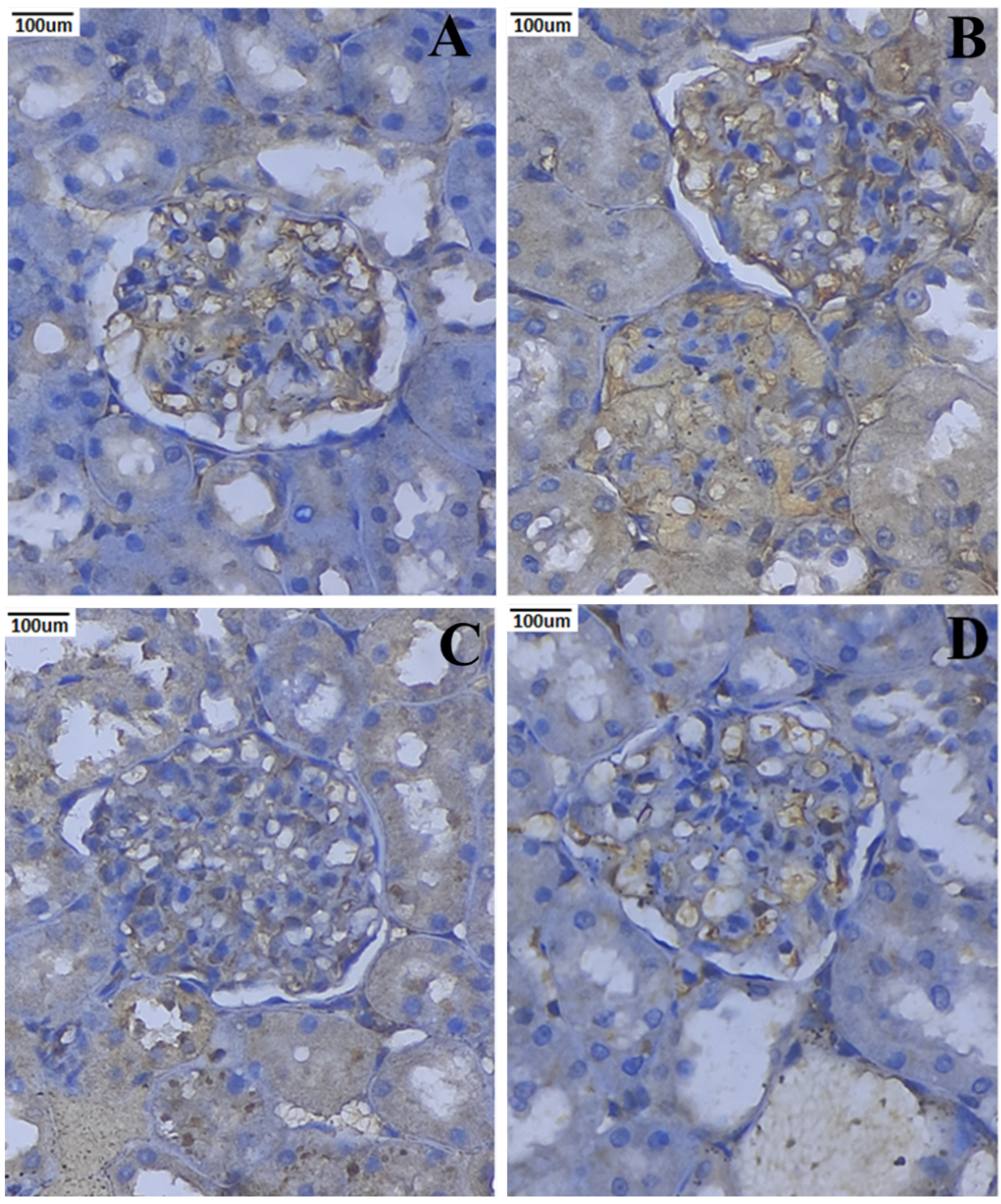
3.10. Immunohistochemical Expression of TNF-α and IL-6
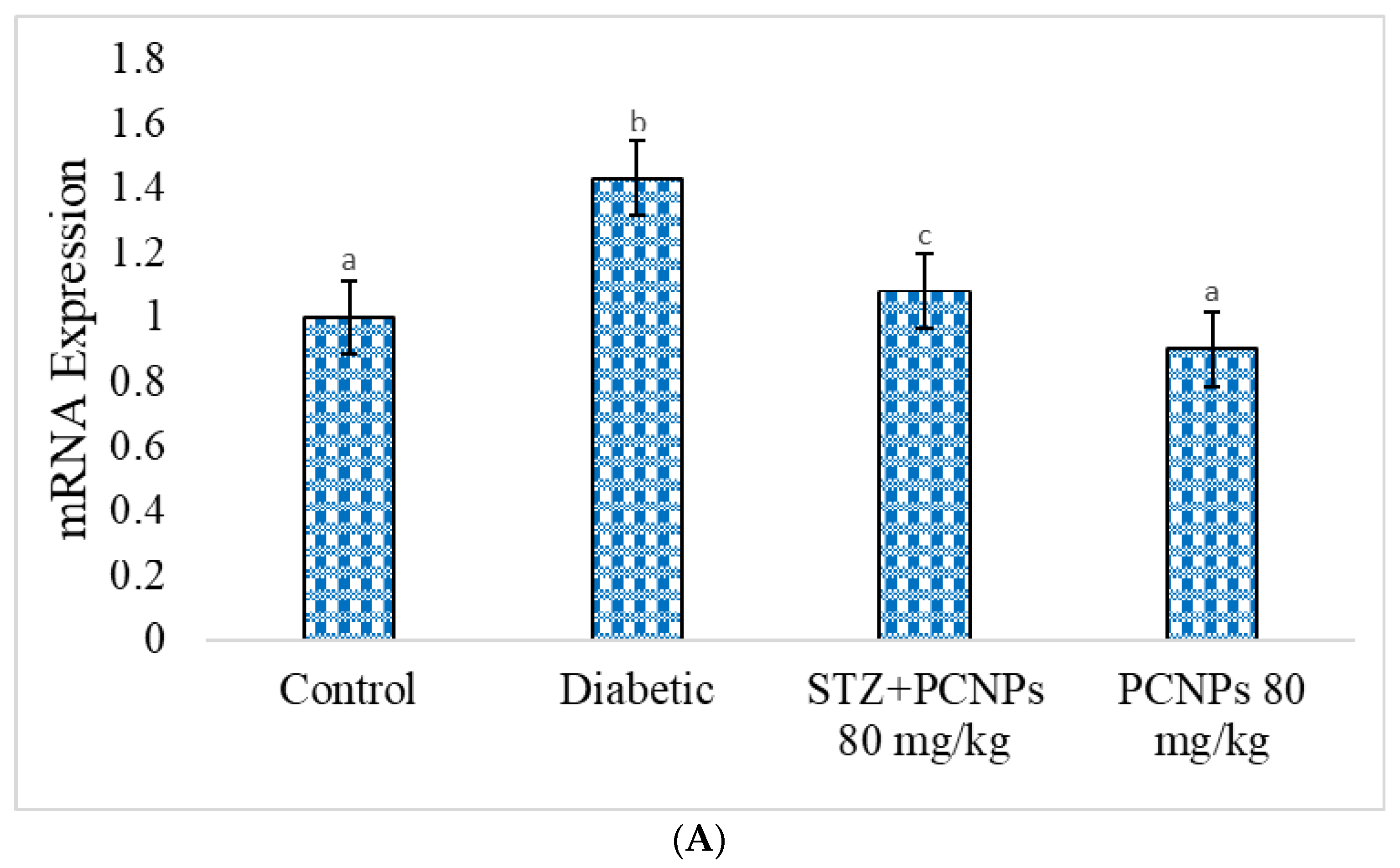
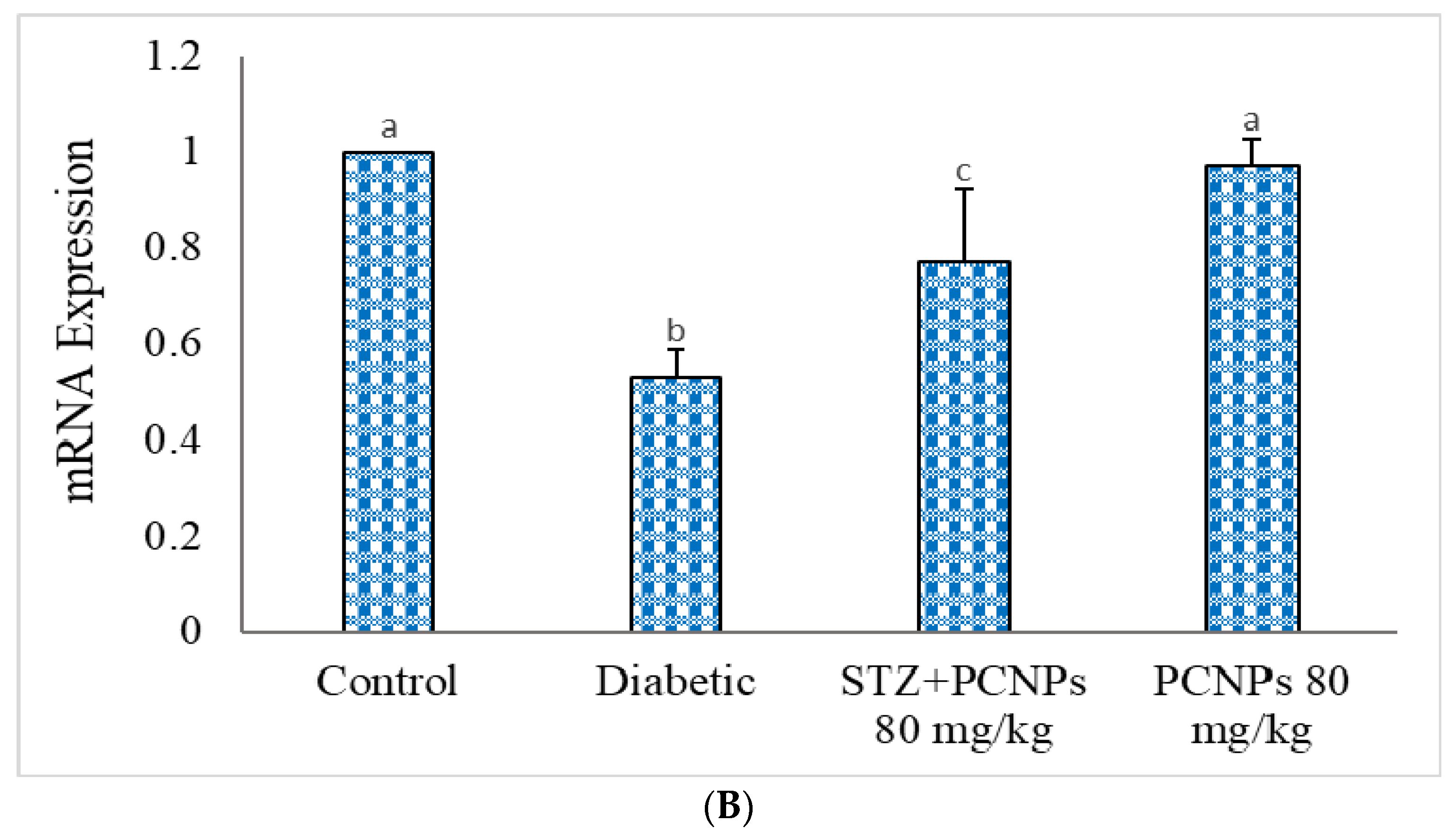
3.11. mRNA Expression of KIM-1 and GLUT-2
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Unnikrishnan, R.; Anjana, R.M.; Mohan, V. Diabetes mellitus and its complications in India. Nat. Rev. Endocrinol. 2016, 12, 357–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ambalavanan, R.; John, A.D.; Selvaraj, A.D. Nephroprotective role of nanoencapsulated tinospora cordifolia (Willd.) using polylactic acid nanoparticles in streptozotocin-induced diabetic nephropathy rats. IET Nanobiotechnol. 2021, 15, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Nishad, R.; Tahaseen, V.; Kavvuri, R.; Motrapu, M.; Singh, A.K.; Peddi, K.; Pasupulati, A.K. Advanced-Glycation End-Products Induce Podocyte Injury and Contribute to Proteinuria. Front. Med. 2021, 8, 685447. [Google Scholar] [CrossRef] [PubMed]
- Strippoli, G.F.; Bonifati, C.; Craig, M.; Navaneethan, S.D.; Craig, J.C. Angiotensin converting enzyme inhibitors and angiotensin II receptor antagonists for preventing the progression of diabetic kidney disease. Cochrane Database Syst. Rev. 2006, 4, CD006257. [Google Scholar] [CrossRef] [PubMed]
- Chawla, T.; Sharma, D.; Singh, A. Role of the renin angiotensin system in diabetic nephropathy. World J. Diabetes 2010, 5, 141–145. [Google Scholar] [CrossRef]
- Kim, S.R.; Lee, Y.H.; Lee, S.G.; Kang, E.S.; Cha, B.S.; Lee, B.W. The renal tubular damage marker urinary N-acetyl-β-D-glucosaminidase may be more closely associated with early detection of atherosclerosis than the glomerular damage marker albuminuria in patients with type 2 diabetes. Cardiovasc. Diabetol. 2017, 16, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bayless, R.L.; Moore, A.R.; Hassel, D.M.; Byer, B.J.; Landolt, G.A.; Nout-Lomas, Y.S. Equine urinary N-acetyl-β-D-glucosaminidase assay validation and correlation with other markers of kidney injury. J. Vet. Diagn. 2019, 31, 688–695. [Google Scholar] [CrossRef]
