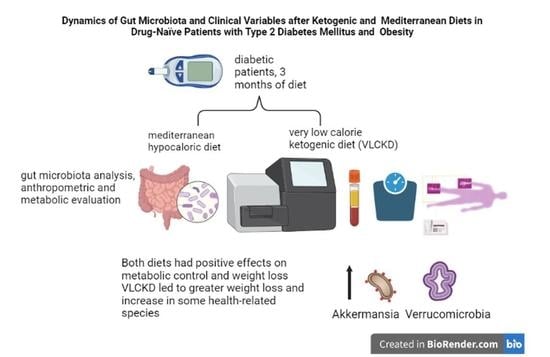
Dynamics of Gut Microbiota and Clinical Variables after Ketogenic and Mediterranean Diets in Drug-Naïve Patients with Type 2 Diabetes Mellitus and Obesity
Abstract
1. Introduction
1.1. Nutritional Treatment in T2DM
1.2. Ketogenic Diet
2. Materials and Methods
2.1. Study Design and Characteristics of the Sample
2.2. Clinical, Metabolic, Anthropometric, Lifestyle, and Health Status Evaluation
2.3. Dietary Protocols
2.4. Gut Microbiota Analysis
2.4.1. Sample Collection
2.4.2. Genomic DNA Extraction, Bacterial DNA Quantification, and 16S Libraries Preparation and Sequencing
2.5. Bioinformatic and Statistical Analysis
3. Results
3.1. Anthropometric, Metabolic, Lifestyle, and Health Status Evaluation
3.2. Gut Microbiota Analysis
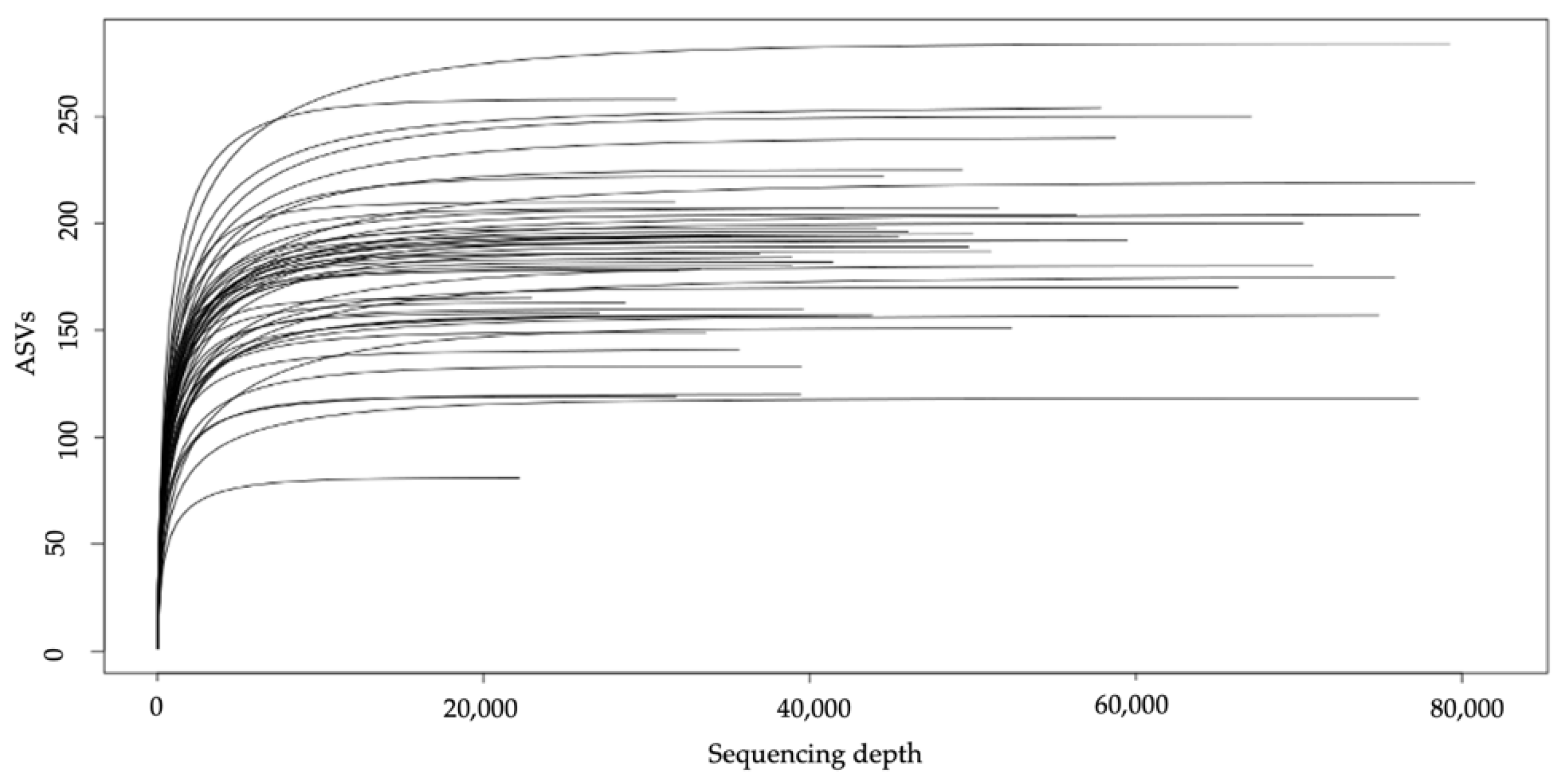
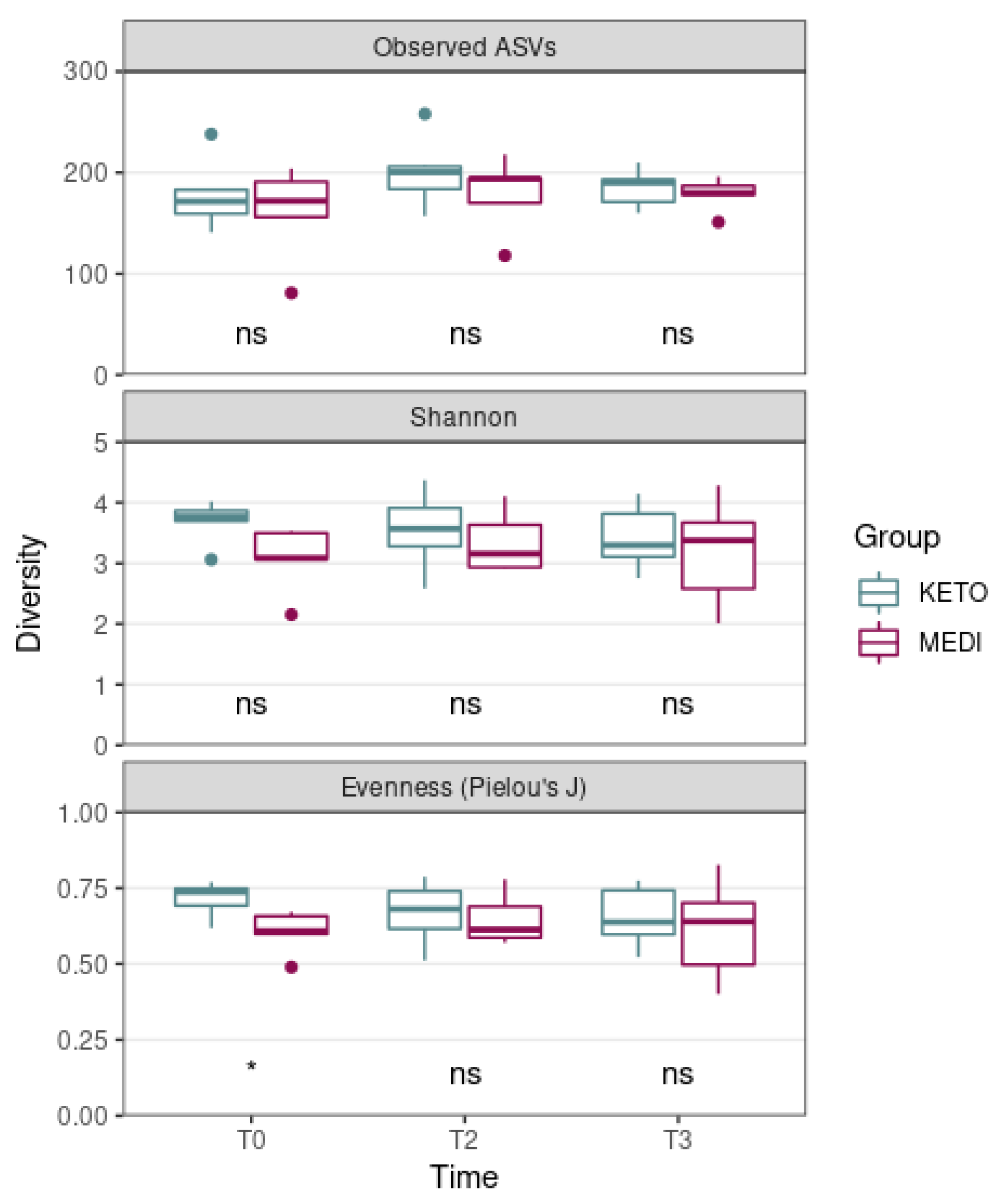
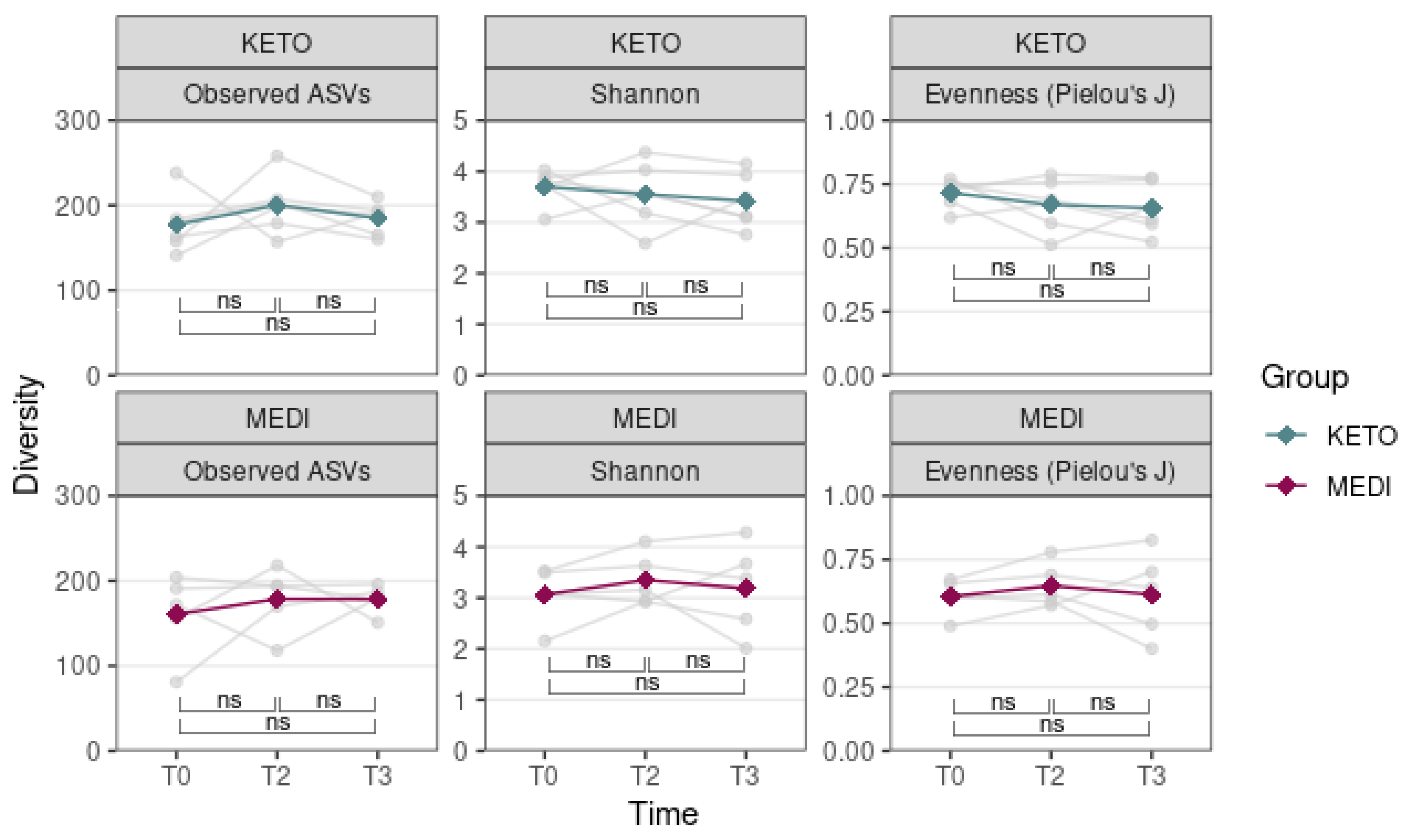
3.2.1. Alpha and Beta Diversity Analysis
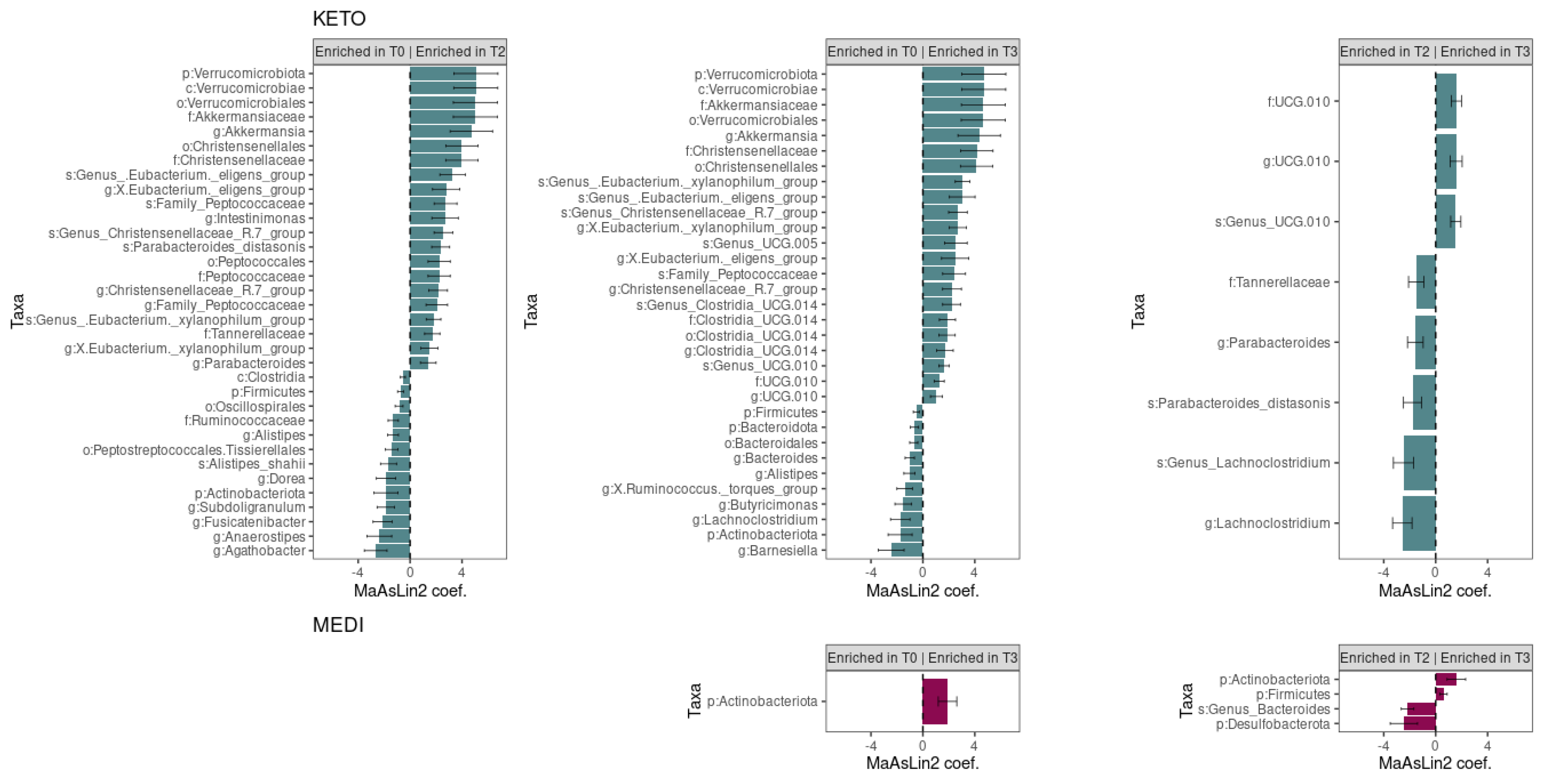
3.2.2. Compositional Analysis of Intestinal Microbiota
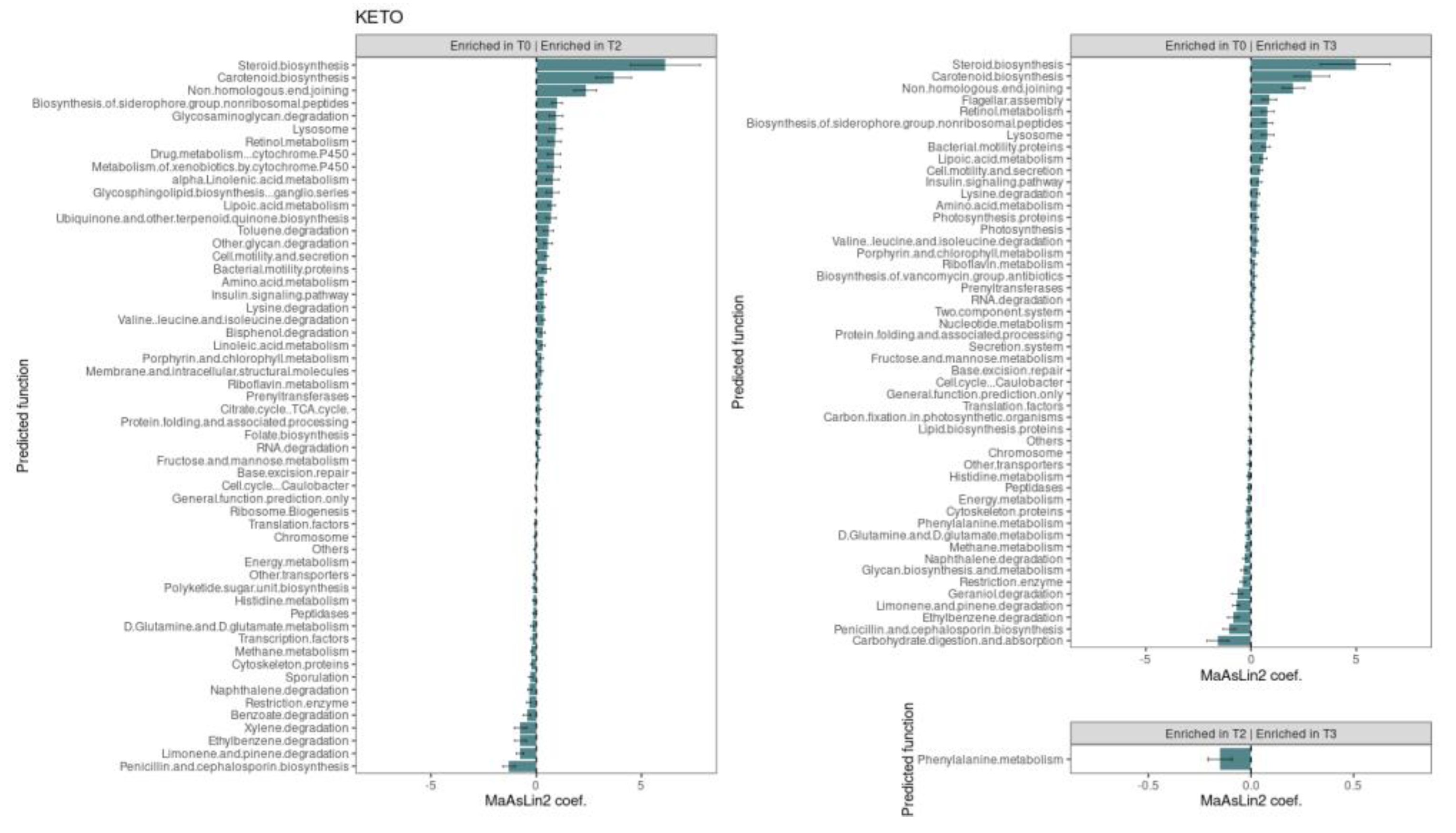
3.2.3. Functional Metagenome Prediction Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reed, J.; Bain, S.; Kanamarlapudi, V. A Review of Current Trends with Type 2 Diabetes Epidemiology, Aetiology, Pathogenesis, Treatments and Future Perspectives. Diabetes Metab. Syndr. Obes. Targets Ther. 2021, 14, 3567–3602. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2021. Diabetes Care 2021, 44, S15–S33. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization; International Diabetes Federation. Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycemia: Report of a WHO/IDF Consultation; World Health Organization: Geneva, Switzerland, 2006. [CrossRef]
- Sirdah, M.M.; Reading, N.S. Genetic predisposition in type 2 diabetes: A promising approach toward a personalized management of diabetes. Clin. Genet. 2020, 98, 525–547. [Google Scholar] [CrossRef] [PubMed]
- Dayeh, T.; Volkov, P.; Salö, S.; Hall, E.; Nilsson, E.; Olsson, A.H.; Kirkpatrick, C.; Wollheim, C.B.; Eliasson, L.; Rönn, T.; et al. Genome-Wide DNA Methylation Analysis of Human Pancreatic Islets from Type 2 Diabetic and Non-Diabetic Donors Identifies Candidate Genes That Influence Insulin Secretion. PLoS Genet. 2014, 10, e1004160. [Google Scholar] [CrossRef] [PubMed]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Tuccinardi, D.; Nicastro, V.; Barrea, L.; Colao, A.; Savastano, S. Sleep disturbances: One of the culprits of obesity-related cardiovascular risk? Int. J. Obes. Suppl. 2020, 10, 62–72. [Google Scholar] [CrossRef]
- Pinna, F.; Sardu, C.; Orrù, W.; Velluzzi, F.; Loviselli, A.; Contu, P.; Carpiniello, B. Psychopathology, psychosocial factors and obesity. Riv. Psichiatr. 2016, 51, 30–36. [Google Scholar] [CrossRef]
- Kolb, H.; Martin, S. Environmental/lifestyle factors in the pathogenesis and prevention of type 2 diabetes. BMC Med. 2017, 15, 1–11. [Google Scholar] [CrossRef]
- Neeland, I.J.; Ross, R.; Després, J.-P.; Matsuzawa, Y.; Yamashita, S.; Shai, I.; Seidell, J.; Magni, P.; Santos, R.D.; Arsenault, B.; et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: A position statement. Lancet Diabetes Endocrinol. 2019, 7, 715–725. [Google Scholar] [CrossRef]
- Okamura, T.; Hashimoto, Y.; Hamaguchi, M.; Obora, A.; Kojima, T.; Fukui, M. Ectopic fat obesity presents the greatest risk for incident type 2 diabetes: A population-based longitudinal study. Int. J. Obes. 2019, 43, 139–148. [Google Scholar] [CrossRef]
- Sesti, G. Pathophysiology of insulin resistance. Best Pract. Res. Clin. Endocrinol. Metab. 2006, 20, 665–679. [Google Scholar] [CrossRef] [PubMed]
- Kalra, S.P.; Kalra, P.S. Neuroendocrine Control of Energy Homeostasis: Update on New Insights. Prog. Brain Res. 2010, 181, 17–33. [Google Scholar] [CrossRef] [PubMed]
- Araujo, E.P.; Moraes, J.C.; Cintra, D.; Velloso, L. Mechanisms in endocrinology: Hypothalamic inflammation and nutrition. Eur. J. Endocrinol. 2016, 175, R97–R105. [Google Scholar] [CrossRef]
- Meek, T.H.; Nelson, J.T.; Matsen, M.E.; Dorfman, M.D.; Guyenet, S.J.; Damian, V.; Allison, M.B.; Scarlett, J.M.; Nguyen, H.T.; Thaler, J.P.; et al. Functional identification of a neurocircuit regulating blood glucose. Proc. Natl. Acad. Sci. USA 2016, 113, E2073-82. [Google Scholar] [CrossRef] [PubMed]
- Manceau, R.; Majeur, D.; Alquier, T. Neuronal control of peripheral nutrient partitioning. Diabetologia 2020, 63, 673–682. [Google Scholar] [CrossRef]
- Zatterale, F.; Longo, M.; Naderi, J.; Raciti, G.A.; Desiderio, A.; Miele, C.; Beguinot, F. Chronic Adipose Tissue Inflammation Linking Obesity to Insulin Resistance and Type 2 Diabetes. Front. Physiol. 2019, 10, 1607. [Google Scholar] [CrossRef]
- Denova-Gutiérrez, E.; Muñoz-Aguirre, P.; Shivappa, N.; Hébert, J.R.; Tolentino-Mayo, L.; Batis, C.; Barquera, S. Dietary Inflammatory Index and Type 2 Diabetes Mellitus in Adults: The Diabetes Mellitus Survey of Mexico City. Nutrients 2018, 10, 385. [Google Scholar] [CrossRef]
