New Oligomeric Dihydrochalcones in the Moss Polytrichum commune: Identification, Isolation, and Antioxidant Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Plant Material and Extraction
2.3. NMR Spectroscopy
2.4. Liquid Chromatography–high-Resolution Mass Spectrometry
2.5. Preparative Chromatography and Fraction Purity Assessment
2.6. In Vitro Antioxidant Capacity Assay (PCL Assay)
2.7. In Silico Biological Activity/Toxicity Assessment
3. Results and Discussion
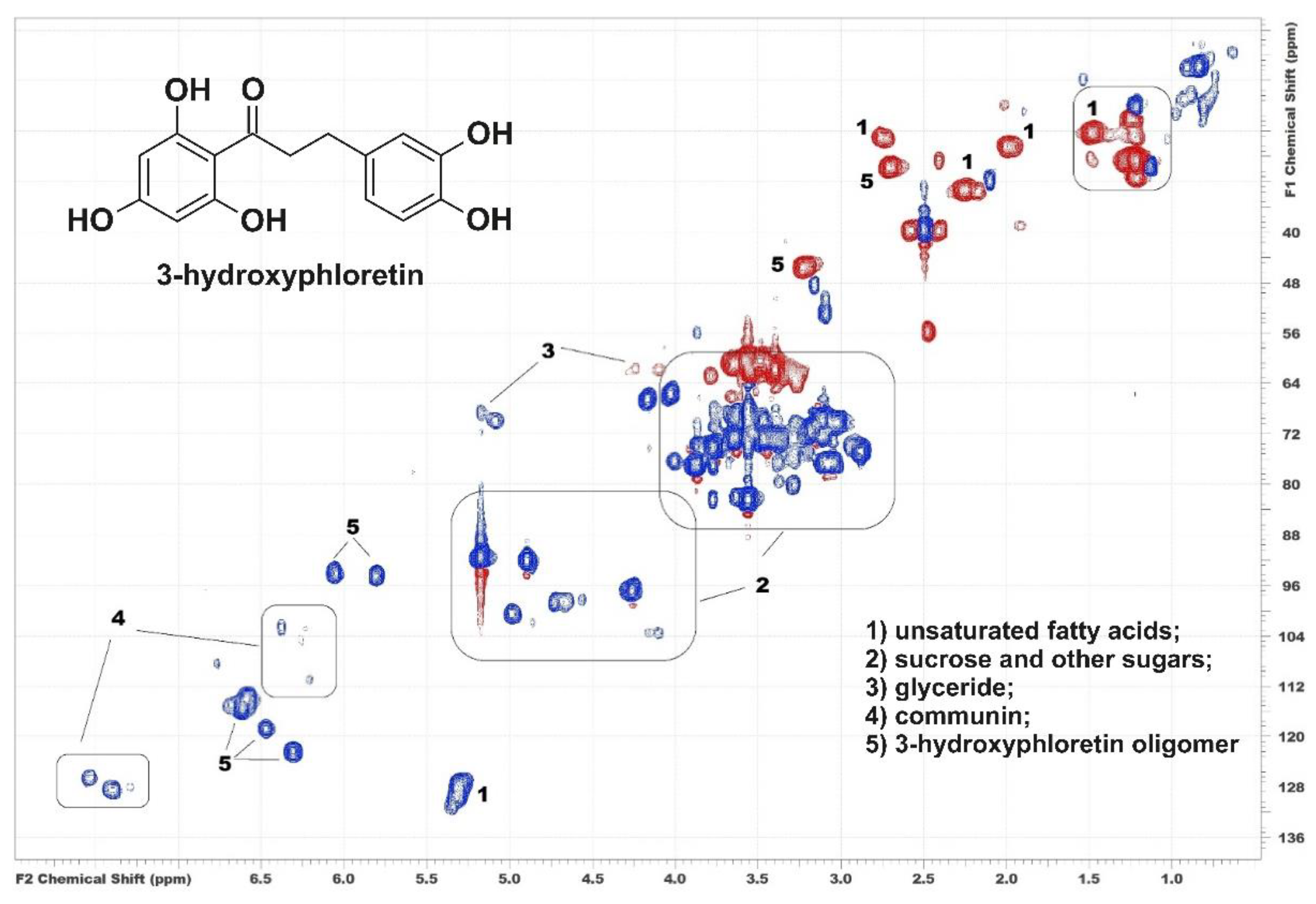
3.1. Two-Dimensional NMR Screening of Plant Extract Chemical Composition
3.2. HPLC-DAD-HRMS Analysis of Phenolic Compounds in the Plant Extract
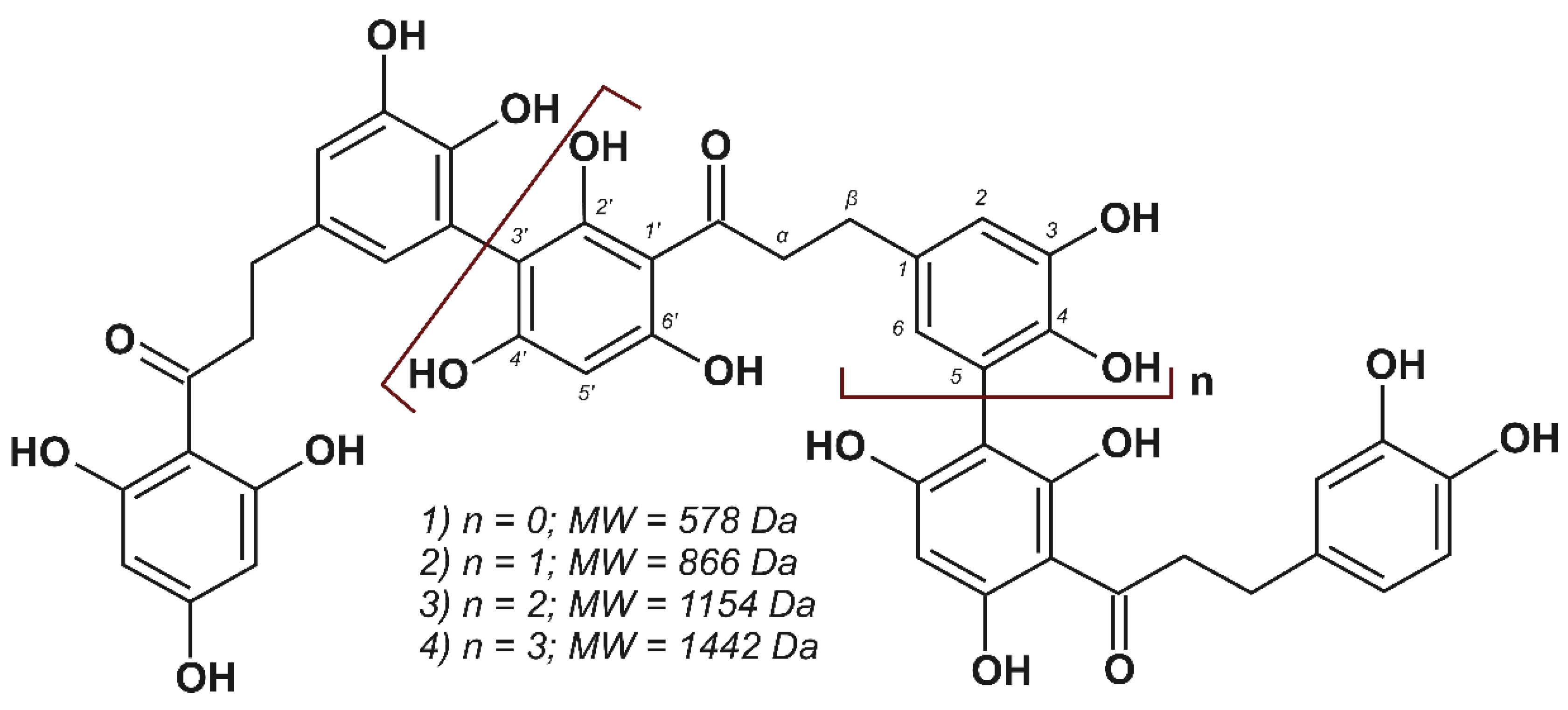
3.3. Isolation and Characterization of Individual Dihydrochalcones
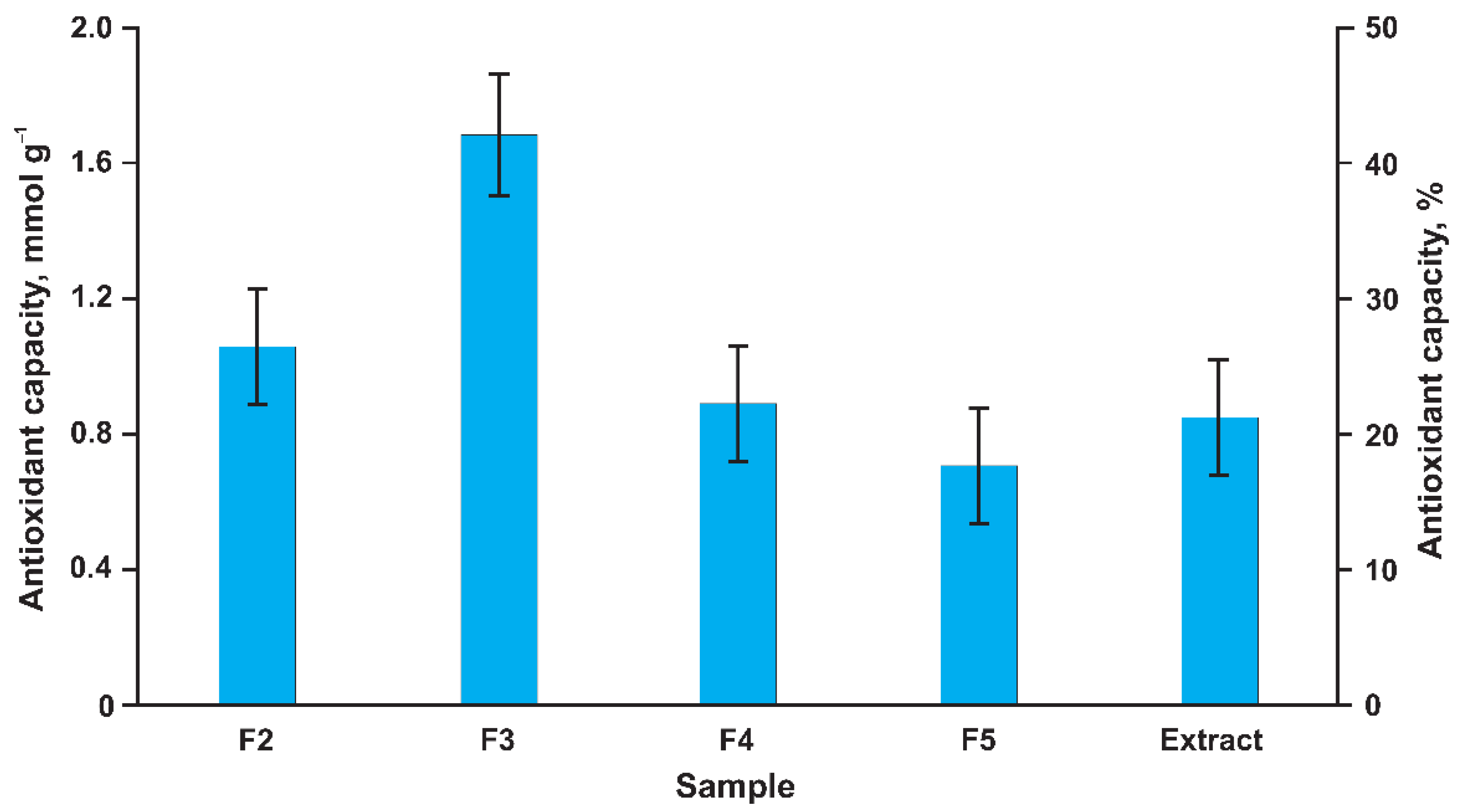
3.4. Antioxidant Activity and Estimated Health Impact
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Asakawa, Y.; Ludwiczuk, A.; Nagashima, F. Chemical Constituents of Bryophyta. In Progress in the Chemistry of Organic Natural Products; Springer: Berlin/Heidelberg, Germany, 2012; pp. 563–605. [Google Scholar] [CrossRef]
- Asakawa, Y.; Ludwiczuk, A.; Nagashima, F. Phytochemical and biological studies of bryophytes. Phytochemistry 2013, 91, 52–80. [Google Scholar] [CrossRef] [PubMed]