- Yu, S.M.; Bonventre, J.V. Acute Kidney Injury and Progression of Diabetic Kidney Disease. Adv. Chronic Kidney Dis. 2018, 25, 166–180. [Google Scholar] [CrossRef] [PubMed]
- Podkowińska, A.; Formanowicz, D. Chronic Kidney Disease as Oxidative Stress- and Inflammatory-Mediated Cardiovascular Disease. Antioxidants 2020, 9, 752. [Google Scholar] [CrossRef] [PubMed]
- Rapa, S.F.; Di Iorio, B.R.; Campiglia, P.; Heidland, A.; Marzocco, S. Inflammation and Oxidative Stress in Chronic Kidney Disease-Potential Therapeutic Role of Minerals, Vitamins and Plant-Derived Metabolites. Int. J. Mol. Sci 2019, 21, 263. [Google Scholar] [CrossRef] [PubMed]
- Amalan, V.; Jose Vinoth Raja, A.; Karthikeyan, B.; Vijayakumar, N.; Kim, S.J. In vitro antioxidant, anti-inflammatory, antimicrobial, and antidiabetic activities of synthesized chitosan-loaded p-Coumaric acid nanoparticles. Curr. Pharm. Biotechnol. 2022. Accepted. [Google Scholar]
- Amalan, V.; Vijayakumar, N.; Indumathi, D.; Ramakrishnan, A. Antidiabetic and antihyperlipidemic activity of p-Coumaric acid in diabetic rats, the role of pancreatic GLUT 2: In vivo approach. Biomed. Pharmacother. 2016, 84, 230–236. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Liu, H.; Li, N.; Zhang, Q.; Zhang, H. The protective effect of fucoidan in rats with streptozotocin-induced diabetic nephropathy. Mar. Drugs 2014, 12, 3292–3306. [Google Scholar] [CrossRef]
- Amalan, V.; Vijayakumar, N.; Ramakrishnan, A. p-Coumaric acid regulates blood glucose and antioxidant levels in streptozotocin induced diabetic rats. J. Chem. Pharm. Res. 2015, 7, 831–839. [Google Scholar]
- Pal, P.B.; Sinha, K.; Sil, P.C. Mangiferin attenuates diabetic nephropathy by Inhibiting oxidative stress mediated signalling cascade, TNFα related and mitochondrial dependent apoptotic pathways in streptozotocin-induced diabetic rats. PLoS ONE 2014, 9, e107220. [Google Scholar] [CrossRef] [Green Version]
