Effect of Myricetin on Lipid Metabolism in Primary Calf Hepatocytes Challenged with Long-Chain Fatty Acids
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Culture of Primary Hepatocytes
2.2. FA Preparation
2.3. Hepatocytes Treatment
2.4. Mesurement of TAG and NEFA Content
2.5. Immunofluorescence of Lipid Droplets
2.6. Determination of Hepatocyte Oxidation and Anti-Oxidant Indices
2.7. Protein Extraction and Western Blotting
2.8. RNA Extraction and Quantitative Real-Time PCR
2.9. Statistical Analysis
3. Results
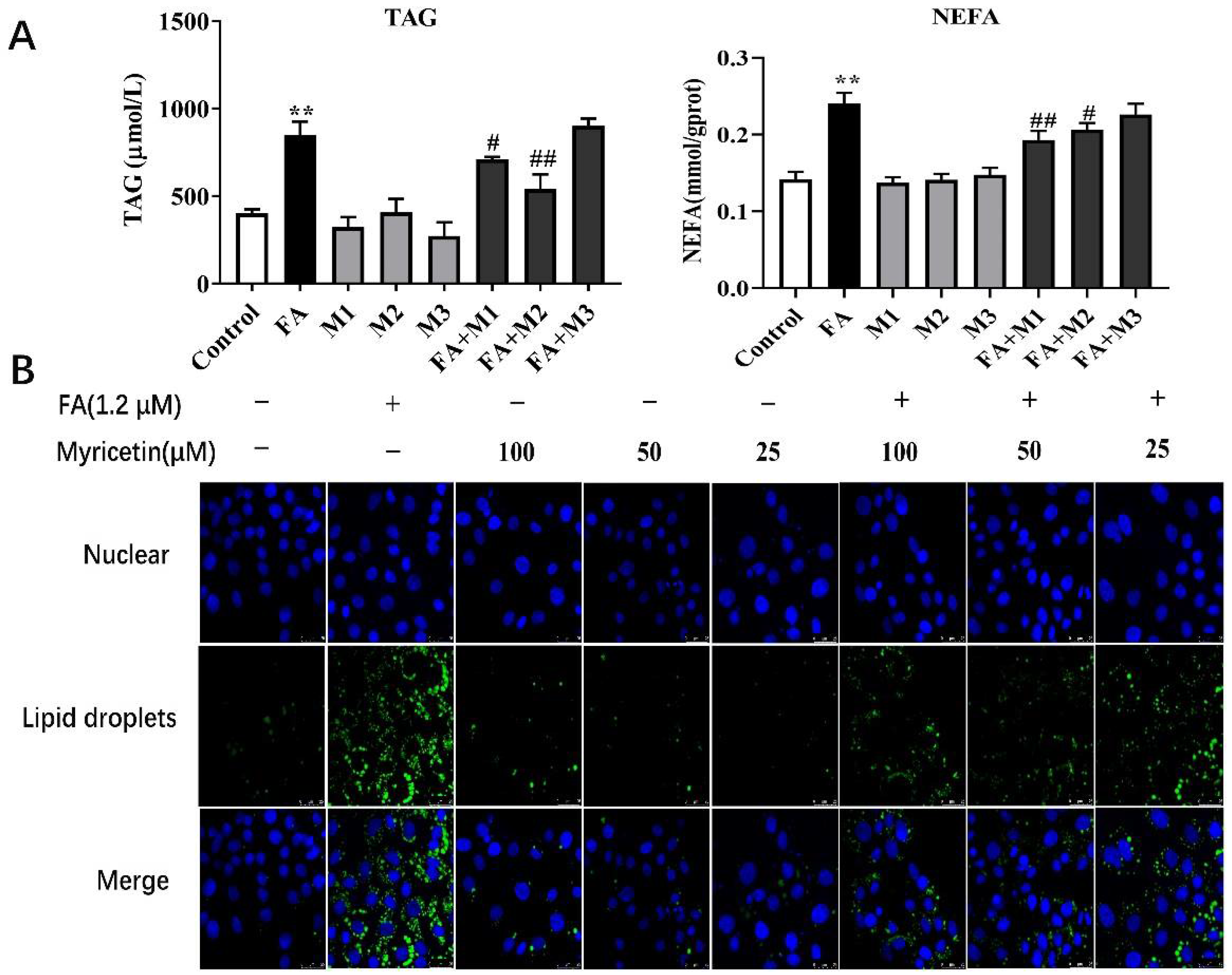
3.1. Effects of Myricetin on Lipid Accumulation
3.2. Effects of Myricetin on Lipid Metabolism
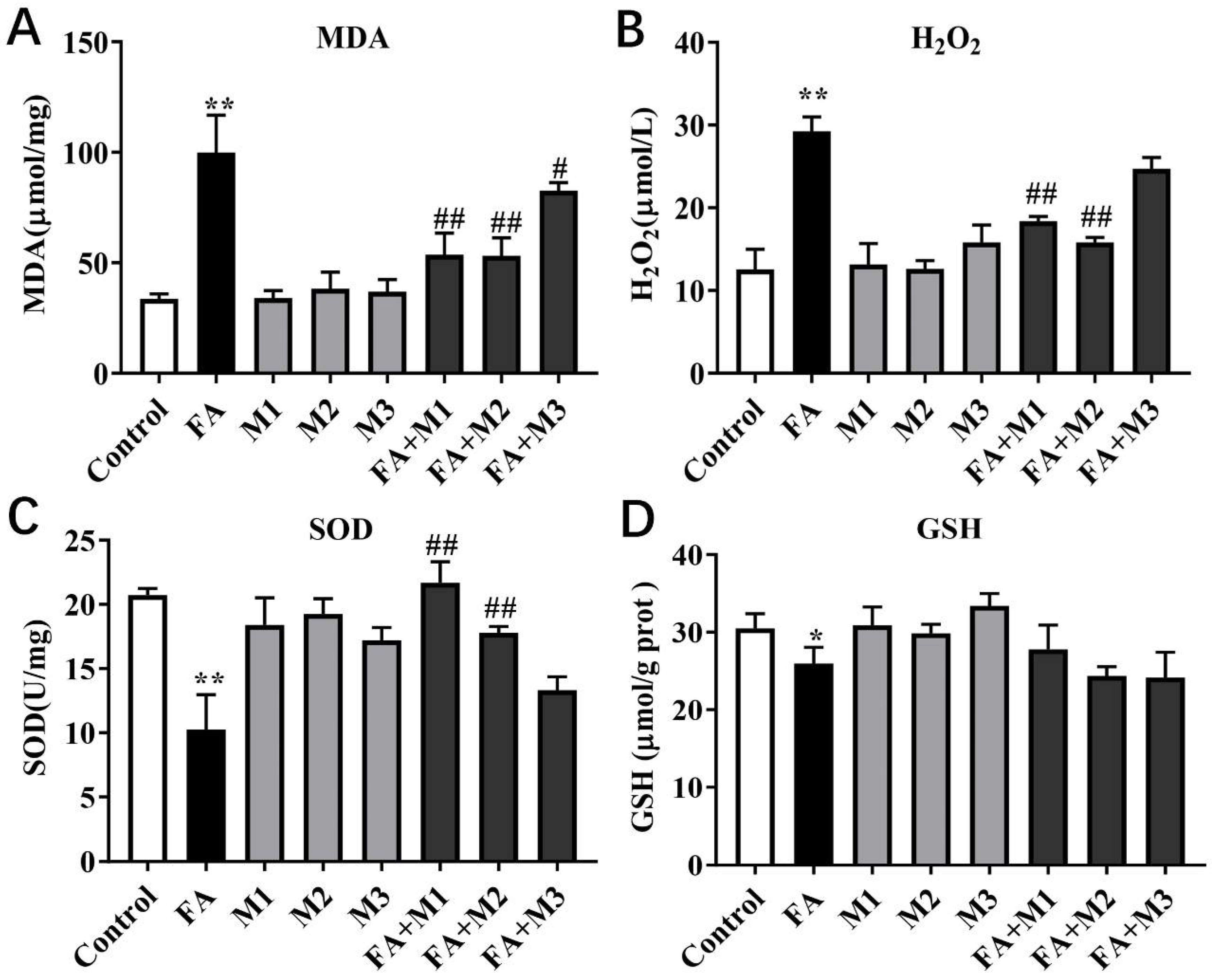
3.3. Effect of Myricetin on the Oxidative and Antioxidant Indexes of Hepatocytes
3.4. Effects of Myricetin on Endoplasmic Reticulum Stress and Inflammation
3.5. Effects of NAC on Lipid Metabolism
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Loor, J.J.; Bionaz, M.; Drackley, J.K. Systems physiology in dairy cattle: Nutritional genomics and beyond. Annu. Rev. Anim. Biosci. 2013, 1, 365–392. [Google Scholar] [CrossRef] [PubMed]
- Bobe, G.; Young, J.W.; Beitz, D.C. Invited review: Pathology, etiology, prevention, and treatment of fatty liver in dairy cows. J. Dairy Sci. 2004, 87, 3105–3124. [Google Scholar] [CrossRef]
- Shahsavari, A.; D’Occhio, M.J.; Al Jassim, R. The role of rumen-protected choline in hepatic function and performance of transition dairy cows. Br. J. Nutr. 2016, 116, 35–44. [Google Scholar] [CrossRef]
- Yang, W.; Li, X.; Liu, G.; Zhang, H.; Chen, Y.; Xia, C.; Xu, C. Development of a Fatty Liver Model by Restricted Feeding of Lactating Sheep. Acta Sci. Vet. 2018, 46, 8. [Google Scholar] [CrossRef]
- Du, X.; Liu, G.; Loor, J.J.; Fang, Z.; Bucktrout, R.; Yang, Y.; Ye, Q.; Shi, Z.; Shen, T.; Wang, X.; et al. Impaired hepatic autophagic activity in dairy cows with severe fatty liver is associated with inflammation and reduced liver function. J. Dairy Sci. 2018, 101, 11175–11185. