Body Weight and Metabolic Rate Changes in Narcolepsy: Current Knowledge and Future Directions
Abstract
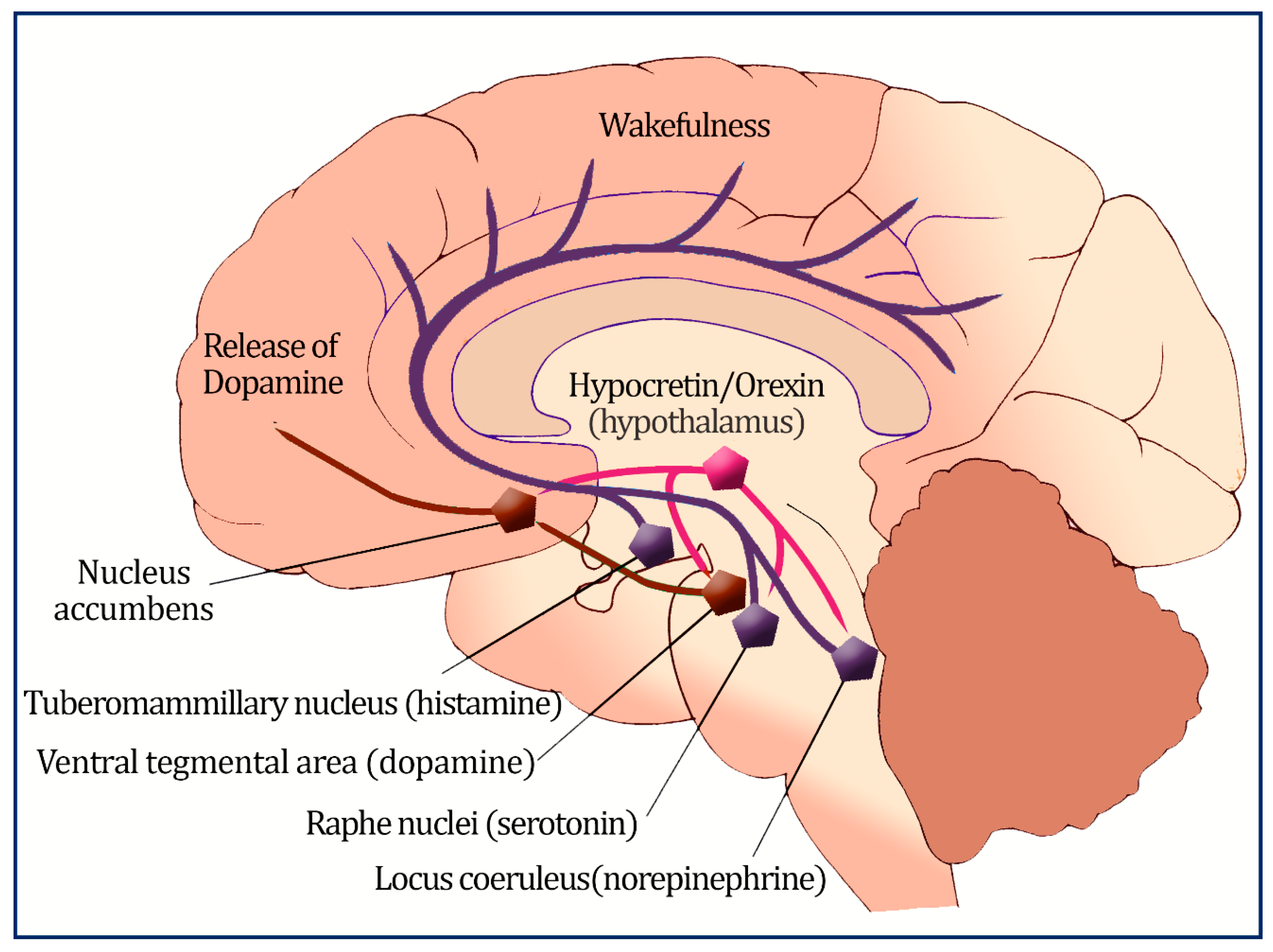
1. Introduction
2. Search Methods
3. Prevalence of Obesity in Patients with Narcolepsy
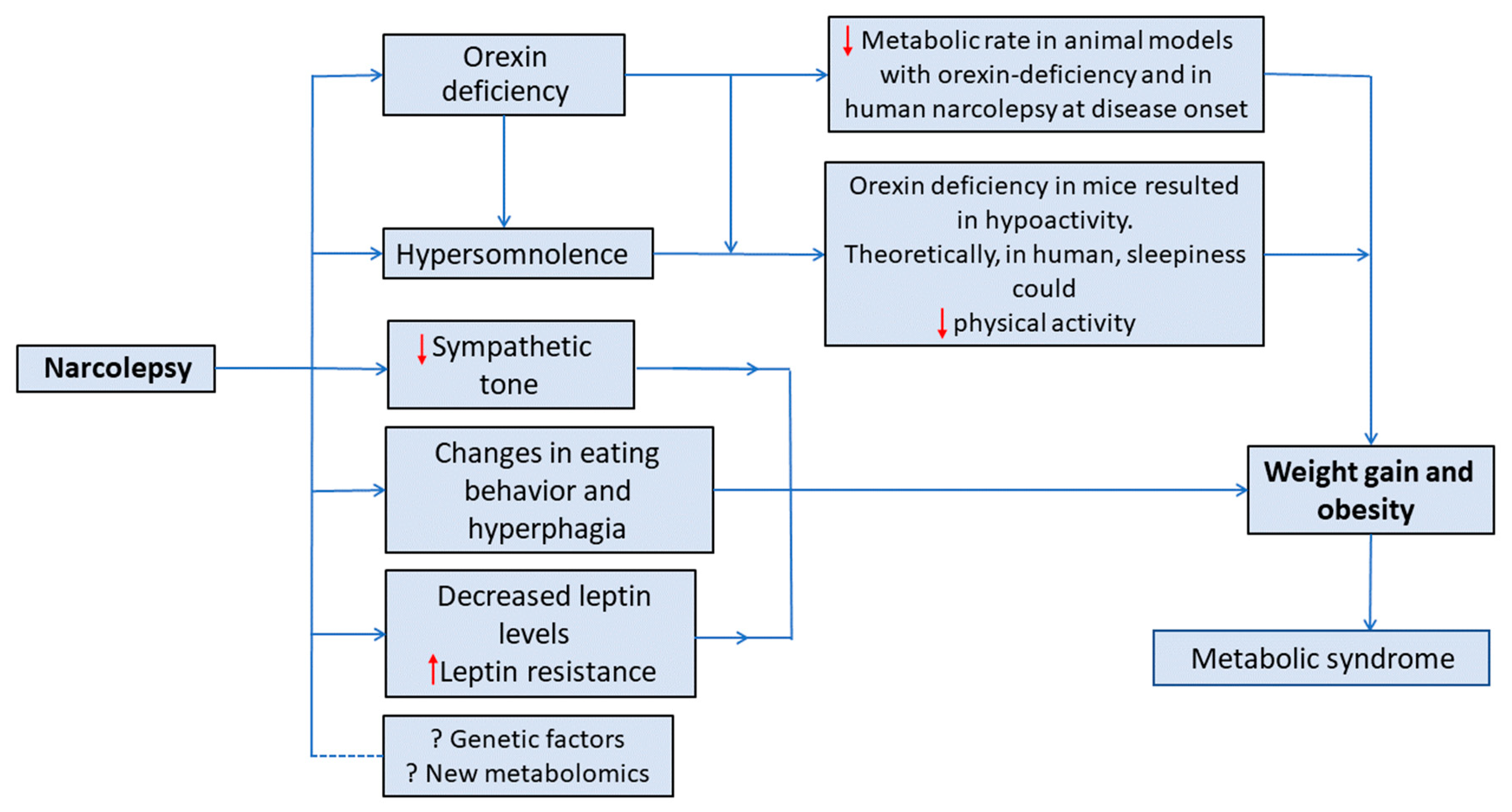
4. Proposed Theories of Weight Gain in Narcolepsy
4.1. Orexin’s Role in Metabolism
4.2. Orexin and Eating Behavior
4.3. Leptin, Ghrelin, and Other Hormonal Changes
4.4. Reduced Sympathetic Tone
4.5. The Role of Diet and Dietary Components
4.6. Physical Activity
4.7. Genetics Factors
4.8. Metabolomics
5. Changes in Metabolic Rate (Energy Expenditure) in Patients with Narcolepsy
6. Narcolepsy Treatment and Weight Loss
7. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Aserinsky, E.; Kleitman, N. Regularly occurring periods of eye motility, and concomitant phenomena, during sleep. Science 1953, 118, 273–274. [Google Scholar] [CrossRef]
- Longstreth, W.T., Jr.; Koepsell, T.D.; Ton, T.G.; Hendrickson, A.F.; van Belle, G. The epidemiology of narcolepsy. Sleep 2007, 30, 13–26. [Google Scholar] [CrossRef]
- American Academy of Sleep Medicine. The International Classification of Sleep Disorders:(ICSD-3); American Academy of Sleep Medicine: Darien, IL, USA, 2014. [Google Scholar]
- Kornum, B.R.; Knudsen, S.; Ollila, H.M.; Pizza, F.; Jennum, P.J.; Dauvilliers, Y.; Overeem, S. Narcolepsy. Nat. Rev. Dis. Primers 2017, 3, 16100. [Google Scholar] [CrossRef]
- Quaedackers, L.; Pillen, S.; Overeem, S. Recognizing the Symptom Spectrum of Narcolepsy to Improve Timely Diagnosis: A Narrative Review. Nat. Sci. Sleep 2021, 13, 1083–1096. [Google Scholar] [CrossRef]
- Mahoney, C.E.; Cogswell, A.; Koralnik, I.J.; Scammell, T.E. The neurobiological basis of narcolepsy. Nat. Rev. Neurosci. 2019, 20, 83–93. [Google Scholar] [CrossRef]
- Siegel, J.M. Narcolepsy: A key role for hypocretins (orexins). Cell 1999, 98, 409–412. [Google Scholar] [CrossRef]
- Li, J.; Hu, Z.; de Lecea, L. The hypocretins/orexins: Integrators of multiple physiological functions. Br. J. Pharmacol. 2014, 171, 332–350. [Google Scholar] [CrossRef]
- Muroya, S.; Funahashi, H.; Yamanaka, A.; Kohno, D.; Uramura, K.; Nambu, T.; Shibahara, M.; Kuramochi, M.; Takigawa, M.; Yanagisawa, M.; et al. Orexins (hypocretins) directly interact with neuropeptide Y, POMC and glucose-responsive neurons to regulate Ca2+ signaling in a reciprocal manner to leptin: Orexigenic neuronal pathways in the mediobasal hypothalamus. Eur. J. Neurosci. 2004, 19, 1524–1534. [Google Scholar] [CrossRef]
- Peyron, C.; Tighe, D.K.; van den Pol, A.N.; de Lecea, L.; Heller, H.C.; Sutcliffe, J.G.; Kilduff, T.S. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J. Neurosci. 1998, 18, 9996–10015. [Google Scholar] [CrossRef]
- Heinonen, M.V.; Purhonen, A.K.; Makela, K.A.; Herzig, K.H. Functions of orexins in peripheral tissues. Acta Physiol. 2008, 192, 471–485. [Google Scholar] [CrossRef]
- Jennum, P.; Thorstensen, E.W.; Pickering, L.; Ibsen, R.; Kjellberg, J. Morbidity and mortality of middle-aged and elderly narcoleptics. Sleep Med. 2017, 36, 23–28. [Google Scholar] [CrossRef]
- Jennum, P.J.; Plazzi, G.; Silvani, A.; Surkin, L.A.; Dauvilliers, Y. Cardiovascular disorders in narcolepsy: Review of associations and determinants. Sleep Med. Rev. 2021, 58, 101440. [Google Scholar] [CrossRef]
- Futenma, K.; Takaesu, Y.; Nakamura, M.; Hayashida, K.; Takeuchi, N.; Inoue, Y. Metabolic-Syndrome-Related Comorbidities in Narcolepsy Spectrum Disorders: A Preliminary Cross-Sectional Study in Japan. Int. J. Environ. Res. Public Health 2022, 19, 6285. [Google Scholar] [CrossRef]
- Paré, G.; Kitsiou, S. Methods for Literature Reviews. In Handbook of eHealth Evaluation: An Evidence-Based Approach; Lau, F., Kuziemsky, C., Eds.; University of Victoria: Victoria, BC, Canada, 2017; pp. 157–179. [Google Scholar]
- Templier, M.; Paré, G. Transparency in literature reviews: An assessment of reporting practices across review types and genres in top IS journals. Eur. J. Inf. Syst. 2018, 27, 503–550. [Google Scholar] [CrossRef]