- Bray, G.A. Energy and Fructose From Beverages Sweetened With Sugar or High-Fructose Corn Syrup Pose a Health Risk for Some People. Adv. Nutr. Int. Rev. J. 2013, 4, 220–225. [Google Scholar] [CrossRef]
- Rahati, S.; Shahraki, M.; Arjomand, G.; Shahraki, T. Food Pattern, Lifestyle and Diabetes Mellitus. Int. J. High Risk Behav. Addict. 2014, 3, e8725. [Google Scholar] [CrossRef]
- Sergi, D.; Boulestin, H.; Campbell, F.M.; Williams, L.M. The Role of Dietary Advanced Glycation End Products in Metabolic Dysfunction. Mol. Nutr. Food Res. 2021, 65, e1900934. [Google Scholar] [CrossRef]
- Szendroedi, J.; Phielix, E.; Roden, M. The role of mitochondria in insulin resistance and type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2011, 8, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Supale, S.; Li, N.; Brun, T.; Maechler, P. Mitochondrial dysfunction in pancreatic β cells. Trends Endocrinol. Metab. 2012, 23, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Deledda, A.; Annunziata, G.; Tenore, G.; Palmas, V.; Manzin, A.; Velluzzi, F. Diet-Derived Antioxidants and Their Role in Inflammation, Obesity and Gut Microbiota Modulation. Antioxidants 2021, 10, 708. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, F.; Aquilina, A.; Vassallo, J.; Pace, N. Bisphenol A and Type 2 Diabetes Mellitus: A Review of Epidemiologic, Functional, and Early Life Factors. Int. J. Environ. Res. Public Health 2021, 18, 716. [Google Scholar] [CrossRef] [PubMed]
- Vanni, R.; Bussuan, R.M.; Rombaldi, R.L.; Arbex, A.K. Endocrine Disruptors and the Induction of Insulin Resistance. Curr. Diabetes Rev. 2021, 17, 1–16. [Google Scholar] [CrossRef]
- Chellappa, S.L.; Qian, J.; Vujovic, N.; Morris, C.J.; Nedeltcheva, A.; Nguyen, H.; Rahman, N.; Heng, S.W.; Kelly, L.; Kerlin-Monteiro, K.; et al. Daytime eating prevents internal circadian misalignment and glucose intolerance in night work. Sci. Adv. 2021, 7, eabg9910. [Google Scholar] [CrossRef]
- Vijay, A.; Valdes, A.M. Role of the gut microbiome in chronic diseases: A narrative review. Eur. J. Clin. Nutr. 2021, 76, 489–501. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, J.; Wang, L. Role and Mechanism of Gut Microbiota in Human Disease. Front. Cell. Infect. Microbiol. 2021, 11, 625913. [Google Scholar] [CrossRef]
- Gurung, M.; Li, Z.; You, H.; Rodrigues, R.; Jump, D.B.; Morgun, A.; Shulzhenko, N. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine 2020, 51, 102590. [Google Scholar] [CrossRef]
- Sierra, A.C.; Ramos-Lopez, O.; Riezu-Boj, J.I.; Milagro, F.I.; Martinez, J.A. Diet, Gut Microbiota, and Obesity: Links with Host Genetics and Epigenetics and Potential Applications. Adv. Nutr. 2019, 10, S17–S30. [Google Scholar] [CrossRef]
- Palmas, V.; Pisanu, S.; Madau, V.; Casula, E.; Deledda, A.; Cusano, R.; Uva, P.; Vascellari, S.; Loviselli, A.; Manzin, A.; et al. Gut microbiota markers associated with obesity and overweight in Italian adults. Sci. Rep. 2021, 11, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Duan, M.; Wang, Y.; Zhang, Q.; Zou, R.; Guo, M.; Zheng, H. Characteristics of gut microbiota in people with obesity. PLoS ONE 2021, 16, e0255446. [Google Scholar] [CrossRef] [PubMed]
- Bornfeldt, K.E. Does Elevated Glucose Promote Atherosclerosis? Pros and Cons. Circ. Res. 2016, 119, 190–193. [Google Scholar] [CrossRef]
- An, J.; Nichols, G.; Qian, L.; Munis, M.; Harrison, T.N.; Li, Z.; Wei, R.; Weiss, T.; Rajpathak, S.; Reynolds, K. Prevalence and incidence of microvascular and macrovascular complications over 15 years among patients with incident type 2 diabetes. BMJ Open Diabetes Res. Care 2021, 9, e001847. [Google Scholar] [CrossRef] [PubMed]
- García-García, P.M.; Getino-Melian, M.A.; Dominguez-Pimentel, V.; Navarro-Gonzalez, J.F. Inflammation in diabetic kidney disease. World J. Diabetes 2014, 5, 431–443. [Google Scholar] [CrossRef]
- Rey-Reñones, C.; Baena-Díez, J.M.; Aguilar-Palacio, I.; Miquel, C.; Grau, M. Type 2 Diabetes Mellitus and Cancer: Epidemiology, Physiopathology and Prevention. Biomedicines 2021, 9, 1429. [Google Scholar] [CrossRef]
- Ferreira, L.S.S.; Fernandes, C.S.; Vieira, M.N.N.; De Felice, F.G. Insulin Resistance in Alzheimer’s Disease. Front. Neurosci. 2018, 12, 830. [Google Scholar] [CrossRef]
- Doyle-Delgado, D.K.; Chamberlain, J.J.; Shubrook, D.J.H.; Skolnik, N.; Trujillo, J. Pharmacologic Approaches to Glycemic Treatment of Type 2 Diabetes: Synopsis of the 2020 American Diabetes Association’s Standards of Medical Care in Diabetes Clinical Guideline. Ann. Intern. Med. 2020, 173, 813–821. [Google Scholar] [CrossRef]
- Linea Guida della Società Italiana di Diabetologia (SID) e dell’Associazione dei Medici Diabetologi (AMD); Sistema Nazionale Linee Guida Dell’istituto Superiore Di Sanità: Rome, Italy, 2021; Available online: https://www.siditalia.it/pdf/LG_379_diabete_2_sid_amd.pdf (accessed on 15 September 2022).