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Li, M.; Yang, W.; Loor, J.J.; Liang, Y.; Wang, S.; Zhao, Y.; Guo, H.; Ma, X.; Yu, L.; et al. Mitochondrial dysfunction and endoplasmic reticulum stress in calf hepatocytes are associated with fatty acid-induced ORAI calcium release-activated calcium modulator 1 signaling. J. Dairy Sci. 2020, 103, 11945–11956. [Google Scholar] [CrossRef]
- Taheri, Y.; Suleria, H.A.R.; Martins, N.; Sytar, O.; Beyatli, A.; Yeskaliyeva, B.; Seitimova, G.; Salehi, B.; Semwal, P.; Painuli, S.; et al. Myricetin bioactive effects: Moving from preclinical evidence to potential clinical applications. BMC Complement. Med. Ther. 2020, 20, 241. [Google Scholar] [CrossRef]
- Song, X.; Tan, L.; Wang, M.; Ren, C.; Guo, C.; Yang, B.; Ren, Y.; Cao, Z.; Li, Y.; Pei, J. Myricetin: A review of the most recent research. Biomed Pharm. 2021, 134, 111017. [Google Scholar] [CrossRef]
- Guo, C.; Xue, G.; Pan, B.; Zhao, M.; Chen, S.; Gao, J.; Chen, T.; Qiu, L. Myricetin Ameliorates Ethanol-Induced Lipid Accumulation in Liver Cells by Reducing Fatty Acid Biosynthesis. Mol. Nutr. Food Res. 2019, 63, 1801393. [Google Scholar] [CrossRef]
- Lou, D.; Bao, S.S.; Li, Y.H.; Lin, Q.M.; Yang, S.F.; He, J.Y. Inhibitory Mechanisms of Myricetin on Human and Rat Liver Cytochrome P450 Enzymes. Eur. J. Drug Metab. Pharm. 2019, 44, 611–618. [Google Scholar] [CrossRef]
- Yamdagni, S.; Schultz, L.H. Fatty acid composition of blood plasma lipids of normal and ketotic cows. J. Dairy Sci. 1970, 53, 1046–1050. [Google Scholar] [CrossRef]
- Li, X.; Li, X.; Bai, G.; Chen, H.; Deng, Q.; Liu, Z.; Zhang, L.; Liu, G.; Wang, Z. Effects of non-esterified fatty acids on the gluconeogenesis in bovine hepatocytes. Mol. Cell. Biochem. 2012, 359, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Yang, W.; Wang, S.; Liu, R.; Loor, J.J.; Dong, Z.; Zhao, Y.; Ma, X.; Xia, C.; Xu, C. Lipid Accumulation and Injury in Primary Calf Hepatocytes Challenged With Different Long-Chain Fatty Acids. Front. Vet. Sci. 2020, 7, 547047. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Wang, S.; Loor, J.J.; Lopes, M.G.; Zhao, Y.; Ma, X.; Li, M.; Zhang, B.; Xu, C. Role of diacylglycerol O-acyltransferase (DGAT) isoforms in bovine hepatic fatty acid metabolism. J. Dairy Sci. 2022, 105, 3588–3600. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Li, M.; Yang, W.; Loor, J.J.; Wang, S.; Zhao, Y.; Guo, H.; Ma, X.; Xia, C.; Xu, C. Orai calcium release-activated calcium modulator 1 (ORAI1) plays a role in endoplasmic reticulum stress in bovine mammary epithelial cells challenged with physiological levels of ketone bodies. J. Dairy Sci. 2020, 103, 4691–4701. [Google Scholar] [CrossRef] [PubMed]
- Gross, J.J.; Schwarz, F.J.; Eder, K.; van Dorland, H.A.; Bruckmaier, R.M. Liver fat content and lipid metabolism in dairy cows during early lactation and during a mid-lactation feed restriction. J. Dairy Sci. 2013, 96, 5008–5017. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.J.; Jacometo, C.B.; Riboni, M.V.; Trevisi, E.; Graugnard, D.E.; Corrêa, M.N.; Loor, J.J. Stress and inflammatory gene networks in bovine liver are altered by plane of dietary energy during late pregnancy. Funct. Integr. Genom. 2015, 15, 563–576. [Google Scholar] [CrossRef]
- Li, M.; Yang, W.; Wen, J.; Loor, J.J.; Aboragah, A.; Wang, J.; Wang, S.; Li, M.; Yu, L.; Hou, X.; et al. Intracellular Ca2+ signaling and ORAI calcium release-activated calcium modulator 1 are associated with hepatic lipidosis in dairy cattle. J. Anim. Sci. 2021, 99, skab184. [Google Scholar] [CrossRef]
- Ashraf, N.U.; Sheikh, T.A. Endoplasmic reticulum stress and Oxidative stress in the pathogenesis of Non-alcoholic fatty liver disease. Free. Radic. Res. 2015, 49, 1405–1418. [Google Scholar] [CrossRef]
- Satapati, S.; Kucejova, B.; Duarte, J.A.; Fletcher, J.A.; Reynolds, L.; Sunny, N.E.; He, T.; Nair, L.A.; Livingston, K.A.; Fu, X.; et al. Mitochondrial metabolism mediates oxidative stress and inflammation in fatty liver. J. Clin. Investig. 2015, 125, 4447–4462. [Google Scholar] [CrossRef]
- Engin, A. Non-Alcoholic Fatty Liver Disease. In Obes. Lipotoxicity; Springer: Berlin/Heidelberg, Germany, 2017; Volume 960, pp. 443–467. [Google Scholar] [CrossRef]
