A New Concept of Associations between Gut Microbiota, Immunity and Central Nervous System for the Innovative Treatment of Neurodegenerative Disorders
Abstract
1. Introduction
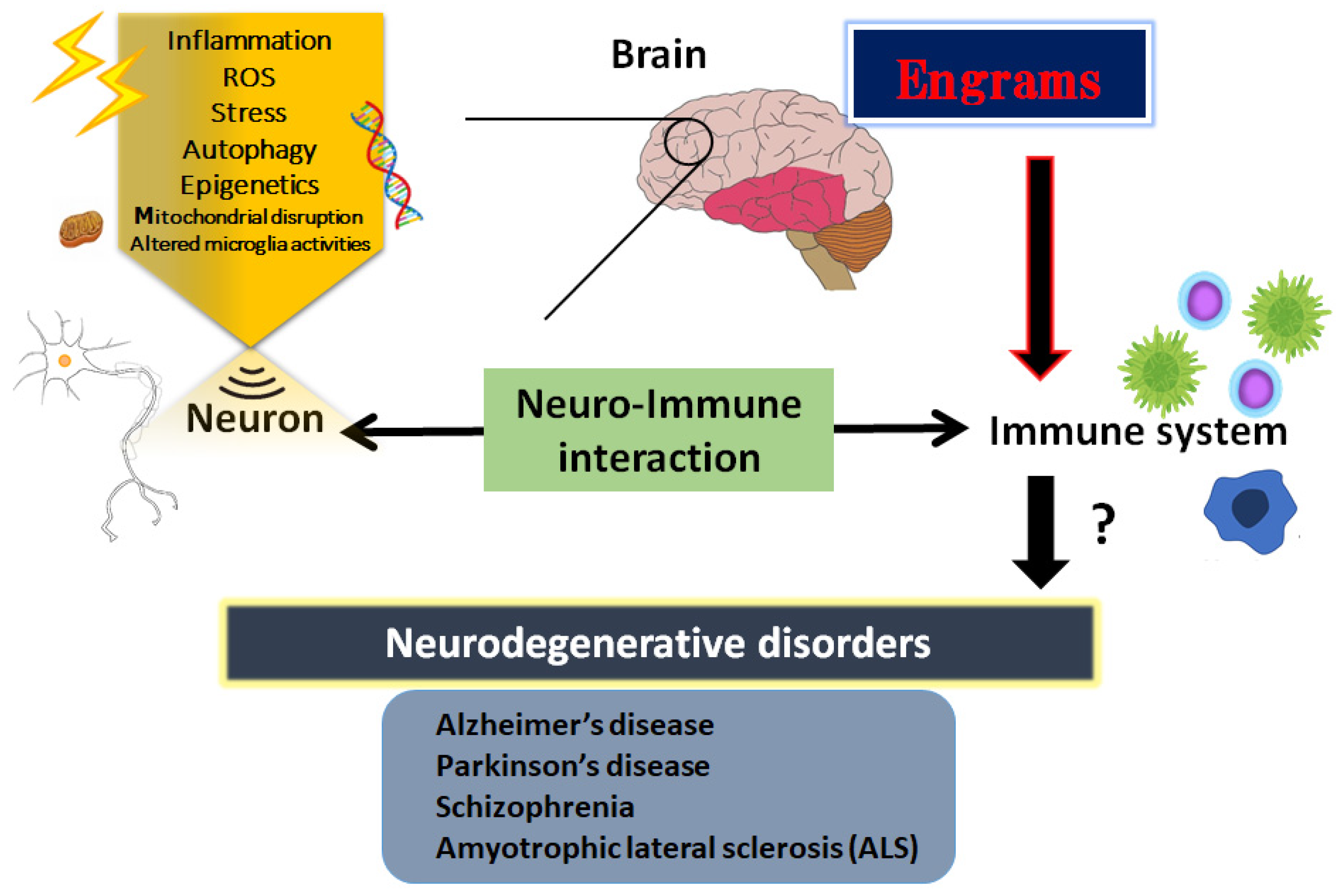
2. Inflammatory Neuro-Immune Responses
3. Engrams and Neuro-Immune Responses in the Pathogenesis of Neurodegenerative Disorders
4. How to Modulate the Engrams
5. Utilization of Gut–Brain Axis for the Treatment of Neurodegenerative Disorders
6. Next Perspectives
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ALS | amyotrophic lateral sclerosis |
| AMP | adenosine mono-phosphate |
| AMPK | AMP-activated protein kinase |
| BBB | blood–brain barrier |
| CNS | central nervous system |
| CSF | cerebrospinal fluid |
| DREADD | designer receptor exclusively activated by designer drugs |
| FMT | fecal microbiota transplantation |
| GABA | gamma amino butyric acid |
| HDACs | histone deacetylases |
| IL | interleukin |
| ROS | reactive oxygen species |
| SCFAs | short-chain fatty acids |
References
- Heneka, M.T.; McManus, R.M.; Latz, E. Inflammasome signalling in brain function and neurodegenerative disease. Nat. Rev. Neurosci. 2018, 19, 610–621. [Google Scholar] [CrossRef] [PubMed]
- Gilodi, M.; Lisi, S.; Dudás, E.F.; Fantini, M.; Puglisi, R.; Louka, A.; Marcatili, P.; Cattaneo, A.; Pastore, A. Selection and Modelling of a New Single-Domain Intrabody Against TDP-43. Front. Mol. Biosci. 2022, 8, 773234. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Nagase, N.; Tsuji, A.; Kitagishi, Y.; Matsuda, S. Neuroprotection by dipeptidyl-peptidase-4 inhibitors and glucagon-like peptide-1 analogs via the modulation of AKT-signaling pathway in Alzheimer’s disease. World. J. Biol. Chem. 2021, 12, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Ogino, M.; Ichimura, M.; Nakano, N.; Minami, A.; Kitagishi, Y.; Matsuda, S. Roles of PTEN with DNA Repair in Parkinson’s Disease. Int. J. Mol. Sci. 2016, 17, 954. [Google Scholar] [CrossRef]
- Kitagishi, Y.; Nakanishi, A.; Ogura, Y.; Matsuda, S. Dietary regulation of PI3K/AKT/GSK-3β pathway in Alzheimer’s disease. Alzheimers. Res. Ther. 2014, 6, 35. [Google Scholar] [CrossRef]
- Abbaszadeh, F.; Fakhri, S.; Khan, H. Targeting apoptosis and autophagy following spinal cord injury: Therapeutic approaches to polyphenols and candidate phytochemicals. Pharmacol. Res. 2020, 160, 105069. [Google Scholar] [CrossRef]
- Matsuda, S.; Ikeda, Y.; Murakami, M.; Nakagawa, Y.; Tsuji, A.; Kitagishi, Y. Roles of PI3K/AKT/GSK3 Pathway Involved in Psychiatric Illnesses. Diseases 2019, 7, 22. [Google Scholar] [CrossRef]
- Kitagishi, Y.; Minami, A.; Nakanishi, A.; Ogura, Y.; Matsuda, S. Neuron membrane trafficking and protein kinases involved in autism and ADHD. Int. J. Mol. Sci. 2015, 16, 3095–3115. [Google Scholar] [CrossRef]
- Singh, A.; Kukretim, R.; Saso, L.; Kukreti, S. Mechanistic Insight into Oxidative Stress-Triggered Signaling Pathways and Type 2 Diabetes. Molecules 2022, 27, 950. [Google Scholar] [CrossRef]
- Hitzeroth, A.; Niehaus, D.J.; Koen, L.; Botes, W.C.; Deleuze, J.F.; Warnich, L. Association between the MnSOD Ala-9Val polymorphism and development of schizophrenia and abnormal involuntary movements in the Xhosa population. Prog. Neuropsychopharmacol. Biol. Psychiatry 2007, 31, 664–672. [Google Scholar] [CrossRef]
- Le Gall, L.; Anakor, E.; Connolly, O.; Vijayakumar, U.G.; Duddy, W.J.; Duguez, S. Molecular and Cellular Mechanisms Affected in ALS. J. Pers. Med. 2020, 10, 101. [Google Scholar] [CrossRef] [PubMed]
- Godoy, J.A.; Rios, J.A.; Picón-Pagès, P.; Herrera-Fernández, V.; Swaby, B.; Crepin, G.; Vicente, R.; Fernández-Fernández, J.M.; Muñoz, F.J. Mitostasis, Calcium and Free Radicals in Health, Aging and Neurodegeneration. Biomolecules 2021, 11, 1012. [Google Scholar] [CrossRef]
- Lee, K.H.; Cha, M.; Lee, B.H. Neuroprotective Effect of Antioxidants in the Brain. Int. J. Mol. Sci. 2020, 21, 7152. [Google Scholar] [CrossRef]