- Leahy, J.L.; Aleppo, G.; Fonseca, V.; Garg, S.K.; Hirsch, I.B.; McCall, A.L.; McGill, J.B.; Polonsky, W.H. Optimizing Postprandial Glucose Management in Adults With Insulin-Requiring Diabetes: Report and Recommendations. J. Endocr. Soc. 2019, 3, 1942–1957. [Google Scholar] [CrossRef]
- Deledda, A.; Pintus, S.; Loviselli, A.; Fosci, M.; Fantola, G.; Velluzzi, F. Nutritional Management in Bariatric Surgery Patients. Int. J. Environ. Res. Public Health 2021, 18, 12049. [Google Scholar] [CrossRef]
- Davies, M.J.; D’Alessio, D.A.; Fradkin, J.; Kernan, W.N.; Mathieu, C.; Mingrone, G.; Rossing, P.; Tsapas, A.; Wexler, D.J.; Buse, J.B. Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018, 41, 2669–2701. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, F.; Grant, P.J.; Aboyans, V.; Bailey, C.J.; Ceriello, A.; Delgado, V.; Federici, M.; Filippatos, G.; Grobbee, D.E.; Hansen, T.B.; et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur. Heart J. 2020, 41, 255–323. [Google Scholar] [CrossRef] [PubMed]
- Arslanian, S.; Bacha, F.; Grey, M.; Marcus, M.D.; White, N.H.; Zeitler, P. Evaluation and Management of Youth-Onset Type 2 Diabetes: A Position Statement by the American Diabetes Association. Diabetes Care 2018, 41, 2648–2668. [Google Scholar] [CrossRef] [PubMed]
- Evert, A.B.; Dennison, M.; Gardner, C.D.; Garvey, W.T.; Lau, K.H.K.; MacLeod, J.; Mitri, J.; Pereira, R.F.; Rawlings, K.; Robinson, S.; et al. Nutrition Therapy for Adults With Diabetes or Prediabetes: A Consensus Report. Diabetes Care 2019, 42, 731–754. [Google Scholar] [CrossRef] [PubMed]
- Leroith, D.; Biessels, G.J.; Braithwaite, S.S.; Casanueva, F.F.; Draznin, B.; Halter, J.B.; Hirsch, I.B.; McDonnell, M.; Molitch, M.E.; Murad, M.H.; et al. TReatment of diabetes in older adults: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2019, 104, 1520–1574. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; Gea, A.; Ruiz-Canela, M. The Mediterranean Diet and Cardiovascular Health. Circ. Res. 2019, 124, 779–798. [Google Scholar] [CrossRef]
- Mazzocchi, A.; Leone, L.; Agostoni, C.; Pali-Schöll, I. The Secrets of the Mediterranean Diet. Does [Only] Olive Oil Matter? Nutrients 2019, 11, 2941. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Schwedhelm, C.; Galbete, C.; Hoffmann, G. Adherence to Mediterranean Diet and Risk of Cancer: An Updated Systematic Review and Meta-Analysis. Nutrients 2017, 9, 1063. [Google Scholar] [CrossRef]
- Laudisio, D.; Barrea, L.; Muscogiuri, G.; Annunziata, G.; Colao, A.; Savastano, S. Breast cancer prevention in premenopausal women: Role of the Mediterranean diet and its components. Nutr. Res. Rev. 2020, 33, 19–32. [Google Scholar] [CrossRef]
- Farinetti, A.; Zurlo, V.; Manenti, A.; Coppi, F.; Mattioli, A.V. Mediterranean diet and colorectal cancer: A systematic review. Nutrition 2017, 43–44, 83–88. [Google Scholar] [CrossRef]
- Katsimbri, P.; Korakas, E.; Kountouri, A.; Ikonomidis, I.; Tsougos, E.; Vlachos, D.; Papadavid, E.; Raptis, A.; Lambadiari, V. The Effect of Antioxidant and Anti-Inflammatory Capacity of Diet on Psoriasis and Psoriatic Arthritis Phenotype: Nutrition as Therapeutic Tool? Antioxidants 2021, 10, 157. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Fabbrocini, G.; Annunziata, G.; Muscogiuri, G.; Donnarumma, M.; Marasca, C.; Colao, A.; Savastano, S. Role of Nutrition and Adherence to the Mediterranean Diet in the Multidisciplinary Approach of Hidradenitis Suppurativa: Evaluation of Nutritional Status and Its Association with Severity of Disease. Nutrients 2018, 11, 57. [Google Scholar] [CrossRef] [PubMed]
- Velluzzi, F.; Anedda, J.; Pisanu, S.; Dell’Antonia, M.; Deledda, A.; Boi, A.; Ferreli, C.; Atzori, L. Mediterranean Diet, Lifestyle and Quality of Life in Sardinian Patients Affected with Hidradenitis Suppurativa. J. Public Health Res. 2021, 11, jphr-2021. [Google Scholar] [CrossRef]
- Garcia-Larsen, V.; Del Giacco, S.R.; Moreira, A.; Bonini, M.; Charles, D.; Reeves, T.; Carlsen, K.-H.; Haahtela, T.; Bonini, S.; Fonseca, J.; et al. Asthma and dietary intake: An overview of systematic reviews. Allergy 2016, 71, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Serra-Majem, L.; Tomaino, L.; Dernini, S.; Berry, E.M.; Lairon, D.; Ngo de la Cruz, J.; Bach-Faig, A.; Donini, L.M.; Medina, F.-X.; Belahsen, R.; et al. Updating the Mediterranean Diet Pyramid towards Sustainability: Focus on Environmental Concerns. Int. J. Environ. Res. Public Health 2020, 17, 8758. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.C.; Hayes, R.; Ahn, J.; Shao, Y.; Silverman, D.T.; Jones, R.R.; Thurston, G.D. Mediterranean Diet and the Association Between Air Pollution and Cardiovascular Disease Mortality Risk. Circulation 2019, 139, 1766–1775. [Google Scholar] [CrossRef]
- Garcia-Mantrana, I.; Selma-Royo, M.; Alcantara, C.; Collado, M.C. Shifts on Gut Microbiota Associated to Mediterranean Diet Adherence and Specific Dietary Intakes on General Adult Population. Front. Microbiol. 2018, 9, 890. [Google Scholar] [CrossRef]
- Pisanu, S.; Palmas, V.; Madau, V.; Casula, E.; Deledda, A.; Cusano, R.; Uva, P.; Vascellari, S.; Boi, F.; Loviselli, A.; et al. Impact of a Moderately Hypocaloric Mediterranean Diet on the Gut Microbiota Composition of Italian Obese Patients. Nutrients 2020, 12, 2707. [Google Scholar] [CrossRef]
- Martín-Peláez, S.; Fito, M.; Castaner, O. Mediterranean Diet Effects on Type 2 Diabetes Prevention, Disease Progression, and Related Mechanisms. A Review. Nutrients 2020, 12, 2236. [Google Scholar] [CrossRef]
- Taylor, R.; Ramachandran, A.; Yancy, W.S.Y., Jr.; Forouhi, N.G. Nutritional basis of type 2 diabetes remission. BMJ 2021, 374, n1449. [Google Scholar] [CrossRef]
- Lean, M.E.; Leslie, W.S.; Barnes, A.C.; Brosnahan, N.; Thom, G.; McCombie, L.; Peters, C.; Zhyzhneuskaya, S.; Al-Mrabeh, A.; Hollingsworth, K.G.; et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): An open-label, cluster-randomised trial. Lancet 2018, 391, 541–551. [Google Scholar] [CrossRef]
- Taheri, S.; Zaghloul, H.; Chagoury, O.; Elhadad, S.; Ahmed, S.H.; El Khatib, N.; Amona, R.A.; El Nahas, K.; Suleiman, N.; Alnaama, A.; et al. Effect of intensive lifestyle intervention on bodyweight and glycaemia in early type 2 diabetes (DIADEM-I): An open-label, parallel-group, randomised controlled trial. Lancet Diabetes Endocrinol. 2020, 8, 477–489. [Google Scholar] [CrossRef]
- Jacob, E.; Avery, A. Energy-restricted interventions are effective for the remission of newly diagnosed type 2 diabetes: A systematic review of the evidence base. Obes. Sci. Pract. 2021, 7, 606–618. [Google Scholar] [CrossRef] [PubMed]
- Churuangsuk, C.; Hall, J.; Reynolds, A.; Griffin, S.J.; Combet, E.; Lean, M.E.J. Diets for weight management in adults with type 2 diabetes: An umbrella review of published meta-analyses and systematic review of trials of diets for diabetes remission. Diabetologia 2022, 65, 14–36. [Google Scholar] [CrossRef]
- Paoli, A.; Rubini, A.; Volek, J.S.; Grimaldi, K.A. Beyond weight loss: A review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets. Eur. J. Clin. Nutr. 2013, 67, 789–796. [Google Scholar] [CrossRef]
- Martin-McGill, K.J.; Jackson, C.F.; Bresnahan, R.; Levy, R.G.; Cooper, P.N. Ketogenic diets for drug-resistant epilepsy. Cochrane Database Syst. Rev. 2018, 11, CD001903. [Google Scholar] [CrossRef]
- Grigolon, R.B.; Gerchman, F.; Schöffel, A.C.; Hawken, E.R.; Gill, H.; Vazquez, G.H.; Mansur, R.B.; McIntyre, R.S.; Brietzke, E. Mental, emotional, and behavioral effects of ketogenic diet for non-epileptic neuropsychiatric conditions. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2020, 102, 109947. [Google Scholar] [CrossRef]
- Casanueva, F.F.; Castellana, M.; Bellido, D.; Trimboli, P.; Castro, A.I.; Sajoux, I.; Carnero, M.G.R.; Gomez-Arbelaez, D.; Crujeiras, A.B.; Olmos, M.M. Ketogenic diets as treatment of obesity and type 2 diabetes mellitus. Rev. Endocr. Metab. Disord. 2020, 21, 381–397. [Google Scholar] [CrossRef]
- Masood, W.; Annamaraju, P.; Uppaluri, K.R. Ketogenic Diet. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021; pp. 158–167. [Google Scholar]
- Gibson, A.A.; Seimon, R.V.; Lee, C.M.Y.; Ayre, J.; Franklin, J.; Markovic, T.P.; Caterson, I.D.; Sainsbury, A. Do ketogenic diets really suppress appetite? A systematic review and meta-analysis. Obes. Rev. 2015, 16, 64–76. [Google Scholar] [CrossRef]
- Muscogiuri, G.; El Ghoch, M.; Colao, A.; Hassapidou, M.; Yumuk, V.; Busetto, L. Obesity Management Task Force (OMTF) of the European Association for the Study of Obesity (EASO) European Guidelines for Obesity Management in Adults with a Very Low-Calorie Ketogenic Diet: A Systematic Review and Meta-Analysis. Obes. Facts 2021, 14, 222–245. [Google Scholar] [CrossRef]
- Ludwig, D.S. The Ketogenic Diet: Evidence for Optimism but High-Quality Research Needed. J. Nutr. 2020, 150, 1354–1359. [Google Scholar] [CrossRef] [PubMed]
- Amerio, M.L.; Angrisani, L.; Annuzzi, G.; Bagnato, C.; Brunani, A.; Buccianti, M.; Busetto, L.; Capodaglio, P.; Carbonelli, M.G.; Caregaro Negrin, L.; et al. Standard Italiani per la Cura dell’Obesità SIO-ADI 2016–2017; SIO (Società Ital. dell’Obesità): Pisa, Italy, 2017; pp. 1–292. [Google Scholar]
- Caprio, M.; Infante, M.; Moriconi, E.; Armani, A.; Fabbri, A.; Mantovani, G.; Mariani, S.; Lubrano, C.; Poggiogalle, E.; Migliaccio, S.; et al. Very-low-calorie ketogenic diet (VLCKD) in the management of metabolic diseases: Systematic review and consensus statement from the Italian Society of Endocrinology (SIE). J. Endocrinol. Investig. 2019, 42, 1365–1386. [Google Scholar] [CrossRef] [PubMed]
- Wheless, J.W. The Ketogenic Diet: An Effective Medical Therapy With Side Effects. J. Child Neurol. 2001, 16, 633–635. [Google Scholar] [CrossRef]
- Qu, C.; Keijer, J.; Adjobo-Hermans, M.J.; van de Wal, M.; Schirris, T.; van Karnebeek, C.; Pan, Y.; Koopman, W.J. The ketogenic diet as a therapeutic intervention strategy in mitochondrial disease. Int. J. Biochem. Cell Biol. 2021, 138, 106050. [Google Scholar] [CrossRef] [PubMed]
- Rawat, K.; Singh, N.; Kumari, P.; Saha, L. A review on preventive role of ketogenic diet (KD) in CNS disorders from the gut microbiota perspective. Rev. Neurosci. 2021, 32, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-Mulero, A.; Tinahones, A.; Bandera, B.; Moreno-Indias, I.; Macías-González, M.; Tinahones, F.J. Keto microbiota: A powerful contributor to host disease recovery. Rev. Endocr. Metab. Disord. 2019, 20, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Vascellari, S.; Palmas, V.; Melis, M.; Pisanu, S.; Cusano, R.; Uva, P.; Perra, D.; Madau, V.; Sarchioto, M.; Oppo, V.; et al. Gut Microbiota and Metabolome Alterations Associated with Parkinson’s Disease. mSystems 2020, 5, e00561-20. [Google Scholar] [CrossRef]
- Hang, H.J.; Battousse, O.; Ramadas, A. Modulation of gut microbiota by dietary macronutrients in type 2 diabetes: A review. Prog. Microbes Mol. Biol. 2021, 4, 1–16. [Google Scholar] [CrossRef]
- Yuan, W.; Lu, W.; Wang, H.; Wu, W.; Zhou, Q.; Chen, Y.; Lee, Y.K.; Zhao, J.; Zhang, H.; Chen, W. A multiphase dietetic protocol incorporating an improved ketogenic diet enhances weight loss and alters the gut microbiome of obese people. Int. J. Food Sci. Nutr. 2021, 73, 238–250. [Google Scholar] [CrossRef]
- Lohman, T.J.; Roache, A.F.; Martorell, R. Anthropometric Standardization Reference Manual. Med. Sci. Sport. Exerc. 1992, 24, 952. Available online: https://journals.lww.com/acsm-msse/Fulltext/1992/08000/Anthropometric_Standardization_Reference_Manual.20.aspx (accessed on 10 September 2022).