- Simon, J.; Nuñez-García, M.; Fernández-Tussy, P.; Barbier-Torres, L.; Fernández-Ramos, D.; Gómez-Santos, B.; Buque, X.; Lopitz-Otsoa, F.; Goikoetxea-Usandizaga, N.; Serrano-Macia, M.; et al. Targeting Hepatic Glutaminase 1 Ameliorates Non-alcoholic Steatohepatitis by Restoring Very-Low-Density Lipoprotein Triglyceride Assembly. Cell Metab. 2020, 31, 605–622.e10. [Google Scholar] [CrossRef]
- Matsuda, S.; Kobayashi, M.; Kitagishi, Y. Roles for PI3K/AKT/PTEN Pathway in Cell Signaling of Nonalcoholic Fatty Liver Disease. Int. Sch. Res. Not. Endocrinol. 2013, 2013, 472432. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Jin, Y.; Wang, Q.; Huang, J.; Wu, X.; Ren, Z. Perilipin 5 Protects against Cellular Oxidative Stress by Enhancing Mitochondrial Function in HepG2 Cells. Cells 2019, 8, 1241. [Google Scholar] [CrossRef] [PubMed]
- Rakotonirina-Ricquebourg, R.; Costa, V.; Teixeira, V. Hello from the other side: Membrane contact of lipid droplets with other organelles and subsequent functional implications. Prog. Lipid. Res. 2022, 85, 101141. [Google Scholar] [CrossRef] [PubMed]
- Tsikas, D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef]
- Graulet, B.; Gruffat, D.; Durand, D.; Bauchart, D. Fatty acid metabolism and very low density lipoprotein secretion in liver slices from rats and preruminant calves. J. Biochem. 1998, 124, 1212–1219. [Google Scholar] [CrossRef]
- Witte, S.; Brockelmann, Y.; Haeger, J.D.; Schmicke, M. Establishing a model of primary bovine hepatocytes with responsive growth hormone receptor expression. J. Dairy Sci. 2019, 102, 7522–7535. [Google Scholar] [CrossRef]
- Hahn, O.; Grönke, S.; Stubbs, T.M.; Ficz, G.; Hendrich, O.; Krueger, F.; Andrews, S.; Zhang, Q.; Wakelam, M.J.; Beyer, A.; et al. Dietary restriction protects from age-associated DNA methylation and induces epigenetic reprogramming of lipid metabolism. Genome Biol. 2017, 18, 56. [Google Scholar] [CrossRef]



| Gene | GeneBank Number | Primer (5′ to 3′) | Length |
|---|---|---|---|
| ACTB | NM_173979.3 | F: GCTAACAGTCCGCCTAGAAGCA R: GTCATCACCATCGGCAATGAG | 403 bp |
