Effects of Softening Dry Food with Water on Stress Response, Intestinal Microbiome, and Metabolic Profile in Beagle Dogs
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Housing
2.2. Diets and Feeding
2.3. Experimental Design
2.4. Chemical Analysis and Digestibility Measurements
2.5. Blood Sample Collection and Analysis
2.6. Microbial Analyses
2.6.1. DNA Extraction, Amplification, and Sequencing
2.6.2. Bioinformatics Analysis
2.7. Fecal Short-Chain Fatty Acids and Branch-Chain Fatty Acids Analyses
2.8. Untargeted Fecal and Serum Metabolomics Analysis
2.9. Statistical Analysis
3. Results
3.1. Effects of SDF on Body Weight, Body Condition Score, and Fecal Score
3.2. Effects of Soften Dry Food on Apparent Total Tract Macronutrient Digestibility
3.3. Effects of SDF on Serum Hormones, Heat Shock Protein-70, IgG, and Inflammatory Factors
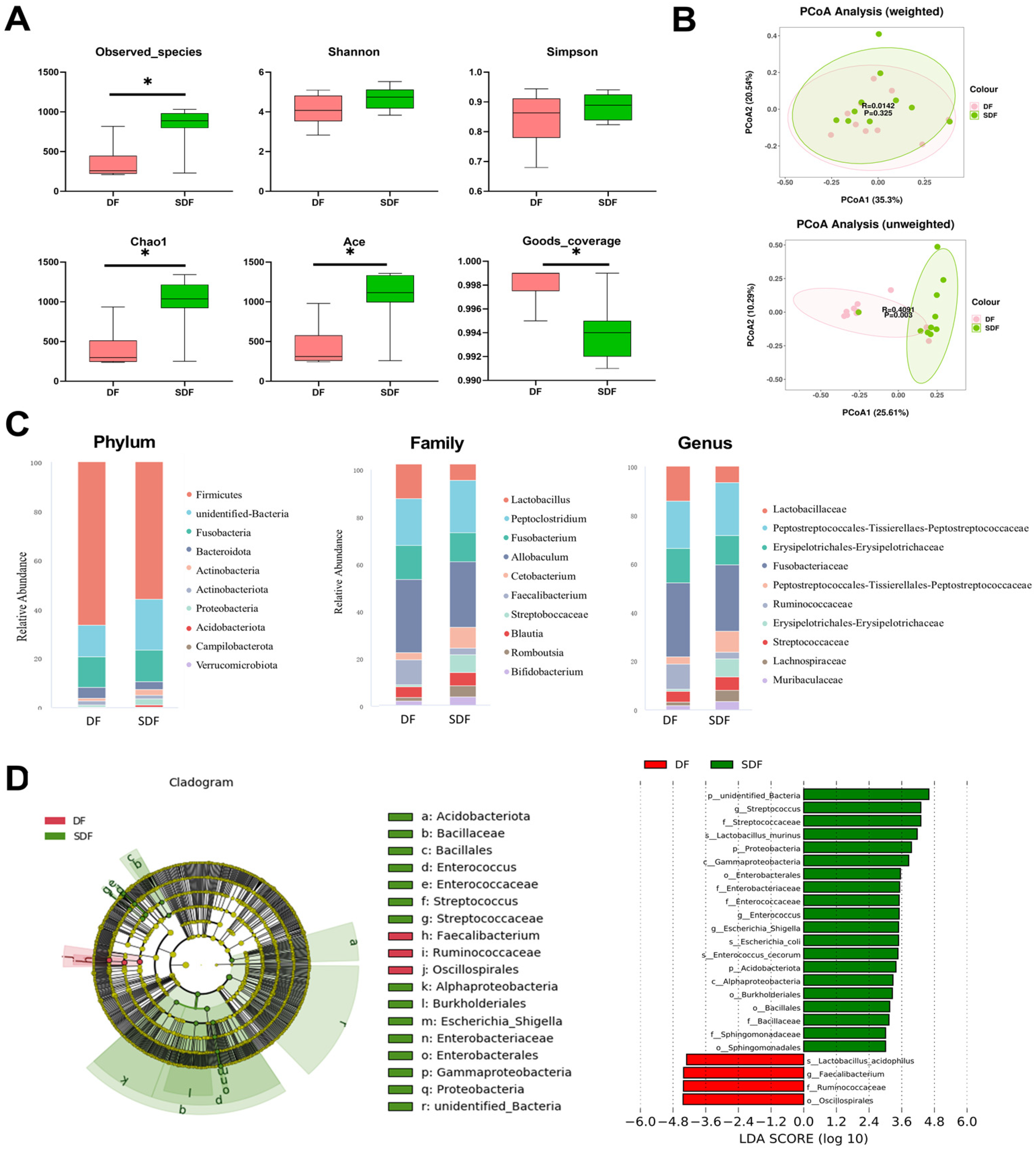
3.4. Effects of SDF on Fecal Microbiota
3.5. Effects of SDF on Fecal Short-Chain Fatty Acids and Branch-Chain Fatty Acids
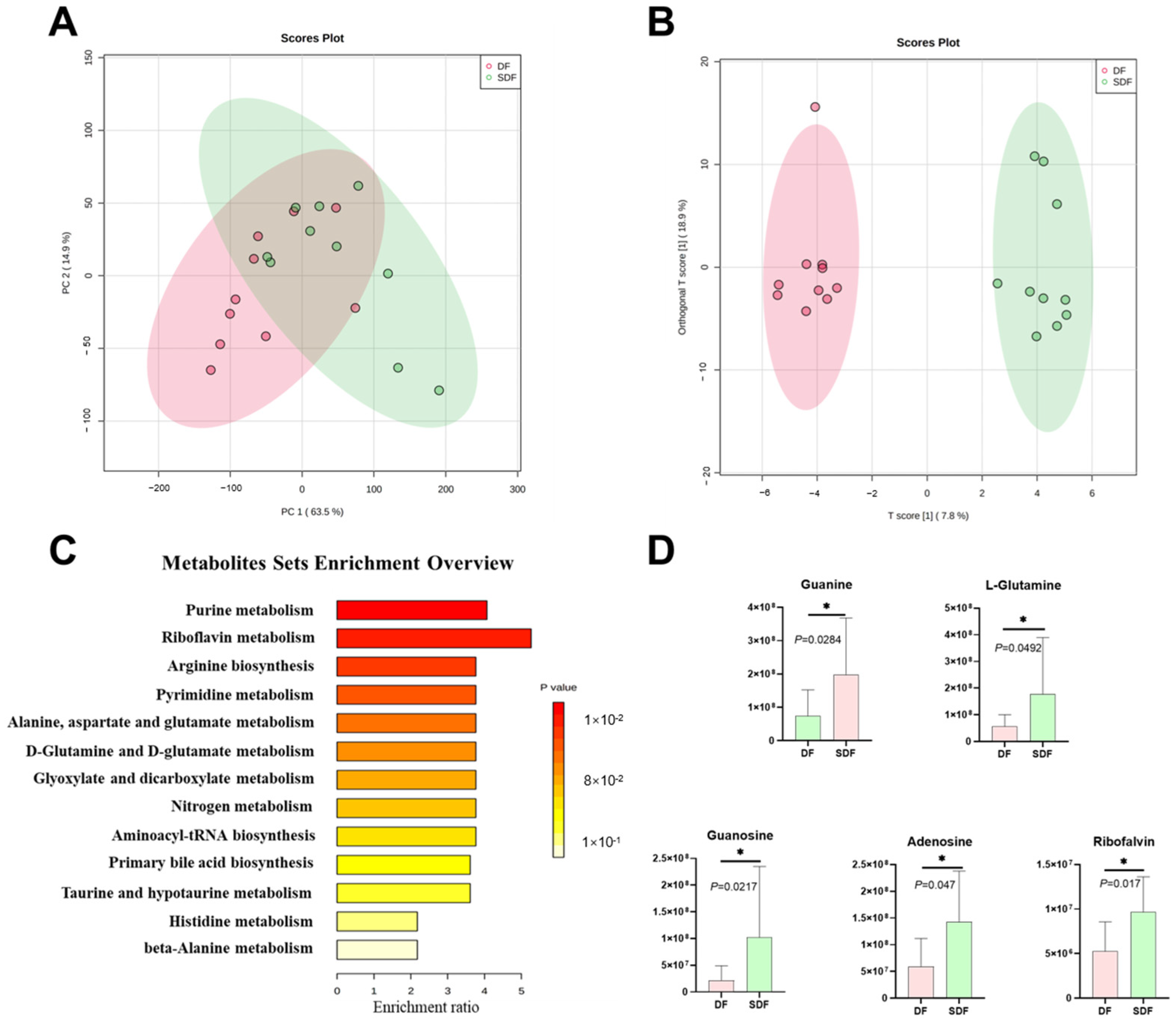
3.6. Effects of SDF on Fecal Metabolome
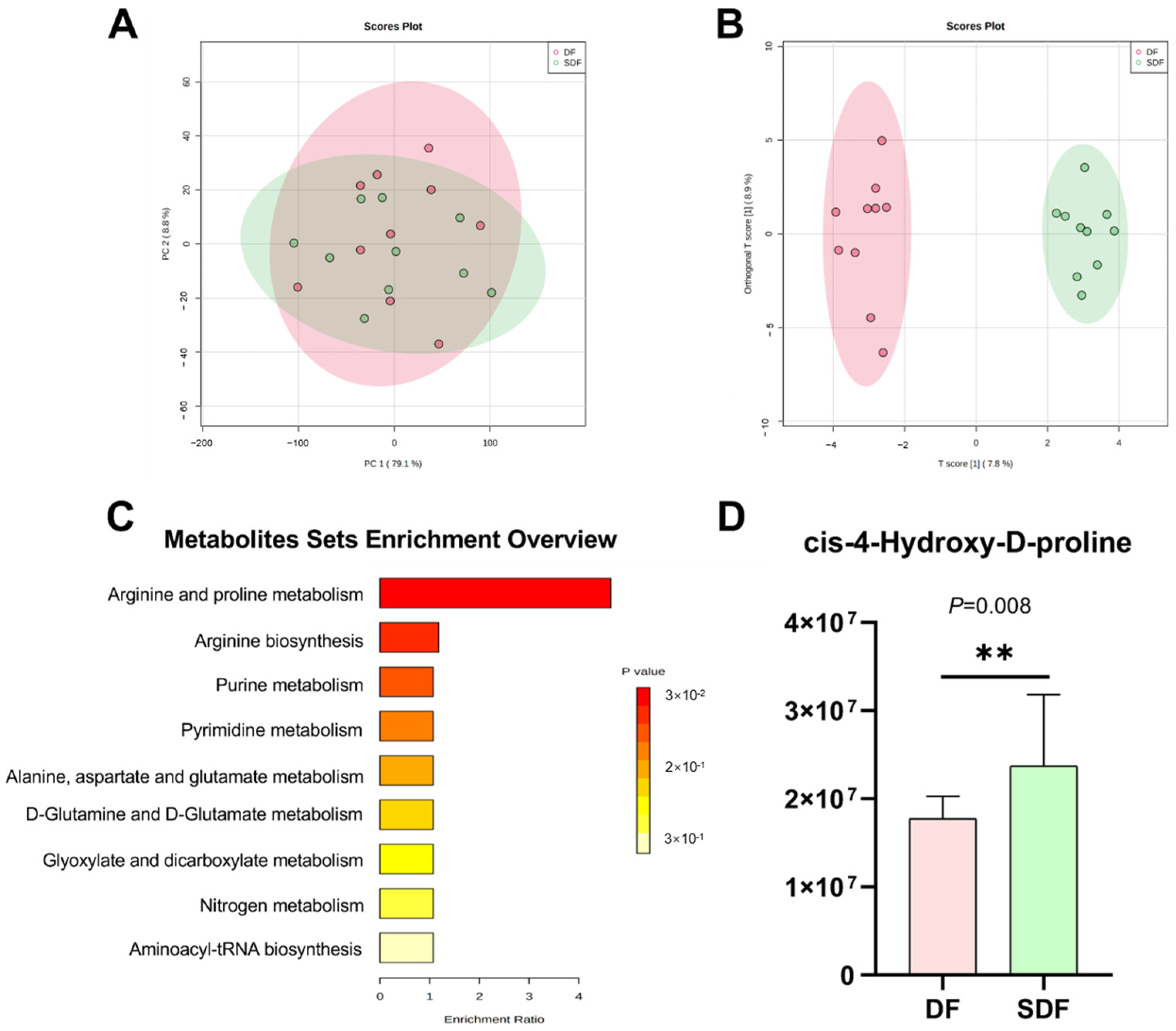
3.7. Effects of SDF on Serum Metabolome
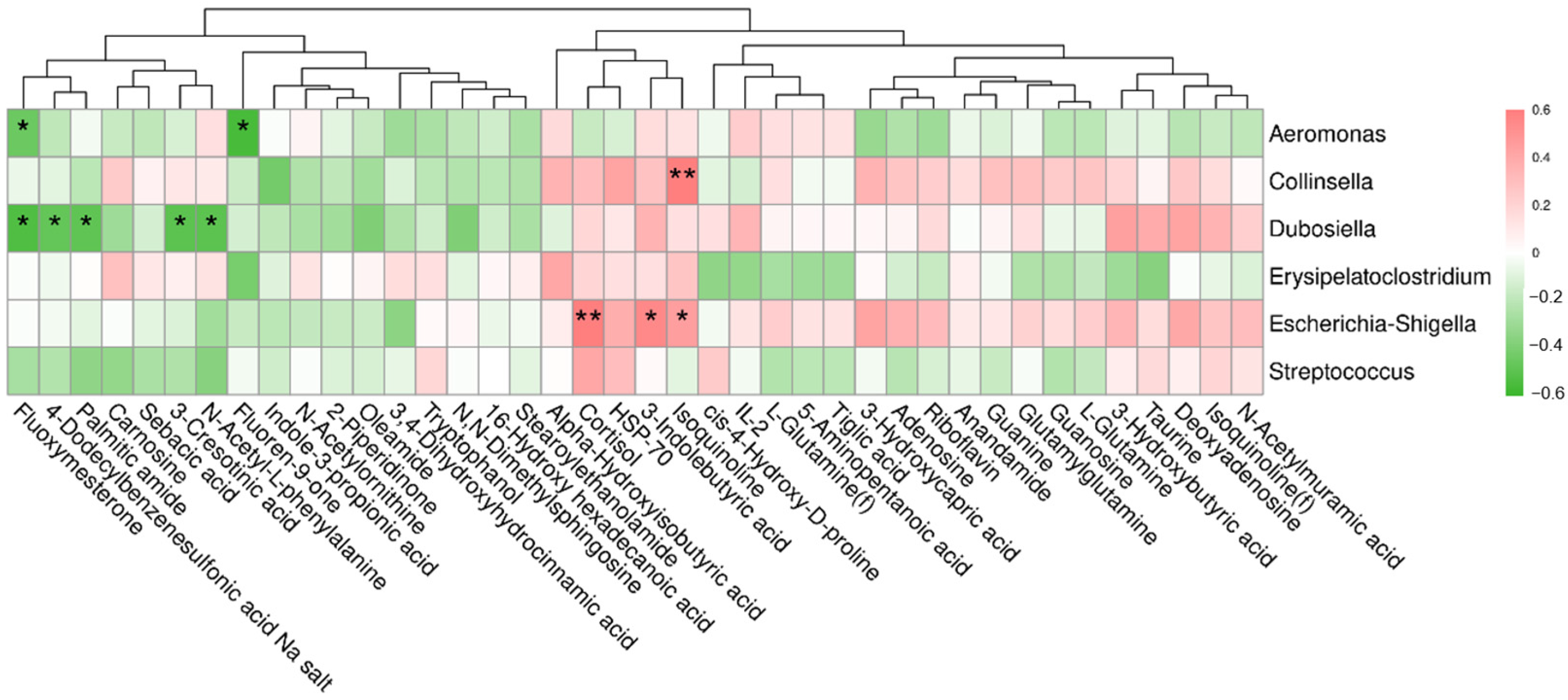
3.8. Correlation between Differential Metabolites and Fecal Bacteria at the Genus Level
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Udell, M.A.; Brubaker, L. Are dogs social generalists? Canine social cognition, attachment, and the dog-human bond. Curr. Dir. Psychol. Sci. 2016, 25, 327–333. [Google Scholar] [CrossRef]
- Christian, H.E.; Westgarth, C.; Bauman, A.; Richards, E.A.; Rhodes, R.E.; Evenson, K.R.; Mayer, J.A.; Thorpe, R.J. Dog ownership and physical activity: A review of the evidence. J. Phy. Act. Health 2013, 10, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Islam, A.; Towell, T. Cat and dog companionship and well-being: A systematic review. Int. J. Appl. Psychol. 2013, 3, 149–155. [Google Scholar]
- Carr, E.C.; Norris, J.M.; Alix Hayden, K.; Pater, R.; Wallace, J.E. A scoping review of the health and social benefits of dog ownership for people who have chronic pain. Anthrozoös 2020, 33, 207–224. [Google Scholar] [CrossRef]
- Dotson, M.J.; Hyatt, E.M. Understanding dog–human companionship. J. Bus. Res. 2008, 61, 457–466. [Google Scholar] [CrossRef]
- Beloshapka, A.N.; Wolff, A.K.; Swanson, K.S. Effects of feeding polydextrose on faecal characteristics, microbiota and fermentative end products in healthy adult dogs. Br. J. Nutr. 2012, 108, 638–644. [Google Scholar] [CrossRef]
- Bordenstein, S.R.; Theis, K.R. Host biology in light of the microbiome: Ten principles of holobionts and hologenomes. PLoS Biol. 2015, 13, e1002226. [Google Scholar] [CrossRef]
- Suchodolski, J.S. Companion animals symposium: Microbes and gastrointestinal health of dogs and cats. J. Anim. Sci. 2011, 89, 1520–1530. [Google Scholar] [CrossRef]
