Relationship between Insulin Resistance Risk Scales and Non-Alcoholic Fatty Liver Disease and Liver Fibrosis Scales in 219,477 Spanish Workers
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Measurements and Data Collection
- -
- TyG index [15]: triglyceride–glucose index.
- -
- Metabolic score-insulin resistance (METS-IR) [16]
- -
- Triglycerides/HDL-c
- -
- Fatty liver index (FLI) [17]
- -
- Hepatic steatosis index (HSI) [18]
- -
- Zhejian University index (ZJU index) [19]
- -
- Fatty liver disease index (FLD) [20]
- -
- Framingham steatosis index (FSI) [21]
- -
- Lipid accumulation product (LAP) [22]
- -
- In men:
- -
- In women:
- -
- BARD scoring [23]
2.3. Statistical Analysis
2.4. Ethical Considerations and/or Aspects
3. Results
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yaribeygi, H.; Farrokhi, F.R.; Butler, A.E.; Sahebkar, A. Insulin resistance: Review of the underlying molecular mechanisms. J. Cell Physiol. 2019, 234, 8152–8161. [Google Scholar] [CrossRef] [PubMed]
- Mastrototaro, L.; Roden, M. Insulin resistance and insulin sensitizing agents. Metabolism 2021, 125, 154892. [Google Scholar] [CrossRef] [PubMed]
- Tanase, D.M.; Gosav, E.M.; Costea, C.F.; Ciocoiu, M.; Lacatusu, C.M.; Maranduca, M.A.; Ouatu, A.; Floria, M. The Intricate Relationship between Type 2 Diabetes Mellitus (T2DM), Insulin Resistance (IR), and Nonalcoholic Fatty Liver Disease (NAFLD). J. Diabetes Res. 2020, 31, 3920196. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.J.; Tao, M.F.; Li, H.P.; Zhao, F.; Wang, F.H. The relationship between patterns of insulin secretion and risks of gestational diabetes mellitus. Int. J. Gynaecol. Obstet. 2020, 150, 318–323. [Google Scholar] [CrossRef]
- Bovolini, A.; Garcia, J.; Andrade, M.A.; Duarte, J.A. Metabolic Syndrome Pathophysiology and Predisposing Factors. Int. J. Sports Med. 2021, 42, 199–214. [Google Scholar] [CrossRef]
- Cifarelli, V.; Appak-Baskoy, S.; Peche, V.S.; Kluzak, A.; Shew, T.; Narendran, R.; Pietka, K.M.; Cella, M.; Walls, C.W.; Czepielewski, R.; et al. Visceral obesity and insulin resistance associate with CD36 deletion in lymphatic endothelial cells. Nat. Commun. 2021, 7, 3350. [Google Scholar] [CrossRef]
- Heeren, J.; Scheja, L. Metabolic-associated fatty liver disease and lipoprotein metabolism. Mol. Metab. 2021, 50, 101238. [Google Scholar] [CrossRef]
- Da Silva, A.A.; do Carmo, J.M.; Li, X.; Wang, Z.; Mouton, A.J.; Hall, J.E. Role of Hyperinsulinemia and Insulin Resistance in Hypertension: Metabolic Syndrome Revisited. Can. J. Cardiol. 2020, 36, 671–682. [Google Scholar] [CrossRef]