- Zhu, D.; Zhang, L.; Cheng, L.; Ren, L.; Tang, J.; Sun, D. Pancreatic kininogenase ameliorates renal fibrosis in streptozotocin induced-diabetic nephropathy rat. Kidney Blood Press. Res. 2016, 41, 9–17. [Google Scholar] [CrossRef]
- Al-Muammar, M.N.; Khan, F. Obesity: The preventive role of the pomegranate (Punica granatum). Nutrition 2012, 28, 595e604. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.N.; Bandwane, D.D.; Mhetre, N.K. Pomegranate (Punica granatum, Linn.) leaves attenuate disturbed glucose homeostasis and hyperglycemia mediated hyperlipidemia and oxidative stress in streptozotocin induced diabetic rats. Eur. J. Integr. Med. 2014, 6, 307e321. [Google Scholar] [CrossRef]
- Mestry, S.N.; Dhodi, J.B.; Kumbhar, S.B.; Juvekar, A.R. Attenuation of diabetic nephropathy in streptozotocin-induced diabetic rats by Punica granatum Linn. leaves extract. J. Tradit. Complement. Med. 2016, 7, 273–280. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, D.S.; Abd El-Maksoud, M.A. Effect of strawberry (Fragaria × ananassa) leaf extract on diabetic nephropathy in rats. Int. J. Exp. Pathol. 2015, 96, 87–93. [Google Scholar] [CrossRef]
- Aramjoo, H.; Mohammadparast-Tabas, P.; Farkhondeh, T.; Zardast, M.; Makhdoumi, M.; Samarghandian, S.; Kiani, Z. Protective effect of Sophora pachycarpa seed extract on carbon tetrachloride-induced toxicity in rats. BMC Complement. Altern. Med. 2022, 22, 76. [Google Scholar] [CrossRef] [PubMed]
- Natelson, S.; Scott, M.L.; Beffa, C.A. Rapid method for the estimation of urea in biologic fluids. Am. J. Clin. Pathol. 1951, 21, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Caraway, W.T. Uric acid. In Standard Methods of Clinical Chemistry; Seligson, D., Ed.; Academic Press: New York, NY, USA, 1963; pp. 239–247. [Google Scholar]
