Liver Fetuin-A at Initiation of Insulin Resistance
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Diets, and Treatment
2.2. Hyperinsulinemic-Euglycemic Clamp Study
2.3. Biochemical Analyses
2.4. Immunofluorescence
2.5. Protein Studies–Western Blotting
2.6. RNA Extraction, Reverse Transcription, and RT-qPCR
2.7. Statistical Analysis
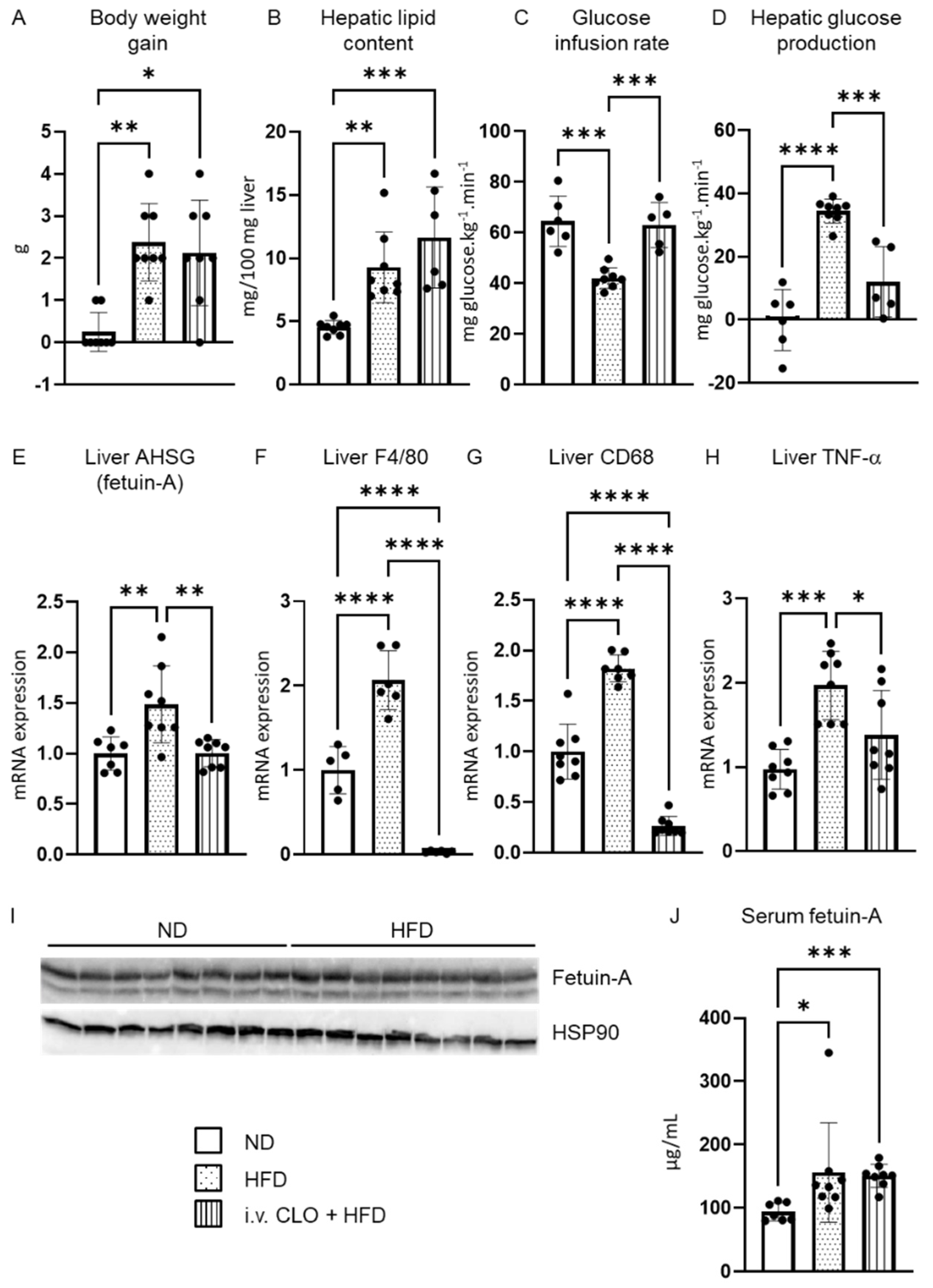
3. Results
3.1. Upregulation of AHSG mRNA Expression in the Liver under Short-Term High-Fat Feeding
3.2. Deletion of Hepatic Macrophages Modulates AHSG Expression
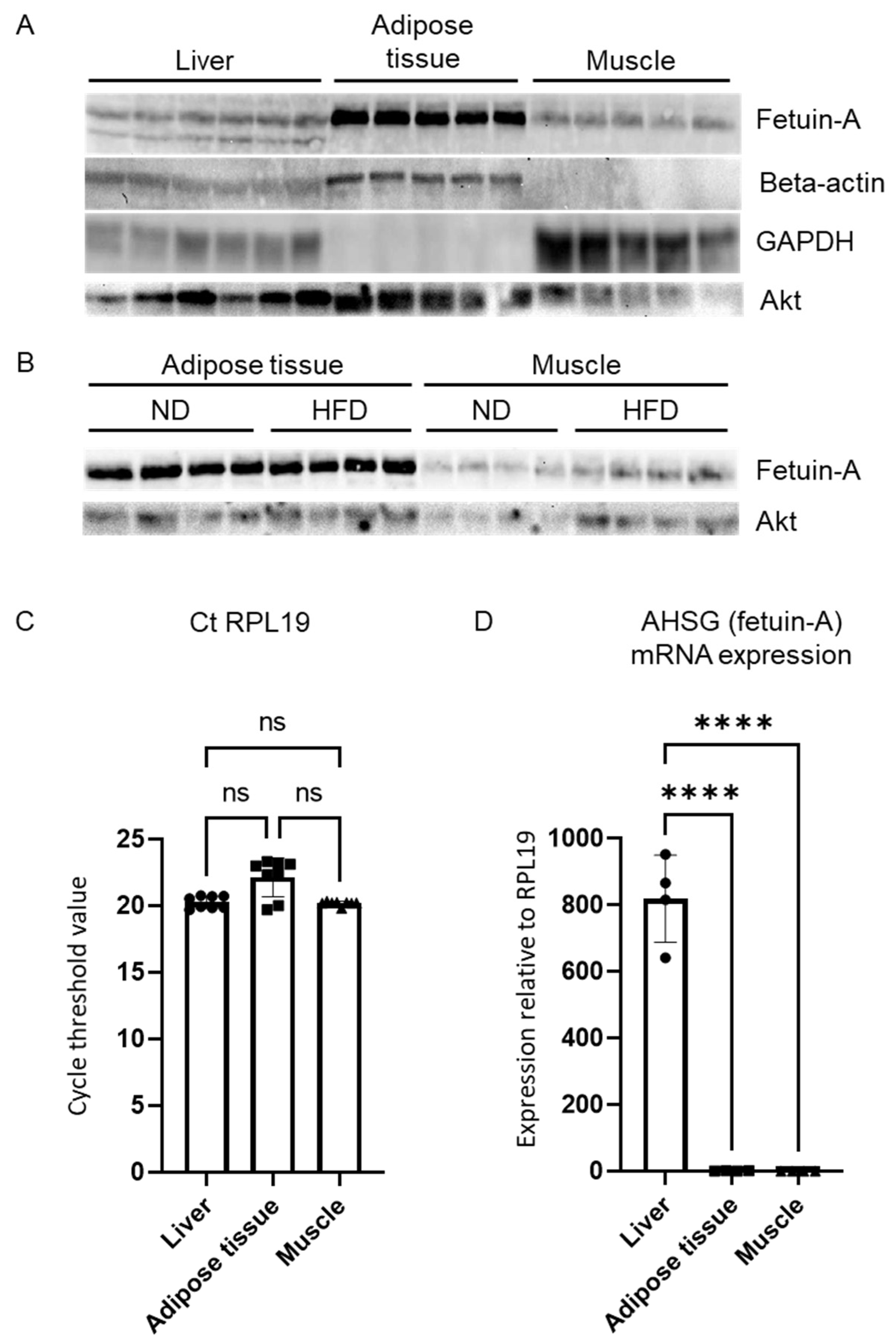
3.3. Fetuin-A Circulating Form and Distribution within the Liver
3.4. Fetuin-A in Other Insulin-Sensitive Tissues
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eslam, M.; Sanyal, A.J.; George, J.; Sanyal, A.; Neuschwander-Tetri, B.; Tiribelli, C.; Kleiner, D.E.; Brunt, E.; Bugianesi, E.; Yki-Järvinen, H.; et al. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology 2020, 158, 1999–2014.e1. [Google Scholar] [CrossRef] [PubMed]
- Francque, S.; Lanthier, N.; Verbeke, L.; Reynaert, H.; van Steenkiste, C.; Vonghia, L.; Kwanten, W.J.; Weyler, J.; Trépo, E.; Cassiman, D.; et al. The Belgian Association for Study of the Liver Guidance Document on the Management of Adult and Paediatric Non-Alcoholic Fatty Liver Disease. Acta Gastroenterol. Belg. 2018, 81, 55–81. [Google Scholar] [PubMed]
- Méndez-Sánchez, N.; Bugianesi, E.; Gish, R.G.; Lammert, F.; Tilg, H.; Nguyen, M.H.; Sarin, S.K.; Fabrellas, N.; Zelber-Sagi, S.; Fan, J.-G.; et al. Global Multi-Stakeholder Endorsement of the MAFLD Definition. Lancet Gastroenterol. Hepatol. 2022, 7, 388–390. [Google Scholar] [CrossRef]
- Lanthier, N.; Vanuytsel, T. Metabolic Dysfunction-Associated Fatty Liver Disease: A New Clearer Nomenclature with Positive Diagnostic Criteria. Acta Gastroenterol. Belg. 2020, 83, 513–515. [Google Scholar]
- Gill, M.G.; Majumdar, A. Metabolic Associated Fatty Liver Disease: Addressing a New Era in Liver Transplantation. World J. Hepatol. 2020, 12, 1168–1181. [Google Scholar] [CrossRef]
