Optimized Fertilization Practices Improved Rhizosphere Soil Chemical and Bacterial Properties and Fresh Waxy Maize Yield
Abstract
1. Introduction
2. Materials and Methods
3. Results
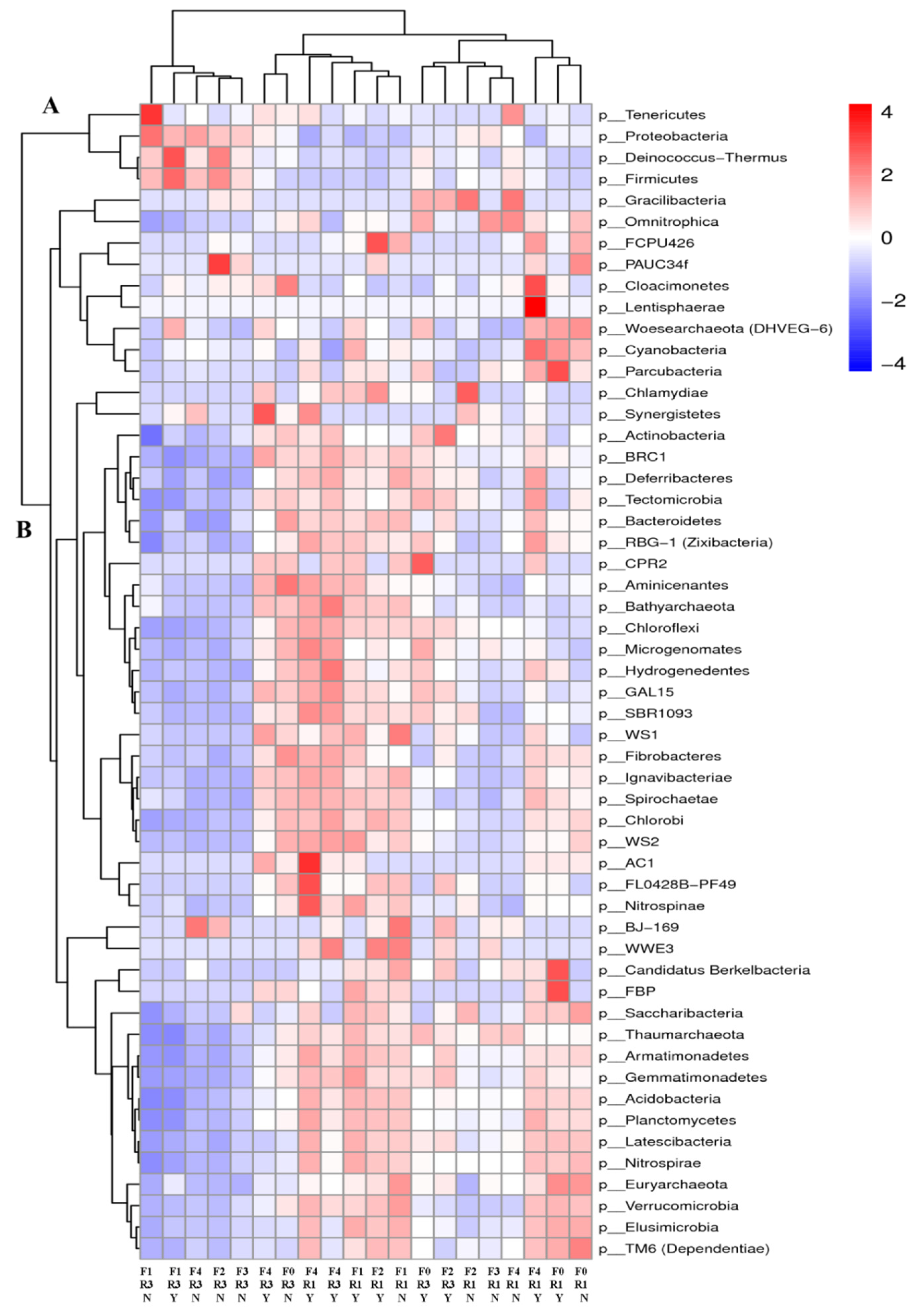
3.1. Abundance of Rhizosphere Soil Bacterial Community
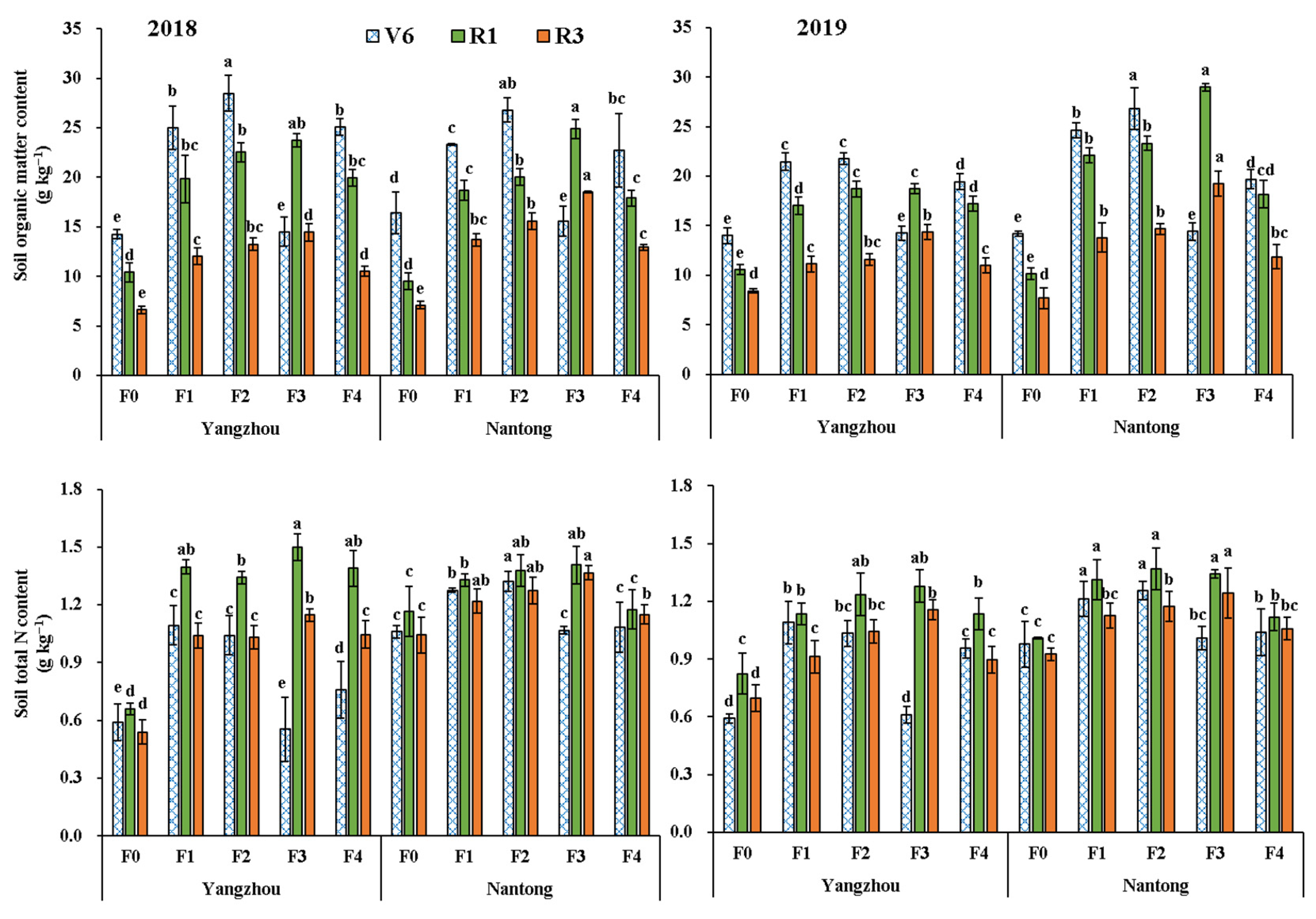
3.2. Rhizosphere Soil Chemical Properties
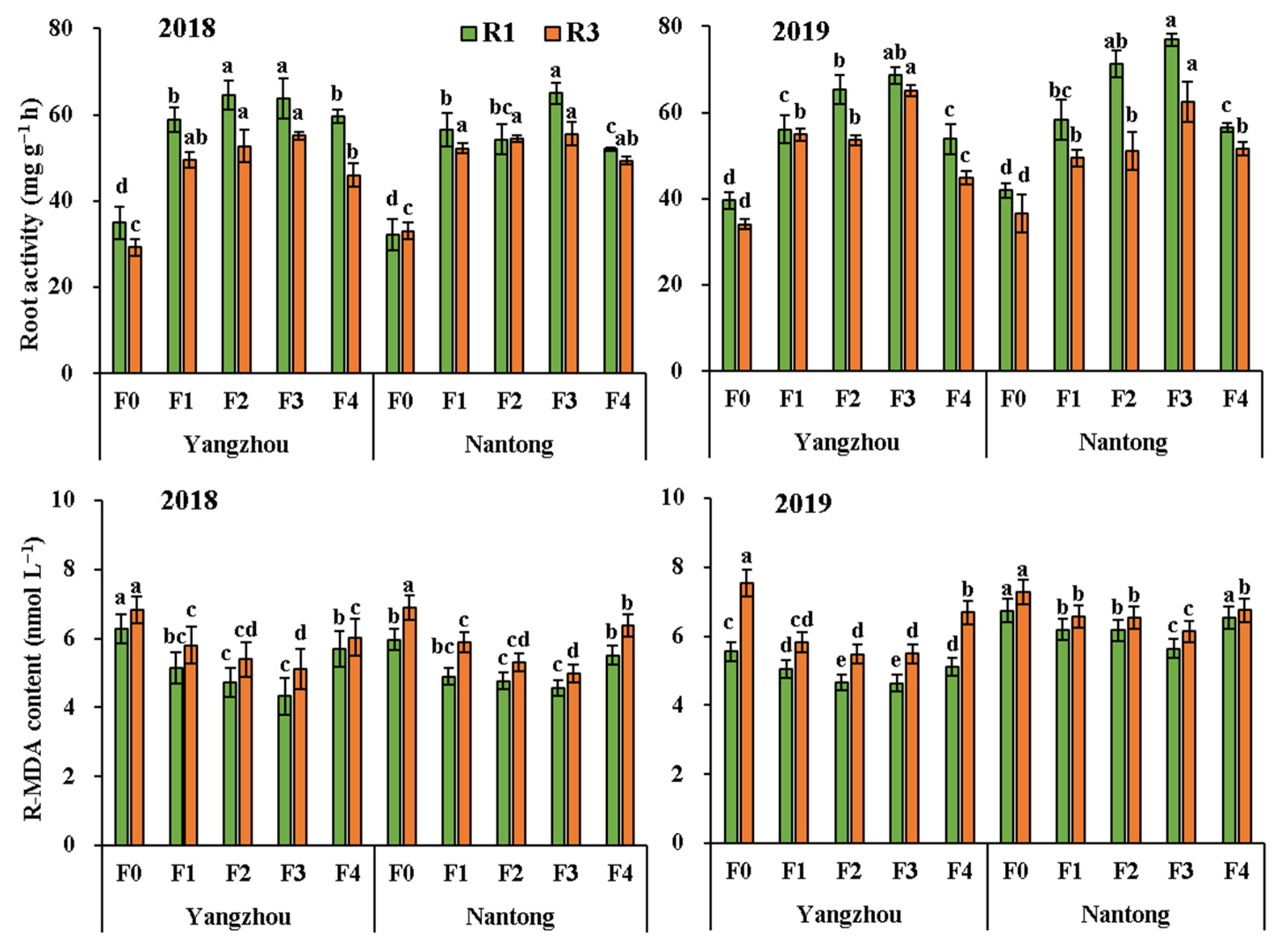
3.3. Root Properties
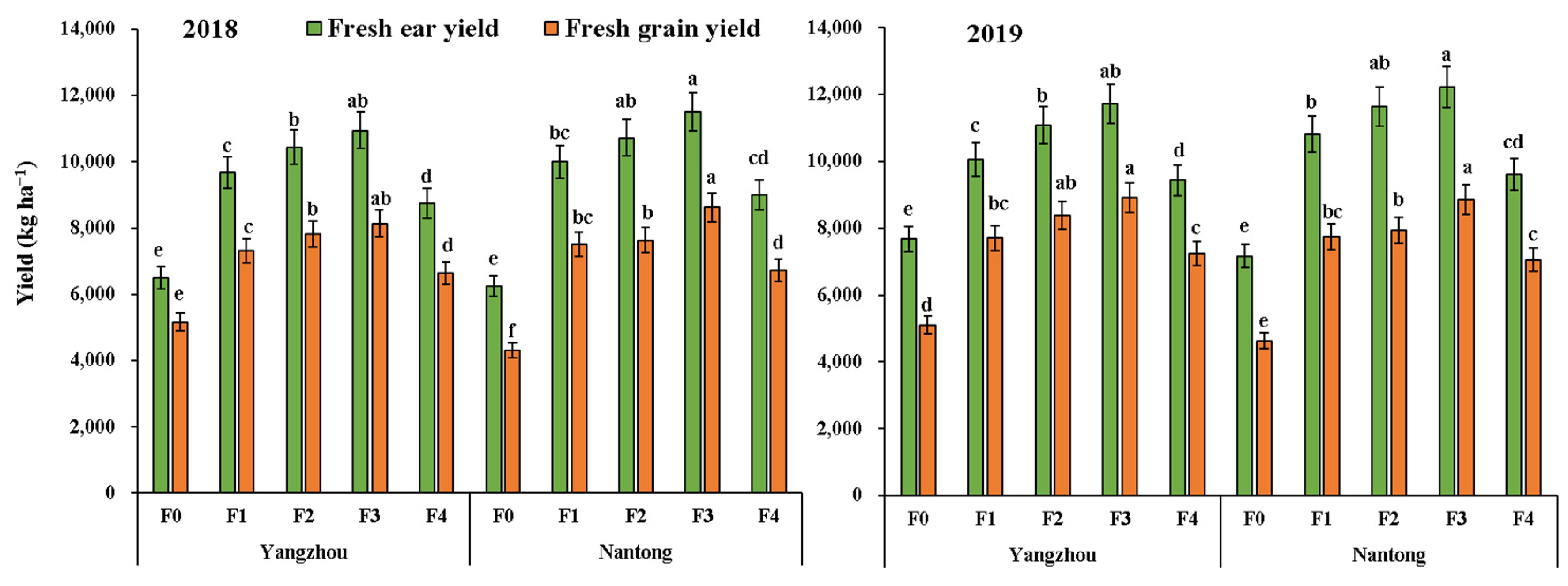
3.4. Yield and Economic Analysis
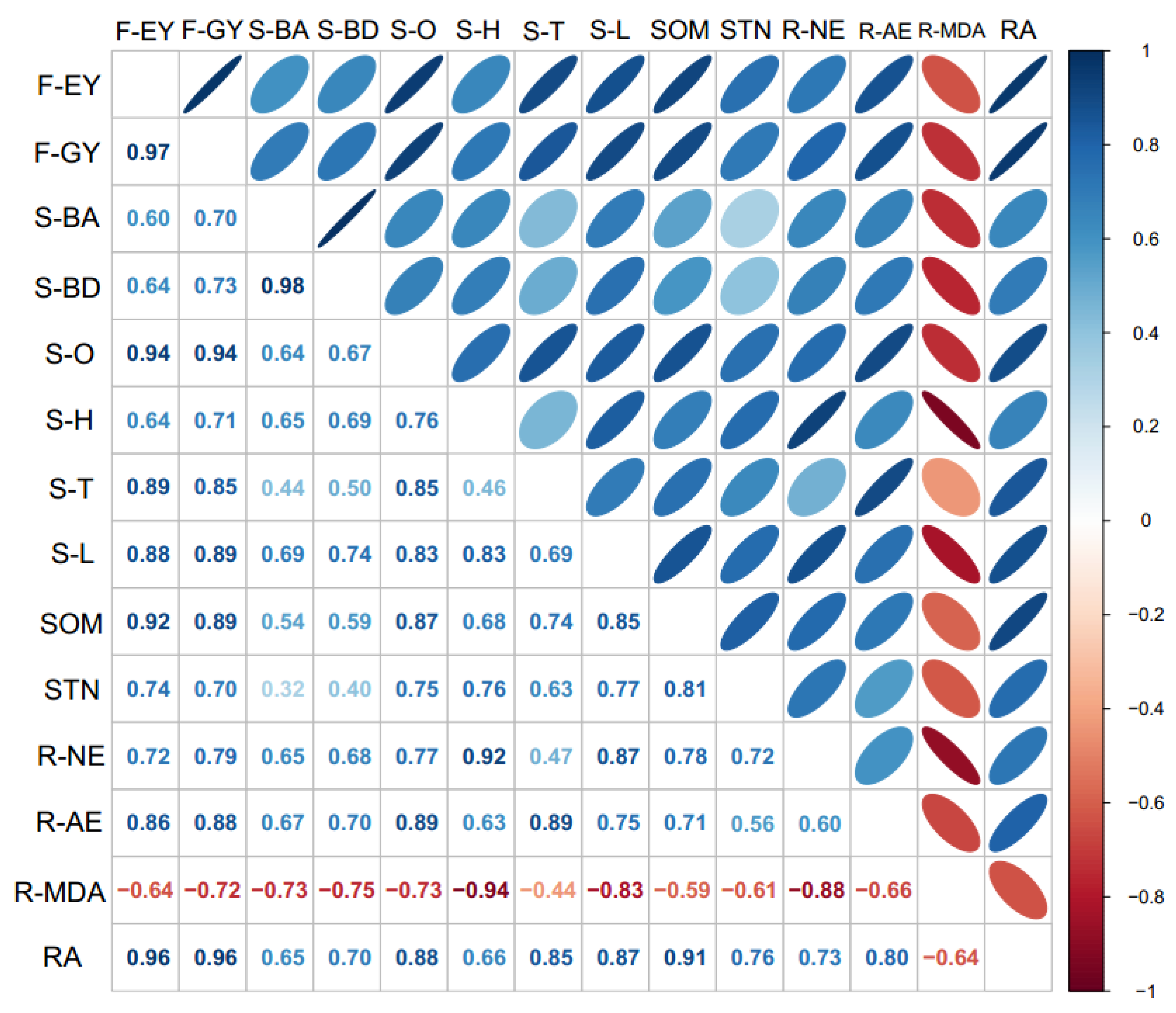
3.5. Spearman Correlation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, B.Q. New Fertilizers; Science Press: Beijing, China, 2013; pp. 14–96. [Google Scholar]
- Lubkowski, K. Environmental impact of fertilizer use and slow release of mineral nutrients as a response to this challenge. Pol. J. Chem Technol. 2016, 18, 72–79. [Google Scholar] [CrossRef]
- Fertahi, S.; Ilsouk, M.; Zeroual, Y.; Oukarroum, A.; Barakat, A. Recent trends in organic coating based on biopolymers and biomass for controlled and slow release fertilizers. J. Control. Release 2021, 330, 341–361. [Google Scholar] [CrossRef] [PubMed]
- Azeem, B.; KuShaari, K.; Man, Z.B.; Basit, A.; Thanh, T.H. Review on materials & methods to produce controlled release coated urea fertilizer. J. Control. Release 2014, 181, 11–21. [Google Scholar] [PubMed]
- Zhang, W.S.; Liang, Z.Y.; He, X.M.; Wang, X.Z.; Shi, X.J.; Zou, C.Q.; Chen, X.P. The effects of controlled release urea on maize productivity and reactive nitrogen losses: A meta-analysis. Environ. Pollut. 2019, 246, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Verburg, K.; Huth, N. Modelling sugarcane nitrogen uptake patterns to inform design of controlled release fertilizer for synchrony of N supply and demand. Field Crops Res. 2017, 213, 51–64. [Google Scholar] [CrossRef]
- Tian, C.; Zhou, X.; Ding, Z.L.; Liu, Q.; Xie, G.Z.; Peng, J.W.; Rong, X.M.; Zhang, Y.P.; Yang, Y.; Eissa, M.A. Controlled-release N fertilizer to mitigate ammonia volatilization from double-cropping rice. Nutr. Cycl. Agroecosys. 2020, 199, 123–137. [Google Scholar] [CrossRef]
- Gao, Y.; Song, X.; Liu, K.; Li, T.; Zheng, W.; Wang, Y.; Liu, Z.; Zhang, M.; Chen, Q.; Li, Z.; et al. Mixture of controlled-release and conventional urea fertilizer application changed soil aggregate stability, humic acid molecular composition, and maize nitrogen uptake. Sci. Total Environ. 2021, 789, 147778. [Google Scholar] [CrossRef]