- Poli, F.; Pizza, F.; Mignot, E.; Ferri, R.; Pagotto, U.; Taheri, S.; Finotti, E.; Bernardi, F.; Pirazzoli, P.; Cicognani, A.; et al. High prevalence of precocious puberty and obesity in childhood narcolepsy with cataplexy. Sleep 2013, 36, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, S.; Moosaie, F.; Saghazadeh, A.; Mahmoudi, M.; Rezaei, N. Metabolic profile in patients with narcolepsy: A systematic review and meta-analysis. Sleep Med. 2021, 81, 268–284. [Google Scholar] [CrossRef]
- Filardi, M.; Demir, N.; Pizza, F.; Vandi, S.; Antelmi, E.; Noce, S.; Bruni, O.; Plazzi, G. Prevalence and neurophysiological correlates of sleep disordered breathing in pediatric type 1 narcolepsy. Sleep Med. 2020, 65, 8–12. [Google Scholar] [CrossRef]
- Barateau, L.; Chenini, S.; Evangelista, E.; Jaussent, I.; Lopez, R.; Dauvilliers, Y. Clinical autonomic dysfunction in narcolepsy type 1. Sleep 2019, 42, zsz187. [Google Scholar] [CrossRef]
- Vandi, S.; Rodolfi, S.; Pizza, F.; Moresco, M.; Antelmi, E.; Ferri, R.; Mignot, E.; Plazzi, G.; Silvani, A. Cardiovascular autonomic dysfunction, altered sleep architecture, and muscle overactivity during nocturnal sleep in pediatric patients with narcolepsy type 1. Sleep 2019, 42, zsz169. [Google Scholar] [CrossRef]
- Morales Drissi, N.; Romu, T.; Landtblom, A.M.; Szakacs, A.; Hallbook, T.; Darin, N.; Borga, M.; Leinhard, O.D.; Engstrom, M. Unexpected Fat Distribution in Adolescents With Narcolepsy. Front. Endocrinol. 2018, 9, 728. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, H.; Stone, W.S.; Zhuang, J.; Qiu, L.; Xu, X.; Wang, Y.; Zhao, Z.; Han, F. Body weight and basal metabolic rate in childhood narcolepsy: A longitudinal study. Sleep Med. 2016, 25, 139–144. [Google Scholar] [CrossRef]
- Kovalska, P.; Kemlink, D.; Nevsimalova, S.; Maurovich Horvat, E.; Jarolimova, E.; Topinkova, E.; Sonka, K. Narcolepsy with cataplexy in patients aged over 60 years: A case-control study. Sleep Med. 2016, 26, 79–84. [Google Scholar] [CrossRef]
- Donadio, V.; Liguori, R.; Vandi, S.; Pizza, F.; Dauvilliers, Y.; Leta, V.; Giannoccaro, M.P.; Baruzzi, A.; Plazzi, G. Lower wake resting sympathetic and cardiovascular activities in narcolepsy with cataplexy. Neurology 2014, 83, 1080–1086. [Google Scholar] [CrossRef]
- Dauvilliers, Y.; Jaussent, I.; Krams, B.; Scholz, S.; Lado, S.; Levy, P.; Pepin, J.L. Non-dipping blood pressure profile in narcolepsy with cataplexy. PLoS ONE 2012, 7, e38977. [Google Scholar] [CrossRef]
- Poli, F.; Plazzi, G.; Di Dalmazi, G.; Ribichini, D.; Vicennati, V.; Pizza, F.; Mignot, E.; Montagna, P.; Pasquali, R.; Pagotto, U. Body mass index-independent metabolic alterations in narcolepsy with cataplexy. Sleep 2009, 32, 1491–1497. [Google Scholar] [CrossRef]
- Arnulf, I.; Lin, L.; Zhang, J.; Russell, I.J.; Ripley, B.; Einen, M.; Nevsimalova, S.; Bassetti, C.; Bourgin, P.; Nishino, S.; et al. CSF versus serum leptin in narcolepsy: Is there an effect of hypocretin deficiency? Sleep 2006, 29, 1017–1024. [Google Scholar] [CrossRef]
- Dahmen, N.; Bierbrauer, J.; Kasten, M. Increased prevalence of obesity in narcoleptic patients and relatives. Eur. Arch. Psychiatry Clin. Neurosci. 2001, 251, 85–89. [Google Scholar] [CrossRef]
- Ponziani, V.; Gennari, M.; Pizza, F.; Balsamo, A.; Bernardi, F.; Plazzi, G. Growing Up with Type 1 Narcolepsy: Its Anthropometric and Endocrine Features. J. Clin. Sleep Med. 2016, 12, 1649–1657. [Google Scholar] [CrossRef]
- Zhang, M.; Thieux, M.; Inocente, C.O.; Vieux, N.; Arvis, L.; Villanueva, C.; Lin, J.S.; Plancoulaine, S.; Guyon, A.; Franco, P. Characterization of rapid weight gain phenotype in children with narcolepsy. CNS Neurosci. Ther. 2022, 28, 829–841. [Google Scholar] [CrossRef]
- Franco, P.; Dauvilliers, Y.; Inocente, C.O.; Guyon, A.; Villanueva, C.; Raverot, V.; Plancoulaine, S.; Lin, J.S. Impaired histaminergic neurotransmission in children with narcolepsy type 1. CNS Neurosci. Ther. 2019, 25, 386–395. [Google Scholar] [CrossRef]
