Metabolic Dysfunction-Associated Fatty Liver Disease in the National Health and Nutrition Examination Survey 2017–2020: Epidemiology, Clinical Correlates, and the Role of Diagnostic Scores
Abstract
1. Introduction
2. Materials and Methods
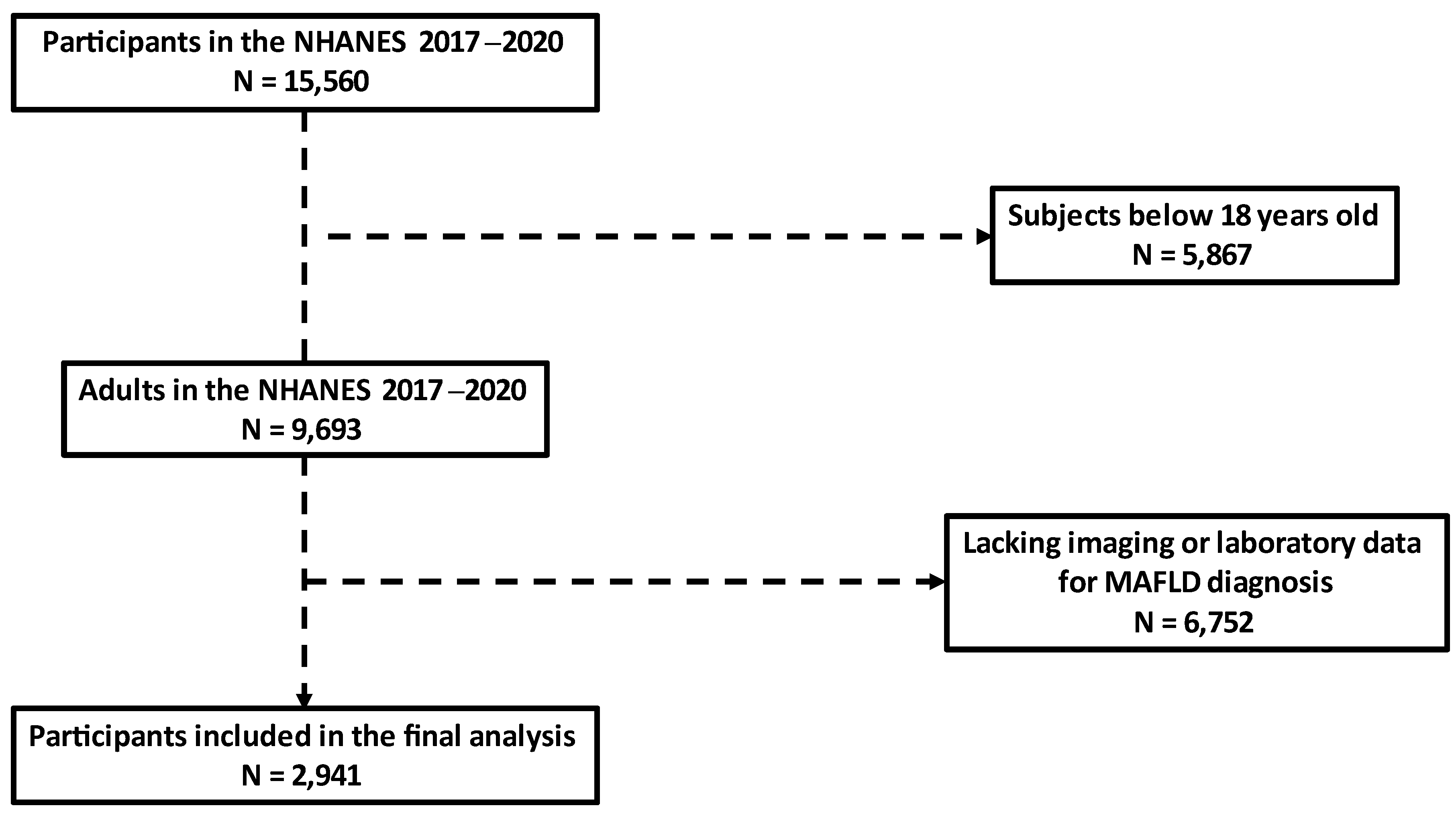
2.1. Study Population
2.2. Assessment of Liver Steatosis and Fibrosis
2.3. Definition of Variables
- Overweight/obesity (body mass index (BMI) ≥ 25 kg/m2 in Caucasians and ≥23 kg/m2 in Asians);
- T2DM (fasting plasma glucose ≥ 126 mg/dL, glycated hemoglobin ≥ 6.5% [15], or known history of the disease);
- At least two metabolic risk abnormalities:
- o
- Waist circumference ≥ 102/88 cm in Caucasian men and women or ≥90/80 cm in Asian men and women;
- o
- Blood pressure ≥ 130/85 mmHg or specific drug treatment;
- o
- Plasma triglycerides ≥ 150 mg/dL or specific drug treatment;
- o
- Plasma high-density lipoprotein cholesterol (HDL-C) < 40 mg/dL in men or <50 mg/dL in women, or specific drug treatment;
- o
- Prediabetes (fasting plasma glucose 100–125 mg/dL or glycated hemoglobin 5.7–6.4%);
- o
- Homeostasis model assessment of insulin resistance (HOMA-IR) ≥ 2.5;
- o
- High-sensitivity C reactive protein (hsCRP) > 2 mg/L.
2.4. Laboratory Evaluations
2.5. Statistical Analysis
3. Results
3.1. Epidemiology of MAFLD and Associations with Other Risk Factors
3.2. Association of MAFLD with Paraclinical Biomarkers
3.3. The Role of Fatty Liver Index in MAFLD Diagnosis
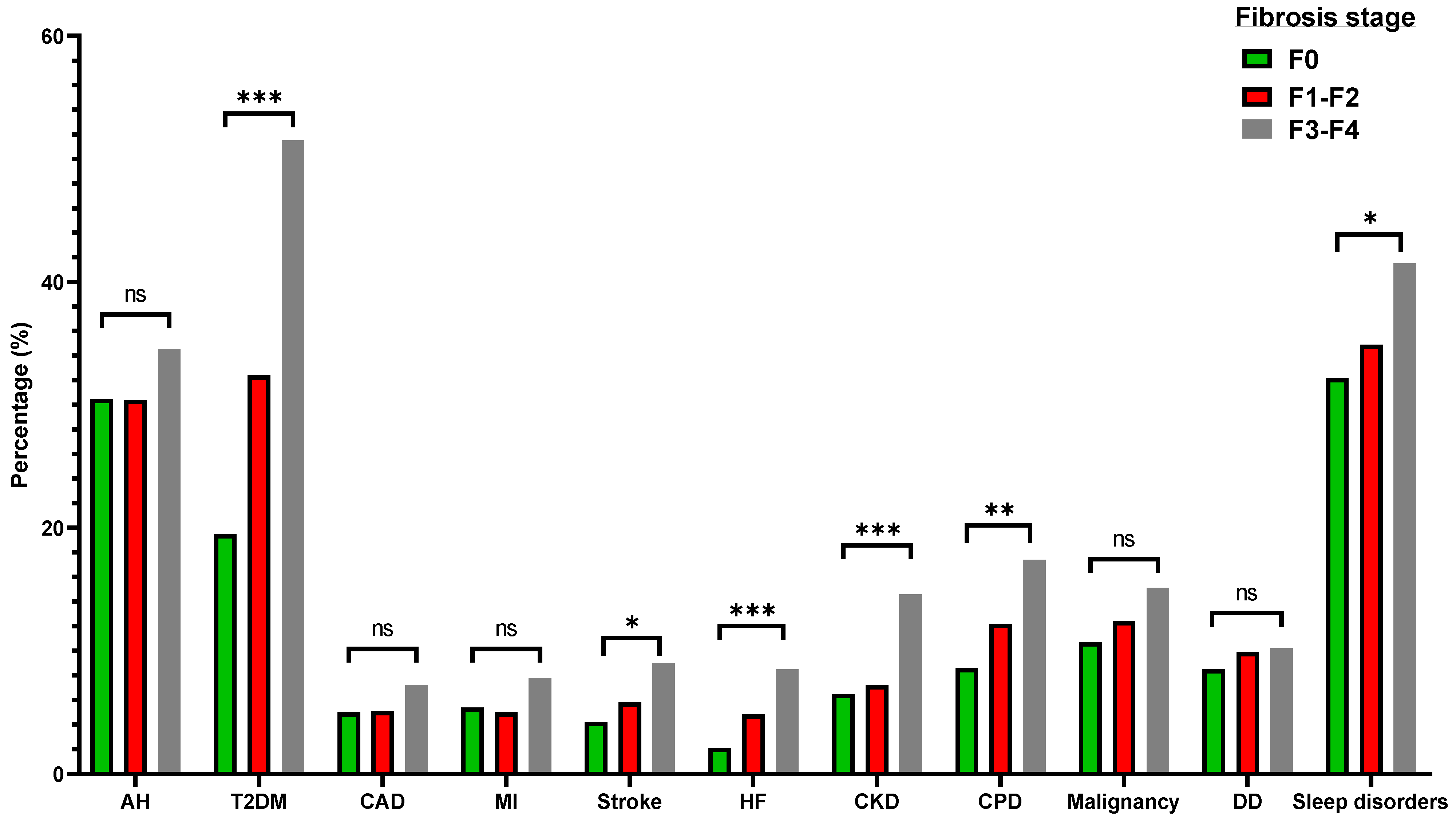
3.4. Elastography-Derived Liver Fibrosis in MAFLD
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bluher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, X.; Wang, Z.; Bancks, M.P.; Carnethon, M.R.; Greenland, P.; Feng, Y.Q.; Wang, H.; Zhong, V.W. Trends in Prevalence of Diabetes and Control of Risk Factors in Diabetes among US Adults, 1999–2018. JAMA 2021, 326, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Le, M.H.; Yeo, Y.H.; Li, X.; Li, J.; Zou, B.; Wu, Y.; Ye, Q.; Huang, D.Q.; Zhao, C.; Zhang, J.; et al. 2019 Global NAFLD Prevalence: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Golabi, P.; de Avila, L.; Paik, J.M.; Srishord, M.; Fukui, N.; Qiu, Y.; Burns, L.; Afendy, A.; Nader, F. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J. Hepatol. 