- Canfora, E.E.; Meex, R.C.; Venema, K.; Blaak, E.E. Gut microbial metabolites in obesity, nafld and t2dm. Nat. Rev. Endocrinol. 2019, 15, 261–273. [Google Scholar] [CrossRef]
- D’Argenio, V.; Salvatore, F. The role of the gut microbiome in the healthy adult status. Clin. Chim. Acta. 2015, 451, 97–102. [Google Scholar] [CrossRef]
- Pereira, A.M.; Clemente, A. Dogs’ microbiome from tip to toe. Top. Companion Anim. Med. 2021, 45, 100584. [Google Scholar] [CrossRef] [PubMed]
- Handl, S.; Dowd, S.E.; Garcia-Mazcorro, J.F.; Steiner, J.M.; Suchodolski, J.S. Massive parallel 16s rrna gene pyrosequencing reveals highly diverse fecal bacterial and fungal communities in healthy dogs and cats. FEMS Microbiol. Ecol. 2011, 76, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.S.; Dowd, S.E.; Suchodolski, J.S.; Middelbos, I.S.; Vester, B.M.; Barry, K.A.; Nelson, K.E.; Torralba, M.; Henrissat, B.; Coutinho, P.M.; et al. Phylogenetic and gene-centric metagenomics of the canine intestinal microbiome reveals similarities with humans and mice. ISME J. 2011, 5, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Mazcorro, J.F.; Dowd, S.E.; Poulsen, J.; Steiner, J.M.; Suchodolski, J.S. Abundance and short-term temporal variability of fecal microbiota in healthy dogs. Microbiol. Open. 2012, 1, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Hand, D.; Wallis, C.; Colyer, A.; Penn, C.W. Pyrosequencing the canine faecal microbiota: Breadth and depth of biodiversity. PLoS ONE 2013, 8, e53115. [Google Scholar] [CrossRef]
- Beloshapka, A.N.; Dowd, S.E.; Suchodolski, J.S.; Steiner, J.M.; Duclos, L.; Swanson, K.S. Fecal microbial communities of healthy adult dogs fed raw meat-based diets with or without inulin or yeast cell wall extracts as assessed by 454 pyrosequencing. FEMS Microbiol. Ecol. 2013, 84, 532–541. [Google Scholar] [CrossRef]
- Sandri, M.; Dal Monego, S.; Conte, G.; Sgorlon, S.; Stefanon, B. Raw meat based diet influences faecal microbiome and end products of fermentation in healthy dogs. BMC Vet. Res. 2016, 13, 65. [Google Scholar] [CrossRef]
- Tanprasertsuk, J.; Perry, L.A.M.; Tate, D.E.; Honaker, R.W.; Shmalberg, J. Apparent total tract nutrient digestibility and metabolizable energy estimation in commercial fresh and extruded dry kibble dog foods. Transl. Anim. Sci. 2021, 5, txabo71. [Google Scholar] [CrossRef]
- Middelbos, I.S.; Fastinger, N.D.; Fahey, G.C. Evaluation of fermentable oligosaccharides in diets fed to dogs in comparison to fiber standards. J. Anim. Sci. 2007, 85, 3033–3044. [Google Scholar] [CrossRef]
- Magoc, T.; Salzberg, S.L. Flash: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. Qiime allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Bokulich, N.A.; Subramanian, S.; Faith, J.J.; Gevers, D.; Gordon, J.I.; Knight, R.; Mills, D.A.; Caporaso, J.G. Quality-filtering vastly improves diversity estimates from illumina amplicon sequencing. Nat. Methods 2013, 10, 57–59. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. Uchime improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef] [PubMed]