- Park, S.Y.; Gautier, J.F.; Chon, S. Assessment of Insulin Secretion and Insulin Resistance in Human. Diabetes Metab. J. 2021, 45, 641–654. [Google Scholar] [CrossRef]
- Fujii, H.; Kawada, N. Japan Study Group Of Nafld Jsg-Nafld. The Role of Insulin Resistance and Diabetes in Nonalcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2020, 29, 3863. [Google Scholar] [CrossRef]
- Roehlen, N.; Crouchet, E.; Baumert, T.F. Liver Fibrosis: Mechanistic Concepts and Therapeutic Perspectives. Cells 2020, 3, 875. [Google Scholar] [CrossRef] [PubMed]
- Busquets-Cortés, C.; Bennasar-Veny, M.; López-González, A.A.; Fresneda, S.; Aguiló, A.; Yanez, A. Fatty liver index and progression to type 2 diabetes: A 5-year longitudinal study in Spanish workers with pre-diabetes. BMJ Open. 2021, 25, e045498. [Google Scholar] [CrossRef] [PubMed]
- Pimpin, L.; Cortez-Pinto, H.; Negro, F.; Corbould, E.; Lazarus, J.V.; Webber, L.; Sheron, N. EASL HEPAHEALTH Steering Committee. Burden of liver disease in Europe: Epidemiology and analysis of risk factors to identify prevention policies. J. Hepatol. 2018, 69, 718–735. [Google Scholar] [CrossRef] [PubMed]
- Gibson, S.; Ashwell, M. A simple cut-off for waist-to-height ratio (0·5) can act as an indicator for cardiometabolic risk: Recent data from adults in the Health Survey for England. Br. J. Nutr. 2020, 28, 681–690. [Google Scholar] [CrossRef]
- Tutunchi, H.; Naeini, F.; Mobasseri, M.; Ostadrahimi, A. Triglyceride glucose (TyG) index and the progression of liver fibrosis: A cross-sectional study. Clin. Nutr. ESPEN 2021, 44, 483–487. [Google Scholar] [CrossRef]
- Wang, P.; Li, Q.; Guo, X.; Zhou, Y.; Li, Z.; Yang, H.; Yu, S.; Sun, G.; Zheng, L.; Sun, Y.; et al. Usefulness of metabolic score for insulin resistance index in estimating the risk of mildly reduced estimate glomerular filtration rate: A cross-sectional study of rural population in China. BMJ Open 2021, 16, e050907. [Google Scholar] [CrossRef]
- Zou, B.; Yeo, Y.H.; Cheung, R.; Ingelsson, E.; Nguyen, M.H. Fatty Liver Index and Development of Cardiovascular Disease: Findings from the UK Biobank. Dig. Dis. Sci. 2021, 66, 2092–2100. [Google Scholar] [CrossRef]
- Chang, J.W.; Lee, H.W.; Kim, B.K.; Park, J.Y.; Ahn, S.H.; Han, K.H.; Kim, S.U. Hepatic Steatosis Index in the Detection of Fatty Liver in Patients with Chronic Hepatitis B Receiving Antiviral Therapy. Gut Liver 2021, 15, 117–127. [Google Scholar] [CrossRef]