- Fakhri, S.; Abbaszadeh, F.; Jorjanim, M. On the therapeutic targets and pharmacological treatments for pain relief following spinal cord injury: A mechanistic review. Biomed. Pharmacother. 2021, 139, 111563. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.R.; Kuang, Q.; Zhang, F.; Chen, B.; Zhong, Z.G. Functional roles of the microbiota-gut-brain axis in Alzheimer’s disease: Implications of gut microbiota-targeted therapy. Transl. Neurosci. 2021, 12, 581–600. [Google Scholar] [CrossRef]
- Dinan, T.G.; Cryan, J.F. Gut instincts: Microbiota as a key regulator of brain development, ageing and neurodegeneration. J. Physiol. 2017, 595, 489–503. [Google Scholar] [CrossRef] [PubMed]
- Bruce-Keller, A.J.; Salbaum, J.M.; Berthoud, H.R. Harnessing Gut Microbes for Mental Health: Getting from Here to There. Biol. Psychiatry 2018, 83, 214–223. [Google Scholar] [CrossRef]
- Martin, S.; Battistini, C.; Sun, J. A Gut Feeling in Amyotrophic Lateral Sclerosis: Microbiome of Mice and Men. Front. Cell. Infect. Microbiol. 2022, 12, 839526. [Google Scholar] [CrossRef]
- Cox, L.M.; Calcagno, N.; Gauthier, C.; Madore, C.; Butovsky, O.; Weiner, H.L. The microbiota restrains neurodegenerative microglia in a model of amyotrophic lateral sclerosis. Microbiome 2022, 10, 47. [Google Scholar] [CrossRef]
- Sawamura, H.; Taniguchi, K.; Ikeda, Y.; Tsuji, A.; Kitagishi, Y.; Matsuda, S. Gut microbiota could modulate the effects of neuro-immune responses and memory traces via the gut-brain-immune axis in schizophrenia. Explor. Neuroprot. Ther. 2022, 2, 74–86. [Google Scholar] [CrossRef]
- Noss, M.M.; Millwood, S.N.; Kuhlman, K.R. Women with lower systemic inflammation demonstrate steeper cognitive decline with age: Results from a large prospective, longitudinal sample. Brain. Behav. Immun. Health 2022, 22, 100465. [Google Scholar] [CrossRef] [PubMed]
- Arsenault, D.; Coulombe, K.; Zhu, A.; Gong, C.; Kil, K.E.; Choi, J.K.; Poutiainen, P.; Brownell, A.L. Loss of Metabotropic Glutamate Receptor 5 Function on Peripheral Benzodiazepine Receptor in Mice Prenatally Exposed to LPS. PLoS ONE 2015, 10, e0142093. [Google Scholar] [CrossRef] [PubMed]
- Chavan, S.S.; Pavlov, V.A.; Tracey, K.J. Mechanisms and Therapeutic Relevance of Neuro-immune Communication. Immunity 2017, 46, 927–942. [Google Scholar] [CrossRef]
- Clark, S.M.; Vaughn, C.N.; Soroka, J.A.; Li, X.; Tonelli, L.H. Neonatal adoptive transfer of lymphocytes rescues social behaviour during adolescence in immune-deficient mice. Eur. J. Neurosci. 2018, 47, 968–978. [Google Scholar] [CrossRef] [PubMed]
- Theodoropoulou, S.; Spanakos, G.; Baxevanis, C.N.; Economou, M.; Gritzapis, A.D.; Papamichail, M.P.; Stefanis, C.N. Cytokine serum levels, autologous mixed lymphocyte reaction and surface marker analysis in never medicated and chronically medicated schizophrenic patients. Schizophr. Res. 2001, 47, 13–25. [Google Scholar] [CrossRef]
- Lupaescu, A.V.; Iavorschi, M.; Covasa, M. The Use of Bioactive Compounds in Hyperglycemia- and Amyloid Fibrils-Induced Toxicity in Type 2 Diabetes and Alzheimer’s Disease. Pharmaceutics 2022, 14, 235. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.C.; Kwak, S.G.; Park, J.S.; Park, D. The effectiveness of nonsteroidal anti-inflammatory drugs and acetaminophen in reduce the risk of amyotrophic lateral sclerosis? A meta-analysis. Sci. Rep. 2020, 10, 14759. [Google Scholar] [CrossRef] [PubMed]
- Csabai, D.; Sebők-Tornai, A.; Wiborg, O.; Czéh, B. A Preliminary Quantitative Electron Microscopic Analysis Reveals Reduced Number of Mitochondria in the Infralimbic Cortex of Rats Exposed to Chronic Mild Stress. Front. Behav. Neurosci. 2022, 16, 885849. [Google Scholar] [CrossRef]
- Karmakar, J.; Mukherjee, K.; Mandal, C. Siglecs Modulate Activities of Immune Cells Through Positive and Negative Regulation of ROS Generation. Front. Immunol. 2021, 12, 758588. [Google Scholar] [CrossRef]