- Petersen, K.F.; Dufour, S.; Feng, J.; Befroy, D.; Dziura, J.; Dalla Man, C.; Cobelli, C.; Shulman, G.I. Increased Prevalence of Insulin Resistance and Nonalcoholic Fatty Liver Disease in Asian-Indian Men. Proc. Natl. Acad. Sci. USA 2006, 103, 18273–18277. [Google Scholar] [CrossRef]
- Kotronen, A.; Yki-Järvinen, H. Fatty Liver: A Novel Component of the Metabolic Syndrome. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 27–38. [Google Scholar] [CrossRef]
- Stefan, N.; Cusi, K. A Global View of the Interplay between Non-Alcoholic Fatty Liver Disease and Diabetes. Lancet Diabetes Endocrinol. 2022, 10, 284–296. [Google Scholar] [CrossRef]
- Samuel, V.T.; Liu, Z.X.; Qu, X.; Elder, B.D.; Bilz, S.; Befroy, D.; Romanelli, A.J.; Shulman, G.I. Mechanism of Hepatic Insulin Resistance in Non-Alcoholic Fatty Liver Disease. J. Biol. Chem. 2004, 279, 32345–32353. [Google Scholar] [CrossRef]
- Lanthier, N.; Molendi-Coste, O.; Horsmans, Y.; van Rooijen, N.; Cani, P.D.; Leclercq, I.A. Kupffer Cell Activation Is a Causal Factor for Hepatic Insulin Resistance. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 298, G107–G116. [Google Scholar] [CrossRef]
- Petersen, K.F.; Dufour, S.; Befroy, D.; Lehrke, M.; Hendler, R.E.; Shulman, G.I. Reversal of Nonalcoholic Hepatic Steatosis, Hepatic Insulin Resistance, and Hyperglycemia by Moderate Weight Reduction in Patients with Type 2 Diabetes. Diabetes 2005, 54, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Seppälä-Lindroos, A.; Vehkavaara, S.; Häkkinen, A.-M.; Goto, T.; Westerbacka, J.; Sovijärvi, A.; Halavaara, J.; Yki-Järvinen, H. Fat Accumulation in the Liver Is Associated with Defects in Insulin Suppression of Glucose Production and Serum Free Fatty Acids Independent of Obesity in Normal Men. J. Clin. Endocrinol. Metab. 2002, 87, 3023–3028. [Google Scholar] [CrossRef] [PubMed]
- Lanthier, N. The Role of the Liver in Insulin Resistance. Treat. Strateg. Hepatol. 2014, 1, 89–95. [Google Scholar]