- Haas, B.J.; Gevers, D.; Earl, A.M.; Feldgarden, M.; Ward, D.V.; Giannoukos, G.; Ciulla, D.; Tabbaa, D.; Highlander, S.K.; Sodergren, E. Chimeric 16s rrna sequence formation and detection in sanger and 454-pyrosequenced pcr amplicons. Genome Res. 2011, 21, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The silva ribosomal rna gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef]
- Edgar, R.C. Muscle: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Yang, K.; Deng, X.; Jian, S.; Zhang, M.; Wen, C.; Xin, Z.; Zhang, L.; Tong, A.; Ye, S.; Deng, B. Gallic acid alleviates gut dysfunction and boosts immune and antioxidant activity in puppies under environmental stress based on microbiome-metabolomics analysis. Front. Immunol. 2022, 5848, 813890. [Google Scholar] [CrossRef]
- Nuli, R.; Azhati, J.; Cai, J.X.; Kadeer, A.; Zhang, B.; Mohemaiti, P. Metagenomics and faecal metabolomics integrative analysis towards the impaired glucose regulation and type 2 diabetes in uyghur-related omics. J. Diabetes Res. 2019, 2019, 2893041. [Google Scholar] [CrossRef]
- Furniss, G. The influence of nutrition on puppy and kitten growth and development. Ir. Vet. J. 2008, 61, 191–194. [Google Scholar]
- Do, S.; Phungviwatnikul, T.; de Godoy, M.R.C.; Swanson, K.S. Nutrient digestibility and fecal characteristics, microbiota, and metabolites in dogs fed human-grade foods. J. Anim. Sci. 2021, 99, skab028. [Google Scholar] [CrossRef]
- Brito, C.B.M.D.; Lima, D.C.D.; Souza, C.M.M.; Vasconcellos, R.S.; Oliveira, S.G.D.; Félix, A.P. Evaluation of dried apple pomace on digestibility and palatability of diets for cats. Braz. J. Anim. Sci. 2020, 49, 1. [Google Scholar] [CrossRef]
- Wakshlag, J.J.; Simpson, K.W.; Struble, A.M.; Dowd, S.E. Negative fecal characteristics are associated with ph and fecal flora alterations during dietary change in dogs. Int. J. Appl. Res. Vet. M 2011, 9, 278–283. [Google Scholar]
- McEwen, B.S. Stress, adaptation, and disease: Allostasis and allostatic load. Ann. N. Y. Acad. Sci. 1998, 840, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Janicki-Deverts, D.; Miller, G.E. Psychological stress and disease. JAMA 2007, 298, 1685–1687. [Google Scholar] [CrossRef]
- Helfand, S.C.; Modiano, J.F.; Nowell, P.C. Immunophysiological studies of interleukin-2 and canine lymphocytes. Vet. Immunol. Immunopathol. 1992, 33, 1–16. [Google Scholar] [CrossRef]
- Hammarqvist, F.; Wernerman, J.; von der Decken, A.; Vinnars, E. Alanyl-glutamine counteracts the depletion of free glutamine and the postoperative decline in protein synthesis in skeletal muscle. Ann. Sur. 1990, 212, 637. [Google Scholar] [CrossRef]