- Shi, M.; Liu, P.; Li, J.; Su, Y.; Zhou, X.; Wu, C.; Chen, X.; Zheng, C. The performance of noninvasive indexes of adults in identification of nonalcoholic fatty liver disease in children. J. Diabetes 2021, 13, 744–753. [Google Scholar] [CrossRef]
- Lee, I.; Cho, J.; Park, J.; Kang, H. Association of hand-grip strength and non-alcoholic fatty liver disease index in older adults. J. Exerc. Nutr. Biochem. 2018, 22, 62–68. [Google Scholar] [CrossRef]
- Jung, T.Y.; Kim, M.S.; Hong, H.P.; Kang, K.A.; Jun, D.W. Comparative Assessment and External Validation of Hepatic Steatosis Formulae in a Community-Based Setting. J. Clin. Med. 2020, 9, 2851. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, R.; Rebekah, G.; Jose, A.; Inbakumari, M.P.; Finney, G.; Thomas, N. Lipid accumulation product (LAP) as a potential index to predict risk of insulin resistance in young, non-obese Asian Indian males from Southern India: Observations from hyperinsulinemic-euglycemic clamp studies. BMJ Open Diabetes Res. Care. 2021, 9, e002414. [Google Scholar] [CrossRef]
- Soresi, M.; Cabibi, D.; Giglio, R.V.; Martorana, S.; Guercio, G.; Porcasi, R.; Terranova, A.; Lazzaro, L.A.; Emma, M.R.; Augello, G.; et al. The Prevalence of NAFLD and Fibrosis in Bariatric Surgery Patients and the Reliability of Noninvasive Diagnostic Methods. Biomed. Res. Int. 2020, 2020, 5023157. [Google Scholar] [CrossRef] [PubMed]
- Ibagon, H.; Tarquino, P.; Barajas-Gamboa, J.S. Are The Risk Scales a Useful Tool In Hospital Services? BJMP 2018, 11, a1111. [Google Scholar]
- Marušić, M.; Paić, M.; Knobloch, M.; Liberati Pršo, A.M. NAFLD, Insulin Resistance, and Diabetes Mellitus Type 2. Can. J. Gastroenterol. Hepatol. 2021, 2021, 6613827. [Google Scholar] [CrossRef] [PubMed]
- Popa, S.G.; Simion, A.M.; Soare, M.; Arcomita, D. Insulin resistance and hepatic steatosis in type 1 diabetes mellitus and their association with diabetic chronic complications. Minerva Endocrinol. 2020. [CrossRef] [PubMed]
- Petta, S.; Ciresi, A.; Bianco, J.; Geraci, V.; Boemi, R.; Galvano, L.; Magliozzo, F.; Merlino, G.; Craxì, A.; Giordano, C. Insulin resistance and hyperandrogenism drive steatosis and fibrosis risk in young females with PCOS. PLoS ONE 2017, 12, e0186136. [Google Scholar] [CrossRef] [PubMed]
- Bullon-Vela, V.; Abete, I.; Tur, J.A.; Konieczna, J.; Romaguera, D.; Pinto, X.; Corbella, E.; Martinez-Gonzalez, M.A.; Sayon-Orea, C.; Toledo, E.; et al. Relationship of visceral adipose tissue with surrogate insulin resistance and liver markers in individuals with metabolic syndrome chronic complications. Ther. Adv. Endocrinol. Metab. 2020, 11, 2042018820958298. [Google Scholar] [CrossRef]