- Kyle, U.G.; Bosaeus, I.; De Lorenzo, A.D.; Deurenberg, P.; Elia, M.; Gómez, J.M.; Heitmann, B.L.; Kent-Smith, L.; Melchior, J.-C.; Pirlich, M.; et al. Bioelectrical impedance analysis—Part II: Utilization in clinical practice. Clin. Nutr. 2004, 23, 1430–1453. [Google Scholar] [CrossRef]
- Panagiotakos, D.B.; Pitsavos, C.; Stefanadis, C. Dietary patterns: A Mediterranean diet score and its relation to clinical and biological markers of cardiovascular disease risk. Nutr. Metab. Cardiovasc. Dis. 2006, 16, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.L.; Yngve, A.; Sallis, J.F.; et al. International Physical Activity Questionnaire: 12-Country Reliability and Validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef] [PubMed]
- Ware, J.E.J.; Sherbourne, C.D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care 1992, 30, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Santoru, M.L.; Piras, C.; Murgia, A.; Palmas, V.; Camboni, T.; Liggi, S.; Ibba, I.; Lai, M.A.; Orrù, S.; Blois, S.; et al. Cross sectional evaluation of the gut-microbiome metabolome axis in an Italian cohort of IBD patients. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Callahan, B.J.; Mcmurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 2016, e2584. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Gregory Caporaso, J. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2’s q2-feature-classifier plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef]
- Ii, M.S.R.; O’Rourke, D.R.; Kaehler, B.D.; Ziemski, M.; Dillon, M.R.; Foster, J.T.; Bokulich, N.A. RESCRIPt: Reproducible sequence taxonomy reference database management. PLoS Comput. Biol. 2021, 17, e1009581. [Google Scholar] [CrossRef]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef] [PubMed]
- Valero-Mora, P.M. ggplot2: Elegant graphics for data analysis. J. Stat. Softw. 2010, 35, 1–3. [Google Scholar] [CrossRef]
- Beule, L.; Karlovsky, P. Improved normalization of species count data in ecology by scaling with ranked subsampling (SRS): Application to microbial communities. PeerJ 2020, 8, e9593. [Google Scholar] [CrossRef] [PubMed]
- Heidrich, V.; Karlovsky, P.; Beule, L. ‘SRS’ R Package and ‘q2-srs’ QIIME 2 Plugin: Normalization of Microbiome Data Using Scaling with Ranked Subsampling (SRS). Appl. Sci. 2021, 11, 11473. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef]
- Robinson, D.; Hayes, A.; Couch, S. Index @ Cran.R-Project.Org. 2020. Available online: https://cran.r-project.org/package=miceadds (accessed on 15 September 2022).
- Mallick, H.; Rahnavard, A.; McIver, L.J.; Ma, S.; Zhang, Y.; Nguyen, L.H.; Tickle, T.L.; Weingart, G.; Ren, B.; Schwager, E.H.; et al. Multivariable association discovery in population-scale meta-omics studies. PLoS Comput. Biol. 2021, 17, e1009442. [Google Scholar] [CrossRef]
- Ross, R.; Neeland, I.J.; Yamashita, S.; Shai, I.; Seidell, J.; Magni, P.; Santos, R.D.; Arsenault, B.; Cuevas, A.; Hu, F.B.; et al. Waist circumference as a vital sign in clinical practice: A Consensus Statement from the IAS and ICCR Working Group on Visceral Obesity. Nat. Rev. Endocrinol. 2020, 16, 177–189. [Google Scholar] [CrossRef]
- Franz, M.J.; Boucher, J.L.; Rutten-Ramos, S.; VanWormer, J.J. Lifestyle Weight-Loss Intervention Outcomes in Overweight and Obese Adults with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. J. Acad. Nutr. Diet. 2015, 115, 1447–1463. [Google Scholar] [CrossRef]
- Huang, X.-L.; Pan, J.-H.; Chen, D.; Chen, J.; Chen, F.; Hu, T.-T. Efficacy of lifestyle interventions in patients with type 2 diabetes: A systematic review and meta-analysis. Eur. J. Intern. Med. 2016, 27, 37–47. [Google Scholar] [CrossRef]
- Villareal, D.T.; Apovian, C.M.; Kushner, R.F.; Klein, S. Obesity in older adults: Technical review and position statement of the American Society for Nutrition and NAASO, The Obesity Society. Am. J. Clin. Nutr. 2005, 82, 923–934. [Google Scholar] [CrossRef] [PubMed]
- Carta, M.G.; Cossu, G.; Pintus, E.; Zoccheddu, R.; Callia, O.; Conti, G.; Pintus, M.; Gonzalez, C.I.A.; Massidda, M.V.; Mura, G.; et al. Active elderly and health—Can moderate exercise improve health and wellbeing in older adults? Protocol for a randomized controlled trial. Trials 2021, 22, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Carta, M.G.; Cossu, G.; Pintus, E.; Zaccheddu, R.; Callia, O.; Conti, G.; Pintus, M.; Gonzalez, C.I.A.; Massidda, M.V.; Mura, G.; et al. Moderate Exercise Improves Cognitive Function in Healthy Elderly People: Results of a Randomized Controlled Trial. Clin. Pract. Epidemiol. Ment. Health 2021, 17, 75–80. [Google Scholar] [CrossRef]
- Fragala, M.S.; Cadore, E.L.; Dorgo, S.; Izquierdo, M.; Kraemer, W.J.; Peterson, M.D.; Ryan, E.D. Resistance Training for Older Adults. J. Strength Cond. Res. 2019, 33, 2019–2052. [Google Scholar] [CrossRef]
- Carta, M.G.; Velluzzi, F.; Monticone, M.; Gonzalez, C.I.A.; Minerba, L.; Pau, M.; Musu, M.; Atzori, L.; Ferreli, C.; Cauli, A.; et al. Exercise Improves the Impact of Chronic Pain in Older Adults: Results of an RCT. Open Pain J. 2022, 15, 1–5. [Google Scholar] [CrossRef]
- Velluzzi, F.; Cossu, G.; Fosci, M.; Montisci, R.; Zaccheddu, R.; Minerba, L.; Musu, M.; Pintus, E.; Fortin, D.; Romano, F.; et al. Effect of a Low-Moderate Exercise Program on Dysmetabolism in Older Adults: Results of a Randomized Controlled Trial. Nutrients 2022, 14, 3337. [Google Scholar] [CrossRef]
- Barrea, L.; Muscogiuri, G.; Frias-Toral, E.; Laudisio, D.; Pugliese, G.; Castellucci, B.; Garcia-Velasquez, E.; Savastano, S.; Colao, A. Nutrition and immune system: From the Mediterranean diet to dietary supplementary through the microbiota. Crit. Rev. Food Sci. Nutr. 2021, 61, 3066–3090. [Google Scholar] [CrossRef] [PubMed]
- Beam, A.; Clinger, E.; Hao, L. Effect of Diet and Dietary Components on the Composition of the Gut Microbiota. Nutrients 2021, 13, 2795. [Google Scholar] [CrossRef] [PubMed]
- Ismael, S.; Silvestre, M.; Vasques, M.; Araújo, J.; Morais, J.; Duarte, M.; Pestana, D.; Faria, A.; Pereira-Leal, J.; Vaz, J.; et al. A Pilot Study on the Metabolic Impact of Mediterranean Diet in Type 2 Diabetes: Is Gut Microbiota the Key? Nutrients 2021, 13, 1228. [Google Scholar] [CrossRef]
- Rondanelli, M.; Gasparri, C.; Peroni, G.; Faliva, M.A.; Naso, M.; Perna, S.; Bazire, P.; Sajoux, I.; Maugeri, R.; Rigon, C. The Potential Roles of Very Low Calorie, Very Low Calorie Ketogenic Diets and Very Low Carbohydrate Diets on the Gut Microbiota Composition. Front. Endocrinol. 2021, 12, 662591. [Google Scholar] [CrossRef]
- Gutiérrez-Repiso, C.; Molina-Vega, M.; Bernal-López, M.; Garrido-Sánchez, L.; García-Almeida, J.; Sajoux, I.; Moreno-Indias, I.; Tinahones, F. Different Weight Loss Intervention Approaches Reveal a Lack of a Common Pattern of Gut Microbiota Changes. J. Pers. Med. 2021, 11, 109. [Google Scholar] [CrossRef] [PubMed]
- Crovesy, L.; Ostrowski, M.; Ferreira, D.M.T.P.; Rosado, E.L.; Soares, M. Effect of Lactobacillus on body weight and body fat in overweight subjects: A systematic review of randomized controlled clinical trials. Int. J. Obes. 2017, 41, 1607–1614. [Google Scholar] [CrossRef] [PubMed]
- Furet, J.-P.; Kong, L.-C.; Tap, J.; Poitou, C.; Basdevant, A.; Bouillot, J.-L.; Mariat, D.; Corthier, G.; Doré, J.; Henegar, C.; et al. Differential Adaptation of Human Gut Microbiota to Bariatric Surgery–Induced Weight Loss. Diabetes 2010, 59, 3049–3057. [Google Scholar] [CrossRef]
- Basciani, S.; Camajani, E.; Contini, S.; Persichetti, A.; Risi, R.; Bertoldi, L.; Strigari, L.; Prossomariti, G.; Watanabe, M.; Mariani, S.; et al. Very-Low-Calorie Ketogenic Diets With Whey, Vegetable, or Animal Protein in Patients With Obesity: A Randomized Pilot Study. J. Clin. Endocrinol. Metab. 2020, 105, 2939–2949. [Google Scholar] [CrossRef] [PubMed]
- Ang, Q.Y.; Alexander, M.; Newman, J.C.; Tian, Y.; Cai, J.; Upadhyay, V.; Turnbaugh, J.A.; Verdin, E.; Hall, K.D.; Leibel, R.L.; et al. Ketogenic Diets Alter the Gut Microbiome Resulting in Decreased Intestinal Th17 Cells. Cell 2020, 181, 1263–1275.e16. [Google Scholar] [CrossRef]