- Karner, J.; Roth, E. Alanyl-glutamine infusions to patients with acute pancreatitis. Clin. Nurt. 1990, 9, 43–44. [Google Scholar] [CrossRef]
- Weiss, Y.G.; Maloyan, A.; Tazelaar, J.; Raj, N.; Deutschman, C.S. Adenoviral transfer of hsp-70 into pulmonary epithelium ameliorates experimental acute respiratory distress syndrome. J. Clin. Invest. 2002, 110, 801–806. [Google Scholar] [CrossRef]
- Inness, V.; McCartney, A.; Khoo, C.; Gross, K.; Gibson, G. Molecular characterisation of the gut microflora of healthy and inflammatory bowel disease cats using fluorescence in situ hybridisation with special reference to desulfovibrio spp. J. Anim. Physiol. Anim. Nutr. 2007, 91, 48–53. [Google Scholar] [CrossRef]
- Janeczko, S.; Atwater, D.; Bogel, E.; Greiter-Wilke, A.; Gerold, A.; Baumgart, M.; Bender, H.; McDonough, P.; McDonough, S.; Goldstein, R. The relationship of mucosal bacteria to duodenal histopathology, cytokine mrna, and clinical disease activity in cats with inflammatory bowel disease. Vet. Microbiol. 2008, 128, 178–193. [Google Scholar] [CrossRef]
- Clavel, T.; Desmarchelier, C.; Haller, D.; Gerard, P.; Rohn, S.; Lepage, P.; Daniel, H. Intestinal microbiota in metabolic diseases from bacterial community structure and functions to species of pathophysiological relevance. Gut Microbes 2014, 5, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; An, J.U.; Kim, W.; Lee, S.; Cho, S. Differences in the gut microbiota of dogs (canis lupus familiaris) fed a natural diet or a commercial feed revealed by the illumina miseq platform. Gut Pathog. 2017, 9, 68. [Google Scholar] [CrossRef] [PubMed]
- Xenoulis, P.G.; Palculict, B.; Allenspach, K.; Steiner, J.M.; Van House, A.M.; Suchodolski, J.S. Molecular-phylogenetic characterization of microbial communities imbalances in the small intestine of dogs with inflammatory bowel disease. FEMS Microbiol. Ecol. 2008, 66, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Thorkildsen, L.T.; Nwosu, F.C.; Avershina, E.; Ricanek, P.; Perminow, G.; Brackmann, S.; Vatn, M.H.; Rudi, K. Dominant fecal microbiota in newly diagnosed untreated inflammatory bowel disease patients. Gastroenterol. Res. Pract. 2013, 2013, 636785. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Arango, L.F.; Barrett, H.L.; Wilkinson, S.A.; Callaway, L.K.; Mcintyre, H.D.; Morrison, M.; Nitert, M.D. Low dietary fiber intake increases collinsella abundance in the gut microbiota of overweight and obese pregnant women. Gut Microbes 2018, 9, 189–201. [Google Scholar] [CrossRef]
- Zhang, H.; DiBaise, J.K.; Zuccolo, A.; Kudrna, D.; Braidotti, M.; Yu, Y.; Parameswaran, P.; Crowell, M.D.; Wing, R.; Rittmann, B.E. Human gut microbiota in obesity and after gastric bypass. Proc. Natl. Acad. Sci. USA 2009, 106, 2365–2370. [Google Scholar] [CrossRef]