2019, 71, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wai-Sun Wong, V.; Dufour, J.F.; Schattenberg, J.M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, C.J.; Ahn, S.H.; Lee, K.S.; Lee, B.K.; Baik, S.J.; Kim, S.U.; Lee, J.I. MAFLD Predicts the Risk of Cardiovascular Disease Better than NAFLD in Asymptomatic Subjects with Health Check-Ups. Dig. Dis. Sci. 2022, 67, 4919–4928. [Google Scholar] [CrossRef]
- Arai, T.; Atsukawa, M.; Tsubota, A.; Kato, K.; Abe, H.; Ono, H.; Kawano, T.; Yoshida, Y.; Tanabe, T.; Okubo, T.; et al. Liver fibrosis is associated with carotid atherosclerosis in patients with liver biopsy-proven nonalcoholic fatty liver disease. Sci. Rep. 2021, 11, 15938. [Google Scholar] [CrossRef]
- Aljawad, M.; Sirpal, S.; Yoshida, E.M.; Chandok, N. Transient elastography in Canada: Current state and future directions. Can. J. Gastroenterol. Hepatol. 2015, 29, 373–376. [Google Scholar] [CrossRef]
- Jun, B.G.; Park, W.Y.; Park, E.J.; Jang, J.Y.; Jeong, S.W.; Lee, S.H.; Kim, S.G.; Cha, S.W.; Kim, Y.S.; Cho, Y.D.; et al. A prospective comparative assessment of the accuracy of the FibroScan in evaluating liver steatosis. PLoS ONE 2017, 12, e0182784. [Google Scholar] [CrossRef]
- Koehler, E.M.; Schouten, J.N.; Hansen, B.E.; Hofman, A.; Stricker, B.H.; Janssen, H.L. External validation of the fatty liver index for identifying nonalcoholic fatty liver disease in a population-based study. Clin. Gastroenterol. Hepatol. 2013, 11, 1201–1204. [Google Scholar] [CrossRef]
- Bedogni, G.; Bellentani, S.; Miglioli, L.; Masutti, F.; Passalacqua, M.; Castiglione, A.; Tiribelli, C. The Fatty Liver Index: A simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006, 6, 33. [Google Scholar] [CrossRef]
- Angulo, P.; Hui, J.M.; Marchesini, G.; Bugianesi, E.; George, J.; Farrell, G.C.; Enders, F.; Saksena, S.; Burt, A.D.; Bida, J.P.; et al. The NAFLD fibrosis score: A noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007, 45, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Sterling, R.K.; Lissen, E.; Clumeck, N.; Sola, R.; Correa, M.C.; Montaner, J.; Mark, S.S.; Torriani, F.J.; Dieterich, D.T.; Thomas, D.L.; et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006, 43, 1317–1325. [Google Scholar] [CrossRef] [PubMed]