- Binet, Q.; Loumaye, A.; Preumont, V.; Thissen, J.P.; Hermans, M.P.; Lanthier, N. Non-Invasive Screening, Staging and Management of Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD) in Type 2 Diabetes Mellitus Patients: What Do We Know so Far? Acta Gastroenterol. Belg. 2022, 85, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Busquets-Cortés, C.; Bennasar-Veny, M.; López-González, A.-A.; Fresneda, S.; Aguiló, A.; Yanez, A. Fatty Liver Index and Progression to Type 2 Diabetes: A 5-Year Longitudinal Study in Spanish Workers with Pre-Diabetes. BMJ Open 2021, 11, e045498. [Google Scholar] [CrossRef]
- Knudsen, C.; Neyrinck, A.M.; Leyrolle, Q.; Baldin, P.; Leclercq, S.; Rodriguez, J.; Beaumont, M.; Cani, P.D.; Bindels, L.B.; Lanthier, N.; et al. Hepatoprotective Effects of Indole, a Gut Microbial Metabolite, in Leptin-Deficient Obese Mice. J. Nutr. 2021, 151, 1507–1516. [Google Scholar] [CrossRef]
- Huang, W.; Metlakunta, A.; Dedousis, N.; Zhang, P.; Sipula, I.; Dube, J.J.; Scott, D.K.; O’Doherty, R.M. Depletion of Liver Kupffer Cells Prevents the Development of Diet-Induced Hepatic Steatosis and Insulin Resistance. Diabetes 2010, 59, 347–357. [Google Scholar] [CrossRef]
- Gadd, V.L.; Skoien, R.; Powell, E.E.; Fagan, K.J.; Winterford, C.; Horsfall, L.; Irvine, K.; Clouston, A.D. The Portal Inflammatory Infiltrate and Ductular Reaction in Human Nonalcoholic Fatty Liver Disease. Hepatology 2014, 59, 1393–1405. [Google Scholar] [CrossRef]
- Brøns, C.; Jensen, C.B.; Storgaard, H.; Hiscock, N.J.; White, A.; Appel, J.S.; Jacobsen, S.; Nilsson, E.; Larsen, C.M.; Astrup, A.; et al. Impact of Short-Term High-Fat Feeding on Glucose and Insulin Metabolism in Young Healthy Men. J. Physiol. 2009, 587, 2387–2397. [Google Scholar] [CrossRef]
- Armandi, A.; Rosso, C.; Caviglia, G.P.; Bugianesi, E. Insulin Resistance across the Spectrum of Nonalcoholic Fatty Liver Disease. Metabolites 2021, 11, 155. [Google Scholar] [CrossRef]
- Lanthier, N.; Leclercq, I.A. Liver and Systemic Insulin Resistance. Hepatology 2014, 60, 1113–1114. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.; Yuan, M.; Frantz, D.F.; Melendez, P.A.; Hansen, L.; Lee, J.; Shoelson, S.E. Local and Systemic Insulin Resistance Resulting from Hepatic Activation of IKK-β and NF-ΚB. Nat. Med. 2005, 11, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Clarembeau, F.; Bale, G.; Lanthier, N. Cirrhosis and Insulin Resistance: Current Knowledge, Pathophysiological Mechanisms, Complications and Potential Treatments. Clin. Sci. 2020, 134, 2117–2135. [Google Scholar] [CrossRef] [PubMed]