- Cox, L.M.; Sohn, J.; Tyrrell, K.L.; Citron, D.M.; Lawson, P.A.; Patel, N.B.; Iizumi, T.; Perez-Perez, G.I.; Goldstein, E.J.; Blaser, M.J. Description of two novel members of the family erysipelotrichaceae: Ileibacterium valens gen. Nov., sp. Nov. And dubosiella newyorkensis, gen. Nov., sp. Nov., from the murine intestine, and emendation to the description of faecalibacterium rodentium. Int. J. Syst. Evol. Microbiol. 2017, 67, 1247. [Google Scholar] [CrossRef]
- Sheng, K.; Zhang, G.; Sun, M.; He, S.; Kong, X.; Wang, J.; Zhu, F.; Zha, X.; Wang, Y. Grape seed proanthocyanidin extract ameliorates dextran sulfate sodium-induced colitis through intestinal barrier improvement, oxidative stress reduction, and inflammatory cytokines and gut microbiota modulation. Food Funct. 2020, 11, 7817–7829. [Google Scholar] [CrossRef]
- Chamorro, S.; Romero, C.; Brenes, A.; Sanchez-Patan, F.; Bartolome, B.; Viveros, A.; Arija, I. Impact of a sustained consumption of grape extract on digestion, gut microbial metabolism and intestinal barrier in broiler chickens. Food Funct. 2019, 10, 1444–1454. [Google Scholar] [CrossRef]
- Vitek, L.; Haluzik, M. The role of bile acids in metabolic regulation. J. Endocrinol. 2016, 228, R85–R96. [Google Scholar] [CrossRef]
- Ciccarelli, R.; Di Iorio, P.; Giuliani, P.; D’Alimonte, I.; Ballerini, P.; Caciagli, F.; Rathbone, M.P. Rat cultured astrocytes release guanine-based purines in basal conditions and after hypoxia/hypoglycemia. Glia 1999, 25, 93–98. [Google Scholar] [CrossRef]
- Cornforth, D.M.; Foster, K.R. Competition sensing: The social side of bacterial stress responses. Nat. Rev. Microbiol. 2013, 11, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Rowlett, V.W.; Mallampalli, V.K.P.S.; Karlstaedt, A.; Dowhan, W.; Taegtmeyer, H.; Margolin, W.; Vitrac, H. Impact of membrane phospholipid alterations in escherichia coli on cellular function and bacterial stress adaptation. J. Bacteriol. 2017, 199, e00849-16. [Google Scholar] [CrossRef] [PubMed]
- Bange, G.; Bedrunka, P. Physiology of guanosine-based second messenger signaling in bacillus subtilis. Biol. Chem. 2020, 401, 1307–1322. [Google Scholar] [CrossRef]
- Haber, A.; Friedman, S.; Lobel, L.; Burg-Golani, T.; Sigal, N.; Rose, J.; Livnat-Levanon, N.; Lewinson, O.; Herskovits, A.A. L-glutamine induces expression of listeria monocytogenes virulence genes. PLoS Pathog. 2017, 13, e1006161. [Google Scholar] [CrossRef]
- Ådén, U.; O’Connor, W.T.; Berman, R.F. Changes in purine levels and adenosine receptors in kindled seizures in the rat. Neuroreport 2004, 15, 1585–1589. [Google Scholar] [CrossRef]
- Bouma, M.G.; van den Wildenberg, F.A.J.M.; Buurman, W.A. The anti-inflammatory potential of adenosine in ischemia-reperfusion injury: Established and putative beneficial actions of a retaliatory metabolite. Shock 1997, 8, 313–320. [Google Scholar] [CrossRef]