- Dauvilliers, Y.; Delallee, N.; Jaussent, I.; Scholz, S.; Bayard, S.; Croyal, M.; Schwartz, J.C.; Robert, P. Normal cerebrospinal fluid histamine and tele-methylhistamine levels in hypersomnia conditions. Sleep 2012, 35, 1359–1366. [Google Scholar] [CrossRef] [PubMed]
- Anaclet, C.; Parmentier, R.; Ouk, K.; Guidon, G.; Buda, C.; Sastre, J.P.; Akaoka, H.; Sergeeva, O.A.; Yanagisawa, M.; Ohtsu, H.; et al. Orexin/hypocretin and histamine: Distinct roles in the control of wakefulness demonstrated using knock-out mouse models. J. Neurosci. 2009, 29, 14423–14438. [Google Scholar] [CrossRef] [PubMed]
- Parmentier, R.; Ohtsu, H.; Djebbara-Hannas, Z.; Valatx, J.L.; Watanabe, T.; Lin, J.S. Anatomical, physiological, and pharmacological characteristics of histidine decarboxylase knock-out mice: Evidence for the role of brain histamine in behavioral and sleep-wake control. J. Neurosci. 2002, 22, 7695–7711. [Google Scholar] [CrossRef] [PubMed]
- Schuld, A.; Beitinger, P.A.; Dalal, M.; Geller, F.; Wetter, T.C.; Albert, E.D.; Hebebrand, J.; Pollmacher, T. Increased body mass index (BMI) in male narcoleptic patients, but not in HLA-DR2-positive healthy male volunteers. Sleep Med. 2002, 3, 335–339. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Ueta, Y.; Date, Y.; Nakazato, M.; Hara, Y.; Serino, R.; Nomura, M.; Shibuya, I.; Matsukura, S.; Yamashita, H. Down regulation of the prepro-orexin gene expression in genetically obese mice. Brain Res. Mol. Brain Res. 1999, 65, 14–22. [Google Scholar] [CrossRef]
- Almeneessier, A.S.; Alballa, N.S.; Alsalman, B.H.; Aleissi, S.; Olaish, A.H.; BaHammam, A.S. A 10-Year Longitudinal Observational Study Of Cataplexy In A Cohort Of Narcolepsy Type 1 Patients. Nat. Sci. Sleep 2019, 11, 231–239. [Google Scholar] [CrossRef]
- Cremaschi, R.C.; Hirotsu, C.; Tufik, S.; Coelho, F.M. Narcolepsy type 1 and type 2—A 10-year follow-up: Body mass index and comorbidities. Sleep Med. 2017, 32, 285–286. [Google Scholar] [CrossRef]
- Abulmeaty, M.M.A.; BaHammam, A.S.; Aljuraiban, G.S.; Almajwal, A.M.; Aldosari, M.S. Measured resting metabolic rate, respiratory quotient, and body composition in patients with narcolepsy: A preliminary report of a case-control study. Sci. Rep. 2020, 10, 11024. [Google Scholar] [CrossRef]
- Parmar, A.; Yeh, E.A.; Korczak, D.J.; Weiss, S.K.; Lu, Z.; Zweerink, A.; Toulany, A.; Murray, B.J.; Narang, I. Depressive symptoms, sleep patterns, and physical activity in adolescents with narcolepsy. Sleep 2019, 42, zsz111. [Google Scholar] [CrossRef]
- Chabas, D.; Foulon, C.; Gonzalez, J.; Nasr, M.; Lyon-Caen, O.; Willer, J.C.; Derenne, J.P.; Arnulf, I. Eating disorder and metabolism in narcoleptic patients. Sleep 2007, 30, 1267–1273. [Google Scholar] [CrossRef]
- Fronczek, R.; Overeem, S.; Reijntjes, R.; Lammers, G.J.; van Dijk, J.G.; Pijl, H. Increased heart rate variability but normal resting metabolic rate in hypocretin/orexin-deficient human narcolepsy. J. Clin. Sleep Med. 2008, 4, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Donjacour, C.E.; Aziz, N.A.; Roelfsema, F.; Frolich, M.; Overeem, S.; Lammers, G.J.; Pijl, H. Effect of sodium oxybate on growth hormone secretion in narcolepsy patients and healthy controls. Am. J. Physiol. Endocrinol. Metab. 2011, 300, E1069–E1075. [Google Scholar] [CrossRef] [PubMed]
- Schuld, A.; Hebebrand, J.; Geller, F.; Pollmacher, T. Increased body-mass index in patients with narcolepsy. Lancet 2000, 355, 1274–1275. [Google Scholar] [CrossRef]
- Chang, X.; Suo, L.; Xu, N.; Zhao, Y. Orexin-A Stimulates Insulin Secretion Through the Activation of the OX1 Receptor and Mammalian Target of Rapamycin in Rat Insulinoma Cells. Pancreas 2019, 48, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, T.; Amemiya, A.; Ishii, M.; Matsuzaki, I.; Chemelli, R.M.; Tanaka, H.; Williams, S.C.; Richardson, J.A.; Kozlowski, G.P.; Wilson, S.; et al. Orexins and orexin receptors: A family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 1998, 92, 573–585. [Google Scholar] [CrossRef]