- Asakawa, Y. Phytochemistry of Bryophytes Biologically Active Terpenoids and Aromatic Compounds from Liverworts. In Phytochemicals in Human Health Protection, Nutrition, and Plant Defense; Romeo, J.T., Ed.; Kluwer Academic: Norwell, MA, USA; Plenum Publishers: New York, NY, USA, 1999; pp. 319–342. [Google Scholar] [CrossRef]
- Chandra, S.; Chandra, D.; Barh, A.; Pankaj Pandey, R.K.; Sharma, I.P. Bryophytes: Hoard of remedies, an ethno-medicinal review. J. Tradit. Complement. Med. 2017, 7, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.-F.; Bi, G.-M.; Zhang, Y.-H.; Li, J.-H.; Meng, D.-L. Rare benzonaphthoxanthenones from Chinese folk herbal medicine Polytrichum commune and their anti-neuroinflammatory activities in vitro. Bioorganic Chem. 2020, 102, 104087. [Google Scholar] [CrossRef]
- Zinsmeister, H.D.; Becker, H.; Eicher, T. Bryophytes, a Source of Biologically Active, Naturally Occurring Material? Angew. Chem. Int. Ed. Engl. 1991, 30, 130–147. [Google Scholar] [CrossRef]
- Asakawa, Y. Chemosystematics of the Hepaticae. Phytochemistry 2004, 65, 623–669. [Google Scholar] [CrossRef] [PubMed]