- Karlas, T.; Petroff, D.; Sasso, M.; Fan, J.G.; Mi, Y.Q.; de Ledinghen, V.; Kumar, M.; Lupsor-Platon, M.; Han, K.H.; Cardoso, A.C.; et al. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J. Hepatol. 2017, 66, 1022–1030. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Professional Practice, C. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2022. Diabetes Care 2022, 45, S17–S38. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef]
- Inker, L.A.; Eneanya, N.D.; Coresh, J.; Tighiouart, H.; Wang, D.; Sang, Y.; Crews, D.C.; Doria, A.; Estrella, M.M.; Froissart, M.; et al. New Creatinine- and Cystatin C-Based Equations to Estimate GFR without Race. N. Engl. J. Med. 2021, 385, 1737–1749. [Google Scholar] [CrossRef]
- Levis, B.; Benedetti, A.; Thombs, B.D.; Collaboration, D.E.S.D. Accuracy of Patient Health Questionnaire-9 (PHQ-9) for screening to detect major depression: Individual participant data meta-analysis. BMJ 2019, 365, l1476. [Google Scholar] [CrossRef]
- Cacho, J.; Sevillano, J.; de Castro, J.; Herrera, E.; Ramos, M.P. Validation of simple indexes to assess insulin sensitivity during pregnancy in Wistar and Sprague-Dawley rats. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E1269–E1276. [Google Scholar] [CrossRef]
- Cordova, C.M.; Schneider, C.R.; Juttel, I.D.; Cordova, M.M. Comparison of LDL-cholesterol direct measurement with the estimate using the Friedewald formula in a sample of 10,664 patients. Arq. Bras. Cardiol. 2004, 83, 476–487. [Google Scholar] [CrossRef]
- Fluss, R.; Faraggi, D.; Reiser, B. Estimation of the Youden Index and its associated cutoff point. Biom. J. 2005, 47, 458–472. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Guo, M.; An, Z.; Meng, J.; Jiang, J.; Song, J.; Wu, W. Prevalence and Risk Factors of Metabolic Associated Fatty Liver Disease in Xinxiang, China. Int. J. Environ. Res. Public Health 2020, 17, 1818. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.W.; Wong, G.L.; Woo, J.; Abrigo, J.M.; Chan, C.K.; Shu, S.S.; Leung, J.K.; Chim, A.M.; Kong, A.P.; Lui, G.C.; et al. Impact of the New Definition of Metabolic Associated Fatty Liver Disease on the Epidemiology of the Disease. Clin. Gastroenterol. Hepatol. 2021, 19, 2161–2171.e2165. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Li, H.; Li, S.; Xu, Z.; Tian, S.; Wu, J.; Liang, X.Y.; Li, X.; Liu, Z.L.; Xiao, J.; et al. Prevalence of and risk factors for metabolic associated fatty liver disease in an urban population in China: A cross-sectional comparative study. BMC Gastroenterol. 2021, 21, 212. [Google Scholar] [CrossRef]
