Fractional Gluconeogenesis: A Biomarker of Dietary Energy Adequacy in a Rat Brain Injury Model
Abstract
:1. Introduction
2. Experimental Design
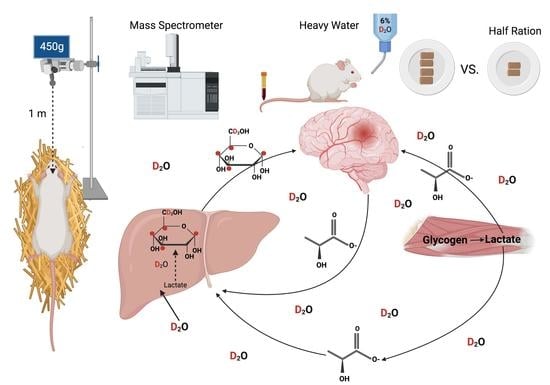
2.1. Traumatic Brain Injury Model
2.2. Labeled Water and Body Water Enrichment Analysis
2.3. Fractional Gluconeogenesis Measurement
2.4. Blood Glucose Analyses
2.5. Kinematic and Behavioral Analyses
2.6. Statistical Analyses
3. Results
3.1. TBI Leads to Alterations in Light Sensitivity 13 Days Post-Injury
3.2. TBI and CR Significantly Alter Body Weight and Voluntary Food Consumption Post-TBI
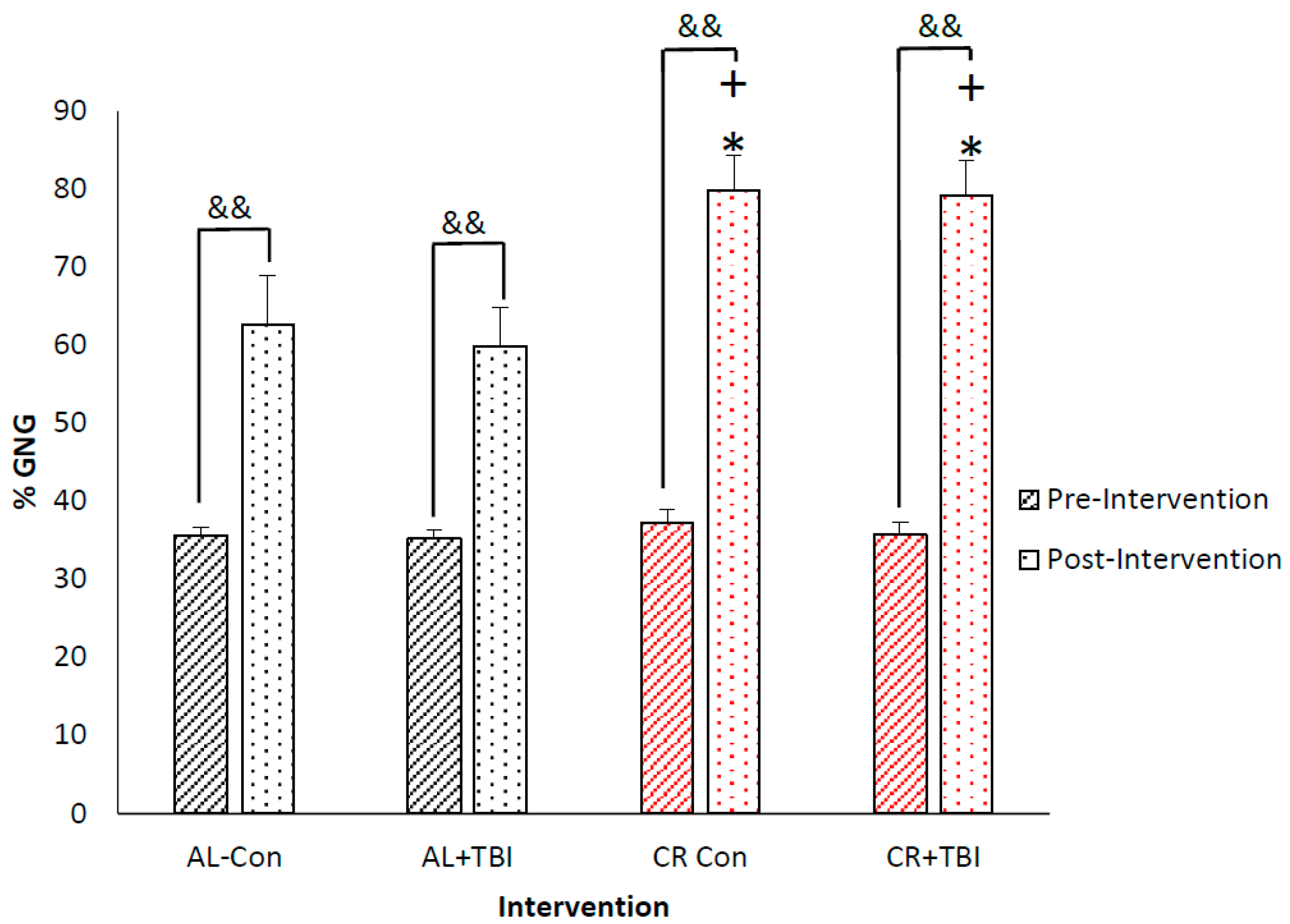
3.3. Caloric Restriction Increases Fractional Production of Endogenous Glucose via Gluconeogenesis with No Effect on Blood Glucose Concentrations
4. Discussion
4.1. TBI and Glucose Control
4.2. Clinical Ramifications of under/over Feeding, Benefits of Using fGNG as a Biomarker
4.3. Limitations
4.4. Future Research Direction
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peterson, A.B.; Thomas, K.E.; Zhou, H. Surveillance Report of Traumatic Brain Injury-related Deaths by Age Group, Sex, and Mechanism of Injury—United States, 2018 and 2019; Centers for Disease Control and Prevention, US Department of Health and Human Services: Atlanta, GA, USA, 2022.
- TalavageThomas, M.; NaumanEric, A.; BreedloveEvan, L.; DyeAnne, E.; MorigakiKatherine, E.; LeverenzLarry, J. Functionally-detected cognitive impairment in high school football players without clinically-diagnosed concussion. J. Neurotrauma 2014, 31, 327–338. [Google Scholar]
- Rattanachaiwong, S.; Singer, P. Indirect calorimetry as point of care testing. Clin. Nutr. 2019, 38, 2531–2544. [Google Scholar] [CrossRef] [PubMed]
- McEvoy, C.T.; Cran, G.W.; Cooke, S.R.; Young, I.S. Resting energy expenditure in non-ventilated, non-sedated patients recovering from serious traumatic brain injury: Comparison of prediction equations with indirect calorimetry values. Clin. Nutr. 2009, 28, 526–532. [Google Scholar] [CrossRef] [Green Version]
- Pepe, J.L.; Barba, C.A. The metabolic response to acute traumatic brain injury and implications for nutritional support. J. Head Trauma Rehabil. 1999, 14, 462–474. [Google Scholar] [CrossRef]
- Glenn, T.C.; Martin, N.A.; McArthur, D.L.; Hovda, D.A.; Vespa, P.; Johnson, M.L.; Horning, M.A.; Brooks, G.A. Endogenous nutritive support after traumatic brain injury: Peripheral lactate production for glucose supply via gluconeogenesis. J. Neurotrauma 2015, 32, 811–819. [Google Scholar] [CrossRef] [Green Version]