- Klavina, L.; Springe, G.; Nikolajeva, V.; Martsinkevich, I.; Nakurte, I.; Dzabijeva, D.; Steinberga, I. Chemical composition analysis, antimicrobial activity and cytotoxicity screening of moss extracts (Moss Phytochemistry). Molecules 2015, 20, 17221–17243. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.-Q.; Ho, D.K.; Elder, P.J.; Stephens, R.E.; Cottrell, C.E.; Cassady, J.M. Ohioensins and pallidisetins: Novel cytotoxic agents from the moss Polytrichum Pallidisetum. J. Nat. Prod. 1994, 57, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Fu, P.; Lin, S.; Shan, L.; Lu, M.; Shen, Y.-H.; Tang, J.; Liu, R.-H.; Zhang, X.; Zhu, R.-L.; Zhang, W.-D. Constituents of the moss Polytrichum commune. J. Nat. Prod. 2009, 72, 1335–1337. [Google Scholar] [CrossRef]
- Byeon, H.-E.; Um, S.H.; Yim, J.H.; Lee, H.K.; Pyo, S. Ohioensin F suppresses TNF-α-induced adhesion molecule expression by inactivation of the MAPK, Akt and NF-κB pathways in vascular smooth muscle cells. Life Sci. 2012, 90, 396–406. [Google Scholar] [CrossRef]
- Hanif, U.; Ali, H.A.; Shahwar, D.; Farid, S.; Ishtiaq, S. Evaluation of two bryophytes (Funaria hygrometrica and Polytrichum commune) as a source of natural antioxidant. Asian J. Chem. 2014, 26, 4339–4343. [Google Scholar] [CrossRef]
- Siegel, S.M. Evidence for the presence of lignin in moss gametophytes. Am. J. Bot. 1969, 56, 175–179. [Google Scholar] [CrossRef]