- Scherz-Shouval, R.; Elazar, Z. Regulation of autophagy by ROS: Physiology and pathology. Trends. Biochem. Sci. 2011, 36, 30–38. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, L. New Insights into the Interplay Among Autophagy, the NLRP3 Inflammasome and Inflammation in Adipose Tissue. Front. Endocrinol. 2022, 13, 739882. [Google Scholar] [CrossRef] [PubMed]
- Marcucci, F.; Bellone, M.; Caserta, C.A.; Corti, A. Pushing tumor cells towards a malignant phenotype: Stimuli from the microenvironment, intercellular communications and alternative roads. Int. J. Cancer. 2014, 135, 1265–1276. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.S.; Zhang, S.F.; Luo, G.; Cheng, B.C.; Zhang, C.; Wang, Y.W.; Qiu, X.Y.; Zhou, X.H.; Wang, Q.G.; Song, X.L.; et al. Schisandrin B mitigates hepatic steatosis and promotes fatty acid oxidation by inducing autophagy through AMPK/mTOR signaling pathway. Metabolism 2022, 131, 155200. [Google Scholar] [CrossRef]
- Nagy, S.; Maurer, G.W.; Hentze, J.L.; Rose, M.; Werge, T.M.; Rewitz, K. AMPK signaling linked to the schizophrenia-associated 1q21.1 deletion is required for neuronal and sleep maintenance. PLoS Genet. 2018, 14, e1007623. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, T.; Fujita, N.; Jang, M.H.; Uematsu, S.; Yang, B.G.; Satoh, T.; Omori, H.; Noda, T.; Yamamoto, N.; Komatsu, M.; et al. Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1beta production. Nature 2008, 456, 264–268. [Google Scholar] [CrossRef]
- De Luca, C.; Colangelo, A.M.; Alberghina, L.; Papa, M. Neuro-Immune Hemostasis: Homeostasis and Diseases in the Central Nervous System. Front. Cell Neurosci. 2018, 12, 459. [Google Scholar] [CrossRef]
- Koren, T.; Yifa, R.; Amer, M.; Krot, M.; Boshnak, N.; Ben-Shaanan, T.L.; Azulay-Debby, H.; Zalayat, I.; Avishai, E.; Hajjo, H.; et al. Insular cortex neurons encode and retrieve specific immune responses. Cell 2021, 184, 5902–5915.e17. [Google Scholar] [CrossRef]
- Gogolla, N. The brain remembers where and how inflammation struck. Cell 2021, 184, 5851–5853. [Google Scholar] [CrossRef]
- Roy, D.S.; Park, Y.G.; Kim, M.E.; Zhang, Y.; Ogawa, S.K.; DiNapoli, N.; Gu, X.; Cho, J.H.; Choi, H.; Kamentsky, L.; et al. Brain-wide mapping reveals that engrams for a single memory are distributed across multiple brain regions. Nat. Commun. 2022, 13, 1799. [Google Scholar] [CrossRef]
- Sakaguchi, M.; Hayashi, Y. Catching the engram: Strategies to examine the memory trace. Mol. Brain. 2012, 5, 32. [Google Scholar] [CrossRef]
- Gebicke-Haerter, P.J. Engram formation in psychiatric disorders. Front. Neurosci. 2014, 8, 118. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Ramos, M.; Alaiz-Noya, M.; Barco, A. Transcriptome and epigenome analysis of engram cells: Next-generation sequencing technologies in memory research. Neurosci. Biobehav. Rev. 2021, 127, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, S.; Linde, J.; Bell, M.; Spehr, M.; Zempel, H.; Zimmer-Bensch, G. DNA Methyltransferase 1 (DNMT1) Shapes Neuronal Activity of Human iPSC-Derived Glutamatergic Cortical Neurons. Int. J. Mol. Sci. 2021, 22, 2034. [Google Scholar] [CrossRef] [PubMed]
- Gulmez Karaca, K.; Kupke, J.; Brito, D.V.C.; Zeuch, B.; Thome, C.; Weichenhan, D.; Lutsik, P.; Plass, C.; Oliveira, A.M.M. Neuronal ensemble-specific DNA methylation strengthens engram stability. Nat. Commun. 2020, 11, 639. [Google Scholar] [CrossRef]
- Niemi, M.B.; Härting, M.; Kou, W.; Del Rey, A.; Besedovsky, H.O.; Schedlowski, M.; Pacheco-López, G. Taste-immunosuppression engram: Reinforcement and extinction. J. Neuroimmunol. 2007, 188, 74–79. [Google Scholar] [CrossRef]
- Pacheco-López, G.; Niemi, M.B.; Kou, W.; Baum, S.; Hoffman, M.; Altenburger, P.; del Rey, A.; Besedovsky, H.O.; Schedlowski, M. Central blockade of IL-1 does not impair taste-LPS associative learning. Neuroimmunomodulation 2007, 14, 150–156. [Google Scholar] [CrossRef]