- Wang, X.; Wu, S.; Yuan, X.; Chen, S.; Fu, Q.; Sun, Y.; Lan, Y.; Hu, S.; Wang, Y.; Lu, Y.; et al. Metabolic Dysfunction-associated Fatty Liver Disease and Mortality among Chinese Adults: A Prospective Cohort Study. J. Clin. Endocrinol. Metab. 2022, 107, e745–e755. [Google Scholar] [CrossRef]
- Lee, H.; Lee, Y.H.; Kim, S.U.; Kim, H.C. Metabolic Dysfunction-Associated Fatty Liver Disease and Incident Cardiovascular Disease Risk: A Nationwide Cohort Study. Clin. Gastroenterol. Hepatol. 2021, 19, 2138–2147.e2110. [Google Scholar] [CrossRef]
- Ciardullo, S.; Perseghin, G. Prevalence of NAFLD, MAFLD and associated advanced fibrosis in the contemporary United States population. Liver Int. 2021, 41, 1290–1293. [Google Scholar] [CrossRef]
- Liu, J.; Ayada, I.; Zhang, X.; Wang, L.; Li, Y.; Wen, T.; Ma, Z.; Bruno, M.J.; de Knegt, R.J.; Cao, W.; et al. Estimating Global Prevalence of Metabolic Dysfunction-Associated Fatty Liver Disease in Overweight or Obese Adults. Clin. Gastroenterol. Hepatol. 2022, 20, e573–e582. [Google Scholar] [CrossRef]
- Lee, H.; Lim, T.S.; Kim, S.U.; Kim, H.C. Long-term cardiovascular outcomes differ across metabolic dysfunction-associated fatty liver disease subtypes among middle-aged population. Hepatol. Int. 2022. [Google Scholar] [CrossRef]
- Quek, J.; Ng, C.H.; Tang, A.S.P.; Chew, N.; Chan, M.; Khoo, C.M.; Wei, C.P.; Chin, Y.H.; Tay, P.; Lim, G.; et al. Metabolic Associated Fatty Liver Disease Increases the Risk of Systemic Complications and Mortality. A Meta-Analysis and Systematic Review of 12,620,736 Individuals. Endocr. Pract. 2022, 28, 667–672. [Google Scholar] [CrossRef]
- Wen, W.; Li, H.; Wang, C.; Chen, C.; Tang, J.; Zhou, M.; Hong, X.; Cheng, Y.; Wu, Q.; Zhang, X.; et al. Metabolic dysfunction-associated fatty liver disease and cardiovascular disease: A meta-analysis. Front. Endocrinol. 2022, 13, 934225. [Google Scholar] [CrossRef] [PubMed]
- Masarone, M.; Rosato, V.; Dallio, M.; Gravina, A.G.; Aglitti, A.; Loguercio, C.; Federico, A.; Persico, M. Role of Oxidative Stress in Pathophysiology of Nonalcoholic Fatty Liver Disease. Oxid. Med. Cell. Longev. 2018, 2018, 9547613. [Google Scholar] [CrossRef] [PubMed]