- Weng, J.-K.; Chapple, C. The origin and evolution of lignin biosynthesis. New Phytol. 2010, 187, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Karmanov, A.P.; Kocheva, L.S.; Karmanova, Y.A. Investigation of lignin from moss Polytrichum commune. Khimiya Rastit. Syr'ya 2014, 4, 109–114. (In Russian) [Google Scholar] [CrossRef][Green Version]
- Rencoret, J.; Gutiérrez, A.; Marques, G.; del Río, J.C.; Tobimatsu, Y.; Lam, P.Y.; Pérez-Boada, M.; Ruiz-Dueñas, F.J.; Barrasa, J.M.; Martínez, A.T. New Insights on Structures Forming the Lignin-Like Fractions of Ancestral Plants. Front. Plant Sci. 2021, 12, 740923. [Google Scholar] [CrossRef]
- Pegg, R.B.; Amarowicz, R.; Naczk, M.; Shahidi, F. PHOTOCHEM® for determination of antioxidant capacity of plant extracts. ACS Symp. Ser. 2007, 956, 140–158. [Google Scholar] [CrossRef]
- Faleva, A.V.; Pikovskoi, I.I.; Pokryshkin, S.A.; Chukhchin, D.G.; Kosyakov, D.S. Features of the Chemical Composition and Structure of Birch Phloem Dioxane Lignin: A Comprehensive Study. Polymers 2022, 14, 964. [Google Scholar] [CrossRef]
- Harnly, J.M.; Bhagwat, S.; Lin, L.-Z. Profiling methods for the determination of phenolic compounds in foods and dietary supplements. Anal. Bioanal. Chem. 2007, 389, 47–61. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Itoigawa, M.; Haruna, M.; Murata, H.; Furukawa, H. Dihydrochalcones from Balanophora tobiracola. Phytochemistry 1980, 19, 476–477. [Google Scholar] [CrossRef]