- Li, G.; Fu, P.; Cheng, G.; Lu, W.; Lu, D. Delaying application time of slow-release fertilizer increases soil rhizosphere nitrogen content, root activity, and grain yield of spring maize. Crop. J. 2022, in press. [CrossRef]
- Zhang, X.Y.; Li, G.H.; Yang, H.; Lu, D.L. Foliar brassinolide sprays ameliorate post-silking heat stress on the accumulation and remobilization of biomass and nitrogen in fresh waxy maize. Agronomy 2022, 12, 1363. [Google Scholar] [CrossRef]
- Hayat, R.; Ali, S.; Amara, U.; Khalid, R.; Ahmed, I. Soil beneficial bacteria and their role in plant growth promotion: A review. Ann. Microbiol. 2010, 60, 579–598. [Google Scholar] [CrossRef]
- Chen, D.; Yuan, L.; Liu, Y.; Ji, J.H.; Hou, H.Q. Long-term application of manures plus chemical fertilizers sustained high rice yield and improved soil chemical and bacterial properties. Eur. J. Agron. 2017, 90, 34–42. [Google Scholar] [CrossRef]
- Lazcano, C.; Gómez-Brandón, M.; Revilla, P.; Domínguez, J. Short-term effects of organic and inorganic fertilizers on microbial community structure and function: A field study with sweet corn. Biol. Fert. Soils. 2013, 49, 723–733. [Google Scholar] [CrossRef]
- Wang, J.; Xie, J.; Li, L.; Luo, Z.; Zhang, R.; Jiang, Y. Nitrogen application increases soil microbial carbon fixation and maize productivity on the semiarid Loess Plateau. Plant Soil 2022. Online. [Google Scholar] [CrossRef]
- Tian, S.; Zhu, B.; Yin, R.; Wang, M.; Jiang, Y.; Zhang, C.; Li, D.; Chen, X.; Kardol, P.; Liu, M. Organic fertilization promotes crop productivity through changes in soil aggregation. Soil Biol. Biochem. 2022, 165, 108533. [Google Scholar] [CrossRef]
- Poffenbarger, H.; Barker, D.; Helmers, M.; Miguez, F.; Olk, D.; Sawyer, J.; Six, J.; Castellano, M. Maximum soil organic carbon storage in Midwest U.S. Cropping systems when crops are optimally nitrogen-fertilized. PLoS ONE. 2017, 12, e0172293. [Google Scholar] [CrossRef]
- Mahal, N.; Osterholz, W.; Miguez, F.; Poffenbarger, H.; Sawyer, J.; Olk, D.; Archontoulis, S.; Castellano, M. Nitrogen fertilizer suppresses mineralization of soil organic matter in maize agroecosystems. Front. Ecol. Evol. 2019, 7, 59. [Google Scholar] [CrossRef]
- Feng, G.; Zhang, Y.; Chen, Y.; Li, Q.; Chen, F.; Gao, Q.; Mi, G. Effects of nitrogen application on root length and grain yield of rain-fed maize under different soil type. Agron. J. 2016, 108, 1656–1665. [Google Scholar] [CrossRef]