- Theofilis, P.; Sagris, M.; Oikonomou, E.; Antonopoulos, A.S.; Siasos, G.; Tsioufis, C.; Tousoulis, D. Inflammatory Mechanisms Contributing to Endothelial Dysfunction. Biomedicines 2021, 9, 781. [Google Scholar] [CrossRef] [PubMed]
- Theofilis, P.; Vordoni, A.; Nakas, N.; Kalaitzidis, R.G. Endothelial Dysfunction in Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Life 2022, 12, 718. [Google Scholar] [CrossRef]
- Qu, W.; Ma, T.; Cai, J.; Zhang, X.; Zhang, P.; She, Z.; Wan, F.; Li, H. Liver Fibrosis and MAFLD: From Molecular Aspects to Novel Pharmacological Strategies. Front. Med. 2021, 8, 761538. [Google Scholar] [CrossRef]
- Theofilis, P.; Sagris, M.; Antonopoulos, A.S.; Oikonomou, E.; Tsioufis, C.; Tousoulis, D. Inflammatory Mediators of Platelet Activation: Focus on Atherosclerosis and COVID-19. Int. J. Mol. Sci. 2021, 22, 11170. [Google Scholar] [CrossRef]
- Oikonomou, E.; Leopoulou, M.; Theofilis, P.; Antonopoulos, A.S.; Siasos, G.; Latsios, G.; Mystakidi, V.C.; Antoniades, C.; Tousoulis, D. A link between inflammation and thrombosis in atherosclerotic cardiovascular diseases: Clinical and therapeutic implications. Atherosclerosis 2020, 309, 16–26. [Google Scholar] [CrossRef]
- Stenvinkel, P.; Chertow, G.M.; Devarajan, P.; Levin, A.; Andreoli, S.P.; Bangalore, S.; Warady, B.A. Chronic Inflammation in Chronic Kidney Disease Progression: Role of Nrf2. Kidney Int. Rep. 2021, 6, 1775–1787. [Google Scholar] [CrossRef]
- Han, A.L. Validation of fatty liver index as a marker for metabolic dysfunction-associated fatty liver disease. Diabetol. Metab. Syndr. 2022, 14, 44. [Google Scholar] [CrossRef]
- Han, A.L.; Lee, H.K. Comparison of the Diagnostic Performance of Steatosis Indices for Discrimination of CT-Diagnosed Metabolic Dysfunction-Associated Fatty Liver Disease. Metabolites 2022, 12, 664. [Google Scholar] [CrossRef]
- Wu, J.; Tian, S.; Li, H.; Xu, Z.; Li, S.; Chen, Y.L.; Liang, X.Y.; Xiao, J.; Song, J.Y.; She, R.L.; et al. Population-specific cut-off points of fatty liver index: A study based on the National Health and Nutrition Examination Survey data. BMC Gastroenterol. 2022, 22, 265. [Google Scholar] [CrossRef] [PubMed]