- Charrueau, C.; Belabed, L.; Besson, V.; Chaumeil, J.-C.; Cynober, L.; Moinard, C. Metabolic response and nutritional support in traumatic brain injury: Evidence for resistance to renutrition. J. Neurotrauma 2009, 26, 1911–1920. [Google Scholar] [CrossRef] [PubMed]
- Moinard, C.; Neveux, N.; Royo, N.; Genthon, C.; Marchand-Verrecchia, C.; Plotkine, M.; Cynober, L. Characterization of the alteration of nutritional state in brain injury induced by fluid percussion in rats. Intensive Care Med. 2005, 31, 281–288. [Google Scholar] [CrossRef]
- Shi, J.; Dong, B.; Mao, Y.; Guan, W.; Cao, J.; Zhu, R.; Wang, S. Traumatic brain injury and hyperglycemia, a potentially modifiable risk factor. Oncotarget 2016, 7, 71052. [Google Scholar] [CrossRef] [Green Version]
- Maxwell, J.; Gwardschaladse, C.; Lombardo, G.; Petrone, P.; Policastro, A.; Karev, D.; Prabhakaran, K.; Betancourt, A.; Marini, C. The impact of measurement of respiratory quotient by indirect calorimetry on the achievement of nitrogen balance in patients with severe traumatic brain injury. Eur. J. Trauma Emerg. Surg. 2017, 43, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, R.N.; Pitts, S.L.; Maish III, G.O.; Schroeppel, T.J.; Magnotti, L.J.; Croce, M.A.; Minard, G.; Brown, R.O. A reappraisal of nitrogen requirements for patients with critical illness and trauma. J. Trauma Acute Care Surg. 2012, 73, 549–557. [Google Scholar] [CrossRef]
- Glenn, T.C.; Martin, N.A.; Horning, M.A.; McArthur, D.L.; Hovda, D.A.; Vespa, P.; Brooks, G.A. Lactate: Brain fuel in human traumatic brain injury: A comparison with normal healthy control subjects. J. Neurotrauma 2015, 32, 820–832. [Google Scholar] [CrossRef]
- Vespa, P.; Bergsneider, M.; Hattori, N.; Wu, H.-M.; Huang, S.-C.; Martin, N.A.; Glenn, T.C.; McArthur, D.L.; Hovda, D.A. Metabolic crisis without brain ischemia is common after traumatic brain injury: A combined microdialysis and positron emission tomography study. J. Cereb. Blood Flow Metab. 2005, 25, 763–774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allick, G.; van der Crabben, S.N.; Ackermans, M.T.; Endert, E.; Sauerwein, H.P. Measurement of gluconeogenesis by deuterated water: The effect of equilibration time and fasting period. Am. J. Physiol.-Endocrinol. Metab. 2006, 290, E1212–E1217. [Google Scholar] [CrossRef] [Green Version]
- Chung, S.T.; Chacko, S.K.; Sunehag, A.L.; Haymond, M.W. Measurements of gluconeogenesis and glycogenolysis: A methodological review. Diabetes 2015, 64, 3996–4010. [Google Scholar] [CrossRef] [Green Version]
- Hellerstein, M.K.; Neese, R.; Linfoot, P.; Christiansen, M.; Turner, S.; Letscher, A. Hepatic gluconeogenic fluxes and glycogen turnover during fasting in humans. A stable isotope study. J. Clin. Investig. 1997, 100, 1305–1319. [Google Scholar]