- Wen, Z.; Shen, J.; Blackwell, M.; Li, H.; Zhao, B.; Yuan, H. Combined application of nitrogen and phosphorus fertilizer with manure increase maize yield and nutrient uptake via stimulating root growth in long term experiment. Pedosphere 2016, 26, 62–73. [Google Scholar] [CrossRef]
- Ordóñez, R.; Castellano, M.; Danalatos, G.; Wright, E.; Hatfield, J.; Burras, L.; Archontoulis, S. Insufficient and excessive N fertilizer input reduces maize root mass across soil types. Field Crops Res. 2019, 267, 108142. [Google Scholar] [CrossRef]
- Geng, J.; Sun, Y.; Zhang, M.; Li, C.; Yang, Y.; Liu, Z.; Li, S. Long-term effects of controlled release urea application on crop yields and soil fertility under rice-oilseed rape rotation system. Field Crops Res. 2015, 184, 65–73. [Google Scholar] [CrossRef]
- Li, R.; Gao, Y.; Chen, Q.; Li, Z.; Gao, F.; Meng, Q.; Li, T.; Liu, A.; Wang, Q.; Wu, L.; et al. Blended controlled-release nitrogen fertilizer with straw returning improved soil nitrogen availability, soil microbial community, and root morphology of wheat. Soil Till. Res. 2021, 212, 105045. [Google Scholar] [CrossRef]
- Shao, G.; Li, Z.; Ning, T.; Zheng, Y. Responses of photosynthesis, chlorophyll fluorescence, and grain yield of maize to controlled-release urea and irrigation after anthesis. J. Plant Nutr. Soil Sc. 2013, 176, 595–602. [Google Scholar] [CrossRef]
- Li, G.; Zhao, B.; Dong, S.; Zhang, J.; Liu, P.; Ren, B.; Lu, D.; Lu, W. Morphological and physiological characteristics of maize roots in response to controlled-release urea under different soil moisture conditions. Agron. J. 2019, 111, 1849–1864. [Google Scholar] [CrossRef]
- Li, G.; Wang, L.; Li, L.; Lu, D.; Lu, W. Effects of fertilizer management strategies on maize yield and nitrogen use efficiencies under different densities. Agron. J. 2020, 112, 368–381. [Google Scholar] [CrossRef]
- Edenborn, S.; Sexstone, A. DGGE fingerprinting of culturable soil bacterial communities complements culture-independent analyses. Soil Biol. Biochem. 2007, 39, 1570–1579. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Liu, H.; Liu, P.; Dong, S.; Zhao, B.; Hwat, B.; Li, G.; Liu, H.; Zhang, J.; Zhao, B. Morphological and physiological characteristics of corn (Zea mays L.) roots from cultivars with different yield potentials. Eur. J. Agron. 2012, 38, 54–63. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, X.; Kuzyakov, Y.; Wang, H.; Fu, X.; Yang, Y.; Chen, F.; Dungait, J.; Green, S.; Fang, X. Responses of C-, N- and P-acquiring hydrolases to P and N fertilizers in a subtropical Chinese fir plantation depend on soil depth. Appl. Soil Ecol. 2020, 150, 103465. [Google Scholar] [CrossRef]