- Kyrke-Smith, M.; Williams, J.M. Bridging Synaptic and Epigenetic Maintenance Mechanisms of the Engram. Front. Mol. Neurosci. 2018, 11, 369. [Google Scholar] [CrossRef]
- Manea, S.A.; Vlad, M.L.; Fenyo, I.M.; Lazar, A.G.; Raicu, M.; Muresian, H.; Simionescu, M.; Manea, A. Pharmacological inhibition of histone deacetylase reduces NADPH oxidase expression, oxidative stress and the progression of atherosclerotic lesions in hypercholesterolemic apolipoprotein E-deficient mice; potential implications for human atherosclerosis. Redox Biol. 2020, 28, 101338. [Google Scholar] [CrossRef]
- Qing, L.; Liu, L.; Zhou, L.; Zhang, F.; Gao, C.; Hu, L.; Nie, S. Sex-dependent association of mineralocorticoid receptor gene (NR3C2) DNA methylation and schizophrenia. Psychiatry Res. 2020, 292, 113318. [Google Scholar] [CrossRef]
- Bostancıklıoğlu, M. An update on memory formation and retrieval: An engram-centric approach. Alzheimers. Dement. 2020, 16, 926–937. [Google Scholar] [CrossRef]
- Wang, C.; Yue, H.; Hu, Z.; Shen, Y.; Ma, J.; Li, J.; Wang, X.D.; Wang, L.; Sun, B.; Shi, P.; et al. Microglia mediate forgetting via complement-dependent synaptic elimination. Science 2020, 367, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Deng, Y.S.; Dai, S.K.; Mi, T.W.; Li, R.Y.; Liu, P.P.; Liu, C.; He, B.D.; He, X.C.; Du, H.Z.; et al. Loss of microglial EED impairs synapse density, learning, and memory. Mol. Psychiatry 2022, 27, 2999–3009. [Google Scholar] [CrossRef]
- Wang, X.; Chang, L.; Wan, X.; Tan, Y.; Qu, Y.; Shan, J.; Yang, Y.; Ma, L.; Hashimoto, K. (R)-ketamine ameliorates demyelination and facilitates remyelination in cuprizone-treated mice: A role of gut-microbiota-brain axis. Neurobiol. Dis. 2022, 165, 105635. [Google Scholar] [CrossRef]
- Ghezzi, L.; Cantoni, C.; Pinget, G.V.; Zhou, Y.; Piccio, L. Targeting the gut to treat multiple sclerosis. J. Clin. Investig. 2021, 131, e143774. [Google Scholar] [CrossRef] [PubMed]
- Klann, E.M.; Dissanayake, U.; Gurrala, A.; Farrer, M.; Shukla, A.W.; Ramirez-Zamora, A.; Mai, V.; Vedam-Mai, V. The Gut-Brain Axis and Its Relation to Parkinson’s Disease: A Review. Front. Aging Neurosci. 2022, 13, 782082. [Google Scholar] [CrossRef] [PubMed]
- Lecomte, A.; Barateau, L.; Pereira, P.; Paulin, L.; Auvinen, P.; Scheperjans, F.; Dauvilliers, Y. Gut microbiota composition is associated with narcolepsy type 1. Neurol. Neuroimmunol. Neuroinflamm. 2020, 7, e896. [Google Scholar] [CrossRef] [PubMed]
- Wiley, N.C.; Cryan, J.F.; Dinan, T.G.; Ross, R.P.; Stanton, C. Production of Psychoactive Metabolites by Gut Bacteria. Mod. Trends Psychiatry 2021, 32, 74–99. [Google Scholar] [PubMed]
- Muller, P.A.; Schneeberger, M.; Matheis, F.; Wang, P.; Kerner, Z.; Ilanges, A.; Pellegrino, K.; Del Mármol, J.; Castro, T.B.R.; Furuichi, M.; et al. Microbiota modulate sympathetic neurons via a gut-brain circuit. Nature 2020, 583, 441–446. [Google Scholar] [CrossRef]
- Janssens, Y.; Debunne, N.; De Spiegeleer, A.; Wynendaele, E.; Planas, M.; Feliu, L.; Quarta, A.; Claes, C.; Van Dam, D.; De Deyn, P.P.; et al. PapRIV, a BV-2 microglial cell activating quorum sensing peptide. Sci. Rep. 2021, 11, 10723. [Google Scholar] [CrossRef]
- Welcome, M.O. Gut Microbiota Disorder, Gut Epithelial and Blood-Brain Barrier Dysfunctions in Etiopathogenesis of Dementia: Molecular Mechanisms and Signaling Pathways. Neuromolecular. Med. 2019, 21, 205–226. [Google Scholar] [CrossRef]
- Li, X.; Fan, X.; Yuan, X.; Pang, L.; Hu, S.; Wang, Y.; Huang, X.; Song, X. The Role of Butyric Acid in Treatment Response in Drug-Naive First Episode Schizophrenia. Front. Psychiatry 2021, 12, 724664. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, T.J.; Gates, E.J.; Ranger, A.L.; Klegeris, A. Short-chain fatty acids (SCFAs) alone or in combination regulate select immune functions of microglia-like cells. Mol. Cell. Neurosci. 2020, 105, 103493. [Google Scholar] [CrossRef] [PubMed]