- Giriş, M.; Depboylu, B.; Doğru-Abbasoğlu, S.; Erbil, Y.; Olgaç, V.; Alış, H.; Aykaç-Toker, G.; Uysal, M. Effect of taurine on oxidative stress and apoptosis-related protein expression in trinitrobenzene sulphonic acid-induced colitis. Clin. Exp. Immunol. 2008, 152, 102–110. [Google Scholar] [CrossRef]
- Franconi, F.; Loizzo, A.; Ghirlanda, G.; Seghieri, G. Taurine supplementation and diabetes mellitus. Curr. Opin. Clin. Nutr. 2006, 9, 32–36. [Google Scholar] [CrossRef]
- Oliveira, M.W.S.; Minotto, J.B.; de Oliveira, M.R.; Zanotto, A.; Behr, G.A.; Rocha, R.F.; Moreira, J.C.F.; Klamt, F. Scavenging and antioxidant potential of physiological taurine concentrations against different reactive oxygen/nitrogen species. Pharmacol. Rep. 2010, 62, 185–193. [Google Scholar] [CrossRef]
- Kim, C.; Cha, Y.-N. Production of reactive oxygen and nitrogen species in phagocytes is regulated by taurine chloramine. Taurine 2009, 7, 463–472. [Google Scholar]
- Hu, J.; Xu, X.; Yang, J.; Wu, G.; Sun, C.; Lv, Q. Antihypertensive effect of taurine in rat. Taurine 2009, 7, 75–84. [Google Scholar]
- Sudhahar, V.; Shaw, S.; Imig, J.D. Mechanisms involved in oleamide-induced vasorelaxation in rat mesenteric resistance arteries. Eur. J. Pharmacol. 2009, 607, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.-M.; Lee, S.A.; Hong, J.H.; Kim, J.-S.; Kim, D.K.; Kim, C.S. Oleamide suppresses inflammatory responses in lps-induced raw264. 7 murine macrophages and alleviates paw edema in a carrageenan-induced inflammatory rat model. Int. Immunopharmacol. 2018, 56, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Kita, M.; Ano, Y.; Inoue, A.; Aoki, J. Identification of p2y receptors involved in oleamide-suppressing inflammatory responses in murine microglia and human dendritic cells. Sci. Rep. 2019, 9, 3135. [Google Scholar] [CrossRef]
- Kim, M.; Kim, M.; Han, J.Y.; Lee, S.-H.; Jee, S.H.; Lee, J.H. The metabolites in peripheral blood mononuclear cells showed greater differences between patients with impaired fasting glucose or type 2 diabetes and healthy controls than those in plasma. Diabetes Vasc. Dis. Res. 2017, 14, 130–138. [Google Scholar] [CrossRef]
- Lombard, G.L.; Dowell, V., Jr. Comparison of three reagents for detecting indole production by anaerobic bacteria in microtest systems. J. Clin. Mic. 1983, 18, 609–613. [Google Scholar] [CrossRef]
- Cai, H.; Li, H.; Yan, X.; Sun, B.; Zhang, Q.; Yan, M.; Zhang, W.-Y.; Jiang, P.; Zhu, R.-H.; Liu, Y.-P. Metabolomic analysis of biochemical changes in the plasma and urine of first-episode neuroleptic-naive schizophrenia patients after treatment with risperidone. J. Proteomoe Res. 2012, 11, 4338–4350. [Google Scholar] [CrossRef]
- Dileep, K.V.; Remya, C.; Tintu, I.; Haridas, M.; Sadasivan, C. Interactions of selected indole derivatives with phospholipase a2: In silico and in vitro analysis. J. Mol. Model. 2013, 19, 1811–1817. [Google Scholar] [CrossRef]