- Bohlen, P.; Groffman, P.; Driscoll, C.; Fahey, T.; Siccama, T. Plant-soil-microbial interactions in a northern hardwood forest. Ecology 2001, 82, 965–978. [Google Scholar]
- Waldrop, M.; Firestone, M. Response of microbial community composition and function to soil climate change. Microb. Ecol. 2006, 52, 716–724. [Google Scholar] [CrossRef]
- Kuzyakov, Y. Priming effects: Interactions between living and dead organic matter. Soil Biol. Biochem. 2010, 42, 1363–1371. [Google Scholar] [CrossRef]
- Wang, H.; Li, J.; Chen, H.; Liu, H.; Nie, M. Enzymic moderations of bacterial and fungal communities on short- and long-term warming impacts on soil organic carbon. Sci. Total Environ. 2022, 804, 150197. [Google Scholar] [CrossRef] [PubMed]
- Balota, E.L.; Filho, A.; Andrade, D.S.; Dick, R.P. Long-term tillage and crop rotation effects on microbial biomass and C and N mineralization in a Brazilian Oxisol. Soil Till. Res. 2004, 77, 137–145. [Google Scholar] [CrossRef]
- Mikanová, O.; Friedlová, M.; Šimon, T. The influence of fertilization and crop rotation on soil microbial characteristics in the long-term field experiment. Plant Soil Environ. 2009, 55, 11–16. [Google Scholar] [CrossRef]
- Liu, J.; Han, L.; Chen, F.; Bao, J.; Zhang, F.; Mi, G. Microarray analysis reveals early responsive genes possibly involved in localized nitrate stimulation of lateral root development in maize. Plant Sci. 2008, 175, 272–282. [Google Scholar] [CrossRef]
- Liu, Y.; Behrens, I.; Muthreich, N.; Schutz, W.; Nordheim, A.; Hochholdinger, F. Regulation of the pericycle proteome in maize (Zea mays L.) primary roots by RUM1 which is required for lateral root initiation. Eur. J. Cell Biol. 2010, 89, 236–241. [Google Scholar] [CrossRef]
- Cui, Z.; Zhang, F.; Miao, Y.; Sun, Q.; Li, F.; Chen, X.; Li, J.; Ye, Y.; Yang, Z.; Zhang, Q.; et al. Soil nitrate-N levels required for high yield maize production in the North China Plain. Nutr. Cycl. Agroecosys. 2008, 82, 187–196. [Google Scholar] [CrossRef]