- Dhar, D.; Baglieri, J.; Kisseleva, T.; Brenner, D.A. Mechanisms of liver fibrosis and its role in liver cancer. Exp. Biol. Med. 2020, 245, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Ostovaneh, M.R.; Ambale-Venkatesh, B.; Fuji, T.; Bakhshi, H.; Shah, R.; Murthy, V.L.; Tracy, R.P.; Guallar, E.; Wu, C.O.; Bluemke, D.A.; et al. Association of Liver Fibrosis with Cardiovascular Diseases in the General Population: The Multi-Ethnic Study of Atherosclerosis (MESA). Circ. Cardiovasc. Imaging 2018, 11, e007241. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Csermely, A.; Petracca, G.; Beatrice, G.; Corey, K.E.; Simon, T.G.; Byrne, C.D.; Targher, G. Non-alcoholic fatty liver disease and risk of fatal and non-fatal cardiovascular events: An updated systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2021, 6, 903–913. [Google Scholar] [CrossRef]
- Ciardullo, S.; Ballabeni, C.; Trevisan, R.; Perseghin, G. Liver Stiffness, Albuminuria and Chronic Kidney Disease in Patients with NAFLD: A Systematic Review and Meta-Analysis. Biomolecules 2022, 12, 105. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.G.; Yan, L.J.; Li, X.; Yao, S.Y.; Su, P.; Li, H.C.; Ding, Z.N.; Wang, D.X.; Dong, Z.R.; Li, T. Comments on validation of conventional non-invasive fibrosis scoring systems in patients with metabolic associated fatty liver disease. World J. Gastroenterol. 2022, 28, 689–692. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.L.; Kumar, R.; Wang, M.F.; Singh, M.; Huang, J.F.; Zhu, Y.Y.; Lin, S. Validation of conventional non-invasive fibrosis scoring systems in patients with metabolic associated fatty liver disease. World J. Gastroenterol. 2021, 27, 5753–5763. [Google Scholar] [CrossRef]
- Fang, J.M.; Cheng, J.; Chang, M.F.; Ahn, J.; Westerhoff, M. Transient elastography versus liver biopsy: Discordance in evaluations for fibrosis and steatosis from a pathology standpoint. Mod. Pathol. 2021, 34, 1955–1962. [Google Scholar] [CrossRef]
- Theofilis, P.; Sagris, M.; Oikonomou, E.; Antonopoulos, A.S.; Siasos, G.; Tsioufis, K.; Tousoulis, D. Pleiotropic effects of SGLT2 inhibitors and heart failure outcomes. Diabetes Res. Clin. Pract. 2022, 188, 109927. [Google Scholar] [CrossRef]
- Theofilis, P.; Sagris, M.; Oikonomou, E.; Antonopoulos, A.S.; Siasos, G.; Tsioufis, K.; Tousoulis, D. The impact of SGLT2 inhibitors on inflammation: A systematic review and meta-analysis of studies in rodents. Int. Immunopharmacol. 2022, 111, 109080. [Google Scholar] [CrossRef]