- Chomczynski, P.; Sacchi, N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Owen, J.A.; Iggo, B.; Scandrett, F.J.; Stewart, C.P. The determination of creatinine in plasma or serum, and in urine; a critical examination. Biochem. J. 1954, 58, 426–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammadi-Karakani, A.; Asgharzadeh-Haghighi, S.; Ghazi-Khansari, M.; Hosseini, R. Determination of urinary enzymes as a marker of Int J Diabetes Dev Ctriesearly renal damage in diabetic patients. J. Clin. Lab. Anal. 2007, 21, 413–417. [Google Scholar] [CrossRef]
- Cheng, D.; Liang, B.; Li, Y. Antihyperglycemic effect of Ginkgo biloba extract in streptozotocin-induced diabetes in rats. Biomed Res. Int. 2013, 2013, 162724. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Ortega, M.; Rodrigues-Diez, R.R.; Lavoz, C.; Rayego-Mateos, S. Special Issue “Diabetic Nephropathy: Diagnosis, Prevention and Treatment”. J. Clin. Med. 2020, 9, 813. [Google Scholar] [CrossRef] [Green Version]
- Cao, M.; Li, Y.; Famurewa, A.C.; Olatunji, O.J. Antidiabetic and nephroprotective effects of polysaccharide extract from the seaweed caulerpa racemosa in high fructose-streptozotocin induced diabetic nephropathy. Diabetes Metab. Syndr. Obes. 2021, 14, 2121–2131. [Google Scholar] [CrossRef]
- Joseph, B.; Jini, D. Antidiabetic effects of momordica charantia (bitter melon) and its medicinal potency. Asian Pac. J. Trop. Dis. 2013, 3, 93–102. [Google Scholar] [CrossRef]
- Czech, M.P. Insulin action and resistance in obesity and type 2 diabetes. Nat. Med. 2017, 23, 804–814. [Google Scholar] [CrossRef]
- Hidayat, R.; Wulandari, P. Effects of Andrographis paniculata (Burm. F.) extract on diabetic nephropathy in rats. Rep. Biochem. Mol. Biol. 2021, 10, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.S.; Hathaway, C.K.; Smithies, O.; Kakoki, M. Transforming growth factor-β1 and diabetic nephropathy. Am. J. Physiol. Ren. Physiol. 2016, 310, F689–F696. [Google Scholar] [CrossRef] [PubMed]
- Zafar, M.; Naqvi, S.N. Effects of STZ-induced diabetes on the relative weights of kidney, liver and pancreas in albino rats: A comparative study. Int. J. Morphol. 2010, 28, 135–142. [Google Scholar] [CrossRef] [Green Version]