- Paoli, A.; Mancin, L.; Bianco, A.; Thomas, E.; Mota, J.F.; Piccini, F. Ketogenic Diet and Microbiota: Friends or Enemies? Genes 2019, 10, 534. [Google Scholar] [CrossRef]
- Gutiérrez-Repiso, C.; Hernández-García, C.; García-Almeida, J.M.; Bellido, D.; Martín-Núñez, G.M.; Sánchez-Alcoholado, L.; Alcaide-Torres, J.; Sajoux, I.; Tinahones, F.J.; Moreno-Indias, I. Effect of Synbiotic Supplementation in a Very-Low-Calorie Ketogenic Diet on Weight Loss Achievement and Gut Microbiota: A Randomized Controlled Pilot Study. Mol. Nutr. Food Res. 2019, 63, 1900167. [Google Scholar] [CrossRef]
- Barengolts, E. Gut Microbiota, Prebiotics, Probiotics, and Synbiotics in Management of Obesity and Prediabetes: Review of Randomized Controlled Trials. Endocr. Pract. 2016, 22, 1224–1234. [Google Scholar] [CrossRef]
- Gardner, C.D.; Landry, M.J.; Perelman, D.; Petlura, C.; Durand, L.R.; Aronica, L.; Crimarco, A.; Cunanan, K.M.; Chang, A.; Dant, C.C.; et al. Effect of a ketogenic diet versus Mediterranean diet on glycated hemoglobin in individuals with prediabetes and type 2 diabetes mellitus: The interventional Keto-Med randomized crossover trial. Am. J. Clin. Nutr. 2022, 116, 640–652. [Google Scholar] [CrossRef]
- Moriconi, E.; Camajani, E.; Fabbri, A.; Lenzi, A.; Caprio, M. Reply to Berk et al. Comment on “Moriconi et al. Very-Low-Calorie Ketogenic Diet as a Safe and Valuable Tool for Long-Term Glycemic Management in Patients with Obesity and Type 2 Diabetes. Nutrients 2021, 13, 758”. Nutrients 2021, 13, 3639. [Google Scholar] [CrossRef]
- Landry, M.; Crimarco, A.; Perelman, D.; Durand, L.; Petlura, C.; Aronica, L.; Robinson, J.; Kim, S.; Gardner, C. Adherence to Ketogenic and Mediterranean Study Diets in a Crossover Trial: The Keto–Med Randomized Trial. Nutrients 2021, 13, 967. [Google Scholar] [CrossRef] [PubMed]
- Pisanu, S.; Deledda, A.; Loviselli, A.; Huybrechts, I.; Velluzzi, F. Validity of Accelerometers for the Evaluation of Energy Expenditure in Obese and Overweight Individuals: A Systematic Review. J. Nutr. Metab. 2020, 2020, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Breymeyer, K.L.; Lampe, J.W.; McGregor, B.A.; Neuhouser, M.L. Subjective mood and energy levels of healthy weight and overweight/obese healthy adults on high-and low-glycemic load experimental diets. Appetite 2016, 107, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Cohen, C.W.; Fontaine, K.R.; Arend, R.C.; Soleymani, T.; Gower, B.A. Favorable Effects of a Ketogenic Diet on Physical Function, Perceived Energy, and Food Cravings in Women with Ovarian or Endometrial Cancer: A Randomized, Controlled Trial. Nutrients 2018, 10, 1187. [Google Scholar] [CrossRef] [PubMed]
- Willis, H.J.; Slavin, J.L. The Influence of Diet Interventions Using Whole, Plant Food on the Gut Microbiome: A Narrative Review. J. Acad. Nutr. Diet. 2020, 120, 608–623. [Google Scholar] [CrossRef] [PubMed]
- Belzer, C.; De Vos, W.M. Microbes inside—From diversity to function: The case of Akkermansia. ISME J. 2012, 6, 1449–1458. [Google Scholar] [CrossRef]
- Ganesh, B.; Klopfleisch, R.; Loh, G.; Blaut, M. Commensal Akkermansia muciniphila Exacerbates Gut Inflammation in Salmonella Typhimurium-Infected Gnotobiotic Mice. PLoS ONE 2013, 8, e74963. [Google Scholar] [CrossRef]
- Johansson, M.E.V.; Larsson, J.M.H.; Hansson, G.C. The two mucus layers of colon are organized by the MUC2 mucin, whereas the outer layer is a legislator of host–microbial interactions. Proc. Natl. Acad. Sci. USA 2011, 108, 4659–4665. [Google Scholar] [CrossRef]
- Dao, M.C.; Everard, A.; Aron-Wisnewsky, J.; Sokolovska, N.; Prifti, E.; Verger, E.O.; Kayser, B.D.; Levenez, F.; Chilloux, J.; Hoyles, L.; et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: Relationship with gut microbiome richness and ecology. Gut 2016, 65, 426–436. [Google Scholar] [CrossRef]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef]
- Greer, R.L.; Dong, X.; de Moraes, A.; Zielke, R.A.; Fernandes, G.R.; Peremyslova, E.; Vasquez-Perez, S.; Schoenborn, A.A.; Gomes, E.P.; Pereira, A.C.; et al. Akkermansia muciniphila mediates negative effects of IFNγ on glucose metabolism. Nat. Commun. 2016, 7, 13329. [Google Scholar] [CrossRef] [PubMed]
- Lukovac, S.; Belzer, C.; Pellis, L.; Keijser, B.J.; de Vos, W.M.; Montijn, R.C.; Roeselers, G. Differential Modulation by Akkermansia muciniphila and Faecalibacterium prausnitzii of Host Peripheral Lipid Metabolism and Histone Acetylation in Mouse Gut Organoids. mBio 2014, 5, e01438-14. [Google Scholar] [CrossRef] [PubMed]
- Depommier, C.; Everard, A.; Druart, C.; Plovier, H.; Van Hul, M.; Vieira-Silva, S.; Falony, G.; Raes, J.; Maiter, D.; Delzenne, N.M.; et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: A proof-of-concept exploratory study. Nat. Med. 2019, 25, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Vaiserman, A.M.; Koliada, A.K.; Marotta, F. Gut microbiota: A player in aging and a target for anti-aging intervention. Ageing Res. Rev. 2017, 35, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Derrien, M.; Belzer, C.; de Vos, W.M. Akkermansia muciniphila and its role in regulating host functions. Microb. Pathog. 2017, 106, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Hua, Y.; Zeng, B.; Ning, R.; Li, Y.; Zhao, J. Gut microbiota signatures of longevity. Curr. Biol. 2016, 26, R832–R833. [Google Scholar] [CrossRef]
- Tuikhar, N.; Keisam, S.; Labala, R.K.; Imrat; Ramakrishnan, P.; Arunkumar, M.C.; Ahmed, G.; Biagi, E.; Jeyaram, K. Comparative analysis of the gut microbiota in centenarians and young adults shows a common signature across genotypically non-related populations. Mech. Ageing Dev. 2019, 179, 23–35. [Google Scholar] [CrossRef]
- Kim, B.-S.; Choi, C.W.; Shin, H.; Jin, S.-P.; Bae, J.-S.; Han, M.; Seo, E.Y.; Chun, J.; Chung, J.H. Comparison of the Gut Microbiota of Centenarians in Longevity Villages of South Korea with Those of Other Age Groups. J. Microbiol. Biotechnol. 2019, 29, 429–440. [Google Scholar] [CrossRef]
- Org, E.; Blum, Y.; Kasela, S.; Mehrabian, M.; Kuusisto, J.; Kangas, A.J.; Soininen, P.; Wang, Z.; Ala-Korpela, M.; Hazen, S.L.; et al. Relationships between gut microbiota, plasma metabolites, and metabolic syndrome traits in the METSIM cohort. Genome Biol. 2017, 18, 1–14. [Google Scholar] [CrossRef]
- Waters, J.L.; Ley, R.E. The human gut bacteria Christensenellaceae are widespread, heritable, and associated with health. BMC Biol. 2019, 17, 1–11. [Google Scholar] [CrossRef]
- Goodrich, J.K.; Waters, J.L.; Poole, A.C.; Sutter, J.L.; Koren, O.; Blekhman, R.; Beaumont, M.; Van Treuren, W.; Knight, R.; Bell, J.T.; et al. Human Genetics Shape the Gut Microbiome. Cell 2014, 159, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Alemán, J.O.; Bokulich, N.A.; Swann, J.R.; Walker, J.M.; De Rosa, J.C.; Battaglia, T.; Costabile, A.; Pechlivanis, A.; Liang, Y.; Breslow, J.L.; et al. Fecal microbiota and bile acid interactions with systemic and adipose tissue metabolism in diet-induced weight loss of obese postmenopausal women. J. Transl. Med. 2018, 16, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Bonder, M.J.; Cenit, M.C.; Tigchelaar, E.F.; Maatman, A.; Dekens, J.A.M.; Brandsma, E.; Marczynska, J.; Imhann, F.; Weersma, R.K.; et al. The Gut Microbiome Contributes to a Substantial Proportion of the Variation in Blood Lipids. Circ. Res. 2015, 117, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Hibberd, A.; Yde, C.; Ziegler, M.; Honoré, A.; Saarinen, M.; Lahtinen, S.; Stahl, B.; Jensen, H.; Stenman, L. Probiotic or synbiotic alters the gut microbiota and metabolism in a randomised controlled trial of weight management in overweight adults. Benef. Microbes 2019, 10, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.Y.; You, H.J.; Yoon, H.S.; Kwon, B.; Lee, J.Y.; Lee, S.; Song, Y.-M.; Lee, K.; Sung, J.; Ko, G. The effect of heritability and host genetics on the gut microbiota and metabolic syndrome. Gut 2017, 66, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Lippert, K.; Kedenko, L.; Antonielli, L.; Gemeier, C.; Leitner, M.; Kautzky-Willer, A.; Paulweber, B.; Hackl, E. Gut microbiota dysbiosis associated with glucose metabolism disorders and the metabolic syndrome in older adults. Benef. Microbes 2017, 8, 545–556. [Google Scholar] [CrossRef]