- Yamanaka, A.; Sakurai, T.; Katsumoto, T.; Yanagisawa, M.; Goto, K. Chronic intracerebroventricular administration of orexin-A to rats increases food intake in daytime, but has no effect on body weight. Brain Res. 1999, 849, 248–252. [Google Scholar] [CrossRef]
- Haynes, A.C.; Jackson, B.; Chapman, H.; Tadayyon, M.; Johns, A.; Porter, R.A.; Arch, J.R. A selective orexin-1 receptor antagonist reduces food consumption in male and female rats. Regul. Pept. 2000, 96, 45–51. [Google Scholar] [CrossRef]
- Yamanaka, A.; Beuckmann, C.T.; Willie, J.T.; Hara, J.; Tsujino, N.; Mieda, M.; Tominaga, M.; Yagami, K.; Sugiyama, F.; Goto, K.; et al. Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron 2003, 38, 701–713. [Google Scholar] [CrossRef]
- Sakurai, T. Orexins and orexin receptors: Implication in feeding behavior. Regul. Pept. 1999, 85, 25–30. [Google Scholar] [CrossRef]
- Almeneessier, A.S.; Alzoghaibi, M.; BaHammam, A.A.; Ibrahim, M.G.; Olaish, A.H.; Nashwan, S.Z.; BaHammam, A.S. The effects of diurnal intermittent fasting on the wake-promoting neurotransmitter orexin-A. Ann. Thorac. Med. 2018, 13, 48–54. [Google Scholar]
- Lubkin, M.; Stricker-Krongrad, A. Independent feeding and metabolic actions of orexins in mice. Biochem. Biophys. Res. Commun. 1998, 253, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, Q.; Liu, A.; Lan, X.; Huang, Y.; Zhao, Z.; Jie, H.; Chen, J.; Zhao, Y. Physiological Implications of Orexins/Hypocretins on Energy Metabolism and Adipose Tissue Development. ACS Omega 2020, 5, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Coborn, J.E.; DePorter, D.P.; Mavanji, V.; Sinton, C.M.; Kotz, C.M.; Billington, C.J.; Teske, J.A. Role of orexin-A in the ventrolateral preoptic area on components of total energy expenditure. Int. J. Obes. 2017, 41, 1256–1262. [Google Scholar] [CrossRef]
- Nakamura, M.; Nagamine, T. Neuroendocrine, Autonomic, and Metabolic Responses to an Orexin Antagonist, Suvorexant, in Psychiatric Patients with Insomnia. Innov. Clin. Neurosci. 2017, 14, 30–37. [Google Scholar] [PubMed]
- Yoshikawa, F.; Shigiyama, F.; Ando, Y.; Miyagi, M.; Uchino, H.; Hirose, T.; Kumashiro, N. Chronotherapeutic efficacy of suvorexant on sleep quality and metabolic parameters in patients with type 2 diabetes and insomnia. Diabetes Res. Clin. Pract. 2020, 169, 108412. [Google Scholar] [CrossRef]
- Toi, N.; Inaba, M.; Kurajoh, M.; Morioka, T.; Hayashi, N.; Hirota, T.; Miyaoka, D.; Emoto, M.; Yamada, S. Improvement of glycemic control by treatment for insomnia with suvorexant in type 2 diabetes mellitus. J. Clin. Transl. Endocrinol. 2019, 15, 37–44. [Google Scholar] [CrossRef]
- Morrison, S.F.; Madden, C.J.; Tupone, D. An orexinergic projection from perifornical hypothalamus to raphe pallidus increases rat brown adipose tissue thermogenesis. Adipocyte 2012, 1, 116–120. [Google Scholar] [CrossRef]
- Zink, A.N.; Bunney, P.E.; Holm, A.A.; Billington, C.J.; Kotz, C.M. Neuromodulation of orexin neurons reduces diet-induced adiposity. Int. J. Obes. 2018, 42, 737–745. [Google Scholar] [CrossRef]
- Kok, S.W.; Overeem, S.; Visscher, T.L.; Lammers, G.J.; Seidell, J.C.; Pijl, H.; Meinders, A.E. Hypocretin deficiency in narcoleptic humans is associated with abdominal obesity. Obes. Res. 2003, 11, 1147–1154. [Google Scholar] [CrossRef]
- Pollak, C.P.; Green, J. Eating and its relationships with subjective alertness and sleep in narcoleptic subjects living without temporal cues. Sleep 1990, 13, 467–478. [Google Scholar] [CrossRef][Green Version]
- Joly-Amado, A.; Cansell, C.; Denis, R.G.; Delbes, A.S.; Castel, J.; Martinez, S.; Luquet, S. The hypothalamic arcuate nucleus and the control of peripheral substrates. Best Pract. Res. Clin. Endocrinol. Metab. 2014, 28, 725–737. [Google Scholar] [CrossRef]