- Ji, B.; Qu, H.; Wang, H.; Wei, H.; Deng, H. The ZJU index: A useful indicator for recognizing insulin resistance in the Chinese general population. Acta Diabetol. 2016, 53, 817–823. [Google Scholar] [CrossRef]
- Chun, H.S.; Lee, J.S.; Lee, H.W.; Kim, B.K.; Park, J.Y.; Kim, D.Y.; Ahn, S.H.; Lee, Y.H.; Kim, Y.D.; Kim, S.U. Association between the severity of liver fibrosis and cardiovascular outcomes in patients with type 2 diabetes. J. Gastroenterol. Hepatol. 2021, 36, 1703–1713. [Google Scholar] [CrossRef]

| Men n = 125.403 | Women n = 94.074 | ||
|---|---|---|---|
| Mean (SD) | Mean (SD) | p-Value | |
| Age | 41.8 (10.5) | 39.9 (10.5) | <0.0001 |
| Height | 175.2 (6.8) | 162.3 (6.3) | <0.0001 |
| Weight | 82.6 (15.0) | 68.0 (14.7) | <0.0001 |
| SBP | 126.1 (15.6) | 115.4 (15.5) | <0.0001 |
| DBP | 77.3 (11.1) | 72.3 (10.5) | <0.0001 |
| Cholesterol | 195.6 (37.9) | 192.1 (35.5) | <0.001 |
| HDL-c | 52.1 (9.8) | 57.2 (10.3) | <0.0001 |
| LDL-c | 118.4 (35.1) | 116.3 (33.5) | <0.001 |
| Triglycerides | 125.7 (76.0) | 93.1 (45.6) | <0.0001 |
| Glycaemia | 93.4 (21.5) | 88.3 (16.0) | <0.0001 |
| AST | 29.0 (17.5) | 18.7 (11.6) | <0.0001 |
| ALT | 24.4 (13.3) | 18.2 (7.9) | <0.0001 |
| GGT | 32.7 (31.8) | 18.8 (16.3) | <0.0001 |
| Creatinine | 0.86 (0.17) | 0.68 (0.14) | <0.0001 |
| % | % | p-value | |
| 18–29 years | 14.4 | 19.4 | <0.0001 |
| 30–39 years | 26.6 | 28.9 | |
| 40–49 years | 33.6 | 32.0 | |
| 50–59 years | 21.5 | 16.8 | |
| 60–69 years | 3.9 | 2.9 | |
| Social class I | 6.1 | 7.5 | <0.0001 |
| Social class II | 14.5 | 20.5 | |
| Social class III | 79.4 | 72.0 | |
| Non-smokers | 67.5 | 66.7 | <0.001 |
| Smokers | 32.5 | 33.3 | |
| Obesity I | 15.4 | 12.7 | <0.001 |
| Obesity II | 3.9 | 4.6 | |
| Obesity III | 1.2 | 1.8 |
| FLI | HSI | ZJU | FLD | FSI | LAP | BARD | ||
|---|---|---|---|---|---|---|---|---|
| Men | n | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) |
| TG/HDL-c normal | 78,591 | 28.8 (21.7) | 35.4 (6.2) | 35.4 (4.9) | 30.4 (4.7) | 0.1 (0.1) | 21.0 (12.3) | 0.7 (0.8) |
| TG/HDL-c high | 46,812 | 57.9 (24.5) | 39.2 (6.9) | 40.1 (5.7) | 34.8 (5.3) | 0.3 (0.2) | 55.0 (35.8) | 1.9 (1.0) |
| TyG index normal | 90,306 | 31.1 (22.7) | 35.7 (6.3) | 35.6 (4.9) | 30.7 (4.8) | 0.1 (0.1) | 22.9 (13.7) | 0.8 (0.8) |
| TyG index high | 35,097 | 61.8 (23.5) | 39.7 (6.9) | 41.0 (5.7) | 35.4 (5.4) | 0.4 (0.2) | 61.3 (38.3) | 2.1 (1.0) |