- Wang, F.; Yu, T.; Huang, G.; Cai, D.; Liang, X.; Su, H.; Zhu, Z.; Li, D.; Yang, Y.; Shen, P.; et al. Gut Microbiota Community and Its Assembly Associated with Age and Diet in Chinese Centenarians. J. Microbiol. Biotechnol. 2015, 25, 1195–1204. [Google Scholar] [CrossRef]
- Biagi, E.; Franceschi, C.; Rampelli, S.; Severgnini, M.; Ostan, R.; Turroni, S.; Consolandi, C.; Quercia, S.; Scurti, M.; Monti, D.; et al. Gut Microbiota and Extreme Longevity. Curr. Biol. 2016, 26, 1480–1485. [Google Scholar] [CrossRef]
- Mukherjee, A.; Lordan, C.; Ross, R.P.; Cotter, P.D. Gut microbes from the phylogenetically diverse genus Eubacterium and their various contributions to gut health. Gut Microbes 2020, 12, 1802866. [Google Scholar] [CrossRef]
- Di, S.; Wang, Y.; Han, L.; Bao, Q.; Gao, Z.; Wang, Q.; Yang, Y.; Zhao, L.; Tong, X. The Intervention Effect of Traditional Chinese Medicine on the Intestinal Flora and Its Metabolites in Glycolipid Metabolic Disorders. Evid.-Based Complement. Altern. Med. 2019, 2019, 1–13. [Google Scholar] [CrossRef]
- Zhu, W.; Gregory, J.C.; Org, E.; Buffa, J.A.; Gupta, N.; Wang, Z.; Li, L.; Fu, X.; Wu, Y.; Mehrabian, M.; et al. Gut Microbial Metabolite TMAO Enhances Platelet Hyperreactivity and Thrombosis Risk. Cell 2016, 165, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Gandy, K.A.O.; Zhang, J.; Nagarkatti, P.; Nagarkatti, M. The role of gut microbiota in shaping the relapse-remitting and chronic-progressive forms of multiple sclerosis in mouse models. Sci. Rep. 2019, 9, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Russell, W.R.; Gratz, S.W.; Duncan, S.H.; Holtrop, G.; Ince, J.; Scobbie, L.; Duncan, G.; Johnstone, A.M.; Lobley, G.E.; Wallace, R.J.; et al. High-protein, reduced-carbohydrate weight-loss diets promote metabolite profiles likely to be detrimental to colonic health. Am. J. Clin. Nutr. 2011, 93, 1062–1072. [Google Scholar] [CrossRef] [PubMed]
- Pataky, Z.; Genton, L.; Spahr, L.; Lazarevic, V.; Terraz, S.; Gaïa, N.; Rubbia-Brandt, L.; Golay, A.; Schrenzel, J.; Pichard, C. Impact of Hypocaloric Hyperproteic Diet on Gut Microbiota in Overweight or Obese Patients with Nonalcoholic Fatty Liver Disease: A Pilot Study. Am. J. Dig. Dis. 2016, 61, 2721–2731. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtit, J.P.; et al. A core gut microbiome in obese and lean twins. Nature 2009, 457, 480–484. [Google Scholar] [CrossRef]
- Parker, B.J.; Wearsch, P.A.; Veloo, A.C.M.; Rodriguez-Palacios, A. The Genus Alistipes: Gut Bacteria With Emerging Implications to Inflammation, Cancer, and Mental Health. Front. Immunol. 2020, 11, 906. [Google Scholar] [CrossRef]
- Candela, M.; Biagi, E.; Soverini, M.; Consolandi, C.; Quercia, S.; Severgnini, M.; Peano, C.; Turroni, S.; Rampelli, S.; Pozzilli, P.; et al. Modulation of gut microbiota dysbioses in type 2 diabetic patients by macrobiotic Ma-Pi 2 diet. Br. J. Nutr. 2016, 116, 80–93. [Google Scholar] [CrossRef]
- Vacca, M.; Celano, G.; Calabrese, F.M.; Portincasa, P.; Gobbetti, M.; De Angelis, M. The Controversial Role of Human Gut Lachnospiraceae. Microorganisms 2020, 8, 573. [Google Scholar] [CrossRef]
- de la Cuesta-Zuluaga, J.; Mueller, N.T.; Álvarez-Quintero, R.; Velásquez-Mejía, E.P.; Sierra, J.A.; Corrales-Agudelo, V.; Carmona, J.A.; Abad, J.M.; Escobar, J.S. Higher Fecal Short-Chain Fatty Acid Levels Are Associated with Gut Microbiome Dysbiosis, Obesity, Hypertension and Cardiometabolic Disease Risk Factors. Nutrients 2018, 11, 51. [Google Scholar] [CrossRef]
- Kim, K.N.; Yao, Y.; Ju, S.Y. Short Chain Fatty Acids and Fecal Microbiota Abundance in Humans with Obesity: A Systematic Review and Meta-Analysis. Nutrients 2019, 11, 2512. [Google Scholar] [CrossRef]
- Tirosh, A.; Calay, E.S.; Tuncman, G.; Claiborn, K.C.; Inouye, K.E.; Eguchi, K.; Alcala, M.; Rathaus, M.; Hollander, K.S.; Ron, I.; et al. The short-chain fatty acid propionate increases glucagon and FABP4 production, impairing insulin action in mice and humans. Sci. Transl. Med. 2019, 11, eaav0120. [Google Scholar] [CrossRef] [PubMed]
- Perry, R.J.; Peng, L.; Barry, N.A.; Cline, G.W.; Zhang, D.; Cardone, R.L.; Petersen, K.F.; Kibbey, R.G.; Goodman, A.L.; Shulman, G.I. Acetate mediates a microbiome–brain–β-cell axis to promote metabolic syndrome. Nature 2016, 534, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Youssef, O.; Lahti, L.; Kokkola, A.; Karla, T.; Tikkanen, M.; Ehsan, H.; Carpelan-Holmström, M.; Koskensalo, S.; Böhling, T.; Rautelin, H.; et al. Stool Microbiota Composition Differs in Patients with Stomach, Colon, and Rectal Neoplasms. Am. J. Dig. Dis. 2018, 63, 2950–2958. [Google Scholar] [CrossRef] [PubMed]
- Chumpitazi, B.P.; Hollister, E.B.; Oezguen, N.; Tsai, C.M.; McMeans, A.R.; Luna, R.A.; Savidge, T.C.; Versalovic, J.; Shulman, R.J. Gut microbiota influences low fermentable substrate diet efficacy in children with irritable bowel syndrome. Gut Microbes 2014, 5, 165–175. [Google Scholar] [CrossRef] [PubMed]
- De Groot, P.F.; Belzer, C.; Aydin, Ö.; Levin, E.; Levels, J.H.; Aalvink, S.; Boot, F.; Holleman, F.; Van Raalte, D.H.; Scheithauer, T.P.; et al. Distinct fecal and oral microbiota composition in human type 1 diabetes, an observational study. PLoS ONE 2017, 12, e0188475. [Google Scholar] [CrossRef]
- Ma, Q.; Zhai, R.; Xie, X.; Chen, T.; Zhang, Z.; Liu, H.; Nie, C.; Yuan, X.; Tu, A.; Tian, B.; et al. Hypoglycemic Effects of Lycium barbarum Polysaccharide in Type 2 Diabetes Mellitus Mice via Modulating Gut Microbiota. Front. Nutr. 2022, 9, 916271. [Google Scholar] [CrossRef]
- Wu, H.; Tremaroli, V.; Schmidt, C.; Lundqvist, A.; Olsson, L.M.; Krämer, M.; Gummesson, A.; Perkins, R.; Bergström, G.; Bäckhed, F. The Gut Microbiota in Prediabetes and Diabetes: A Population-Based Cross-Sectional Study. Cell Metab. 2020, 32, 379–390.e3. [Google Scholar] [CrossRef]
- Cunningham, A.L.; Stephens, J.W.; Harris, D.A. Gut microbiota influence in type 2 diabetes mellitus (T2DM). Gut Pathog. 2021, 13, 1–13. [Google Scholar] [CrossRef]
- Vitale, M.; Giacco, R.; Laiola, M.; Della Pepa, G.; Luongo, D.; Mangione, A.; Salamone, D.; Vitaglione, P.; Ercolini, D.; Rivellese, A.A. Acute and chronic improvement in postprandial glucose metabolism by a diet resembling the traditional Mediterranean dietary pattern: Can SCFAs play a role? Clin. Nutr. 2020, 40, 428–437. [Google Scholar] [CrossRef]
- Biddle, A.; Stewart, L.; Blanchard, J.L.; Leschine, S. Untangling the Genetic Basis of Fibrolytic Specialization by Lachnospiraceae and Ruminococcaceae in Diverse Gut Communities. Diversity 2013, 5, 627–640. [Google Scholar] [CrossRef]
- Bedu-Ferrari, C.; Biscarrat, P.; Langella, P.; Cherbuy, C. Prebiotics and the Human Gut Microbiota: From Breakdown Mechanisms to the Impact on Metabolic Health. Nutrients 2022, 14, 2096. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhou, X.; Wang, C.; Zhang, J.; Chang, D.; Liu, W.; Zhu, M.; Zhuang, S.; Shi, H.; Wang, X.; et al. Effect of Tanshinone IIA on Gut Microbiome in Diabetes-Induced Cognitive Impairment. Front. Pharmacol. 2022, 13, 890444. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; He, X.; Liu, J.; Zeng, F.; Chen, L.; Xu, W.; Shao, R.; Huang, Y.; Farag, M.A.; Capanoglu, E.; et al. Amelioration of type 2 diabetes by the novel 6, 8-guanidyl luteolin quinone-chromium coordination via biochemical mechanisms and gut microbiota interaction. J. Adv. Res. 2022. S2090-1232(22)00129-1. [Google Scholar] [CrossRef] [PubMed]
- Del Chierico, F.; Abbatini, F.; Russo, A.; Quagliariello, A.; Reddel, S.; Capoccia, D.; Caccamo, R.; Ginanni Corradini, S.; Nobili, V.; De Peppo, F.; et al. Gut Microbiota Markers in Obese Adolescent and Adult Patients: Age-Dependent Differential Patterns. Front. Microbiol. 2018, 9, 1210. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Liao, M.; Zhou, N.; Bao, L.; Ma, K.; Zheng, Z.; Wang, Y.; Liu, C.; Wang, W.; Wang, J.; et al. Parabacteroides distasonis Alleviates Obesity and Metabolic Dysfunctions via Production of Succinate and Secondary Bile Acids. Cell Rep. 2019, 26, 222–235.e5. [Google Scholar] [CrossRef]