- Kesika, P.; Suganthy, N.; Sivamaruthi, B.S.; Chaiyasut, C. Role of gut-brain axis, gut microbial composition, and probiotic intervention in Alzheimer’s disease. Life Sci. 2021, 264, 118627. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Huang, T.; Debelius, J.W.; Fang, F. Gut microbiome and amyotrophic lateral sclerosis: A systematic review of current evidence. J. Intern. Med. 2021, 290, 758–788. [Google Scholar] [CrossRef] [PubMed]
- Mou, Y.; Du, Y.; Zhou, L.; Yue, J.; Hu, X.; Liu, Y.; Chen, S.; Lin, X.; Zhang, G.; Xiao, H.; et al. Gut Microbiota Interact with the Brain Through Systemic Chronic Inflammation: Implications on Neuroinflammation, Neurodegeneration, and Aging. Front. Immunol. 2022, 13, 796288. [Google Scholar] [CrossRef]
- Håvik, B.; Røkke, H.; Dagyte, G.; Stavrum, A.K.; Bramham, C.R.; Steen, V.M. Synaptic activity-induced global gene expression patterns in the dentate gyrus of adult behaving rats: Induction of immunity-linked genes. Neuroscience 2007, 148, 925–936. [Google Scholar] [CrossRef]
- Rudzki, L.; Maes, M. From “Leaky Gut” to Impaired Glia-Neuron Communication in Depression. Adv. Exp. Med. Biol. 2021, 1305, 129–155. [Google Scholar]
- Caputi, V.; Popov, J.; Giron, M.C.; O’Mahony, S. Gut Microbiota as a Mediator of Host Neuro-Immune Interactions: Implications in Neuroinflammatory Disorders. Mod. Trends. Psychiatry 2021, 32, 40–57. [Google Scholar]
- Benakis, C.; Martin-Gallausiaux, C.; Trezzi, J.P.; Melton, P.; Liesz, A.; Wilmes, P. The microbiome-gut-brain axis in acute and chronic brain diseases. Curr. Opin. Neurobiol. 2020, 61, 1–9. [Google Scholar] [CrossRef]
- Castanon, N.; Luheshi, G.; Layé, S. Role of neuroinflammation in the emotional and cognitive alterations displayed by animal models of obesity. Front. Neurosci. 2015, 9, 229. [Google Scholar] [CrossRef]
- Hertzberg, V.S.; Singh, H.; Fournier, C.N.; Moustafa, A.; Polak, M.; Kuelbs, C.A.; Torralba, M.G.; Tansey, M.G.; Nelson, K.E.; Glass, J.D. Gut microbiome differences between amyotrophic lateral sclerosis patients and spouse controls. Amyotroph. Lateral. Scler. Frontotemporal. Degener. 2022, 23, 91–99. [Google Scholar] [CrossRef]
- Kim, H.S.; Son, J.; Lee, D.; Tsai, J.; Wang, D.; Chocron, E.S.; Jeong, S.; Kittrell, P.; Murchison, C.F.; Kennedy, R.E.; et al. Gut- and oral-dysbiosis differentially impact spinal- and bulbar-onset ALS, predicting ALS severity and potentially determining the location of disease onset. BMC. Neurol. 2022, 22, 62. [Google Scholar] [CrossRef] [PubMed]
- Chidambaram, S.B.; Essa, M.M.; Rathipriya, A.G.; Bishir, M.; Ray, B.; Mahalakshmi, A.M.; Tousif, A.H.; Sakharkar, M.K.; Kashyap, R.S.; Friedland, R.P.; et al. Gut dysbiosis, defective autophagy and altered immune responses in neurodegenerative diseases: Tales of a vicious cycle. Pharmacol. Ther. 2022, 231, 107988. [Google Scholar] [CrossRef] [PubMed]
- Trujillo-Del Río, C.; Tortajada-Pérez, J.; Gómez-Escribano, A.P.; Casterá, F.; Peiró, C.; Millán, J.M.; Herrero, M.J.; Vázquez-Manrique, R.P. Metformin to treat Huntington disease: A pleiotropic drug against a multi-system disorder. Mech. Ageing Dev. 2022, 204, 111670. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, S.; Nakagawa, Y.; Amano, K.; Ikeda, Y.; Tsuji, A.; Kitagishi, Y. By using either endogenous or transplanted stem cells, which could you prefer for neural regeneration? Neural. Regen. Res. 2018, 13, 1731–1732. [Google Scholar] [CrossRef]
- Taniguchi, K.; Ikeda, Y.; Nagase, N.; Tsuji, A.; Kitagishi, Y.; Matsuda, S. Implications of Gut-Brain axis in the pathogenesis of Psychiatric disorders. AIMS. Bioeng. 2021, 8, 243–256. [Google Scholar] [CrossRef]
- Matsuda, S.; Nakagawa, Y.; Kitagishi, Y.; Nakanishi, A.; Murai, T. Reactive Oxygen Species, Superoxide Dimutases, and PTEN-p53-AKT-MDM2 Signaling Loop Network in Mesenchymal Stem/Stromal Cells Regulation. Cells 2018, 7, 36. [Google Scholar] [CrossRef]