| METS-IR normal | 112,656 | 34.1 (22.8) | 35.6 (5.9) | 35.9 (4.5) | 30.9 (4.3) | 0.2 (0.1) | 28.6 (22.2) | 1.0 (1.0) |
| METS-IR high | 12,747 | 81.5 (14.1) | 45.6 (6.3) | 46.2 (5.3) | 40.6 (5.0) | 0.5 (0.2) | 71.4 (43.1) | 2.4 (0.9) |
| Women | n | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) |
| TG/HDL-c normal | 81,396 | 15.8 (19.1) | 35.6 (6.5) | 36.1 (5.6) | 29.3 (5.4) | 0.1 (0.1) | 15.4 (11.7) | 0.48 (0.67) |
| TG/HDL-c high | 12,678 | 42.7 (27.9) | 40.8 (7.6) | 42.0 (6.8) | 34.9 (6.5) | 0.3 (0.2) | 44.2 (29.5) | 1.65 (0.98) |
| TyG index normal | 81,315 | 15.8 (19.2) | 35.6 (6.5) | 36.0 (5.5) | 29.3 (5.4) | 0.1 (0.1) | 15.4 (11.8) | 0.5 (0.7) |
| TyG index high | 12,759 | 42.7 (27.8) | 40.8 (7.7) | 42.4 (6.8) | 34.9 (6.5) | 0.3 (0.2) | 44.1 (29.4) | 1.7 (1.0) |
| METS-IR normal | 87,048 | 18.6 (21.1) | 36.1 (6.6) | 36.7 (5.8) | 29.8 (5.5) | 0.1 (0.1) | 18.6 (17.0) | 0.6 (0.8) |
| METS-IR high | 7026 | 91.6 (7.9) | 55.5 (5.5) | 56.2 (5.0) | 48.7 (4.7) | 0.7 (0.2) | 72.5 (34.5) | 1.8 (0.9) |
| FLI High | HSI High | ZJU High | FLD High | LAP High | BARD High | ||
|---|---|---|---|---|---|---|---|
| Men | n | % | % | % | % | % | % |
| TG/HDL normal | 78,591 | 11.1 | 39.7 | 24.8 | 60.2 | 19.4 | 16.5 |
| TG/HDL high | 46,812 | 49.2 | 66.0 | 60.9 | 63.0 | 78.2 | 62.8 |
| TyG index normal | 90,306 | 13.5 | 42.0 | 26.7 | 61.3 | 24.7 | 18.5 |
| TyG index high | 35,097 | 55.7 | 68.7 | 68.0 | 61.2 | 84.2 | 73.0 |
| METS-IR normal | 112,656 | 17.2 | 43.9 | 31.3 | 7.3 | 35.1 | 27.9 |
| METS-IR high | 12,747 | 96.9 | 99.2 | 100.0 | 67.4 | 96.3 | 85.7 |
| Women | n | % | % | % | % | % | % |
| TG/HDL normal | 81,396 | 5.3 | 40.4 | 30.1 | 43.1 | 20.6 | 8.0 |
| TG/HDL high | 12,678 | 29.7 | 72.3 | 69.9 | 53.3 | 77.2 | 55.3 |
| TyG index normal | 81,315 | 5.3 | 40.4 | 29.7 | 43.1 | 20.6 | 7.6 |
| TyG index high | 12,759 | 29.2 | 72.3 | 72.1 | 53.4 | 77.0 | 57.6 |
| METS-IR normal | 87,048 | 2.3 | 40.3 | 30.3 | 1.6 | 22.7 | 11.4 |
| METS-IR high | 7026 | 85.8 | 99.9 | 100.0 | 48.0 | 96.2 | 50.9 |
| FLI High | HSI High | ZJU High | FLD High | LAP High | BARD High | |
|---|---|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| TG/HDL normal | 1 | 1 | 1 | 1 | 1 | 1 |
| TG/HDL high | 3.95 (3.79–4.11) | 1.67 (1.63–1.72) | 1.63 (1.58–1.68) | 2.37 (2.30–2.45) | 5.40 (5.24–5.56) | 3.46 (3.35–3.57) |
| TyG index normal | 1 | 1 | 1 | 1 | 1 | 1 |
| TyG index high | 2.86 (2.74–2.98) | 1.63 (1.57–1.68) | 3.07 (2.97–3.18) | 1.35 (1.30–1.39) | 4.33 (4.18–4.48) | 5.07 (4.90–5.23) |
| METS-IR normal | 1 | 1 | 1 | 1 | 1 | 1 |