- Leu, S.J.; Lin, Y.P.; Lin, R.D.; Wen, C.L.; Cheng, K.T.; Hsu, F.L.; Lee, M.H. Phenolic constituents of Malus doumeri var. formosana in the field of skin care. Biol. Pharm. Bull. 2006, 29, 740–745. [Google Scholar] [CrossRef] [PubMed]
- Yao, G.-M.; Ding, Y.; Zuo, J.-P.; Wang, H.-B.; Wang, Y.-B.; Ding, B.-Y.; Chiu, P.; Qin, G.-W. Dihydrochalcones from the leaves of Pieris japonica. J. Nat. Prod. 2005, 68, 392–396. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Lee, W.; Ahn, G. Marine algal flavonoids and phlorotannins; an intriguing frontier of biofunctional secondary metabolites. Crit. Rev. Biotechnol. 2022, 42, 23–45. [Google Scholar] [CrossRef] [PubMed]
- Nimse, S.B.; Pal, D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef]
- Rivière, C. Dihydrochalcones: Occurrence in the Plant Kingdom, Chemistry and Biological Activities. Stud. Nat. Prod. Chem. 2016, 51, 253–381. [Google Scholar] [CrossRef]
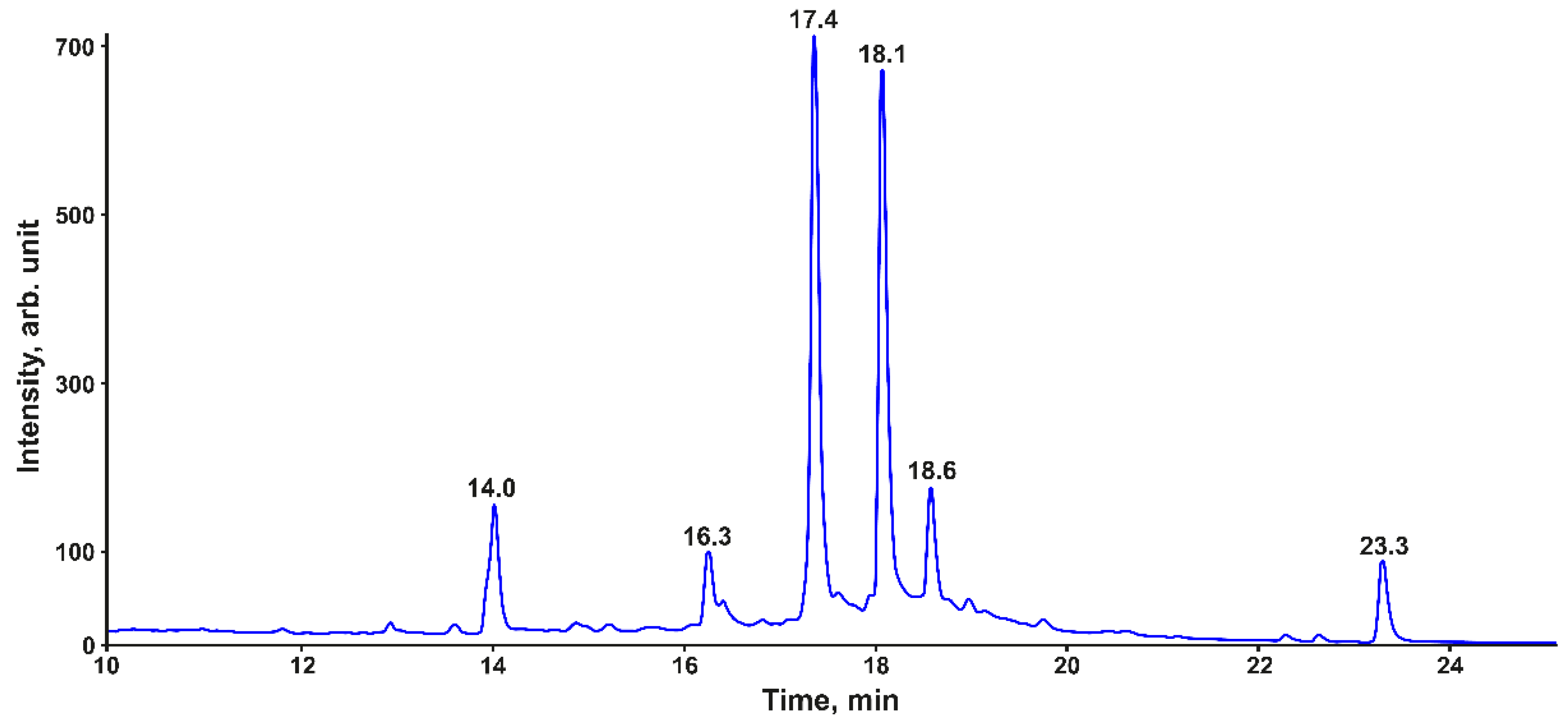
| No | Compound | tR, (min) | m/z [M + H]+ | Elemental Composition | ∆, ppm |
|---|---|---|---|---|---|
| 1 | 3-hydroxyphloretin dimer | 16.3 | 579.1505 | C30H26O12 | 1.4 |
| 2 | 3-hydroxyphloretin trimer | 17.4 | 867.2144 | C45H38O18 | 1.5 |