- Meex, R.C.R.; Watt, M.J. Hepatokines: Linking Nonalcoholic Fatty Liver Disease and Insulin Resistance. Nat. Rev. Endocrinol. 2017, 13, 509–520. [Google Scholar] [CrossRef]
- Watt, M.J.; Miotto, P.M.; de Nardo, W.; Montgomery, M.K. The Liver as an Endocrine Organ—Linking NAFLD and Insulin Resistance. Endocr. Rev. 2019, 40, 1367–1393. [Google Scholar] [CrossRef]
- Pal, D.; Dasgupta, S.; Kundu, R.; Maitra, S.; Das, G.; Mukhopadhyay, S.; Ray, S.; Majumdar, S.S.; Bhattacharya, S. Fetuin-A Acts as an Endogenous Ligand of TLR4 to Promote Lipid-Induced Insulin Resistance. Nat. Med. 2012, 18, 1279–1285. [Google Scholar] [CrossRef]
- Mathews, S.T.; Rakhade, S.; Zhou, X.; Parker, G.C.; Coscina, D.V.; Grunberger, G. Fetuin-Null Mice Are Protected against Obesity and Insulin Resistance Associated with Aging. Biochem. Biophys. Res. Commun. 2006, 350, 437–443. [Google Scholar] [CrossRef]
- Mathews, S.T.; Singh, G.P.; Ranalletta, M.; Cintron, V.J.; Qiang, X.; Goustin, A.S.; Jen, K.L.C.; Charron, M.J.; Jahnen-Dechent, W.; Grunberger, G. Improved Insulin Sensitivity and Resistance to Weight Gain in Mice Null for the Ahsg Gene. Diabetes 2002, 51, 2450–2458. [Google Scholar] [CrossRef]
- Lanthier, N.; Leclercq, I.A. Adipose Tissues as Endocrine Target Organs. Best Pract. Res. Clin. Gastroenterol. 2014, 28, 545–558. [Google Scholar] [CrossRef]
- Kim, T.H.; Hong, D.-G.; Yang, Y.M. Hepatokines and Non-Alcoholic Fatty Liver Disease: Linking Liver Pathophysiology to Metabolism. Biomedicines 2021, 9, 1903. [Google Scholar] [CrossRef]
- Etienne, Q.; Lebrun, V.; Komuta, M.; Navez, B.; Thissen, J.-P.; Leclercq, I.A.; Lanthier, N. Fetuin-A in Activated Liver Macrophages Is a Key Feature of Non-Alcoholic Steatohepatitis. Metabolites 2022, 12, 625. [Google Scholar] [CrossRef] [PubMed]