| METS-IR high | 32.35 (31.10–33.61) | 18.12 (17.70–18.54) | 7.95 (7.78–8.13) | 22.13 (21.94–22.32) | 42.20 (39.10–45.56) | 5.73 (5.51–5.95) |
| FLI High | HSI High | FLD High | LAP High | BARD High | |
|---|---|---|---|---|---|
| AUC (95% CI) | AUC (95% CI) | AUC (95% CI) | AUC (95% CI) | AUC (95% CI) | |
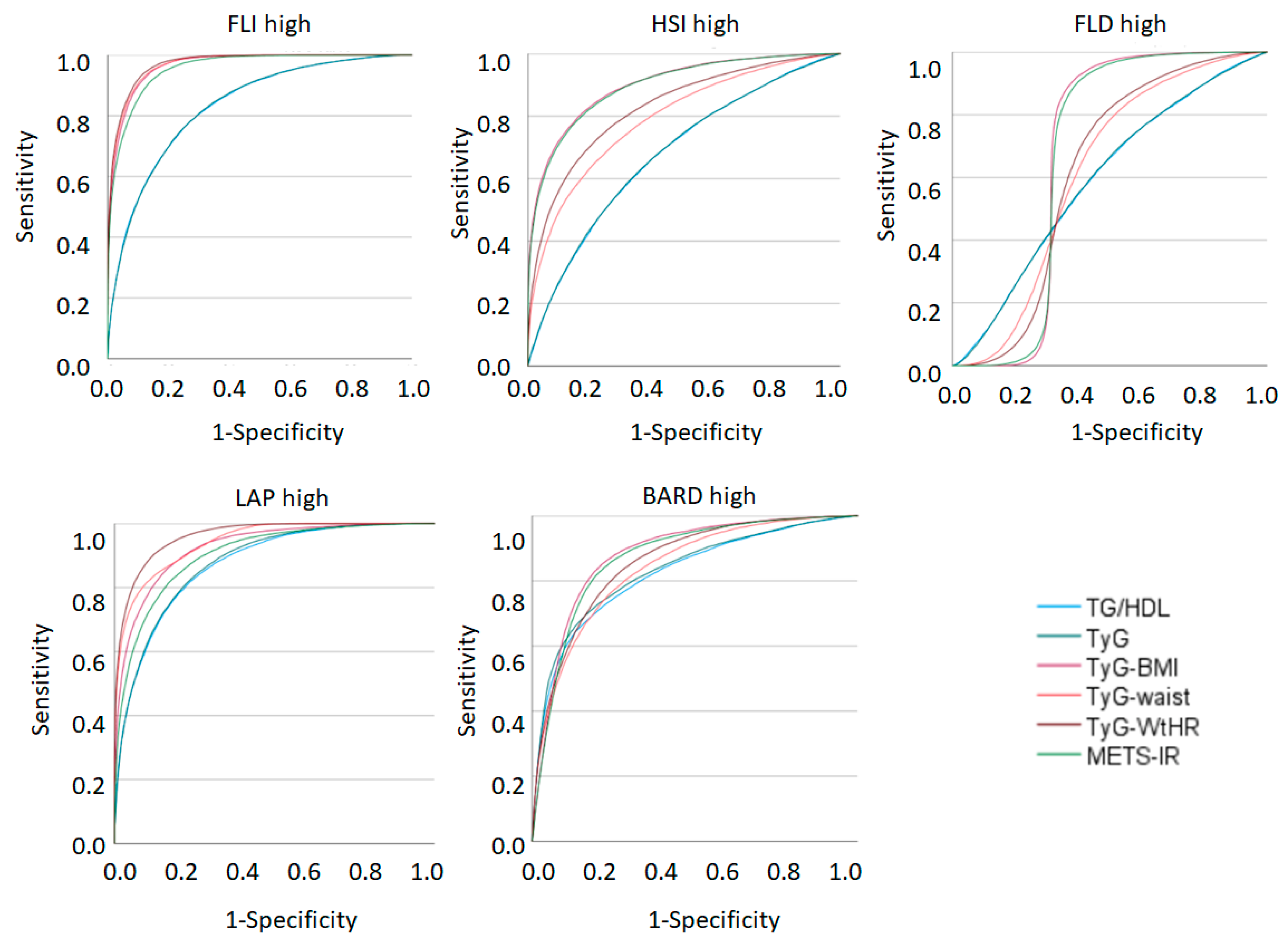
| TG/HDL | 0.832 (0.830–0.835) | 0.678 (0.676–0.680) | 0.592 (0.590–0.595) | 0.874 (0.872–0.875) | 0.824 (0.821–0.826) |
| TyG index | 0.832 (0.830–0.835) | 0.679 (0.677–0.681) | 0.593 (0.590–0.595) | 0.878 (0.876–0.879) | 0.832 (0.830–0.835) |
| TyG-BMI | 0.966 (0.965–0.967) | 0.901 (0.900–0.902) | 0.673 (0.670–0.676) | 0.927 (0.926–0.928) | 0.880 (0.878–0.882) |
| TyG-waist | 0.969 (0.968–0.970) | 0.793 (0.791–0.795) | 0.615 (0.612–0.617) | 0.943 (0.942–0.944) | 0.840 (0.838–0.842) |
| TyG-WtHR | 0.972 (0.971–0.972) | 0.829 (0.827–0.831) | 0.619 (0.616–0.621) | 0.962 (0.961–0.963) | 0.858 (0.856–0.859) |
| METS-IR | 0.957 (0.956–0.957) | 0.897 (0.896–0.899) | 0.669 (0.667–0.672) | 0.905 (0.903–0.906) | 0.871 (0.869–0.873) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez-Manent, J.I.; Martínez-Almoyna, E.; López, C.; Busquets-Cortés, C.; González San Miguel, H.; López-González, Á.A. Relationship between Insulin Resistance Risk Scales and Non-Alcoholic Fatty Liver Disease and Liver Fibrosis Scales in 219,477 Spanish Workers. Metabolites 2022, 12, 1093. https://doi.org/10.3390/metabo12111093
Ramírez-Manent JI, Martínez-Almoyna E, López C, Busquets-Cortés C, González San Miguel H, López-González ÁA. Relationship between Insulin Resistance Risk Scales and Non-Alcoholic Fatty Liver Disease and Liver Fibrosis Scales in 219,477 Spanish Workers. Metabolites. 2022; 12(11):1093. https://doi.org/10.3390/metabo12111093
Chicago/Turabian StyleRamírez-Manent, José Ignacio, Emilio Martínez-Almoyna, Carlos López, Carla Busquets-Cortés, Hilda González San Miguel, and Ángel Arturo López-González. 2022. "Relationship between Insulin Resistance Risk Scales and Non-Alcoholic Fatty Liver Disease and Liver Fibrosis Scales in 219,477 Spanish Workers" Metabolites 12, no. 11: 1093. https://doi.org/10.3390/metabo12111093
APA StyleRamírez-Manent, J. I., Martínez-Almoyna, E., López, C., Busquets-Cortés, C., González San Miguel, H., & López-González, Á. A. (2022). Relationship between Insulin Resistance Risk Scales and Non-Alcoholic Fatty Liver Disease and Liver Fibrosis Scales in 219,477 Spanish Workers. Metabolites, 12(11), 1093. https://doi.org/10.3390/metabo12111093