- Bendotti, G.; Montefusco, L.; Lunati, M.E.; Usuelli, V.; Pastore, I.; Lazzaroni, E.; Assi, E.; Seelam, A.J.; El Essawy, B.; Jang, J.; et al. The anti-inflammatory and immunological properties of GLP-1 Receptor Agonists. Pharmacol. Res. 2022, 182, 106320. [Google Scholar] [CrossRef] [PubMed]
- Zafar, Y.; Rashid, A.M.; Siddiqi, A.K.; Ellahi, A.; Ahmed, A.; Hussain, H.U.; Ahmed, F.; Menezes, R.G.; Siddiqi, T.J.; Maniya, M.T. Effect of novel glucose lowering agents on non-alcoholic fatty liver disease: A systematic review and meta-analysis. Clin. Res. Hepatol. Gastroenterol. 2022, 46, 101970. [Google Scholar] [CrossRef] [PubMed]

| MAFLD (−) (N = 1217) | MAFLD (+) (N = 1724) | p | |
|---|---|---|---|
| Clinical characteristics | |||
| Age, years | 46 (31, 62) | 56 (43, 66) | <0.001 |
| Male sex, % | 53.0 | 55.9 | 0.13 |
| BMI, kg/m2 | 25.1 (22.3, 28.4) | 31.2 (27.2, 36.1) | <0.001 |
| Waist circumference, cm | 88.9 (80.6, 98.3) | 106.5 (97.8, 117.8) | <0.001 |
| Systolic blood pressure, mmHg | 118 (109, 131) | 124 (114, 137) | <0.001 |
| Diastolic blood pressure, mmHg | 71 (65, 78) | 76 (69, 84) | <0.001 |
| Medical history | |||
| Arterial hypertension, % | 19.8 | 30.9 | <0.001 |
| Type 2 diabetes mellitus, % | 7.1 | 27.3 | <0.001 |
| Coronary artery disease, % | 3.0 | 5.3 | 0.003 |
| Myocardial infarction, % | 2.9 | 5.5 | <0.001 |
| Stroke, % | 4.1 | 5.2 | 0.15 |
| Heart failure, % | 2.2 | 3.7 | 0.02 |
| Chronic kidney disease, % | 6.1 | 7.5 | 0.13 |
| Chronic pulmonary disease, % | 6.9 | 10.8 | <0.001 |
| Malignancy, % | 9.3 | 11.7 | 0.04 |
| Sleep disorders, % | 25.0 | 34.1 | <0.001 |
| Depressive disorder, % | 7.2 | 9.2 | 0.05 |
| MAFLD (−) (N = 1217) | MAFLD (+) (N = 1724) | p | |
|---|---|---|---|
| Glucose-insulin homeostasis | |||
| Fasting plasma glucose, mg/dL | 92 (86, 98) | 101 (93, 117) | <0.001 |
| Glycated hemoglobin (%) | 5.4 (5.2, 5.7) | 5.8 (5.4, 6.3) | <0.001 |
| HOMA-IR | 1.5 (1.0, 2.4) | 3.6 (2.2, 5.9) | <0.001 |
| Renal function | |||
| eGFR, ml/min/1.73 m2 | 98.1 (82.4, 111.7) | 95.6 (78.9, 108.2) | <0.001 |
| Urinary albumin-to-creatinine ratio | 6.6 (4.4, 11.0) | 7.8 (5.0, 15.4) | <0.001 |
| Liver biochemistry | |||
| AST, IU/L | 19 (16, 23) | 20 (16, 25) | 0.002 |
| ALT, IU/L | 15 (12, 22) | 21 (15, 30) | <0.001 |
| ALP, IU/L | 71 (59, 86) | 76 (64, 92) | <0.001 |