- Lanthier, N. Targeting Kupffer Cells in Non-Alcoholic Fatty Liver Disease/Non-Alcoholic Steatohepatitis: Why and How? World J. Hepatol. 2015, 7, 2184. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, I.A.; Lebrun, V.A.; Stärkel, P.; Horsmans, Y.J. Intrahepatic Insulin Resistance in a Murine Model of Steatohepatitis: Effect of PPARγ Agonist Pioglitazone. Lab. Investig. 2007, 87, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Lanthier, N.; Horsmans, Y.; Leclercq, I.A. Clodronate Liposomes: All Sites of Injection Are Not Equal. Hepatology 2010, 51, 721–722. [Google Scholar] [CrossRef] [PubMed]
- Lebrun, V.; Molendi-Coste, O.; Lanthier, N.; Sempoux, C.; Cani, P.D.; van Rooijen, N.; Stärkel, P.; Horsmans, Y.; Leclercq, I.A. Impact of PPAR-α Induction on Glucose Homoeostasis in Alcohol-Fed Mice. Clin. Sci. 2013, 125, 501–511. [Google Scholar] [CrossRef]
- Lanthier, N.; Molendi-Coste, O.; Cani, P.D.; Rooijen, N.; Horsmans, Y.; Leclercq, I.A. Kupffer Cell Depletion Prevents but Has No Therapeutic Effect on Metabolic and Inflammatory Changes Induced by a High-fat Diet. FASEB J. 2011, 25, 4301–4311. [Google Scholar] [CrossRef]
- Leclercq, I.A.; Farrell, G.C.; Field, J.; Bell, D.R.; Gonzalez, F.J.; Robertson, G.R. CYP2E1 and CYP4A as Microsomal Catalysts of Lipid Peroxides in Murine Nonalcoholic Steatohepatitis. J. Clin. Investig. 2000, 105, 1067–1075. [Google Scholar] [CrossRef]
- Sun, Q.; Cornelis, M.C.; Manson, J.A.E.; Hu, F.B. Plasma Levels of Fetuin-A and Hepatic Enzymes and Risk of Type 2 Diabetes in Women in the U.S. Diabetes 2013, 62, 49–55. [Google Scholar] [CrossRef][Green Version]
- Mori, K.; Emoto, M.; Yokoyama, H.; Araki, T.; Teramura, M.; Koyama, H.; Shoji, T.; Inaba, M.; Nishizawa, Y. Association of Serum Fetuin-A with Insulin Resistance in Type 2 Diabetic and Nondiabetic Subjects. Diabetes Care 2006, 29, 468. [Google Scholar] [CrossRef]
- Song, A.; Xu, M.; Bi, Y.; Xu, Y.; Huang, Y.; Li, M.; Wang, T.; Wu, Y.; Liu, Y.; Li, X.; et al. Serum Fetuin-A Associates with Type 2 Diabetes and Insulin Resistance in Chinese Adults. PLoS ONE 2011, 6, e19228. [Google Scholar] [CrossRef]