- McNaught, K.S.P.; Carrupt, P.-A.; Altomare, C.; Cellamare, S.; Carotti, A.; Testa, B.; Jenner, P.; Marsden, C.D. Isoquinoline derivatives as endogenous neurotoxins in the aetiology of parkinson’s disease. Biochem. Pharmacol. 1998, 56, 921–933. [Google Scholar] [CrossRef]
| Group 1 | N (Male: Female) | Age (M) | Weight (kg) |
|---|---|---|---|
| DF | 10 (4:6) | 5.38 ± 0.35 | 7.33 ± 0.12 |
| SDF | 10 (4:6) | 5.62 ± 0.29 | 7.25 ± 0.18 |
| Items 1 | Basal diet 2 |
|---|---|
| DM (%) | 91.19 |
| OM (% DM) | 91.57 |
| CP (% DM) | 27.69 |
| AHF (% DM) | 11.21 |
| TDF (% DM) | 3.63 |
| Item 1 | DF 2 | SDF 3 | p Value |
|---|---|---|---|
| BW (kg) | 8.01 ± 0.14 | 7.87 ± 0.18 | 0.540 |
| BCS | 5.05 ± 0.17 | 4.65 ± 0.11 | 0.660 |
| TFS | 2.55 ± 0.14 | 2.62 ± 0.25 | 0.028 |
| TSFR (%) | 0.48 | 0.48 | - |
| TDR (%) | 0.95 | 3.33 | - |
| Item | DF 1 | SDF 2 | p Value |
|---|---|---|---|
| Food intake 3, g/d (DM basis) | 184.00 | 184.00 | - |
| Fecal output, g/d (as is) | 91.07 ± 4.78 | 89.00 ± 1.81 | 0.693 |
| Fecal output, g/d (DM basis) | 24.90 ± 1.27 | 23.82 ± 1.73 | 0.628 |
| Digestibility, % | |||
| Dry matter | 88.55 ± 0.01 | 89.04 ± 0.01 | 0.628 |
| %DM basis | |||
| Organic matter | 81.28 ± 0.26 | 82.39 ± 0.10 | 0.693 |
| Crude protein | 83.88 ± 0.01 | 85.52 ± 0.01 | 0.301 |
| Acid hydrolyzation fat | 94.83 ± 0.01 | 95.84 ± 0.01 | 0.104 |
| Total dietary fiber | 70.73 ± 0.02 | 66.82 ± 0.01 | 0.202 |
| Item 1 | DF 2 | SDF 3 | p Value |
|---|---|---|---|
| IgG | 258.88 ± 16.04 | 227.38 ± 14.90 | 0.167 |
| IFN-γ | 42.10 ± 1.39 | 45.23 ± 1.23 | 0.108 |
| IL-6 | 541.77 ± 20.15 | 567.00 ± 21.69 | 0.405 |
| IL-4 | 163.72 ± 14.20 | 146.66 ± 9.65 | 0.334 |
| IL-2 | 254.86 ± 11.54 | 287.23 ± 11.42 | 0.062 |
| TNF-α | 77.08 ± 4.15 | 77.12 ± 4.69 | 0.995 |
| GC | 321.91 ± 13.13 | 318.80 ± 9.23 | 0.887 |
| ACTH | 67.23 ± 3.31 | 68.10 ± 2.45 | 0.836 |
| COR | 394.24 ± 13.13 | 438.50 ± 9.23 | 0.014 |
| HSP-70 | 334.50 ± 18.05 | 376.07 ± 15.42 | 0.097 |
| Item (µg/g) | DF 1 | SDF 2 | p Value |
|---|---|---|---|
| Acetic acid | 1476.31 ± 35.88 | 1479.08 ± 54.89 | 0.968 |
| Propionic acid | 1079.94 ± 23.41 | 1063.59 ± 43.24 | 0.751 |
| Butyric acid | 442.42 ± 19.53 | 443.47 ± 13.93 | 0.891 |
| Total SCFAs 3 | 2998.66 ± 72.02 | 2986.13 ± 103.37 | 0.924 |
| Isobutyric acid | 92.21 ± 7.12 | 91.06 ± 9.95 | 0.955 |
| Isovaleric acid | 142.52 ± 12.76 | 141.41 ± 14.25 | 0.550 |
| Valeric acid | 19.70 ± 3.26 | 17.35 ± 2.20 | 0.891 |
| Total BCFAs 4 | 254.43 ± 20.20 | 249.82 ± 25.68 | 0.922 |
| Metabolites | p Value | VIP 1 | KEGG 2 | Trend (SDF 3 vs. DF 4) |
|---|---|---|---|---|
| 16-Hydroxy hexadecenoic acid | 0.000 | 2.03 | - | Down |
| Stearoylethanolamide | 0.000 | 1.96 | - | Down |
| Isoquinoline | 0.002 | 2.01 | C06323 | Up |
| Tryptophanol | 0.002 | 2.26 | C00955 | Down |
| N, N-Dimethylsphingosine | 0.002 | 2.17 | C13914 | Down |
| N-Acetylmuramic acid | 0.004 | 1.58 | C02713 | Up |