- Micioni Di Bonaventura, E.; Botticelli, L.; Tomassoni, D.; Tayebati, S.K.; Micioni Di Bonaventura, M.V.; Cifani, C. The Melanocortin System behind the Dysfunctional Eating Behaviors. Nutrients 2020, 12, 3502. [Google Scholar] [CrossRef]
- Fortuyn, H.A.; Swinkels, S.; Buitelaar, J.; Renier, W.O.; Furer, J.W.; Rijnders, C.A.; Hodiamont, P.P.; Overeem, S. High prevalence of eating disorders in narcolepsy with cataplexy: A case-control study. Sleep 2008, 31, 335–341. [Google Scholar] [CrossRef]
- Dimitrova, A.; Fronczek, R.; Van der Ploeg, J.; Scammell, T.; Gautam, S.; Pascual-Leone, A.; Lammers, G.J. Reward-seeking behavior in human narcolepsy. J. Clin. Sleep. Med. 2011, 7, 293–300. [Google Scholar] [CrossRef]
- van Holst, R.J.; van der Cruijsen, L.; van Mierlo, P.; Lammers, G.J.; Cools, R.; Overeem, S.; Aarts, E. Aberrant Food Choices after Satiation in Human Orexin-Deficient Narcolepsy Type 1. Sleep 2016, 39, 1951–1959. [Google Scholar] [CrossRef]
- Kelly, N.R.; Shomaker, L.B.; Radin, R.M.; Thompson, K.A.; Cassidy, O.L.; Brady, S.; Mehari, R.; Courville, A.B.; Chen, K.Y.; Galescu, O.A.; et al. Associations of sleep duration and quality with disinhibited eating behaviors in adolescent girls at-risk for type 2 diabetes. Eat. Behav. 2016, 22, 149–155. [Google Scholar] [CrossRef]
- van Holst, R.J.; Janssen, L.K.; van Mierlo, P.; Lammers, G.J.; Cools, R.; Overeem, S.; Aarts, E. Enhanced food-related responses in the ventral medial prefrontal cortex in narcolepsy type 1. Sci. Rep. 2018, 8, 16391. [Google Scholar] [CrossRef]
- Mehr, J.B.; Mitchison, D.; Bowrey, H.E.; James, M.H. Sleep dysregulation in binge eating disorder and "food addiction": The orexin (hypocretin) system as a potential neurobiological link. Neuropsychopharmacology 2021, 46, 2051–2061. [Google Scholar] [CrossRef]
- Barson, J.R. Orexin/hypocretin and dysregulated eating: Promotion of foraging behavior. Brain Res. 2020, 1731, 145915. [Google Scholar] [CrossRef]
- Gonzalez, J.A.; Jensen, L.T.; Iordanidou, P.; Strom, M.; Fugger, L.; Burdakov, D. Inhibitory Interplay between Orexin Neurons and Eating. Curr. Biol. 2016, 26, 2486–2491. [Google Scholar] [CrossRef]
- Hara, J.; Beuckmann, C.T.; Nambu, T.; Willie, J.T.; Chemelli, R.M.; Sinton, C.M.; Sugiyama, F.; Yagami, K.; Goto, K.; Yanagisawa, M.; et al. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron 2001, 30, 345–354. [Google Scholar] [CrossRef]
- Dauvilliers, Y.; Montplaisir, J.; Molinari, N.; Carlander, B.; Ondze, B.; Besset, A.; Billiard, M. Age at onset of narcolepsy in two large populations of patients in France and Quebec. Neurology 2001, 57, 2029–2033. [Google Scholar] [CrossRef]
- Dahmen, N.; Becht, J.; Engel, A.; Thommes, M.; Tonn, P. Prevalence of eating disorders and eating attacks in narcolepsy. Neuropsychiatr. Dis. Treat. 2008, 4, 257–261. [Google Scholar]
- Del Bianco, C.; Ulivi, M.; Liguori, C.; Pisani, A.; Mercuri, N.B.; Placidi, F.; Izzi, F. Alexithymia, impulsiveness, emotion, and eating dyscontrol: Similarities and differences between narcolepsy type 1 and type 2. Sleep Biol. Rhythms. 2022. [Google Scholar] [CrossRef]
- Alzoghaibi, M.A.; Pandi-Perumal, S.R.; Sharif, M.M.; BaHammam, A.S. Diurnal intermittent fasting during Ramadan: The effects on leptin and ghrelin levels. PLoS ONE 2014, 9, e92214. [Google Scholar] [CrossRef]
- Villano, I.; La Marra, M.; Di Maio, G.; Monda, V.; Chieffi, S.; Guatteo, E.; Messina, G.; Moscatelli, F.; Monda, M.; Messina, A. Physiological Role of Orexinergic System for Health. Int. J. Environ. Res. Public Health 2022, 19, 8353. [Google Scholar] [CrossRef]
- Dahmen, N.; Engel, A.; Helfrich, J.; Manderscheid, N.; Lobig, M.; Forst, T.; Pfutzner, A.; Tonn, P. Peripheral leptin levels in narcoleptic patients. Diabetes Technol. Ther. 2007, 9, 348–353. [Google Scholar] [CrossRef]
- Donjacour, C.E.; Pardi, D.; Aziz, N.A.; Frolich, M.; Roelfsema, F.; Overeem, S.; Pijl, H.; Lammers, G.J. Plasma total ghrelin and leptin levels in human narcolepsy and matched healthy controls: Basal concentrations and response to sodium oxybate. J. Clin. Sleep Med. 2013, 9, 797–803. [Google Scholar] [CrossRef][Green Version]