- Stefan, N.; Hennige, A.M.; Staiger, H.; Machann, J.; Schick, F.; Kröber, S.M.; Machicao, F.; Fritsche, A.; Häring, H.U. A2-Heremans-Schmid Glycoprotein/Fetuin-A Is Associated with Insulin Resistance and Fat Accumulation in the Liver in Humans. Diabetes Care 2006, 29, 853–857. [Google Scholar] [CrossRef] [PubMed]
- Filardi, T.; Panimolle, F.; Tiberti, C.; Crescioli, C.; Lenzi, A.; Pallotta, N.; Morano, S. Circulating Levels of Fetuin-A Are Associated with Moderate–Severe Hepatic Steatosis in Young Adults. J. Endocrinol. Investig. 2021, 44, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Willis, S.A.; Sargeant, J.A.; Yates, T.; Takamura, T.; Takayama, H.; Gupta, V.; Brittain, E.; Crawford, J.; Parry, S.A.; Thackray, A.E.; et al. Acute Hyperenergetic, High-Fat Feeding Increases Circulating FGF21, LECT2, and Fetuin-A in Healthy Men. J. Nutr. 2020, 150, 1076–1085. [Google Scholar] [CrossRef] [PubMed]
- Van der Meer, R.W.; Hammer, S.; Lamb, H.J.; Frölich, M.; Diamant, M.; Rijzewijk, L.J.; de Roos, A.; Romijn, J.A.; Smit, J.W.A. Effects of Short-Term High-Fat, High-Energy Diet on Hepatic and Myocardial Triglyceride Content in Healthy Men. J. Clin. Endocrinol. Metab. 2008, 93, 2702–2708. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Huang, X.; Ding, L.; Wang, P.; Peng, K.; Chen, Y.; Dai, M.; Zhang, D.; Xu, M.; Bi, Y.; et al. Serum Fetuin-A Associated With Fatty Liver Index, Early Indicator of Nonalcoholic Fatty Liver Disease. Medicine 2015, 94, e1517. [Google Scholar] [CrossRef]
- Kahraman, A.; Sowa, J.P.; Schlattjan, M.; Sydor, S.; Pronadl, M.; Wree, A.; Beilfuss, A.; Kilicarslan, A.; Altinbas, A.; Bechmann, L.P.; et al. Fetuin-A MRNA Expression Is Elevated in NASH Compared with NAFL Patients. Clin. Sci. 2013, 125, 391–400. [Google Scholar] [CrossRef]
- Yokota, S.; Fahimi, H.D. Immunocytochemical Localization of Albumin in the Secretory Apparatus of Rat Liver Parenchymal Cells. Proc. Natl. Acad. Sci. USA 1981, 78, 4970–4974. [Google Scholar] [CrossRef]