- Chipomho, J.; Rugare, J.; Mabasa, S.; Zingore, S.; Mashingaidze, A.; Chikowo, R. Short-term impacts of soil nutrient management on maize (Zea mays L.) productivity and weed dynamics along a toposequence in Eastern Zimbabwe. Heliyon 2020, 6, e05223. [Google Scholar] [CrossRef]
- Borowska, K.; Koper, J. The effect of long-term organic–mineral fertilization on selenium content and chosen oxidoreductases activity under winter wheat cultivation. Chem. Ecol. 2010, 26, 111–116. [Google Scholar] [CrossRef]
- Geng, J.; Chen, J.; Sun, Y.; Zheng, W.; Tian, F.; Yang, Y.; Li, C.; Zhang, M. Controlled-release urea improved nitrogen use efficiency and yield of wheat and corn. Agron. J. 2016, 108, 1666–1673. [Google Scholar] [CrossRef]
- Nacry, P.; Bouguyon, E.; Gojon, A. Nitrogen acquisition by roots: Physiological and developmental mechanisms ensuring plant adaptation to a fluctuating resource. Plant Soil. 2013, 370, 1–29. [Google Scholar] [CrossRef]
- Lynch, J.; Wojciechowski, T. Opportunities and challenges in the subsoil: Pathways to deeper rooted crops. J. Exp. Bot. 2015, 66, 2199–2210. [Google Scholar] [CrossRef] [PubMed]
- Schneider, H.; Lynch, J. Functional implications of root cortical senescence for soil resource capture. Plant Soil. 2018, 423, 13–26. [Google Scholar] [CrossRef]
- Chilundo, M.; Joel, A.; Wesström, I.; Rui, B.; Messing, I. Response of maize root growth to irrigation and nitrogen management strategies in semi-arid loamy sandy soil. Field Crops Res. 2017, 200, 143–162. [Google Scholar] [CrossRef]
- Motte, H.; Vanneste, S.; Beeckman, T. Molecular and environmental regulation of root development. Annu. Rev. Plant Biol. 2019, 70, 465–488. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Cheng, G.; Lu, D.; Lu, W. Differences of yield and nitrogen use efficiency under different applications of slow release fertilizer in spring maize. J. Integr. Agr. 2021, 20, 554–564. [Google Scholar] [CrossRef]
- Mueller, S.M.; Vyn, T.J. Maize plant resilience to N stress and post-silking N capacity changes over time: A Review. Front. Plant Sci. 2016, 7, 53. [Google Scholar] [CrossRef]
- Zhao, B.; Dong, S.T.; Zhang, J.W.; Liu, P. Effects of controlled-release fertilizer on nitrogen use efficiency in summer maize. PLoS ONE 2013, 8, e70569. [Google Scholar]
- Farmaha, B.; Sims, A. The influence of PCU and urea fertilizer mixtures on spring wheat protein concentrations and economic returns. Agron. J. 2012, 105, 1328–1334. [Google Scholar] [CrossRef]