- Stoppe, C.; Wendt, S.; Mehta, N.M.; Compher, C.; Preiser, J.-C.; Heyland, D.K.; Kristof, A.S. Biomarkers in critical care nutrition. Crit. Care 2020, 24, 499. [Google Scholar] [CrossRef] [PubMed]
- Glenn, T.C.; Kelly, D.F.; Boscardin, W.J.; McArthur, D.L.; Vespa, P.; Oertel, M.; Hovda, D.A.; Bergsneider, M.; Hillered, L.; Martin, N.A. Energy dysfunction as a predictor of outcome after moderate or severe head injury: Indices of oxygen, glucose, and lactate metabolism. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2003, 23, 1239–1250. [Google Scholar] [CrossRef] [Green Version]
- Brooks, G.A.; Martin, N.A. Cerebral metabolism following traumatic brain injury: New discoveries with implications for treatment. Front. Neurosci. 2015, 8, 408. [Google Scholar] [CrossRef]
- Orendorff, R.; Peck, A.J.; Zheng, B.; Shirazi, S.N.; Ferguson, R.M.; Khandhar, A.P.; Kemp, S.J.; Goodwill, P.; Krishnan, K.M.; Brooks, G.A. First in vivo traumatic brain injury imaging via magnetic particle imaging. Phys. Med. Biol. 2017, 62, 3501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Council, N. Nutrient requirements of laboratory animals. In The National Academies; National Academies Press: Washington, DC, USA, 1995. [Google Scholar]
- Miller, B.F.; Reid, J.J.; Price, J.C.; Lin, H.-J.L.; Atherton, P.J.; Smith, K. CORP: The use of deuterated water for the measurement of protein synthesis. J. Appl. Physiol. 2020, 128, 1163–1176. [Google Scholar] [CrossRef]
- Abbott, C.B.; Lawrence, M.M.; Kobak, K.A.; Lopes, E.B.P.; Peelor, F.F., III; Donald, E.J.; Van Remmen, H.; Griffin, T.M.; Miller, B.F. A novel stable isotope approach demonstrates surprising degree of age-related decline in skeletal muscle collagen proteostasis. Function 2021, 2, zqab028. [Google Scholar] [CrossRef] [PubMed]
- Chacko, S.K.; Sunehag, A.L.; Sharma, S.; Sauer, P.J.; Haymond, M.W. Measurement of gluconeogenesis using glucose fragments and mass spectrometry after ingestion of deuterium oxide. J. Appl. Physiol. 2008, 104, 944–951. [Google Scholar] [CrossRef] [PubMed]
- Tagge, C.A. Effects of Concussive Impact Injury Assessed in a New Murine Neurotrauma Model. Doctoral Dissertation, Boston University, Boston, MA, USA, 2016. [Google Scholar]
- Thiels, E.; Hoffman, E.K.; Gorin, M.B. A reliable behavioral assay for the assessment of sustained photophobia in mice. Curr. Eye Res. 2008, 33, 483–491. [Google Scholar] [CrossRef]
- Brooks, G.A. The precious few grams of glucose during exercise. Int. J. Mol. Sci. 2020, 21, 5733. [Google Scholar] [CrossRef]