- Adnyana, I.K.; Sukandar, E.Y.; Yuniarto, A.; Finna, S. Anti-obesity effect of the pomegranate leaves ethanol extract (Punica granatum L.) in high fat diet induced mice. Int. J. Pharm. Pharm. Sci. 2014, 6, 626–631. [Google Scholar]
- Akinlade, O.M.; Owoyele, B.V.; Soladoye, A.O. Streptozotocin-induced type 1 and 2 diabetes in rodents: A model for studying diabetic cardiac autonomic neuropathy. Afr. Health Sci. 2021, 21, 719–727. [Google Scholar] [CrossRef]
- Guo, W.; Zhou, Q.; Jia, Y.; Xu, J. Increased Levels of Glycated Hemoglobin A1c and Iron Deficiency Anemia: A Review. Med. Sci. Monit. 2019, 7, 8371–8378. [Google Scholar] [CrossRef]
- Sherwani, S.I.; Khan, H.A.; Ekhzaimy, A.; Masood, A.; Sakharkar, M.K. Significance of HbA1c Test in Diagnosis and Prognosis of Diabetic Patients. Biomark. Insights 2016, 11, 95–104. [Google Scholar] [CrossRef]
- Minakami, H. Clinical evaluation of N-acetyl-beta-D-glucosaminidase on prediction of diabetic nephropathy. Hokkaido Igaky Zasshi 1992, 67, 234–246. [Google Scholar]
- Liu, W.J.; Xu, B.H.; Ye, L.; Liang, D.; Wu, H.L.; Zheng, Y.Y.; Deng, K.; Li, B.; Liuz, H.F. Urinary proteins induce lysosomal membrane permeabilization and lysosomal dysfunction in renal tubular epithelial cells. Am. J. Physiol. Renal. Physiol. 2015, 15, F639–F649. [Google Scholar] [CrossRef] [Green Version]
- Walmsley, S.J.; Broeckling, C.; Hess, A.; Prenni, J.; Curthoys, N.P. Proteomic analysis of brush-border membrane vesicles isolated from purified proximal convoluted tubules. Am. J. Physiol. Renal. Physiol. 2010, 298, F1323–F1331. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.R.; Lee, Y.H.; Lee, S.G.; Kang, E.S.; Cha, B.S.; Kim, J.H.; Lee, B.W. Urinary N-acetyl-β-D-glucosaminidase, an early marker of diabetic kidney disease, might reflect glucose excursion in patients with type 2 diabetes. Medicine 2016, 95, e4114. [Google Scholar] [CrossRef] [PubMed]