- Ikeda, Y.; Taniguchi, K.; Nagase, N.; Tsuji, A.; Kitagishi, Y.; Matsuda, S. Reactive oxygen species may influence on the crossroads of stemness, senescence, and carcinogenesis in a cell via the roles of APRO family proteins. Explor. Med. 2021, 2, 443–454. [Google Scholar] [CrossRef]
- Zhang, L.; Qian, Y.; Li, J.; Zhou, X.; Xu, H.; Yan, J.; Xiang, J.; Yuan, X.; Sun, B.; Sisodia, S.S.; et al. BAD-mediated neuronal apoptosis and neuroinflammation contribute to Alzheimer’s disease pathology. iScience 2021, 24, 102942. [Google Scholar] [CrossRef]
- Acosta, S.; Jernberg, J.; Sanberg, C.D.; Sanberg, P.R.; Small, B.J.; Gemma, C.; Bickford, P.C. NT-020, a natural therapeutic approach to optimize spatial memory performance and increase neural progenitor cell proliferation and decrease inflammation in the aged rat. Rejuvenation Res. 2010, 13, 581–588. [Google Scholar] [CrossRef]
- Fond, G.B.; Lagier, J.C.; Honore, S.; Lancon, C.; Korchia, T.; Sunhary De Verville, P.-L.; Llorca, P.M.; Auquier, P.; Guedj, E.; Boyer, L. Microbiota-Orientated Treatments for Major Depression and Schizophrenia. Nutrients 2020, 12, 1024. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, K.; Chung, S.K.; Vanamala, J.; Xu, B. Causal Relationship between Diet-Induced Gut Microbiota Changes and Diabetes: A Novel Strategy to Transplant Faecalibacterium prausnitzii in Preventing Diabetes. Int. J. Mol. Sci. 2018, 19, 3720. [Google Scholar] [CrossRef] [PubMed]
- Grün, D.; Zimmer, V.C.; Kauffmann, J.; Spiegel, J.; Dillmann, U.; Schwiertz, A.; Faßbender, K.; Fousse, M.; Unger, M.M. Impact of oral COMT-inhibitors on gut microbiota and short chain fatty acids in Parkinson’s disease. Parkinsonism. Relat. Disord. 2020, 70, 20–22. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.T.; Huang, H.L.; Xu, H.M.; Luo, Q.L.; He, J.; Li, Y.Q.; Zhou, Y.L.; Nie, Y.Q.; Zhou, Y.J. Fecal microbiota transplantation ameliorates active ulcerative colitis. Exp. Ther. Med. 2020, 19, 2650–2660. [Google Scholar] [CrossRef]
- Zhu, F.; Guo, R.; Wang, W.; Ju, Y.; Wang, Q.; Ma, Q.; Sun, Q.; Fan, Y.; Xie, Y.; Yang, Z.; et al. Transplantation of microbiota from drug-free patients with schizophrenia causes schizophrenia-like abnormal behaviors and dysregulated kynurenine metabolism in mice. Mol. Psychiatry 2020, 25, 2905–2918. [Google Scholar] [CrossRef]
- Dong, S.; Sun, M.; He, C.; Cheng, H. Brain-gut-microbiota axis in Parkinson’s disease: A historical review and future perspective. Brain Res. Bull. 2022, 183, 84–93. [Google Scholar] [CrossRef]
- Casani-Cubel, J.; Benlloch, M.; Sanchis-Sanchis, C.E.; Marin, R.; Lajara-Romance, J.M.; de la Rubia Orti, J.E. The Impact of Microbiota on the Pathogenesis of Amyotrophic Lateral Sclerosis and the Possible Benefits of Polyphenols. An Overview. Metabolites 2021, 11, 120. [Google Scholar] [CrossRef]
- Torquati, L.; Gajanand, T.; Cox, E.R.; Willis, C.; Zaugg, J.; Keating, S.E.; Coombes, J.S. Effects of exercise intensity on gut microbiome composition and function in people with type 2 diabetes. Eur. J. Sport. Sci. 2022, 23, 1–12. [Google Scholar] [CrossRef]
- Nocera, A.; Nasrallah, H.A. The Association of the Gut Microbiota with Clinical Features in Schizophrenia. Behav. Sci. 2022, 12, 89. [Google Scholar] [CrossRef]
- Xu, Y.; Cai, Z.; Fang, C.; Zheng, J.; Shan, J.; Yang, Y. Impact of aerobic exercise on cognitive function in patients with schizophrenia during daily care: A meta-analysis. Psychiatry Res. 2022, 312, 114560. [Google Scholar] [CrossRef]
- Jopowicz, A.; Wiśniowska, J.; Tarnacka, B. Cognitive and Physical Intervention in Metals’ Dysfunction and Neurodegeneration. Brain. Sci. 2022, 12, 345. [Google Scholar] [CrossRef] [PubMed]