- Gomez-Nguyen, A.; Basson, A.R.; Dark-Fleury, L.; Hsu, K.; Osme, A.; Menghini, P.; Pizarro, T.T.; Cominelli, F. Parabacteroides distasonis induces depressive-like behavior in a mouse model of Crohn’s disease. Brain Behav. Immun. 2021, 98, 245–250. [Google Scholar] [CrossRef]
- Zhao, J.-D.; Li, Y.; Sun, M.; Yu, C.-J.; Li, J.-Y.; Wang, S.-H.; Yang, D.; Guo, C.-L.; Du, X.; Zhang, W.-J.; et al. Effect of berberine on hyperglycaemia and gut microbiota composition in type 2 diabetic Goto-Kakizaki rats. World J. Gastroenterol. 2021, 27, 708–724. [Google Scholar] [CrossRef]
- Magzal, F.; Shochat, T.; Haimov, I.; Tamir, S.; Asraf, K.; Tuchner-Arieli, M.; Even, C.; Agmon, M. Increased physical activity improves gut microbiota composition and reduces short-chain fatty acid concentrations in older adults with insomnia. Sci. Rep. 2022, 12, 1–14. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, M.; Guo, Y.; Wang, Z.; Liu, Q.; Yan, R.; Wang, Y.; Wu, Q.; Yuan, K.; Sun, W. The Profile and Function of Gut Microbiota in Diabetic Nephropathy. Diabetes Metab. Syndr. Obes. Targets Ther. 2021, 14, 4283–4296. [Google Scholar] [CrossRef]
- Murphy, R.; Tsai, P.; Jüllig, M.; Liu, A.; Plank, L.; Booth, M. Differential Changes in Gut Microbiota After Gastric Bypass and Sleeve Gastrectomy Bariatric Surgery Vary According to Diabetes Remission. Obes. Surg. 2017, 27, 917–925. [Google Scholar] [CrossRef]
- Merkevičius, K.; Kundelis, R.; Maleckas, A.; Veličkienė, D. Microbiome Changes after Type 2 Diabetes Treatment: A Systematic Review. Medicina 2021, 57, 1084. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.J.; Florea, L.; Sears, C.L.; Maruthur, N.; Potter, J.J.; Schweitzer, M.; Magnuson, T.; Clark, J.M. Changes in Gut Microbiome after Bariatric Surgery Versus Medical Weight Loss in a Pilot Randomized Trial. Obes. Surg. 2019, 29, 3239–3245. [Google Scholar] [CrossRef] [PubMed]
- LE, T.K.C.; Hosaka, T.; Nguyen, T.T.; Kassu, A.; Dang, T.O.; Tran, H.B.; Pham, T.P.; Tran, Q.B.; Da Pham, X. Bifidobacterium species lower serum glucose, increase expressions of insulin signaling proteins, and improve adipokine profile in diabetic mice. Biomed. Res. 2015, 36, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; O’Riordan, M.X.D. Regulation of Bacterial Pathogenesis by Intestinal Short-Chain Fatty Acids. In Advances in Applied Microbiology; Elsevier: Amsterdam, The Netherlands, 2013; Volume 85, pp. 93–118. ISBN 978-0-12-407672-3. [Google Scholar]
- Özdemir, Ö. RETRACTED: Preventative and Therapeutic Role of Probiotics in Various Allergic and Autoimmune Disorders: An Up-to-Date Literature Review of Essential Experimental and Clinical Data. J. Evid.-Based Integr. Med. 2013, 18, 121–151. [Google Scholar] [CrossRef]
- Sishc, B.J.; Davis, A.J. The Role of the Core Non-Homologous End Joining Factors in Carcinogenesis and Cancer. Cancers 2017, 9, 81. [Google Scholar] [CrossRef]
- Coutinho, A.E.; Chapman, K.E. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol. Cell. Endocrinol. 2011, 335, 2–13. [Google Scholar] [CrossRef]
- Bonaventura, A.; Montecucco, F. Steroid-induced hyperglycemia: An underdiagnosed problem or clinical inertia? A narrative review. Diabetes Res. Clin. Pract. 2018, 139, 203–220. [Google Scholar] [CrossRef]
- Geer, E.B.; Islam, J.; Buettner, C. Mechanisms of Glucocorticoid-Induced Insulin Resistance. Endocrinol. Metab. Clin. N. Am. 2014, 43, 75–102. [Google Scholar] [CrossRef]
- Aberer, F.; Hochfellner, D.; Sourij, H.; Mader, J. A Practical Guide for the Management of Steroid Induced Hyperglycaemia in the Hospital. J. Clin. Med. 2021, 10, 2154. [Google Scholar] [CrossRef]
- Al Kury, L.T.; Abdoh, A.; Ikbariah, K.; Sadek, B.; Mahgoub, M. In Vitro and In Vivo Antidiabetic Potential of Monoterpenoids: An Update. Molecules 2021, 27, 182. [Google Scholar] [CrossRef]
- Hou, Y.-P.; He, Q.-Q.; Ouyang, H.-M.; Peng, H.-S.; Wang, Q.; Li, J.; Lv, X.-F.; Zheng, Y.-N.; Li, S.-C.; Liu, H.-L.; et al. Human Gut Microbiota Associated with Obesity in Chinese Children and Adolescents. BioMed Res. Int. 2017, 2017, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Palmas, V.; Pisanu, S.; Madau, V.; Casula, E.; Deledda, A.; Cusano, R.; Uva, P.; Loviselli, A.; Velluzzi, F.; Manzin, A. Gut Microbiota Markers and Dietary Habits Associated with Extreme Longevity in Healthy Sardinian Centenarians. Nutrients 2022, 14, 2436. [Google Scholar] [CrossRef] [PubMed]
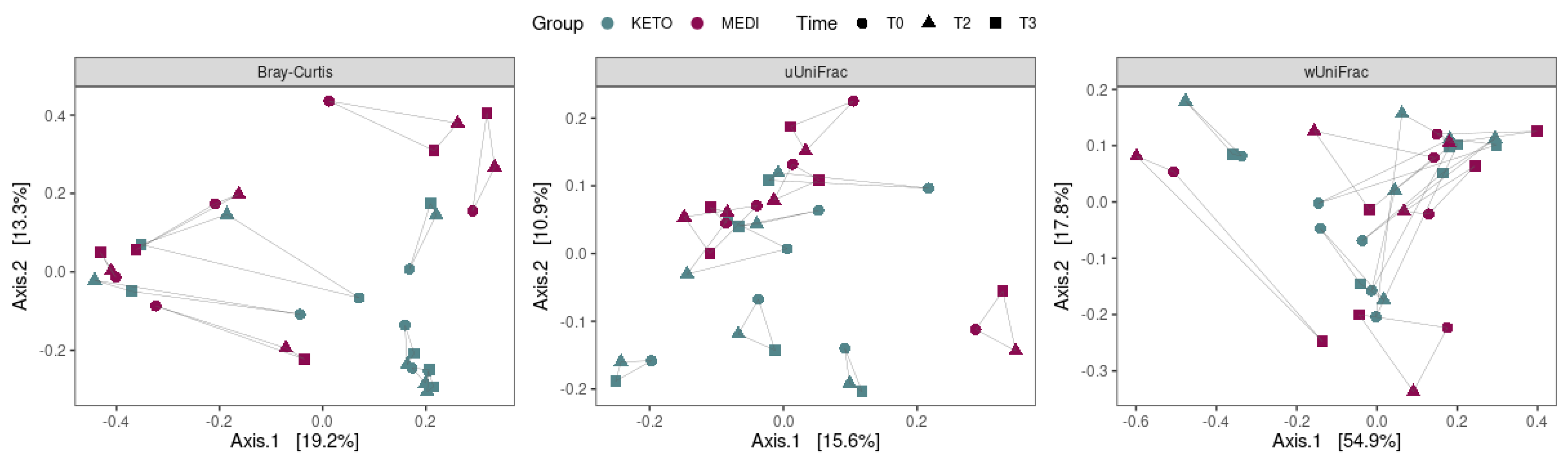
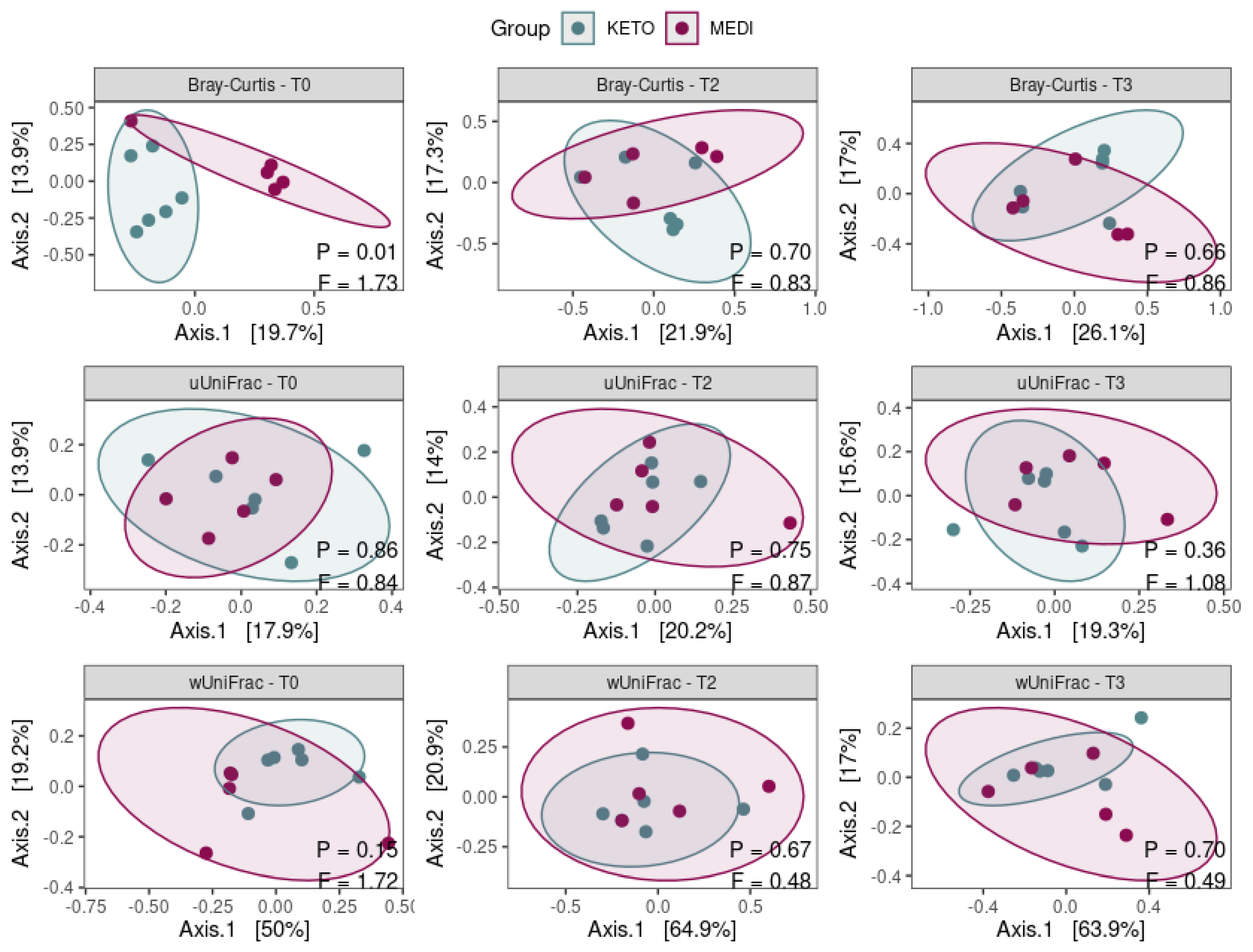
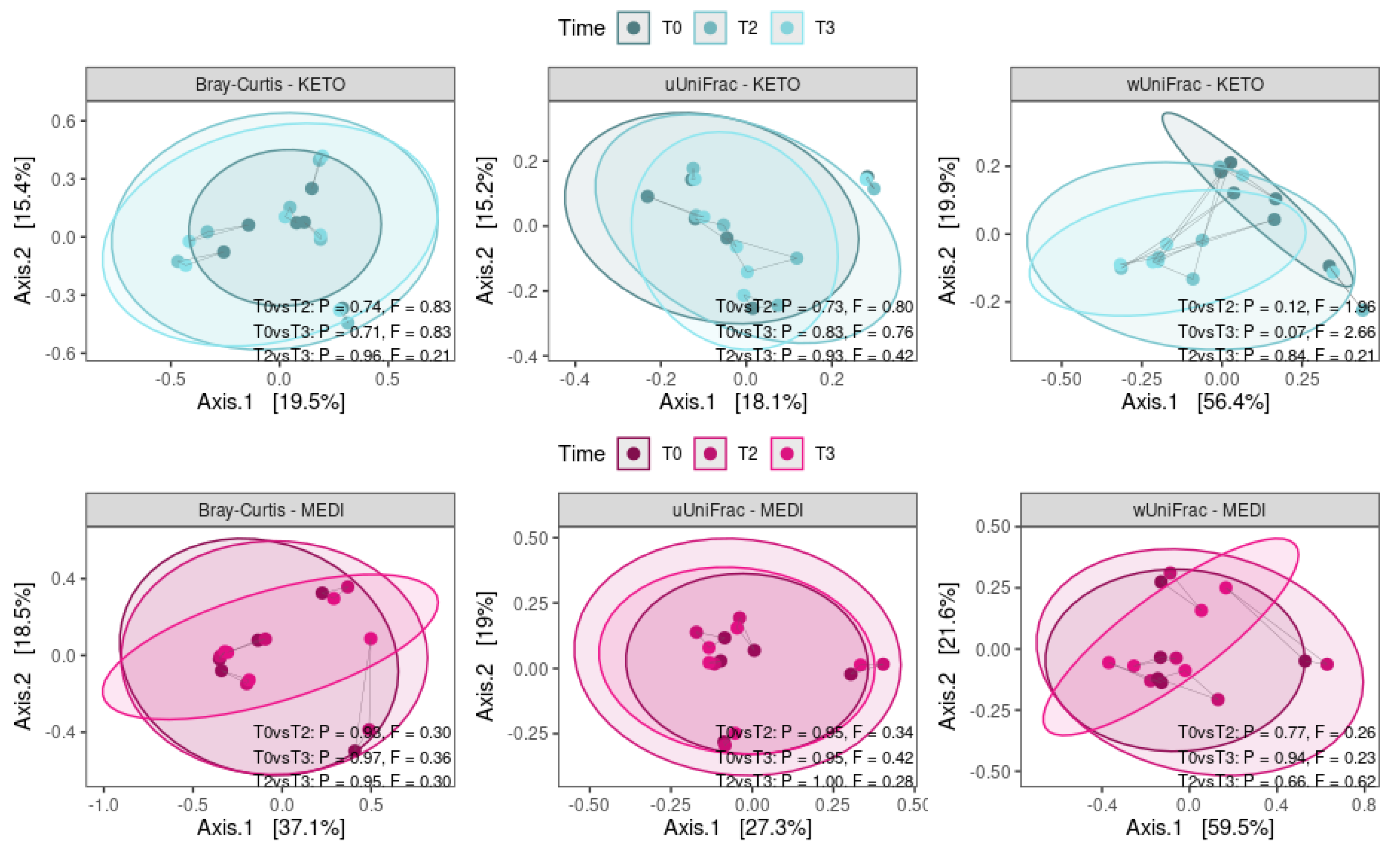
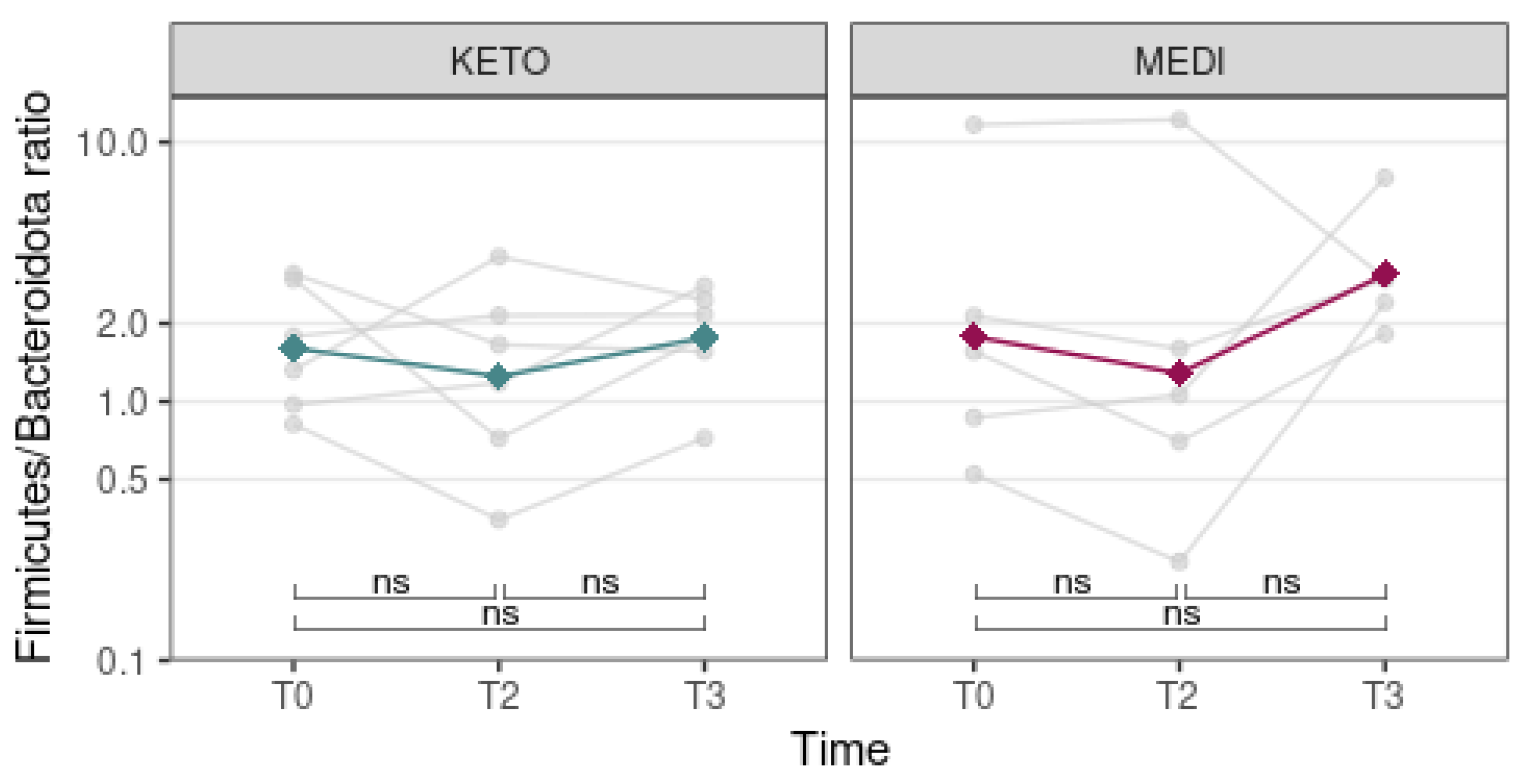

| Variable (M ± SD) | KETO | MEDI | p-Value |
|---|---|---|---|
| Body weight (kg) | 95.2 ± 15.2 | 86.7 ± 16.8 | 0.4 |
| BMI (kg/m2) | 35.3 ± 4.3 | 30.2 ± 4.2 | 0.07 |
| WC (cm) | 115.8 ± 6.6 | 109 ± 8.6 | 0.2 |
| FM (%) | 38.5 ± 8.2 | 34.8 ± 8.6 | 0.5 |
| FFM (kg) | 59.1 ± 15.6 | 56.5 ± 12.3 | 0.8 |
| phA (°) | 6.1 ± 0.4 | 5.6 ± 0.5 | 0.07 |
| FPG (mg/dL) | 127.7 ± 29.4 | 133.8 ± 10.2 | 0.7 |
| HbA1c (%) | 6.6 ± 0.9 | 7.1 ± 0.9 | 0.4 |
| Total Cholesterol (mg/dL) | 215.5 ± 66.3 | 219.2 ± 11.6 | 0.9 |
| LDL Cholesterol (mg/dL) | 137.3 ± 57.0 | 139.8 ± 12.8 | 0.9 |
| HDL Cholesterol (mg/dL) | 48.7 ± 11.9 | 53.4 ± 9.2 | 0.5 |
| Triglycerides (mg/dL) | 146.3 ± 82.7 | 130.4 ± 73.1 | 0.7 |
| SBP (mmHg) | 133 ± 15.4 | 151.0 ± 11.4 | 0.06 |
| DBP (mmHg) | 78.5 ± 9.0 | 81.0 ± 10.8 | 0.7 |
| MDS | 25.7 ± 7.3 | 26.8 ± 4.6 | 0.8 |
| PAL(METs/week) | 915.8 ± 1190.8 | 708.0 ± 559.9 | 0.8 |
| Daily sitting time (h/day) | 6.0 ± 2.3 | 7.2 ±2.7 | 0.4 |
| SF-36 PCS | 40.8 ± 9.6 | 50.4 ± 4.2 | 0.07 |
| SF-36 MCS | 46.3 ± 11.9 | 49.0 ± 15.0 | 0.7 |
| Variable (M ± SD) | KETO | MEDI | ||||
|---|---|---|---|---|---|---|
| T0 | T3 | p-Value | T0 | T3 | p-Value | |
| Body weight (kg) | 95.2 ± 15.1 | 80.9 ± 12.3 | <0.0001 | 86.7 ± 16.8 | 83.6 ± 18.3 | 0.02 |
| BMI (kg/m2) | 35.3 ± 4.3 | 30.0 ± 3.4 | <0.0001 | 30.2 ± 4.2 | 29.1 ± 4.8 | 0.02 |
| WC (cm) | 115.8 ± 6.6 | 103.5 ± 7.8 | <0.0002 | 109 ± 8.6 | 104.3 ± 9.5 | 0.004 |
| FM (%) | 38.5 ± 8.2 | 31.5 ± 9.3 | 0.001 | 34.8 ± 8.6 | 31.8 ± 9.6 | 0.07 |
| FFM (kg) | 59.1 ± 15.6 | 56.2 ± 15.0 | 0.01 | 56.5 ± 12.3 | 56.7 ± 13.2 | 0.88 |
| phA (°) | 6.1 ± 0.4 | 5.9 ± 0.5 | 0.36 | 5.6 ± 0.5 | 5.9 ± 0.6 | 0.25 |
| FPG (mg/dL) | 127.7 ± 29.4 | 102.8 ± 17.5 | 0.08 | 133.8 ± 10.2 | 140.6 ± 23.5 | 0.57 |
| HbA1c (%) | 6.6 ± 0.9 | 5.5 ± 0.5 | 0.012 | 7.1 ± 0,9 | 6.4 ± 0.8 | 0.22 |
| Total Cholesterol (mg/dL) | 215.5 ± 66.3 | 168 ± 40.7 | 0.08 | 219.2 ± 11.6 | 210.2 ± 20.7 | 0.36 |
| LDL Cholesterol (mg/dL) | 137.3 ± 57.0 | 103.6 ± 39.1 | 0.13 | 139.8 ± 12.8 | 124.2 ± 12.4 | 0.09 |
| HDL Cholesterol (mg/dL) | 48.7 ± 11.9 | 48.8 ± 9.1 | 0.9 | 53.4 ± 9.2 | 59.6 ± 8.4 | 0.16 |
| Triglycerides (mg/dL) | 146.3 ± 82.7 | 62.5 ± 19.4 | 0.08 | 130.4 ± 73.1 | 131.6 ± 96.4 | 0.92 |
| SBP (mmHg) | 133.0 ± 15.4 | 139.0 ± 15.9 | 0.14 | 151.0 ± 11.4 | 147.0 ± 17.2 | 0.33 |
| DBP (mmHg) | 78.5 ± 9.0 | 80.8 ± 3.8 | 0.45 | 81.0 ± 10.8 | 85.0 ± 13.5 | 0.5 |
| MDS | 25.7 ± 7.3 | 30 ± 5.7 | 0.2 | 26.8 ± 4.6 | 30.4 ± 5.6 | 0.4 |
| PAL (METs/week) | 915.8 ± 1190.8 | 755 ± 590.4 | 0.78 | 708.0 ± 559.9 | 754 ± 501 | 0.92 |
| Daily sitting time (h/day) | 6 ± 2.3 | 5.7 ± 1.9 | 0.57 | 7.2 ± 2.7 | 6.6 ± 3.3 | 0.6 |
| SF-36 PCS | 40.8 ± 9.6 | 46.8 ± 8.8 | 0.05 | 50.4 ± 4.2 | 46.6 ± 8.1 | 0.25 |
| SF-36 MCS | 46.3 ± 11.9 | 53.9 ± 8.8 | 0.03 | 49.0 ± 15.0 | 40.8 ± 9.6 | 0.23 |
| Variable (Δ T0–T3) | KETO | MEDI | p-Value |
|---|---|---|---|
| Body weight (kg) | −14.3 ± 3.1 | −3.04 ± 1.9 | <0.0001 |
| BMI (kg/m2) | −5.3 ± 0.9 | −1.1 ± 0.7 | <0.0001 |
| WC (cm) | −12.9 ± 3.1 | −4.7 ± 1.8 | 0.0006 |
| FM (%) | −7 ± 2.7 | −3.1 ± 2.7 | 0.03 |
| FFM (kg) | −2.8 ± 1.8 | +0. 2 ± 2.8 | 0,053 |
| phA (°) | −0.2 ± 0.2 | +0.3 ± 0.5 | 0.04 |
| FPG (mg/dL) | −24.8 ± 27.9 | +6.8 ± 25.3 | 0.08 |
| HbA1c (%) | −1.15 ± 0.7 | −0.7 ± 1.1 | 0.45 |
| Total Cholesterol (mg/dL) | −47.5 ± 54.9 | −3.8 ± 21.7 | 0.13 |
| LDL Cholesterol (mg/dL) | −30.5 ± 47.2 | −15.6 ± 16.3 | 0.52 |
| HDL Cholesterol (mg/dL) | +0.17 ± 6.1 | +6.2 ± 8.1 | 0.19 |
| Triglycerides (mg/dL) | −83.8 ± 96.4 | −1.2 ± 27 | 0.09 |
| SBP (mmHg) | +6.5 ± 9.3 | −4.0 ± 8.2 | 0.08 |
| DBP (mmHg) | +4.0 ± 7.6 | +4.0 ± 13.9 | 0.99 |