| GAPDH | NM_001034034.2 | F: GTCTTCACTACCATGGAGAAGG R: TCATGGATGACCTTGGCCAG | 197 bp |
| DGAT1 | XM_025001414.1 | F: ACGCCGTGAAGTATAACCCT R: CCAAAAATCGCTTGTCCCTT | 101 bp |
| DGAT2 | NM_205793.2 | F: ACCCTCATAGCCGCCTACTC R: GCCAAGTGACAGAAAACAGGT | 239 bp |
| SREBF1 | NM_001113302.1 | F: GCAGCCCATTCATCAGCCAGACC R: CGACACCACCAGCATCAACCACG | 119 bp |
| CPT1A | NM_001304989.2 | F: ACGCCGTGAAGTATAACCCT R: CCAAAAATCGCTTGTCCCTT | 119 bp |
| FASN | NM_001012669.1 | F: ACAGCCTCTTCCTGTTTGACG R: CTCTGCACGATCAGCTCGAC | 144 bp |
| GRP78 | XM_024998380.1 | F: GCATCGACCTGGGTACCACCTA R: CCCTTCAGGAGTGAAAGCCACA | 122 bp |
| PERK | XM_010810067.3 | F: GCCGCTCAGCTCTCCTAGTCC R: TGGCTCTCGGATGAACTGGTCTG | 165 bp |
| NF-κB | NM_001113302.1 | F: AGGACCAACCAGACCG R: TGTCACCAGGCGAGTTAT | 204 bp |
| TNF-α | NM_001205408.1 | F: CTGCCGGACTACCTGGACTAT R: CCTCACTTCCCTACATCCCTAA | 144 bp |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, W.; Yang, M.; Tian, Y.; Jiang, Q.; Loor, J.J.; Cao, J.; Wang, S.; Gao, C.; Fan, W.; Zhang, B.; et al. Effect of Myricetin on Lipid Metabolism in Primary Calf Hepatocytes Challenged with Long-Chain Fatty Acids. Metabolites 2022, 12, 1071. https://doi.org/10.3390/metabo12111071
Yang W, Yang M, Tian Y, Jiang Q, Loor JJ, Cao J, Wang S, Gao C, Fan W, Zhang B, et al. Effect of Myricetin on Lipid Metabolism in Primary Calf Hepatocytes Challenged with Long-Chain Fatty Acids. Metabolites. 2022; 12(11):1071. https://doi.org/10.3390/metabo12111071
Chicago/Turabian StyleYang, Wei, Mingmao Yang, Yan Tian, Qianming Jiang, Juan J. Loor, Jie Cao, Shuang Wang, Changhong Gao, Wenwen Fan, Bingbing Zhang, and et al. 2022. "Effect of Myricetin on Lipid Metabolism in Primary Calf Hepatocytes Challenged with Long-Chain Fatty Acids" Metabolites 12, no. 11: 1071. https://doi.org/10.3390/metabo12111071
APA StyleYang, W., Yang, M., Tian, Y., Jiang, Q., Loor, J. J., Cao, J., Wang, S., Gao, C., Fan, W., Zhang, B., & Xu, C. (2022). Effect of Myricetin on Lipid Metabolism in Primary Calf Hepatocytes Challenged with Long-Chain Fatty Acids. Metabolites, 12(11), 1071. https://doi.org/10.3390/metabo12111071