- Aridegbe, T.; Kandler, R.; Walters, S.J.; Walsh, T.; Shaw, P.J.; McDermott, C.J. The natural history of motor neuron disease: Assessing the impact of specialist care. Amyotroph. Lateral. Scler. Frontotemporal. Degener. 2013, 14, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Van Es, M.A.; Hardiman, O.; Chio, A.; Al-Chalabi, A.; Pasterkamp, R.J.; Veldink, J.H.; van den Berg, L.H. Amyotrophic lateral sclerosis. Lancet 2017, 390, 2084–2098. [Google Scholar] [CrossRef]
- Scott, A. Drug therapy: On the treatment trail for ALS. Nature 2017, 550, S120–S121. [Google Scholar] [CrossRef]
- Wobst, H.J.; Mack, K.L.; Brown, D.G.; Brandon, N.J.; Shorter, J. The clinical trial landscape in amyotrophic lateral sclerosis-Past, present, and future. Med. Res. Rev. 2020, 40, 1352–1384. [Google Scholar] [CrossRef] [PubMed]
- Bellingham, M.C. A review of the neural mechanisms of action and clinical efficiency of riluzole in treating amyotrophic lateral sclerosis: What have we learned in the last decade? CNS Neurosci. Ther. 2011, 17, 4–31. [Google Scholar] [CrossRef]
- Andrews, J.A.; Jackson, C.E.; Heiman-Patterson, T.D.; Bettica, P.; Brooks, B.R.; Pioro, E.P. Real-world evidence of riluzole effectiveness in treating amyotrophic lateral sclerosis. Amyotroph. Lateral. Scler. Frontotemporal. Degener. 2020, 21, 509–518. [Google Scholar] [CrossRef]
- West, R.J.H.; Ugbode, C.; Fort-Aznar, L.; Sweeney, S.T. Neuroprotective activity of ursodeoxycholic acid in CHMP2BIntron5 models of frontotemporal dementia. Neurobiol. Dis. 2020, 144, 105047. [Google Scholar] [CrossRef]
- Sala, G.; Arosio, A.; Conti, E.; Beretta, S.; Lunetta, C.; Riva, N.; Ferrarese, C.; Tremolizzo, L. Riluzole Selective Antioxidant Effects in Cell Models Expressing Amyotrophic Lateral Sclerosis Endophenotypes. Clin. Psychopharmacol. Neurosci. 2019, 17, 438–442. [Google Scholar] [CrossRef]
- Rothstein, J.D. Edaravone: A new drug approved for ALS. Cell 2017, 171, 725. [Google Scholar] [CrossRef]
- Sawada, H. Clinical efficacy of edaravone for the treatment of amyotrophic lateral sclerosis. Expert Opin. Pharmacother. 2017, 18, 735–738. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Wu, H.T.; Li, X.X.; Yu, Y.; Gu, R.Z.; Lan, R.; Qin, X.Y. Edaravone protects rat astrocytes from oxidative or neurotoxic inflammatory insults by restoring Akt/Bcl-2/Caspase-3 signaling axis. IBRO Rep. 2020, 8, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, S.; Murozono, M.; Kanazawa, M.; Nara, T.; Ozawa, T.; Watanabe, Y. Edaravone and cyclosporine A as neuroprotective agents for acute ischemic stroke. Acute Med. Surg. 2018, 5, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Bu, F.; Chen, T.; Shi, G.; Yuan, X.; Feng, Z.; Duan, Z.; Wang, R.; Zhang, S.; Wang, Q.; et al. A next-generation probiotic: Akkermansia muciniphila ameliorates chronic stress-induced depressive-like behavior in mice by regulating gut microbiota and metabolites. Appl. Microbiol. Biotechnol. 2021, 105, 8411–8426. [Google Scholar] [CrossRef]
- Xu, X.; Shen, D.; Gao, Y.; Zhou, Q.; Ni, Y.; Meng, H.; Shi, H.; Le, W.; Chen, S.; Chen, S. A perspective on therapies for amyotrophic lateral sclerosis: Can disease progression be curbed? Transl. Neurodegener. 2021, 10, 29. [Google Scholar] [CrossRef]
- Arthur, K.C.; Calvo, A.; Price, T.R.; Geiger, J.T.; Chiò, A.; Traynor, B.J. Projected increase in amyotrophic lateral sclerosis from 2015 to 2040. Nat. Commun. 2016, 7, 12408. [Google Scholar] [CrossRef]
- Oleskin, A.V.; Shenderov, B.A. Neuromodulatory effects and targets of the SCFAs and gasotransmitters produced by the human symbiotic microbiota. Microb. Ecol. Health Dis. 2016, 27, 30971. [Google Scholar] [CrossRef]
- Oleskin, A.V.; Shenderov, B.A.; Rogovsky, V.S. Role of Neurochemicals in the Interaction between the Microbiota and the Immune and the Nervous System of the Host Organism. Probiotics Antimicrob. Proteins 2017, 9, 215–234. [Google Scholar] [CrossRef]
- Oleskin, A.V.; Shenderov, B.A. Probiotics and Psychobiotics: The Role of Microbial Neurochemicals. Probiotics Antimicrob. Proteins 2019, 11, 1071–1085. [Google Scholar] [CrossRef]