- Omozee, E.B.; Okaka, E.I.; Edo, A.E.; Obika, L.F. Urinary N-acetyl-beta-d-glucosaminidase levels in diabetic adults. J. Lab. Med. 2019, 11, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Dollah, M.A.; Parhizkar, S.; Izwan, M. Effect of Nigella sativa on the kidney function in rats. Avicenna J. Phytomed. 2013, 3, 152. [Google Scholar] [PubMed]
- Phong, N.V.; Zhao, Y.; Min, B.S.; Yang, S.Y.; Kim, J.A. Inhibitory Activity of Bioactive Phloroglucinols from the Rhizomes of Dryopteris crassirhizoma on Escherichia coli β-Glucuronidase: Kinetic Analysis and Molecular Docking Studies. Metabolites 2022, 12, 938. [Google Scholar] [CrossRef]
- Biernawska, J.; Kotfis, K.; Szymańska-Pasternak, J.; Bogacka, A.; Bober, J. Long-Term Consequences of Increased Activity of Urine Enzymes After Cardiac Surgery-A Prospective Observational Study. Ther. Clin. Risk. Manag. 2022, 18, 867–877. [Google Scholar] [CrossRef]
- Subramanian, P.; Anandan, R.; Jayapalan, J.J.; Hashim, O.H. Hesperidin protects gentamicin-induced nephrotoxicity via Nrf2/HO-1 signaling and inhibits inflammation mediated by NF-κB in rats. J. Funct. Foods 2015, 13, 89–99. [Google Scholar] [CrossRef]
- Al Hroob, A.M.; Abukhalil, M.H.; Alghonmeen, R.D.; Mahmoud, A.M. Ginger alleviates hyperglycemia-induced oxidative stress, inflammation and apoptosis and protects rats against diabetic nephropathy. Biomed. Pharmacother. 2018, 106, 381–389. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 7023. [Google Scholar] [CrossRef] [Green Version]
- Balamash, K.S.; Alkreathy, H.M.; Al Gahdali, E.H.; Khoja, S.O.; Ahmad, A. Comparative Biochemical and Histopathological Studies on the Efficacy of Metformin and Virgin Olive Oil against Streptozotocin-Induced Diabetes in Sprague-Dawley Rats. J. Diabetes Res. 2018, 2018, 4692197. [Google Scholar] [CrossRef]
- Maric-Bilkan, C.; Flynn, E.R.; Chade, A.R. Microvascular disease precedes the decline in renal function in the streptozotocin-induced diabetic rat. Am. J. Physiol. Cell Physiol. 2012, 302, F308–F315. [Google Scholar] [CrossRef] [Green Version]