- Heier, M.S.; Jansson, T.S.; Gautvik, K.M. Cerebrospinal fluid hypocretin 1 deficiency, overweight, and metabolic dysregulation in patients with narcolepsy. J. Clin. Sleep Med. 2011, 7, 653–658. [Google Scholar] [CrossRef]
- Huda, M.S.; Mani, H.; Durham, B.H.; Dovey, T.M.; Halford, J.C.; Aditya, B.S.; Pinkney, J.H.; Wilding, J.P.; Hart, I.K. Plasma obestatin and autonomic function are altered in orexin-deficient narcolepsy, but ghrelin is unchanged. Endocrine 2013, 43, 696–704. [Google Scholar] [CrossRef]
- Kok, S.W.; Meinders, A.E.; Overeem, S.; Lammers, G.J.; Roelfsema, F.; Frolich, M.; Pijl, H. Reduction of plasma leptin levels and loss of its circadian rhythmicity in hypocretin (orexin)-deficient narcoleptic humans. J. Clin. Endocrinol. Metab. 2002, 87, 805–809. [Google Scholar] [CrossRef][Green Version]
- Nishino, S.; Ripley, B.; Overeem, S.; Nevsimalova, S.; Lammers, G.J.; Vankova, J.; Okun, M.; Rogers, W.; Brooks, S.; Mignot, E. Low cerebrospinal fluid hypocretin (Orexin) and altered energy homeostasis in human narcolepsy. Ann. Neurol. 2001, 50, 381–388. [Google Scholar] [CrossRef]
- Tsuneki, H.; Tokai, E.; Nakamura, Y.; Takahashi, K.; Fujita, M.; Asaoka, T.; Kon, K.; Anzawa, Y.; Wada, T.; Takasaki, I.; et al. Hypothalamic orexin prevents hepatic insulin resistance via daily bidirectional regulation of autonomic nervous system in mice. Diabetes 2015, 64, 459–470. [Google Scholar] [CrossRef]
- Tsuneki, H.; Murata, S.; Anzawa, Y.; Soeda, Y.; Tokai, E.; Wada, T.; Kimura, I.; Yanagisawa, M.; Sakurai, T.; Sasaoka, T. Age-related insulin resistance in hypothalamus and peripheral tissues of orexin knockout mice. Diabetologia 2008, 51, 657–667. [Google Scholar] [CrossRef]
- Shiuchi, T.; Haque, M.S.; Okamoto, S.; Inoue, T.; Kageyama, H.; Lee, S.; Toda, C.; Suzuki, A.; Bachman, E.S.; Kim, Y.B.; et al. Hypothalamic orexin stimulates feeding-associated glucose utilization in skeletal muscle via sympathetic nervous system. Cell Metab. 2009, 10, 466–480. [Google Scholar] [CrossRef]
- Engel, A.; Helfrich, J.; Manderscheid, N.; Musholt, P.B.; Forst, T.; Pfutzner, A.; Dahmen, N. Investigation of insulin resistance in narcoleptic patients: Dependent or independent of body mass index? Neuropsychiatr. Dis. Treat. 2011, 7, 351–356. [Google Scholar] [CrossRef]
- Dergacheva, O.; Philbin, K.; Bateman, R.; Mendelowitz, D. Hypocretin-1 (orexin A) prevents the effects of hypoxia/hypercapnia and enhances the GABAergic pathway from the lateral paragigantocellular nucleus to cardiac vagal neurons in the nucleus ambiguus. Neuroscience 2011, 175, 18–23. [Google Scholar] [CrossRef][Green Version]
- Zhang, W.; Sakurai, T.; Fukuda, Y.; Kuwaki, T. Orexin neuron-mediated skeletal muscle vasodilation and shift of baroreflex during defense response in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 290, R1654–R1663. [Google Scholar] [CrossRef]
- Schwimmer, H.; Stauss, H.M.; Abboud, F.; Nishino, S.; Mignot, E.; Zeitzer, J.M. Effects of sleep on the cardiovascular and thermoregulatory systems: A possible role for hypocretins. J. Appl. Physiol. 2010, 109, 1053–1063. [Google Scholar] [CrossRef][Green Version]
- Sorensen, G.L.; Knudsen, S.; Petersen, E.R.; Kempfner, J.; Gammeltoft, S.; Sorensen, H.B.; Jennum, P. Attenuated heart rate response is associated with hypocretin deficiency in patients with narcolepsy. Sleep 2013, 36, 91–98. [Google Scholar] [CrossRef]
- Bastianini, S.; Silvani, A.; Berteotti, C.; Elghozi, J.L.; Franzini, C.; Lenzi, P.; Lo Martire, V.; Zoccoli, G. Sleep related changes in blood pressure in hypocretin-deficient narcoleptic mice. Sleep 2011, 34, 213–218. [Google Scholar] [CrossRef]
- Grimaldi, D.; Calandra-Buonaura, G.; Provini, F.; Agati, P.; Pierangeli, G.; Franceschini, C.; Barletta, G.; Plazzi, G.; Montagna, P.; Cortelli, P. Abnormal sleep-cardiovascular system interaction in narcolepsy with cataplexy: Effects of hypocretin deficiency in humans. Sleep 2012, 35, 519–528. [Google Scholar] [CrossRef]