- Banting, F.G.; Best, C.H.; Collip, J.B.; Campbell, W.R.; Fletcher, A.A. Pancreatic extracts in the treatment of diabetes mellitus. Can. Med. Assoc. J. 1922, 12, 141. [Google Scholar]
- Van den Berghe, G.; Wouters, P.; Weekers, F.; Verwaest, C.; Bruyninckx, F.; Schetz, M.; Vlasselaers, D.; Ferdinande, P.; Lauwers, P.; Bouillon, R. Intensive Insulin Therapy in Critically Ill Patients. N. Engl. J. Med. 2001, 345, 1359–1367. [Google Scholar] [CrossRef]
- Investigators, N.-S.S. Intensive versus conventional glucose control in critically ill patients. N. Engl. J. Med. 2009, 360, 1283–1297. [Google Scholar]
- Oddo, M.; Schmidt, J.M.; Carrera, E.; Badjatia, N.; Connolly, E.S.; Presciutti, M.; Ostapkovich, N.D.; Levine, J.M.; Le Roux, P.; Mayer, S.A. Impact of tight glycemic control on cerebral glucose metabolism after severe brain injury: A microdialysis study. Crit. Care Med. 2008, 36, 3233–3238. [Google Scholar] [CrossRef] [PubMed]
- Dhandapani, S.; Dhandapani, M.; Agarwal, M.; Chutani, A.M.; Subbiah, V.; Sharma, B.S.; Mahapatra, A.K. The prognostic significance of the timing of total enteral feeding in traumatic brain injury. Surg. Neurol. Int. 2012, 3, 31. [Google Scholar] [CrossRef] [Green Version]
- Casaer, M.P.; Van den Berghe, G. Nutrition in the acute phase of critical illness. N. Engl. J. Med. 2014, 370, 1227–1236. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.A.; Benedict, F.G. A biometric study of human basal metabolism. Proc. Natl. Acad. Sci. USA 1918, 4, 370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bharadwaj, S.; Ginoya, S.; Tandon, P.; Gohel, T.D.; Guirguis, J.; Vallabh, H.; Jevenn, A.; Hanouneh, I. Malnutrition: Laboratory markers vs nutritional assessment. Gastroenterol. Rep. 2016, 4, 272–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parent, B.; Seaton, M.; O’Keefe, G.E. Biochemical markers of nutrition support in critically ill trauma victims. J. Parenter. Enter. Nutr. 2018, 42, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, R.D. Too much of a good thing: The curse of overfeeding. Crit. Care 2007, 11, 176. [Google Scholar] [CrossRef]
- Gerich, J.E.; Meyer, C.; Woerle, H.J.; Stumvoll, M. Renal gluconeogenesis: Its importance in human glucose homeostasis. Diabetes Care 2001, 24, 382–391. [Google Scholar] [CrossRef] [Green Version]
- Bergman, B.C.; Horning, M.A.; Casazza, G.A.; Wolfel, E.E.; Butterfield, G.E.; Brooks, G.A. Endurance training increases gluconeogenesis during rest and exercise in men. Am. J. Physiol.-Endocrinol. Metab. 2000, 278, E244–E251. [Google Scholar] [CrossRef] [Green Version]