- Misu, H.; Takamura, T.; Takayama, H.; Hayashi, H.; Matsuzawa-Nagata, N.; Kurita, S.; Ishikura, K.; Ando, H.; Takeshita, Y.; Ota, T.; et al. A Liver-Derived Secretory Protein, Selenoprotein P, Causes Insulin Resistance. Cell Metab. 2010, 12, 483–495. [Google Scholar] [CrossRef]
- Khadir, A.; Kavalakatt, S.; Madhu, D.; Hammad, M.; Devarajan, S.; Tuomilehto, J.; Tiss, A. Fetuin-A Levels Are Increased in the Adipose Tissue of Diabetic Obese Humans but Not in Circulation. Lipids Health Dis. 2018, 17, 291. [Google Scholar] [CrossRef]
- Jenkins, N.T.; McKenzie, J.A.; Hagberg, J.M.; Witkowski, S. Plasma Fetuin-A Concentrations in Young and Older High- and Low-Active Men. Metabolism 2011, 60, 265–271. [Google Scholar] [CrossRef][Green Version]
- Lanthier, N.; Delzenne, N. Targeting the Gut Microbiome to Treat Metabolic Dysfunction-Associated Fatty Liver Disease: Ready for Prime Time? Cells 2022, 11, 2718. [Google Scholar] [CrossRef] [PubMed]
- Lefere, S.; Tacke, F. Macrophages in Obesity and Non-Alcoholic Fatty Liver Disease: Crosstalk with Metabolism. JHEP Rep. 2019, 1, 30–43. [Google Scholar] [CrossRef] [PubMed]
- Alharthi, J.; Latchoumanin, O.; George, J.; Eslam, M. Macrophages in Metabolic Associated Fatty Liver Disease. World J. Gastroenterol. 2020, 26, 1861–1878. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lanthier, N.; Lebrun, V.; Molendi-Coste, O.; van Rooijen, N.; Leclercq, I.A. Liver Fetuin-A at Initiation of Insulin Resistance. Metabolites 2022, 12, 1023. https://doi.org/10.3390/metabo12111023
Lanthier N, Lebrun V, Molendi-Coste O, van Rooijen N, Leclercq IA. Liver Fetuin-A at Initiation of Insulin Resistance. Metabolites. 2022; 12(11):1023. https://doi.org/10.3390/metabo12111023
Chicago/Turabian StyleLanthier, Nicolas, Valérie Lebrun, Olivier Molendi-Coste, Nico van Rooijen, and Isabelle A. Leclercq. 2022. "Liver Fetuin-A at Initiation of Insulin Resistance" Metabolites 12, no. 11: 1023. https://doi.org/10.3390/metabo12111023
APA StyleLanthier, N., Lebrun, V., Molendi-Coste, O., van Rooijen, N., & Leclercq, I. A. (2022). Liver Fetuin-A at Initiation of Insulin Resistance. Metabolites, 12(11), 1023. https://doi.org/10.3390/metabo12111023






