Three New Compounds, Licopyranol A–C, Together with Eighteen Known Compounds Isolated from Glycyrrhiza glabra L. and Their Antitumor Activities
Abstract
1. Introduction
2. Materials and Methods
2.1. General Experimental Procedures
2.2. Plant Material
2.3. Extraction and Isolation
2.4. Cell lines and Culture
2.5. Cell Viability Assay
2.6. Cell Proliferation Assay
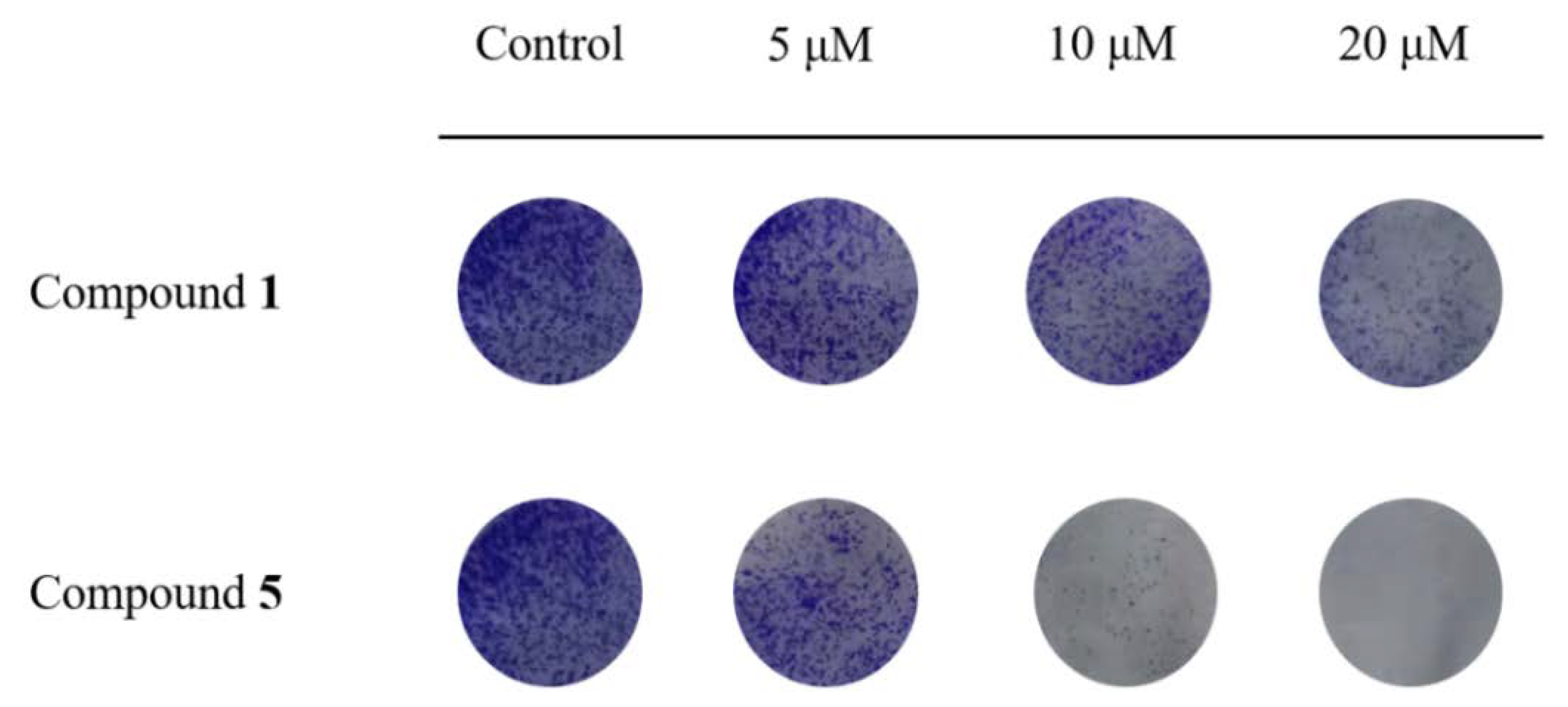
2.7. Cell Cloning Assay
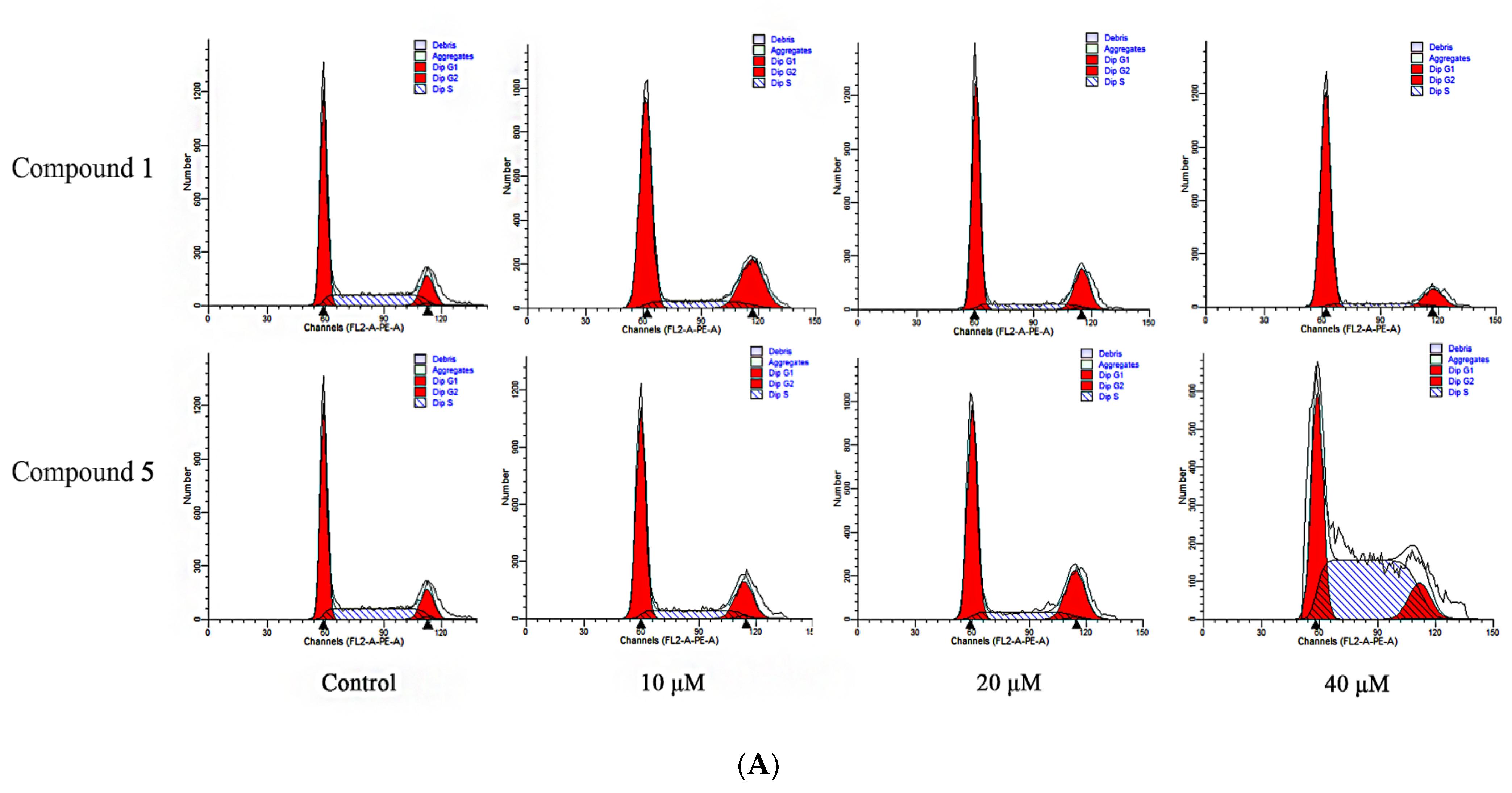
2.8. Cell Cycle Assay