| 3 | 3-hydroxyphloretin tetramer | 18.1 | 1155.278 | C60H50O24 | 1.7 |
| 4 | 3-hydroxyphloretin pentamer | 18.6 | 1443.342 | C75H62O30 | 1.6 |
| No | δC *, ppm | δH, ppm (J, Hz) | HMBC Correlation | |
|---|---|---|---|---|
| End groups | ||||
| 1 | qC | 134.7 | - | |
| 2 | CH | 116.4 | 6.69, (m, overlapped) | 1, 3, 6, β |
| 3 | qC | 145.9 | - | |
| 4 | qC | 144.0 | - | |
| 5 | CH | ND | ND | |
| 6 | CH | 120.5 | 6.57, (m, overlapped) | 2, 4, β |
| 1′ | qC | 105.0 | - | |
| 2′ | qC | 165.8 | - | |
| 3′ | CH | 95.7 | 5.82, s | 1′, 2′, 5′, 6′ |
| 4′ | qC | 168.9 | - | |
| 5′ | CH | 95.7 | 5.82, s | 1′, 2′ 3′, 6′ |
| 6′ | qC | 165.8 | - | |
| C=O | 206.7 | - | ||
| α | CH2 | 47.4 | 3.32, t (overlapped) | β, C=O, 1 |
| β | CH2 | 31.7 | 2.83, t (overlapped) | α, C=O, 1, 2, 6 |
| Conjugated links | ||||
| 1 | qC | 134.7 | - | |
| 2 | CH | 115.7 | 6.73, (m, overlapped) | 1, 3, 6, β |
| 3 | qC | 146.8 | - | |
| 4 | qC | 142.5 | - | |
| 5 | qC | ND | - | |
| 6 | CH | 124.3 | 6.54, (m, overlapped) | 2, 4, β, 3′ |
| 1′ | qC | 105.8 | - | |
| 2′ | qC | 163.5 ** | - | |
| 3′ | qC | 106.2 | - | |
| 4′ | qC | ND | - | |
| 5′ | CH | 95.6 | 6.03, s | 1′, 2′ 3′, 6′ |
| 6′ | qC | 163.5 ** | - | |
| C=O | 206.7 | - | ||
| α | CH2 | 47.2 | 3.37, t (overlapped) | β, C=O, 1 |