- Yuvashree, M.; Ganesh, R.N.; Viswanathan, P. Potential application of nanoemulsified garlic oil blend in mitigating the progression of type 2 diabetes-mediated nephropathy in Wistar rats. Biotech 2020, 10, 272. [Google Scholar] [CrossRef] [PubMed]
- Popko, K.; Gorska, E.; Stelmaszczyk-Emmel, A.; Plywaczewski, R.; Stoklosa, A.; Gorecka, D.; Pyrzak, B.; Demkow, U. Proinflammatory cytokines Il-6 and TNF-α and the development of inflammation in obese subjects. Eur. J. Med. Res. 2010, 4, 120–122. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Lei, C.T.; Zhang, C. Interleukin-6 Signaling Pathway and Its Role in Kidney Disease: An Update. Front. Immunol. 2017, 8, 405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, W.Y.; Bai, B.; Luo, C.; Yang, K.; Li, D.; Wu, D.; Félétou, M.; Villeneuve, N.; Zhou, Y.; Yang, J.; et al. Lipocalin-2 derived from adipose tissue mediates aldosterone-induced renal injury. JCI Insight 2018, 6, e120196. [Google Scholar] [CrossRef] [PubMed]
- Hinden, L.; Udi, S.; Drori, A.; Gammal, A.; Nemirovski, A.; Hadar, R.; Baraghithy, S.; Permyakova, A.; Geron, M.; Cohen, M.; et al. Modulation of Renal GLUT2 by the Cannabinoid-1 Receptor: Implications for the Treatment of Diabetic Nephropathy. J. Am. Soc. Nephrol. 2018, 29, 34–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, Z.K.; Tian, P.X.; Wang, X.Z.; Xue, W.J.; Ding, X.M.; Zheng, J.; Ding, C.G.; Mao, T.C.; Duan, W.L.; Xi, M. Kidney injury molecule-1 and osteopontin: New markers for prediction of early kidney transplant rejection. Mol. Immunol. 2013, 54, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Chen, X.; Zhang, Y.; George, J.; Cobbs, A.; Wang, G.; Emmett, N. Kidney Injury Molecule-1 Is Upregulated in Renal Lipotoxicity and Mediates Palmitate-Induced Tubular Cell Injury and Inflammatory Response. Int. J. Mol. Sci. 2019, 20, 3406. [Google Scholar] [CrossRef]

| S.No. | Gene | Sequence of Primers | Accession Numbers |