| GGT, IU/L | 18 (13, 26) | 25 (18, 38) | <0.001 |
| Lipid profile | |||
| Total cholesterol, mg/dL | 179 (155, 206) | 183 (158, 213) | 0.002 |
| LDL-Cholesterol, mg/dL | 103 (83, 126) | 115 (93, 139) | <0.001 |
| HDL-Cholesterol, mg/dL | 56 (48, 66) | 46 (40, 54) | <0.001 |
| Triglycerides, mg/dL | 84 (64, 114) | 126 (90, 175) | <0.001 |
| Uric acid, mg/dL | 5.1 (4.3, 6.0) | 5.7 (4.8, 6.8) | <0.001 |
| Inflammatory markers | |||
| hsCRP, mg/L | 1.1 (0.6, 2.7) | 2.6 (1.2, 5.2) | <0.001 |
| hsCRP/albumin ratio | 0.28 (0.13, 0.65) | 0.65 (0.30, 1.35) | <0.001 |
| Ferritin, ng/mL | 103 (53, 185) | 133 (65, 233) | <0.001 |
| White blood cells (K, μL) | 6000 (4900, 7200) | 6700 (5600, 8200) | <0.001 |
| Score | AUROC Curve | 95% Confidence Interval | p | Cutoff | Sensitivity | Specificity |
|---|---|---|---|---|---|---|
| FLI | 0.781 | 0.75–0.82 | <0.001 | ≥91.0 | 71.3 | 73.4 |
| NFS | 0.731 | 0.69–0.77 | <0.001 | ≥−0.72 | 70.2 | 65.7 |
| FIB4 | 0.605 | 0.56–0.65 | <0.001 | ≥1.26 | 48.0 | 69.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Theofilis, P.; Vordoni, A.; Kalaitzidis, R.G. Metabolic Dysfunction-Associated Fatty Liver Disease in the National Health and Nutrition Examination Survey 2017–2020: Epidemiology, Clinical Correlates, and the Role of Diagnostic Scores. Metabolites 2022, 12, 1070. https://doi.org/10.3390/metabo12111070
Theofilis P, Vordoni A, Kalaitzidis RG. Metabolic Dysfunction-Associated Fatty Liver Disease in the National Health and Nutrition Examination Survey 2017–2020: Epidemiology, Clinical Correlates, and the Role of Diagnostic Scores. Metabolites. 2022; 12(11):1070. https://doi.org/10.3390/metabo12111070
Chicago/Turabian StyleTheofilis, Panagiotis, Aikaterini Vordoni, and Rigas G. Kalaitzidis. 2022. "Metabolic Dysfunction-Associated Fatty Liver Disease in the National Health and Nutrition Examination Survey 2017–2020: Epidemiology, Clinical Correlates, and the Role of Diagnostic Scores" Metabolites 12, no. 11: 1070. https://doi.org/10.3390/metabo12111070
APA StyleTheofilis, P., Vordoni, A., & Kalaitzidis, R. G. (2022). Metabolic Dysfunction-Associated Fatty Liver Disease in the National Health and Nutrition Examination Survey 2017–2020: Epidemiology, Clinical Correlates, and the Role of Diagnostic Scores. Metabolites, 12(11), 1070. https://doi.org/10.3390/metabo12111070