- Noellsch, A.; Motavalli, P.; Nelson, K.; Kitchen, N. Corn response to conventional and slow-release nitrogen fertilizers across a claypan landscape. Agron. J. 2009, 101, 607–614. [Google Scholar] [CrossRef]
- Guo, J.; Wang, Y.; Blaylock, A.; Chen, X. Mixture of controlled release and normal urea to optimize nitrogen management for high-yielding (>15 Mg ha−1) maize. Field Crops Res. 2017, 204, 23–30. [Google Scholar] [CrossRef]
- Guo, J.; Fan, J.; Zhang, F.; Yan, S.; Zheng, J.; Wu, Y.; Li, J.; Wang, Y.; Sun, X.; Liu, X.; et al. Blending urea and slow-release nitrogen fertilizer increases dryland maize yield and nitrogen use efficiency while mitigating ammonia volatilization. Sci. Total Environ. 2021, 790, 148058. [Google Scholar] [CrossRef] [PubMed]

| ANOVA | SOM g kg−1) | Total N (g kg−1) | Deh (U g−1 FW) | POD (U g−1 FW) | CAT (U g−1 FW) | NR (U g−1 FW) | Inv (U g−1 FW) | Amy (U g−1 FW) | Ure (U g−1 FW) | Pho (U g−1 FW) | Tsa (U g−1 FW) | TGS (U g−1 FW) | ASPD (U g−1 FW) | GAD (U g−1 FW) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Year (Y) | 2018 | 15.6 a | 1.17 a | 0.792 a | 0.169 b | 0.121 b | 0.190 b | 7.53 a | 0.576 b | 10.70 a | 8.08 a | 3.11 b | 0.258 b | 1.92 b | 3.45 a |
| 2019 | 15.4 a | 1.10 ab | 0.741 ab | 0.205 a | 0.145 a | 0.214 a | 6.78 b | 0.633 a | 8.39 b | 7.54 b | 3.46 ab | 0.302 a | 2.13 a | 3.05 b | |
| Site (S) | Yangzhou | 14.6 b | 1.06 ab | 0.776 a | 0.178 b | 0.132 a | 0.200 a | 7.22 a | 0.598 b | 10.00 a | 7.66 a | 3.20 a | 0.276 b | 2.07 a | 3.25 a |
| Nantong | 16.4 a | 1.20 a | 0.757 a | 0.196 a | 0.134 a | 0.204 a | 7.10 b | 0.611 a | 9.09 b | 7.95 a | 3.36 a | 0.284 a | 1.98 b | 3.25 a | |
| Fertilization (F) | F0 | 8.8 d | 0.85 c | 0.605 d | 0.148 d | 0.105 d | 0.175 c | 5.80 e | 0.453 d | 7.56 c | 6.29 d | 2.76 d | 0.235 d | 1.71 c | 2.59 e |
| F1 | 16.0 bc | 1.15 b | 0.800 bc | 0.194 b | 0.136 b | 0.208 ab | 7.27 c | 0.621 b | 9.69 b | 7.90 b | 3.32 b | 0.282 bc | 2.07 b | 3.24 c | |
| F2 | 17.5 b | 1.23 ab | 0.826 b | 0.200 ab | 0.145 ab | 0.215 a | 7.74 b | 0.667 ab | 10.40 ab | 8.46 ab | 3.47 ab | 0.291 b | 2.16 ab | 3.52 b | |
| F3 | 20.4 a | 1.31 a | 0.868 a | 0.210 a | 0.150 a | 0.217 a | 8.29 a | 0.690 a | 10.88 a | 8.79 a | 3.67 a | 0.320 a | 2.25 a | 3.97 a | |
| F4 | 15.0 c | 1.13 b | 0.734 c | 0.183 c | 0.128 c | 0.194 b | 6.68 d | 0.590 c | 9.22 b | 7.60 bc | 3.19 c | 0.271 c | 1.94 b | 2.94 d | |