|---|---|---|---|
| 1 | KIM-1 | Forward: 5′-GAGTTCATTAGAGCCATTTCCACTCC-3′ Reverse: 5′-GAAAGCCTGTGTCCTGCTCTCTCT-3′ | NM_173149.2 |
| 2 | GLUT-2 | Forward: 5′-CTC GGG CCT TAC GTG TTC TTC CTT-3′ Reverse: 5′-TGG TTC CCT TCT GGT CTG TTC CTG-3′ | XM_039101783.1 |
| 3 | β-actin | Forward: 5′-TACAGCTTCACCACCACAGC-3′ Reverse: 5′-TCTCCAGGGAGGAAGAGGAT-3′ | NM_031144.3 |
| Groups | Body Weight (g) | Kidney Weight (g) | Kidney Hypertrophy Index (%) | |
|---|---|---|---|---|
| Initial | Final | |||
| Control | 225.30 | 233.50 a | 1.85 a | 0.79 a |
| Diabetic | 230.50 | 162.43 b | 2.38 b | 1.46 b |
| STZ + PCNPs 80 mg/kg | 235.65 | 193.67 c | 2.13 c | 1.09 c |
| PCNPs 80 mg/kg | 220.55 | 230.15 a | 1.90 a | 0.82 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venkatesan, A.; Roy, A.; Kulandaivel, S.; Natesan, V.; Kim, S.-J. p-Coumaric Acid Nanoparticles Ameliorate Diabetic Nephropathy via Regulating mRNA Expression of KIM-1 and GLUT-2 in Streptozotocin-Induced Diabetic Rats. Metabolites 2022, 12, 1166. https://doi.org/10.3390/metabo12121166
Venkatesan A, Roy A, Kulandaivel S, Natesan V, Kim S-J. p-Coumaric Acid Nanoparticles Ameliorate Diabetic Nephropathy via Regulating mRNA Expression of KIM-1 and GLUT-2 in Streptozotocin-Induced Diabetic Rats. Metabolites. 2022; 12(12):1166. https://doi.org/10.3390/metabo12121166
Chicago/Turabian StyleVenkatesan, Amalan, Anitha Roy, Srinivasan Kulandaivel, Vijayakumar Natesan, and Sung-Jin Kim. 2022. "p-Coumaric Acid Nanoparticles Ameliorate Diabetic Nephropathy via Regulating mRNA Expression of KIM-1 and GLUT-2 in Streptozotocin-Induced Diabetic Rats" Metabolites 12, no. 12: 1166. https://doi.org/10.3390/metabo12121166
APA StyleVenkatesan, A., Roy, A., Kulandaivel, S., Natesan, V., & Kim, S.-J. (2022). p-Coumaric Acid Nanoparticles Ameliorate Diabetic Nephropathy via Regulating mRNA Expression of KIM-1 and GLUT-2 in Streptozotocin-Induced Diabetic Rats. Metabolites, 12(12), 1166. https://doi.org/10.3390/metabo12121166