- Lammers, G.J.; Pijl, H.; Iestra, J.; Langius, J.A.; Buunk, G.; Meinders, A.E. Spontaneous food choice in narcolepsy. Sleep 1996, 19, 75–76. [Google Scholar] [CrossRef]
- Husain, A.M.; Yancy, W.S., Jr.; Carwile, S.T.; Miller, P.P.; Westman, E.C. Diet therapy for narcolepsy. Neurology 2004, 62, 2300–2302. [Google Scholar] [CrossRef]
- Moriguchi, T.; Sakurai, T.; Nambu, T.; Yanagisawa, M.; Goto, K. Neurons containing orexin in the lateral hypothalamic area of the adult rat brain are activated by insulin-induced acute hypoglycemia. Neurosci. Lett. 1999, 264, 101–104. [Google Scholar] [CrossRef]
- Tabuchi, S.; Tsunematsu, T.; Black, S.W.; Tominaga, M.; Maruyama, M.; Takagi, K.; Minokoshi, Y.; Sakurai, T.; Kilduff, T.S.; Yamanaka, A. Conditional ablation of orexin/hypocretin neurons: A new mouse model for the study of narcolepsy and orexin system function. J. Neurosci. 2014, 34, 6495–6509. [Google Scholar] [CrossRef]
- Hara, J.; Yanagisawa, M.; Sakurai, T. Difference in obesity phenotype between orexin-knockout mice and orexin neuron-deficient mice with same genetic background and environmental conditions. Neurosci. Lett. 2005, 380, 239–242. [Google Scholar] [CrossRef]
- Bornstein, A.; Hedstrom, A.; Wasling, P. Actigraphy measurement of physical activity and energy expenditure in narcolepsy type 1, narcolepsy type 2 and idiopathic hypersomnia: A Sensewear Armband study. J. Sleep Res. 2021, 30, e13038. [Google Scholar] [CrossRef]
- Filardi, M.; Pizza, F.; Antelmi, E.; Pillastrini, P.; Natale, V.; Plazzi, G. Physical Activity and Sleep/Wake Behavior, Anthropometric, and Metabolic Profile in Pediatric Narcolepsy Type 1. Front. Neurol. 2018, 9, 707. [Google Scholar] [CrossRef]
- Dauvilliers, Y.; Barateau, L.; Middleton, B.; van der Veen, D.R.; Skene, D.J. Metabolomics Signature of Patients With Narcolepsy. Neurology 2022, 98, e493–e505. [Google Scholar] [CrossRef]
- Sellayah, D.; Bharaj, P.; Sikder, D. Orexin is required for brown adipose tissue development, differentiation, and function. Cell Metab. 2011, 14, 478–490. [Google Scholar] [CrossRef] [PubMed]
- Kakizaki, M.; Tsuneoka, Y.; Takase, K.; Kim, S.J.; Choi, J.; Ikkyu, A.; Abe, M.; Sakimura, K.; Yanagisawa, M.; Funato, H. Differential Roles of Each Orexin Receptor Signaling in Obesity. iScience 2019, 20, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Haugen, H.A.; Chan, L.N.; Li, F. Indirect calorimetry: A practical guide for clinicians. Nutr. Clin. Pract. 2007, 22, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Compher, C.; Frankenfield, D.; Keim, N.; Roth-Yousey, L. Best practice methods to apply to measurement of resting metabolic rate in adults: A systematic review. J. Am. Diet. Assoc. 2006, 106, 881–903. [Google Scholar] [CrossRef]
- Dahmen, N.; Tonn, P.; Messroghli, L.; Ghezel-Ahmadi, D.; Engel, A. Basal metabolic rate in narcoleptic patients. Sleep 2009, 32, 962–964. [Google Scholar]
- Daniels, L.E. Narcolepsy. Medicine 1934, 13, 1–122. [Google Scholar] [CrossRef]
- Ellis, A.C.; Hyatt, T.C.; Hunter, G.R.; Gower, B.A. Respiratory quotient predicts fat mass gain in premenopausal women. Obesity 2010, 18, 2255–2259. [Google Scholar] [CrossRef]
- Ponziani, V.; Pizza, F.; Zenesini, C.; Vignatelli, L.; Pession, A.; Plazzi, G. BMI changes in pediatric type 1 narcolepsy under sodium oxybate treatment. Sleep 2021, 44, zsaa295. [Google Scholar] [CrossRef]
- Husain, A.M.; Ristanovic, R.K.; Bogan, R.K. Weight loss in narcolepsy patients treated with sodium oxybate. Sleep Med. 2009, 10, 661–663. [Google Scholar] [CrossRef]
- Aguilar, A.C.; Frange, C.; Pimentel Filho, L.H.; Reis, M.J.; Tufik, S.; Coelho, F.M.S. Lisdexamfetamine to improve excessive daytime sleepiness and weight management in narcolepsy: A case series. Braz. J. Psychiatry 2020, 42, 314–316. [Google Scholar] [CrossRef]