| Y | 0.1 | 3.7 * | 7.7 * | 178.7 ** | 97.6 ** | 15.3 ** | 33.0 ** | 106.9 ** | 132.7 ** | 9.9 ** | 18.9 ** | 144.9 ** | 59.3 ** | 27.7 ** | |
| S | 19.8 ** | 9.0 * | 1.4 | 41.7 ** | 6.0 | 0.3 | 0.1 | 17.5 ** | 20.0 ** | 5.2 | 6.6 * | 3.5 | 9.8 * | 0.2 | |
| F | 66.8 ** | 87.3 ** | 118.7 ** | 96.83 ** | 88.1 ** | 60.8 ** | 54.6 ** | 143.9 ** | 121.7 ** | 109.8 ** | 100.9 ** | 86.2 ** | 69.8 ** | 120.7 ** | |
| Y × S | 3.1 | 0.1 | 34.2 ** | 80.6 ** | 19.6 ** | 0.1 | 0.1 | 1.0 | 6.7 * | 5.3 ** | 4.2 | 0.1 | 40.5 ** | 1.1 | |
| Y × F | 0.4 | 4.5 | 1.1 | 0.6 | 2.9 | 16.4 ** | 1.5 | 0.22 | 1.1 | 3.5 * | 3.3 | 3.8 * | 1.2 | 2.4 | |
| S × F | 4.1 * | 12.8 ** | 4.7 * | 4.8 * | 5.5 * | 6.7 * | 1.5 | 1.8 | 11.1 ** | 7.7 * | 2.8 | 12.4 ** | 2.0 | 1.9 | |
| Y × S × F | 1.2 | 4.2 * | 7.0 * | 1.2 | 1.0 | 8.4 * | 1.2 | 1.2 | 1.7 | 5.1 * | 3.1 | 0.3 | 4.5 * | 5.5 * | |
| ANOVA | R-NR (U g−1 FW) | R-GOGAT (U g−1 FW) | R-GS (U g−1 FW) | Root Activity (mg g−1 h) | R-MDA (nmol L−1) | R-SOD (U g−1 FW) | R-POD (U g−1 FW) | R-CAT (U g−1 FW) | |
|---|---|---|---|---|---|---|---|---|---|
| Year (Y) | 2018 | 1.767 a | 0.854 a | 0.520 a | 50.97 b | 5.526 b | 32.03 b | 0.239 b | 0.083 b |
| 2019 | 1.652 b | 0.576 b | 0.463 b | 54.59 a | 6.029 a | 35.09 a | 0.277 a | 0.091 a | |
| Site (S) | Yangzhou | 1.757 a | 0.720 a | 0.491 a | 52.52 a | 5.569 ab | 33.93 a | 0.256 a | 0.086 a |
| Nantong | 1.662 b | 0.710 a | 0.492 a | 53.04 a | 5.986 a | 33.19 a | 0.260 a | 0.087 a | |
| Fertilization (F) | F0 | 1.278 e | 0.555 d | 0.346 e | 35.20 e | 6.637 a | 26.32 d | 0.207 e | 0.066 d |
| F1 | 1.811 c | 0.719 c | 0.505 c | 54.49 c | 5.668 c | 34.14 b | 0.262 c | 0.089 bc | |
| F2 | 1.881 b | 0.769 b | 0.545 b | 58.42 b | 5.379 cd | 36.79 ab | 0.280 b | 0.095 ab | |
| F3 | 1.967 a | 0.839 a | 0.597 a | 64.07 a | 5.115 d | 38.25 a | 0.298 a | 0.100 a | |
| F4 | 1.611 d | 0.691 c | 0.466 d | 51.72 d | 6.088 b | 32.30 c | 0.243 d | 0.083 c | |
| Y | 18.6 ** | 317.2 ** | 26.0 ** | 5.4 * | 6.8 * | 42.1 ** | 78.4 ** | 35.5 ** | |
| S | 7.4 * | 0.1 | 0.2 | 0.3 | 4.9 * | 0.3 | 0.6 | 1.1 | |
| F | 313.2 ** | 138.0 ** | 148.3 ** | 131.0 ** | 38.6 ** | 50.9 ** | 86.8 ** | 135.1 ** | |
| Y × S | 1.8 | 0.1 | 0.3 | 2.0 | 3.3 | 9.7 * | 0.3 | 38.8 ** | |
| Y × F | 15.1 ** | 15.3 ** | 6.6 * | 4.5 * | 2.3 | 2.0 | 6.4 * | 5.4 * | |
| S × F | 1.1 | 2.0 | 0.7 | 1.8 | 1.5 | 4.6 * | 2.4 | 5.3 * | |