| MDS | +6 ± 5.9 | +3.6 ± 8.6 | 0.59 |
| PAL (METs/week) | +19.2 ± 1263 | +46 ± 1001 | 0.97 |
| Daily sitting time (h/day) | −0.3 ±1.4 | −0.6 ± 2.4 | 0.8 |
| SF-36 PCS | +6.2 ± 3.1 | −3.8 ± 6.4 | 0.007 |
| SF-36 MCS | +7.5 ± 6.8 | −8.2 ± 13.3 | 0.03 |
| Phylum | Class | Order | Family | Genus | Species | Ref. Group | p | q | ↓/↑ | Coeff. | Std.Err. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Actinobacteriota | T0–T2 | 0.073 | 0.218 | ↓ | −1.85 | 0.92 | |||||
| Actinobacteriota | T0–T3 | 0.087 | 0.224 | ↓ | −1.75 | 0.92 | |||||
| Bacteroidota | T0–T3 | 0.068 | 0.218 | ↓ | −0.64 | 0.32 | |||||
| Bacteroidia | Bacteroidales | T0–T3 | 0.048 | 0.242 | ↓ | −0.70 | 0.31 | ||||
| Tannerellaceae | T0–T2 | 0.016 | 0.136 | ↑ | 1.71 | 0.59 | |||||
| Tannerellaceae | T2–T3 | 0.029 | 0.179 | ↓ | −1.50 | 0.59 | |||||
| Parabacteroides | T0–T2 | 0.043 | 0.220 | ↑ | 1.40 | 0.60 | |||||
| Parabacteroides | T2–T3 | 0.027 | 0.204 | ↓ | −1.57 | 0.60 | |||||
| Parabacteroides_distasonis | T0–T2 | 0.007 | 0.113 | ↑ | 2.36 | 0.69 | |||||
| Parabacteroides_distasonis | T2–T3 | 0.027 | 0.226 | ↓ | −1.79 | 0.69 | |||||
| Bacteroidaceae | Bacteroides | T0–T3 | 0.018 | 0.203 | ↓ | −1.01 | 0.36 | ||||
| Barnesiellaceae | Barnesiella | T0–T3 | 0.034 | 0.204 | ↓ | −2.44 | 1.00 | ||||
| Rikenellaceae | Alistipes | T0–T2 | 0.011 | 0.203 | ↓ | −1.31 | 0.42 | ||||
| Rikenellaceae | Alistipes | T0–T3 | 0.032 | 0.204 | ↓ | −1.05 | 0.42 | ||||
| Alistipes_shahii | T0–T2 | 0.023 | 0.199 | ↓ | −1.66 | 0.62 | |||||
| Marinifilaceae | Butyricimonas | T0–T3 | 0.041 | 0.220 | ↓ | −1.51 | 0.64 | ||||
| Firmicutes | T0–T2 | 0.009 | 0.120 | ↓ | −0.74 | 0.23 | |||||
| Firmicutes | T0–T3 | 0.050 | 0.218 | ↓ | −0.51 | 0.23 | |||||
| Clostridia | T0–T2 | 0.015 | 0.162 | ↓ | −0.55 | 0.19 | |||||
| Peptostreptococcales.Tissierellales | T0–T2 | 0.013 | 0.129 | ↓ | −1.42 | 0.47 | |||||
| Christensenellales | T0–T2 | 0.010 | 0.129 | ↑ | 4.00 | 1.25 | |||||
| Christensenellales | T0–T3 | 0.008 | 0.129 | ↑ | 4.14 | 1.25 | |||||
| Christensenellaceae | T0–T2 | 0.010 | 0.131 | ↑ | 3.99 | 1.25 | |||||
| Christensenellaceae | T0–T3 | 0.008 | 0.131 | ↑ | 4.16 | 1.25 | |||||
| Christensenellaceae_R.7_group | T0–T2 | 0.014 | 0.203 | ↑ | 2.16 | 0.72 | |||||
| Christensenellaceae_R.7_group | T0–T3 | 0.011 | 0.203 | ↑ | 2.24 | 0.72 | |||||
| Genus_Christensenellaceae_R.7_group | T0–T2 | 0.005 | 0.110 | ↑ | 2.57 | 0.72 | |||||
| Genus_Christensenellaceae_R.7_group | T0–T3 | 0.004 | 0.110 | ↑ | 2.70 | 0.72 | |||||
| Clostridia_UCG.014 | T0–T3 | 0.013 | 0.129 | ↑ | 1.87 | 0.62 | |||||
| Clostridia_UCG.014 | T0–T3 | 0.012 | 0.136 | ↑ | 1.89 | 0.62 | |||||
| Clostridia_UCG.014 | T0–T3 | 0.024 | 0.204 | ↑ | 1.70 | 0.64 | |||||
| Genus_Clostridia_UCG.014 | T0–T3 | 0.009 | 0.113 | ↑ | 2.21 | 0.68 | |||||
| Oscillospirales | T0–T2 | 0.019 | 0.135 | ↓ | −0.85 | 0.30 | |||||
| Ruminococcaceae | T0–T2 | 0.008 | 0.131 | ↓ | −1.30 | 0.39 | |||||
| Subdoligranulum | T0–T2 | 0.019 | 0.203 | ↓ | −1.88 | 0.67 | |||||
| UCG.010 | T0–T3 | 0.009 | 0.131 | ↑ | 1.26 | 0.39 | |||||
| UCG.010 | T2–T3 | 0.002 | 0.131 | ↑ | 1.60 | 0.39 | |||||
| UCG.010 | T0–T3 | 0.048 | 0.226 | ↑ | 1.05 | 0.46 | |||||
| UCG.010 | T2–T3 | 0.007 | 0.203 | ↑ | 1.57 | 0.46 | |||||
| Genus_UCG.010 | T0–T3 | 0.002 | 0.110 | ↑ | 1.62 | 0.39 | |||||
| Genus_UCG.010 | T2–T3 | 0.003 | 0.110 | ↑ | 1.55 | 0.39 | |||||
| Oscillospiraceae | Intestinimonas | T0–T2 | 0.025 | 0.204 | ↑ | 2.69 | 1.02 | ||||
| UCG.005 | Genus_UCG.005 | T0–T3 | 0.016 | 0.157 | ↑ | 2.54 | 0.87 | ||||
| Lachnospiraceae | X.Eubacterium._xylanophilum_group | T0–T2 | 0.046 | 0.226 | ↑ | 1.49 | 0.65 | ||||
| Lachnospiraceae | X.Eubacterium._xylanophilum_group | T0–T3 | 0.002 | 0.203 | ↑ | 2.69 | 0.65 | ||||
| Genus_.Eubacterium._xylanophilum_group | T0–T2 | 0.009 | 0.113 | ↑ | 1.81 | 0.56 | |||||
| Lachnospiraceae | Genus_.Eubacterium._xylanophilum_group | T0–T3 | 0.000 | 0.039 | ↑ | 3.06 | 0.56 | ||||
| Lachnospiraceae | X.Eubacterium._eligens_group | T0–T2 | 0.018 | 0.203 | ↑ | 2.78 | 1.05 | ||||
| Lachnospiraceae | X.Eubacterium._eligens_group | T0–T3 | 0.032 | 0.204 | ↑ | 2.49 | 1.05 | ||||
| Lachnospiraceae | Genus_.Eubacterium._eligens_group | T0–T2 | 0.005 | 0.110 | ↑ | 3.28 | 0.99 | ||||
| Genus_.Eubacterium._eligens_group | T0–T3 | 0.008 | 0.113 | ↑ | 3.03 | 0.99 | |||||
| Lachnospiraceae | Lachnoclostridium | T0–T3 | 0.042 | 0.220 | ↓ | −1.74 | 0.75 | ||||
| Lachnospiraceae | Lachnoclostridium | T2–T3 | 0.006 | 0.203 | ↓ | −2.57 | 0.75 | ||||
| Genus_Lachnoclostridium | T2–T3 | 0.009 | 0.113 | ↓ | −2.48 | 0.77 | |||||
| Lachnospiraceae | Anaerostipes | T0–T2 | 0.033 | 0.204 | ↓ | −2.37 | 0.96 | ||||
| Lachnospiraceae | Dorea | T0–T2 | 0.032 | 0.204 | ↓ | −1.85 | 0.74 | ||||
| Lachnospiraceae | Agathobacter | T0–T2 | 0.013 | 0.203 | ↓ | −2.65 | 0.87 | ||||
| Lachnospiraceae | Fusicatenibacter | T0–T2 | 0.016 | 0.203 | ↓ | −2.12 | 0.73 | ||||
| Lachnospiraceae | X.Ruminococcus._torques_group | T0–T3 | 0.041 | 0.220 | ↓ | −1.40 | 0.60 | ||||
| Peptococcales | T0–T2 | 0.026 | 0.143 | ↑ | 2.24 | 0.85 | |||||
| Peptococcaceae | T0–T2 | 0.025 | 0.175 | ↑ | 2.24 | 0.85 | |||||
| Family_Peptococcaceae | T0–T2 | 0.033 | 0.204 | ↑ | 2.06 | 0.83 | |||||
| Family_Peptococcaceae | T0–T2 | 0.011 | 0.123 | ↑ | 2.73 | 0.88 | |||||
| Family_Peptococcaceae | T0–T3 | 0.021 | 0.198 | ↑ | 2.41 | 0.88 | |||||
| Verrucomicrobiota | T0–T2 | 0.014 | 0.120 | ↑ | 5.07 | 1.70 | |||||
| Verrucomicrobiota | T0–T3 | 0.020 | 0.120 | ↑ | 4.69 | 1.70 | |||||
| Verrucomicrobiae | T0–T2 | 0.014 | 0.162 | ↑ | 5.06 | 1.70 | |||||
| Verrucomicrobiae | T0–T3 | 0.020 | 0.162 | ↑ | 4.69 | 1.70 | |||||
| Verrucomicrobiales | T0–T2 | 0.014 | 0.129 | ↑ | 5.03 | 1.70 | |||||
| Verrucomicrobiales | T0–T3 | 0.021 | 0.135 | ↑ | 4.65 | 1.70 | |||||
| Akkermansiaceae | T0–T2 | 0.014 | 0.136 | ↑ | 5.02 | 1.69 | |||||
| Akkermansiaceae | T0–T3 | 0.020 | 0.157 | ↑ | 4.66 | 1.69 | |||||
| Akkermansia | T0–T2 | 0.016 | 0.203 | ↑ | 4.72 | 1.63 | |||||
| Akkermansia | T0–T3 | 0.024 | 0.204 | ↑ | 4.34 | 1.63 |
| Phylum | Class | Order | Family | Genus | Species | Ref. Group | p | q | ↓/↑ | Coeff. | Std._Err. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Actinobacteriota | T0-T3 | 0.029 | 0.212 | ↑ | 1.90 | 0.71 | |||||
| Actinobacteriota | T2-T3 | 0.056 | 0.212 | ↑ | 1.59 | 0.71 | |||||
| Firmicutes | T2-T3 | 0.057 | 0.212 | ↑ | 0.61 | 0.27 | |||||
| Desulfobacterota | T2-T3 | 0.048 | 0.212 | ↓ | −2.45 | 1.05 | |||||
| Bacteroidota | Bacteroidia | Bacteroidales | Bacteroidaceae | Bacteroides | Genus_Bacteroides | T2-T3 | 0.002 | 0.246 | ↓ | −2.17 | 0.49 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deledda, A.; Palmas, V.; Heidrich, V.; Fosci, M.; Lombardo, M.; Cambarau, G.; Lai, A.; Melis, M.; Loi, E.; Loviselli, A.; et al. Dynamics of Gut Microbiota and Clinical Variables after Ketogenic and Mediterranean Diets in Drug-Naïve Patients with Type 2 Diabetes Mellitus and Obesity. Metabolites 2022, 12, 1092. https://doi.org/10.3390/metabo12111092
Deledda A, Palmas V, Heidrich V, Fosci M, Lombardo M, Cambarau G, Lai A, Melis M, Loi E, Loviselli A, et al. Dynamics of Gut Microbiota and Clinical Variables after Ketogenic and Mediterranean Diets in Drug-Naïve Patients with Type 2 Diabetes Mellitus and Obesity. Metabolites. 2022; 12(11):1092. https://doi.org/10.3390/metabo12111092
Chicago/Turabian StyleDeledda, Andrea, Vanessa Palmas, Vitor Heidrich, Michele Fosci, Mauro Lombardo, Giulia Cambarau, Alessio Lai, Marietta Melis, Elisabetta Loi, Andrea Loviselli, and et al. 2022. "Dynamics of Gut Microbiota and Clinical Variables after Ketogenic and Mediterranean Diets in Drug-Naïve Patients with Type 2 Diabetes Mellitus and Obesity" Metabolites 12, no. 11: 1092. https://doi.org/10.3390/metabo12111092
APA StyleDeledda, A., Palmas, V., Heidrich, V., Fosci, M., Lombardo, M., Cambarau, G., Lai, A., Melis, M., Loi, E., Loviselli, A., Manzin, A., & Velluzzi, F. (2022). Dynamics of Gut Microbiota and Clinical Variables after Ketogenic and Mediterranean Diets in Drug-Naïve Patients with Type 2 Diabetes Mellitus and Obesity. Metabolites, 12(11), 1092. https://doi.org/10.3390/metabo12111092