| Riboflavin | 0.004 | 2.28 | C00255 | Up |
| 4-Dodecylbenzenesulfonic acid Na salt | 0.004 | 2.24 | - | Down |
| Oleamide | 0.005 | 2.03 | C19670 | Down |
| Anandamide | 0.005 | 1.63 | C11695 | Down |
| Guanosine | 0.006 | 1.98 | C00387 | Up |
| Sebacic acid | 0.006 | 2.15 | C08277 | Down |
| Glutamylglutamine | 0.012 | 1.92 | - | Up |
| 2-Piperidinone | 0.020 | 2.12 | - | Down |
| Carnosine | 0.027 | 2.08 | C00386 | Down |
| Tiglic acid | 0.027 | 1.73 | C08279 | Up |
| Palmitic amide | 0.023 | 1.82 | - | Down |
| 5-Aminopentanoic acid | 0.028 | 1.72 | C00431 | Up |
| Taurine | 0.029 | 1.61 | C00245 | Up |
| Adenosine | 0.034 | 1.51 | C00212 | Down |
| L-Glutamine | 0.035 | 1.56 | C00064 | Up |
| Fluoxymesterone | 0.036 | 1.57 | - | Down |
| Guanine | 0.039 | 1.89 | C00242 | Up |
| Deoxyadenosine | 0.040 | 1.51 | C00559 | Up |
| Metabolites | p Value | VIP 1 | KEGG 2 | Trend (SDF 3 vs. DF 4) |
|---|---|---|---|---|
| 3-Hydroxycapric acid | 0.001 | 2.49 | - | Up |
| N-Acetylornithine | 0.004 | 2.13 | C00437 | Down |
| 3-Indolebutyric acid | 0.006 | 2.16 | C11284 | Up |
| cis-4-Hydroxy-D-proline | 0.008 | 2.02 | C03440 | Up |
| 3-Hydroxybutyric acid | 0.011 | 1.94 | C01089 | Up |
| Isoquinoline | 0.011 | 2.05 | C06323 | Up |
| Fluoren-9-one | 0.012 | 1.87 | C06712 | Down |
| 3-Cresotinic acid | 0.022 | 1.89 | C14088 | Down |
| 3,4-Dihydroxyhydrocinnamic acid | 0.024 | 1.84 | C10447 | Down |
| N-Acetyl-L-phenylalanine | 0.026 | 1.85 | C03519 | Down |
| Alpha-Hydroxyisobutyric acid | 0.027 | 1.90 | - | Up |
| L-Glutamine | 0.030 | 1.76 | C00064 | Up |
| Indole-3-propionic acid | 0.042 | 1.83 | - | Down |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Yang, K.; Jian, S.; Xin, Z.; Wen, C.; Zhang, L.; Huang, J.; Deng, B.; Deng, J. Effects of Softening Dry Food with Water on Stress Response, Intestinal Microbiome, and Metabolic Profile in Beagle Dogs. Metabolites 2022, 12, 1124. https://doi.org/10.3390/metabo12111124
Zhang L, Yang K, Jian S, Xin Z, Wen C, Zhang L, Huang J, Deng B, Deng J. Effects of Softening Dry Food with Water on Stress Response, Intestinal Microbiome, and Metabolic Profile in Beagle Dogs. Metabolites. 2022; 12(11):1124. https://doi.org/10.3390/metabo12111124
Chicago/Turabian StyleZhang, Limeng, Kang Yang, Shiyan Jian, Zhongquan Xin, Chaoyu Wen, Lingna Zhang, Jian Huang, Baichuan Deng, and Jinping Deng. 2022. "Effects of Softening Dry Food with Water on Stress Response, Intestinal Microbiome, and Metabolic Profile in Beagle Dogs" Metabolites 12, no. 11: 1124. https://doi.org/10.3390/metabo12111124
APA StyleZhang, L., Yang, K., Jian, S., Xin, Z., Wen, C., Zhang, L., Huang, J., Deng, B., & Deng, J. (2022). Effects of Softening Dry Food with Water on Stress Response, Intestinal Microbiome, and Metabolic Profile in Beagle Dogs. Metabolites, 12(11), 1124. https://doi.org/10.3390/metabo12111124