| Y × S × F | 2.9 | 2.6 | 4.2 | 0.6 | 1.0 | 1.7 | 1.7 | 8.4 * | |
| Year (Y) | Site (S) | Fertilization (F) | Gross Return | Fertilizer Cost | Fertilization Cost | Net Return |
|---|---|---|---|---|---|---|
| 2018 | Yangzhou | F0 | 13,000 g | 0 | 0 | 9880 g |
| F1 | 19,334 d | 1917 | 750 | 13,547 de | ||
| F2 | 20,888 c | 1917 | 750 | 15,101 cd | ||
| F3 | 21,890 b | 1917 | 750 | 16,103 bc | ||
| F4 | 17,490 e | 1652 | 1500 | 11,218 f | ||
| Nantong | F0 | 12,504 g | 0 | 0 | 9384 g | |
| F1 | 19,994 cd | 1917 | 750 | 14,207 d | ||
| F2 | 21,450 bc | 1917 | 750 | 15,663 c | ||
| F3 | 23,010 ab | 1917 | 750 | 17,223 b | ||
| F4 | 18,008 e | 1652 | 1500 | 11,736 f | ||
| 2019 | Yangzhou | F0 | 15,356 f | 0 | 0 | 12,236 ef |
| F1 | 20,112 cd | 1917 | 750 | 14,325 d | ||
| F2 | 22,172 b | 1917 | 750 | 16,385 bc | ||
| F3 | 23,438 ab | 1917 | 750 | 17,651 ab | ||
| F4 | 18,858 de | 1652 | 1500 | 12,586 e | ||
| Nantong | F0 | 14,340 f | 0 | 0 | 11,220 f | |
| F1 | 21,630 bc | 1917 | 750 | 15,843 c | ||
| F2 | 23,280 ab | 1917 | 750 | 17,493 ab | ||
| F3 | 24,450 a | 1917 | 750 | 18,663 a | ||
| F4 | 19,228 d | 1652 | 1500 | 12,956 e | ||
| Y | 176.7 ** | 219.8 ** | ||||
| S | 35.8 * | 83.4 ** | ||||
| F | 190.6 ** | 198.1 ** | ||||
| Y × S | 28.8 ** | 27.7 ** | ||||
| Y × F | 26.0 ** | 33.1 ** | ||||
| S × F | 18.6 ** | 47.2 ** | ||||
| Y × S × F | 15.5 ** | 32.0 ** | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, G.; Li, W.; Zhang, S.; Lu, W.; Lu, D. Optimized Fertilization Practices Improved Rhizosphere Soil Chemical and Bacterial Properties and Fresh Waxy Maize Yield. Metabolites 2022, 12, 935. https://doi.org/10.3390/metabo12100935
Li G, Li W, Zhang S, Lu W, Lu D. Optimized Fertilization Practices Improved Rhizosphere Soil Chemical and Bacterial Properties and Fresh Waxy Maize Yield. Metabolites. 2022; 12(10):935. https://doi.org/10.3390/metabo12100935
Chicago/Turabian StyleLi, Guanghao, Wei Li, Shibo Zhang, Weiping Lu, and Dalei Lu. 2022. "Optimized Fertilization Practices Improved Rhizosphere Soil Chemical and Bacterial Properties and Fresh Waxy Maize Yield" Metabolites 12, no. 10: 935. https://doi.org/10.3390/metabo12100935
APA StyleLi, G., Li, W., Zhang, S., Lu, W., & Lu, D. (2022). Optimized Fertilization Practices Improved Rhizosphere Soil Chemical and Bacterial Properties and Fresh Waxy Maize Yield. Metabolites, 12(10), 935. https://doi.org/10.3390/metabo12100935