| β | CH2 | 31.8 | 2.87, t (overlapped) | α, C=O, 1, 2, 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faleva, A.V.; Ul’yanovskii, N.V.; Falev, D.I.; Onuchina, A.A.; Budaev, N.A.; Kosyakov, D.S. New Oligomeric Dihydrochalcones in the Moss Polytrichum commune: Identification, Isolation, and Antioxidant Activity. Metabolites 2022, 12, 974. https://doi.org/10.3390/metabo12100974
Faleva AV, Ul’yanovskii NV, Falev DI, Onuchina AA, Budaev NA, Kosyakov DS. New Oligomeric Dihydrochalcones in the Moss Polytrichum commune: Identification, Isolation, and Antioxidant Activity. Metabolites. 2022; 12(10):974. https://doi.org/10.3390/metabo12100974
Chicago/Turabian StyleFaleva, Anna V., Nikolay V. Ul’yanovskii, Danil I. Falev, Aleksandra A. Onuchina, Nikolay A. Budaev, and Dmitry S. Kosyakov. 2022. "New Oligomeric Dihydrochalcones in the Moss Polytrichum commune: Identification, Isolation, and Antioxidant Activity" Metabolites 12, no. 10: 974. https://doi.org/10.3390/metabo12100974
APA StyleFaleva, A. V., Ul’yanovskii, N. V., Falev, D. I., Onuchina, A. A., Budaev, N. A., & Kosyakov, D. S. (2022). New Oligomeric Dihydrochalcones in the Moss Polytrichum commune: Identification, Isolation, and Antioxidant Activity. Metabolites, 12(10), 974. https://doi.org/10.3390/metabo12100974