- Callahan, M.L.; Lim, M.M. Sensory sensitivity in TBI: Implications for chronic disability. Curr. Neurol. Neurosci. Rep. 2018, 18, 56. [Google Scholar] [CrossRef]
- Tayek, J.A.; Katz, J. Glucose production, recycling, and gluconeogenesis in normals and diabetics: A mass isotopomer [U-13C] glucose study. Am. J. Physiol.-Endocrinol. Metab. 1996, 270, E709–E717. [Google Scholar] [CrossRef]


| Group | Number of Animals | Pre-Intervention | 24 h Post-Intervention | 13 Days Post-Intervention | Total Weight Change |
|---|---|---|---|---|---|
| AL-Con | 3 | 249.3 ± 2.1 g | 248.7 ± 0.6 g | 294.3 ± 6.7 g | 45.0 ± 6.2 g |
| AL+TBI | 6 | 252.2 ± 15.8 g | 241.2 ± 15.5 g * | 285.3 ± 16.1 g | 33.2 ± 20.6 g |
| CR-Con | 6 | 292.16 ± 14.8 g *+^ | 291.5 ± 15.3 g | 278.3 ± 10.6 g | −13.83 ± 13.2 g *+ |
| CR+TBI | 5 | 244.8 ± 13.9 g | 233.0 ± 12.1 g * | 216.6015.8 g *$+ | −28.20 ± 7.0 g *+ |
| Group | Number of Animals | Pre-Intervention | 24 Hours Post-Intervention | 13 Days Post-Intervention |
|---|---|---|---|---|
| AL-Con | 3 | 22.5 ± 0.8 g | 18.0 ± 2.6 g | 21.1 ± 1.4 g |
| AL+TBI | 6 | 22.7 ± 1.2 g | 12.3 ± 4.5 g *& | 21.0 ± 1.5 g |
| CR Con | 6 | 25.0 ± 1.2 g | 12.8 ± 0.4 g *& | 12.8 ± 0.4 g *+& |
| CR+TBI | 5 | 21.9 ± 1.9 g | 11.0 ± 1.0 g *& | 11.0 ± 1.0 g *+& |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Curl, C.C.; Kumar, A.; Peck, A.J.; Arevalo, J.A.; Gleason, A.; Leija, R.G.; Osmond, A.D.; Duong, J.J.; Miller, B.F.; Horning, M.A.; et al. Fractional Gluconeogenesis: A Biomarker of Dietary Energy Adequacy in a Rat Brain Injury Model. Metabolites 2022, 12, 1163. https://doi.org/10.3390/metabo12121163
Curl CC, Kumar A, Peck AJ, Arevalo JA, Gleason A, Leija RG, Osmond AD, Duong JJ, Miller BF, Horning MA, et al. Fractional Gluconeogenesis: A Biomarker of Dietary Energy Adequacy in a Rat Brain Injury Model. Metabolites. 2022; 12(12):1163. https://doi.org/10.3390/metabo12121163
Chicago/Turabian StyleCurl, Casey C., Anika Kumar, Austin J. Peck, Jose A. Arevalo, Allison Gleason, Robert G. Leija, Adam D. Osmond, Justin J. Duong, Benjamin F. Miller, Michael A. Horning, and et al. 2022. "Fractional Gluconeogenesis: A Biomarker of Dietary Energy Adequacy in a Rat Brain Injury Model" Metabolites 12, no. 12: 1163. https://doi.org/10.3390/metabo12121163
APA StyleCurl, C. C., Kumar, A., Peck, A. J., Arevalo, J. A., Gleason, A., Leija, R. G., Osmond, A. D., Duong, J. J., Miller, B. F., Horning, M. A., & Brooks, G. A. (2022). Fractional Gluconeogenesis: A Biomarker of Dietary Energy Adequacy in a Rat Brain Injury Model. Metabolites, 12(12), 1163. https://doi.org/10.3390/metabo12121163