- Romigi, A.; Vitrani, G.; Lo Giudice, T.; Centonze, D.; Franco, V. Profile of pitolisant in the management of narcolepsy: Design, development, and place in therapy. Drug Des. Devel. Ther. 2018, 12, 2665–2675. [Google Scholar] [CrossRef] [PubMed]
- Kotanska, M.; Kuder, K.J.; Szczepanska, K.; Sapa, J.; Kiec-Kononowicz, K. The histamine H3 receptor inverse agonist pitolisant reduces body weight in obese mice. Naunyn Schmiedebergs Arch. Pharmacol. 2018, 391, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Funato, H.; Tsai, A.L.; Willie, J.T.; Kisanuki, Y.; Williams, S.C.; Sakurai, T.; Yanagisawa, M. Enhanced orexin receptor-2 signaling prevents diet-induced obesity and improves leptin sensitivity. Cell Metab. 2009, 9, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, T.; Hara, H.; Kawano, A.; Kimura, H. Danavorexton, a selective orexin 2 receptor agonist, provides a symptomatic improvement in a narcolepsy mouse model. Pharmacol. Biochem. Behav. 2022, 220, 173464. [Google Scholar] [CrossRef]
| Study/Year Country | Study Design | No of Cases | Prevalence of Overweight (OW), Obesity (OB)%, or Mean BMI ± SD in Cases | No of Controls | Prevalence of Overweight (OW), Obesity (OB)%, or Mean BMI ± SD in Controls | p-Value |
|---|---|---|---|---|---|---|
| Filardi [19], 2020, Italy | Case-control | 38 | OW 28.95 OB 39.47 | 21 | OW 4.76 OB 28.57 | <0.05 |
| Barateau [20], 2019, France | Case-control | 92 | OW 38.04 OB 23.91 | 109 | OW 27.52 OB 4.59 | <0.000 |
| Vandi [21], 2019, Italy | Case-control | 27 | OW 62.96 OB 18.5 | 19 | OW 21.1 OB 21.1 | 0.010 |
| Drissi [22], 2018, Sweden | Case-control | 19 | 24.72 ± 6.37 | 17 | 21.22 ± 2.42 | 0.039 |
| Wang [23], 2016, China | Case-control | 65 | OW 26.15 OB 38.46 | 79 | OW 5.06 OB 3.80 | 0.002 <0.001 |
| Kovalska [24], 2016, Czech Republic | Case-control | 42 | 31.47 ± 5.41 | 46 | 27.53 ± 6.11 | 0.0021 |
| Donadio [25], 2014, Italy | Case-control | 19 | 25 ± 4 | 19 | 23 ± 3 | <0.05 |
| Dauvillers [26], 2012, France | Case-control | 50 | BMI 25.11 [17.01–38.30] | 42 | BMI 21.40 [18.30–29.10] | 0.0001 |
| Poli [27],2009, Italy | Case-control | 14 | 28 ± 4.4 | 14 | 24.2 ± 2.8 | 0.012 |
| Arnulf [28], 2006, France | Case-control | 93 | 27.6 ± 0.6 | 111 | 25.0 ± 0.4 | <0.05 |
| Dahmen [29], 2001, Germany | Case-control | 132 | 28.2 ± 5.5 | 104 | 24.5 ± 4.7 | <0.0001 |
| Study/Year Country | Study Design | No of Cases | Mean Age of Cases | No of Controls | Mean Age of Controls | BMR/RMR |
|---|---|---|---|---|---|---|
| Abulmeaty [40], 2020, Saudi Arabia | Case-control | 7 | 25.29 | 14 | 27.86 | RMR; No significant difference |
| Wang [23], 2016, China | Case-control | 18 | 13.75 | 16 | 14.03 | BMR; Less by 25% in cases at 6 months follow up/normal at 36 months |
| Dahmen [107], 2009, Germany | Case-control | 13 | 36.54 | 30 | 36.37 | BMR; No significant difference. Lower BMR was detected in non-obese cases compared with controls |
| Fronczek [43], 2008, The Netherlands | Case-control | 15 | 36.3 | 15 | 35.6 | RMR; No significant difference |
| Chabas [42], 2007, France | Case-control | 13 | 26.25 | 9 | 29 | RMR was less in cases compared with controls |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhafar, H.O.; BaHammam, A.S. Body Weight and Metabolic Rate Changes in Narcolepsy: Current Knowledge and Future Directions. Metabolites 2022, 12, 1120. https://doi.org/10.3390/metabo12111120
Dhafar HO, BaHammam AS. Body Weight and Metabolic Rate Changes in Narcolepsy: Current Knowledge and Future Directions. Metabolites. 2022; 12(11):1120. https://doi.org/10.3390/metabo12111120
Chicago/Turabian StyleDhafar, Hamza O., and Ahmed S. BaHammam. 2022. "Body Weight and Metabolic Rate Changes in Narcolepsy: Current Knowledge and Future Directions" Metabolites 12, no. 11: 1120. https://doi.org/10.3390/metabo12111120
APA StyleDhafar, H. O., & BaHammam, A. S. (2022). Body Weight and Metabolic Rate Changes in Narcolepsy: Current Knowledge and Future Directions. Metabolites, 12(11), 1120. https://doi.org/10.3390/metabo12111120