- Cheng, L.H.; Liu, Y.W.; Wu, C.C.; Wang, S.; Tsai, Y.C. Psychobiotics in mental health, neurodegenerative and neurodevelopmental disorders. J Food Drug Anal. 2019, 27, 632–648. [Google Scholar] [CrossRef]
- Akbari, E.; Asemi, Z.; Daneshvar Kakhaki, R.; Bahmani, F.; Kouchaki, E.; Tamtaji, O.R.; Hamidi, G.A.; Salami, M. Effect of Probiotic Supplementation on Cognitive Function and Metabolic Status in Alzheimer’s Disease: A Randomized, Double-Blind and Controlled Trial. Front Aging Neurosci. 2016, 8, 256. [Google Scholar] [CrossRef] [PubMed]
- Tamtaji, O.R.; Heidari-Soureshjani, R.; Mirhosseini, N.; Kouchaki, E.; Bahmani, F.; Aghadavod, E.; Tajabadi-Ebrahimi, M.; Asemi, Z. Probiotic and selenium co-supplementation, and the effects on clinical, metabolic and genetic status in Alzheimer’s disease: A randomized, double-blind, controlled trial. Clin Nutr. 2019, 38, 2569–2575. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Liu, X.; Jiang, R.; Yan, X.; Ling, Z. Roles and Mechanisms of Gut Microbiota in Patients With Alzheimer’s Disease. Front Aging Neurosci. 2021, 13, 650047. [Google Scholar] [CrossRef] [PubMed]
- Westfall, S.; Lomis, N.; Kahouli, I.; Dia, S.Y.; Singh, S.P.; Prakash, S. Microbiome, probiotics and neurodegenerative diseases: Deciphering the gut brain axis. Cell Mol Life Sci. 2017, 74, 3769–3787. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, K.; Li, X.; Xu, L.; Yang, Z. Sodium butyrate ameliorates the impairment of synaptic plasticity by inhibiting the neuroinflammation in 5XFAD mice. Chem. Biol. Interact. 2021, 341, 109452. [Google Scholar] [CrossRef] [PubMed]
- Roberts, B.; Theunissen, F.; Mastaglia, F.L.; Akkari, P.A.; Flynn, L.L. Synucleinopathy in Amyotrophic Lateral Sclerosis: A Potential Avenue for Antisense Therapeutics? Int. J. Mol. Sci. 2022, 23, 9364. [Google Scholar] [CrossRef]
- Agorastos, A.; Bozikas, V.P. Gut microbiome and adaptive immunity in schizophrenia. Psychiatriki 2019, 30, 189–192. [Google Scholar] [CrossRef]
- Wang, L.; Wei, X. T Cell-Mediated Autoimmunity in Glaucoma Neurodegeneration. Front. Immunol. 2021, 12, 803485. [Google Scholar] [CrossRef]
- Taniguchi, K.; Sawamura, H.; Ikeda, Y.; Tsuji, A.; Kitagishi, Y.; Matsuda, S. D-Amino Acids as a Biomarker in Schizophrenia. Diseases 2022, 10, 9. [Google Scholar] [CrossRef]
- Varma-Doyle, A.V.; Lukiw, W.J.; Zhao, Y.; Lovera, J.; Devier, D. A hypothesis-generating scoping review of miRs identified in both multiple sclerosis and dementia, their protein targets, and miR signaling pathways. J. Neurol. Sci. 2021, 420, 117202. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshikawa, S.; Taniguchi, K.; Sawamura, H.; Ikeda, Y.; Tsuji, A.; Matsuda, S. A New Concept of Associations between Gut Microbiota, Immunity and Central Nervous System for the Innovative Treatment of Neurodegenerative Disorders. Metabolites 2022, 12, 1052. https://doi.org/10.3390/metabo12111052
Yoshikawa S, Taniguchi K, Sawamura H, Ikeda Y, Tsuji A, Matsuda S. A New Concept of Associations between Gut Microbiota, Immunity and Central Nervous System for the Innovative Treatment of Neurodegenerative Disorders. Metabolites. 2022; 12(11):1052. https://doi.org/10.3390/metabo12111052
Chicago/Turabian StyleYoshikawa, Sayuri, Kurumi Taniguchi, Haruka Sawamura, Yuka Ikeda, Ai Tsuji, and Satoru Matsuda. 2022. "A New Concept of Associations between Gut Microbiota, Immunity and Central Nervous System for the Innovative Treatment of Neurodegenerative Disorders" Metabolites 12, no. 11: 1052. https://doi.org/10.3390/metabo12111052
APA StyleYoshikawa, S., Taniguchi, K., Sawamura, H., Ikeda, Y., Tsuji, A., & Matsuda, S. (2022). A New Concept of Associations between Gut Microbiota, Immunity and Central Nervous System for the Innovative Treatment of Neurodegenerative Disorders. Metabolites, 12(11), 1052. https://doi.org/10.3390/metabo12111052