2.9. Cell Apoptosis Assay
2.10. Western Blotting Assay
2.11. Statistical Analysis
3. Results
3.1. Structure Identification of Isolated Compounds
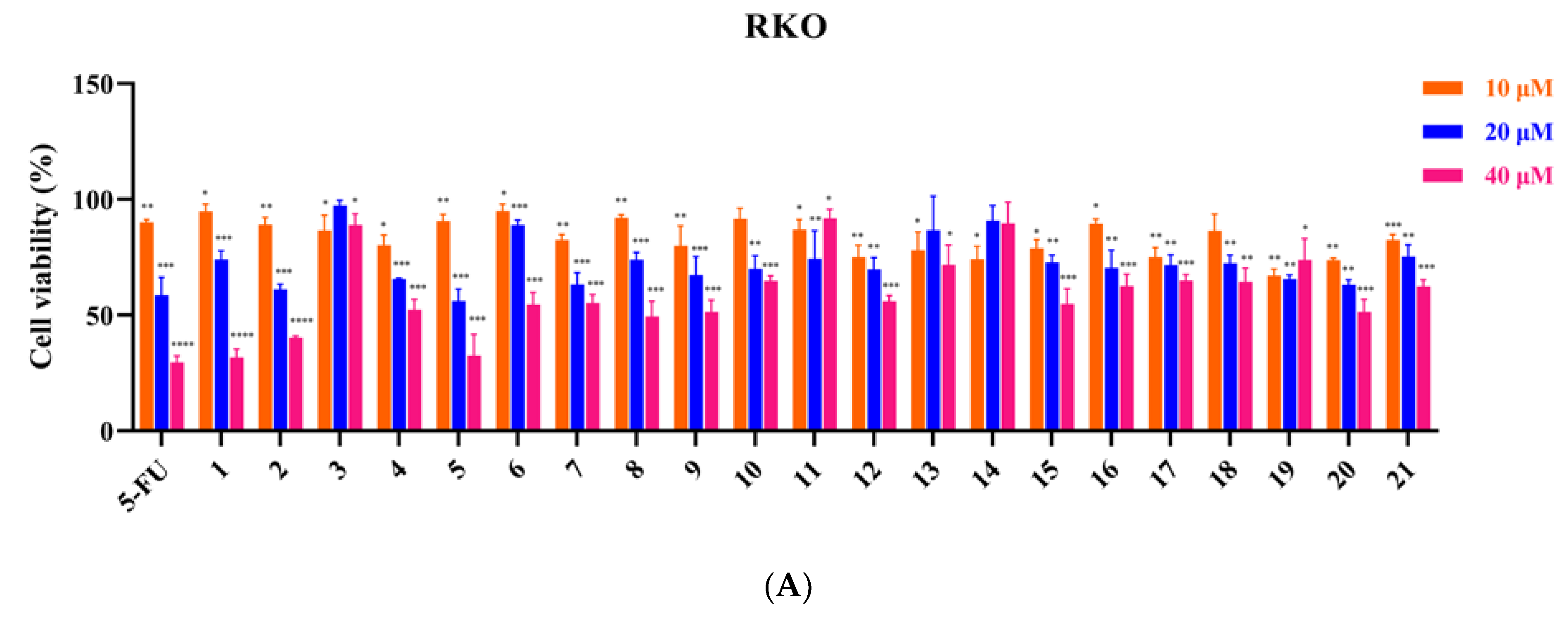
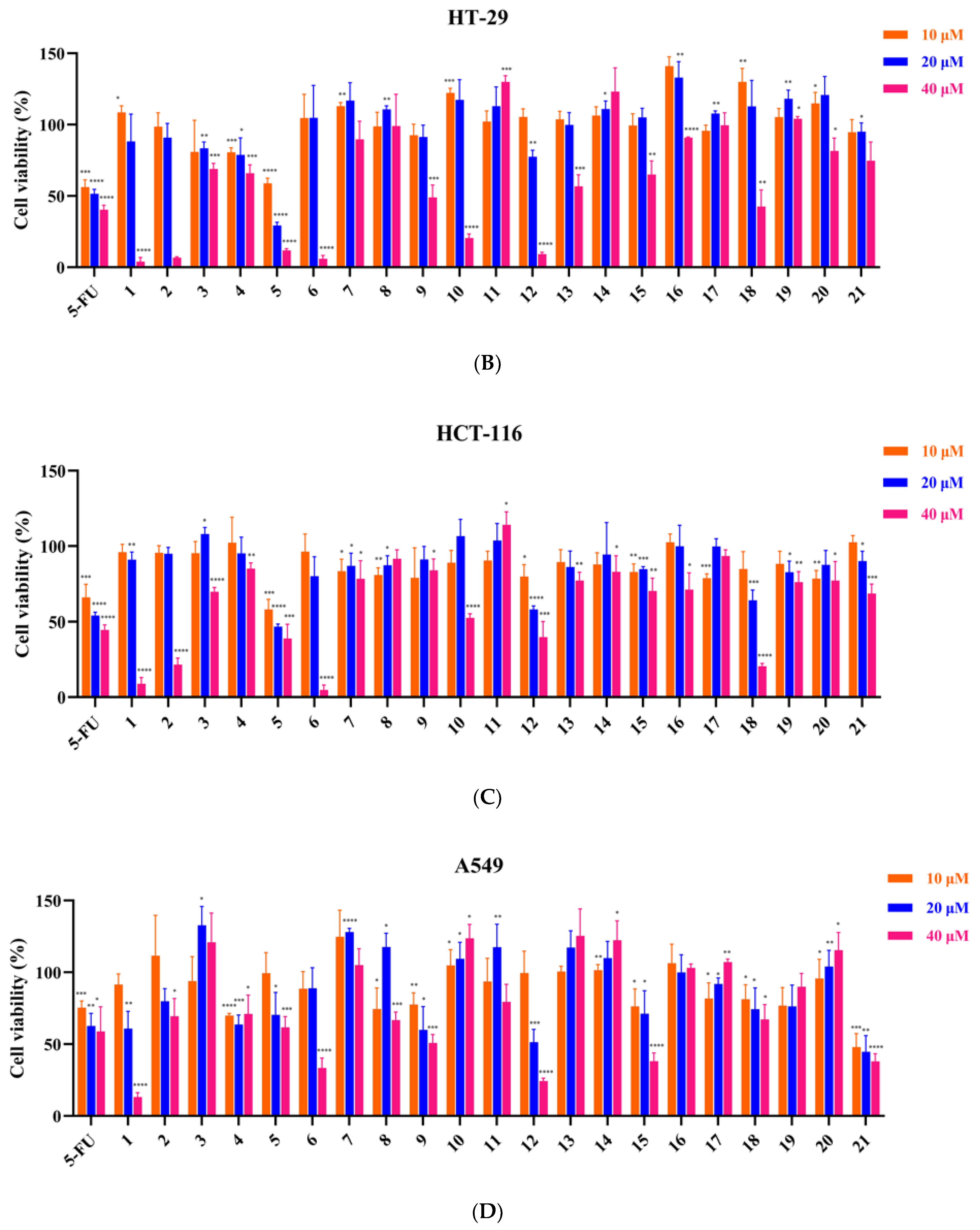
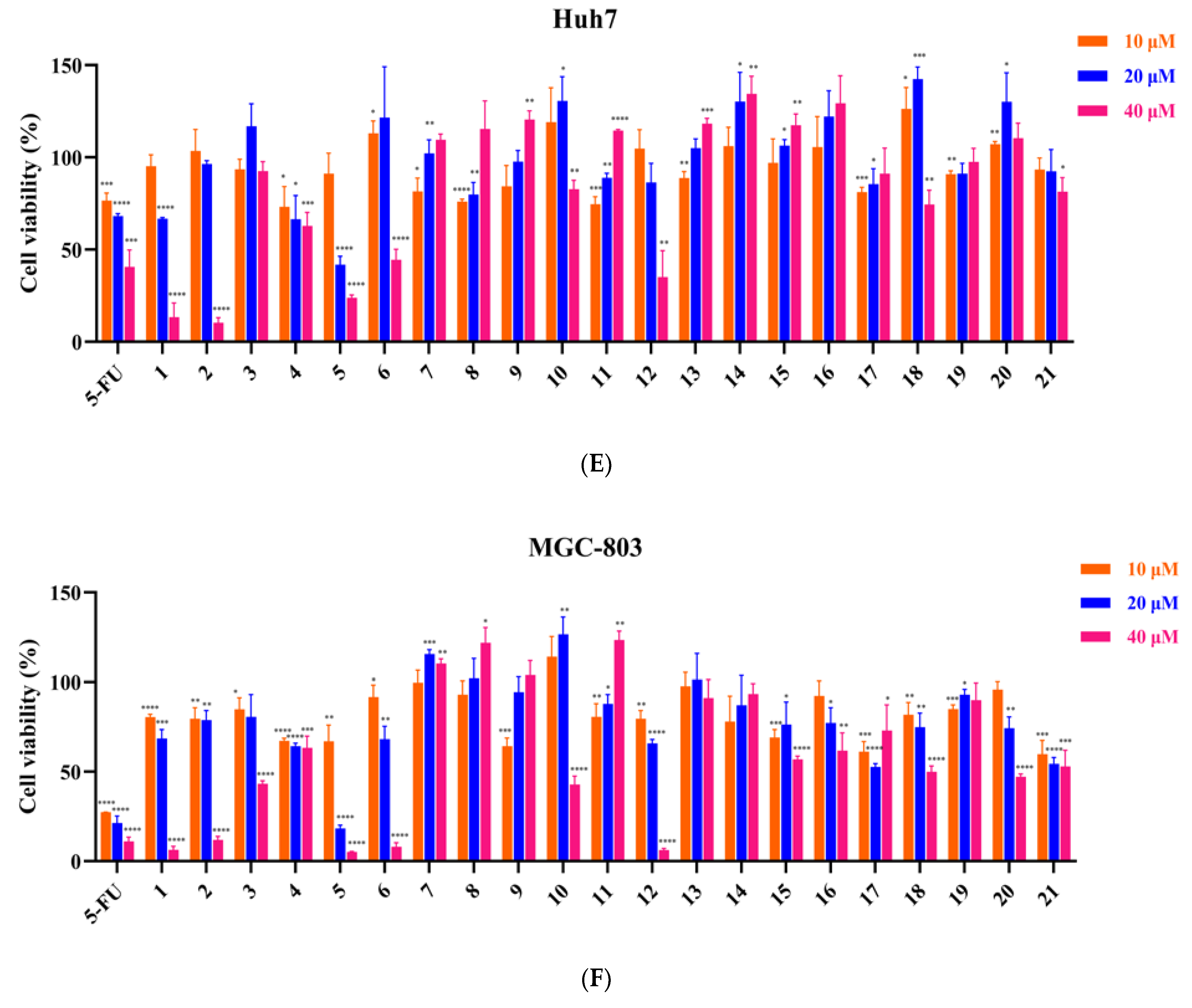
3.2. Inhibitory Effect of 21 Compounds on 6 Tumor Cell Lines
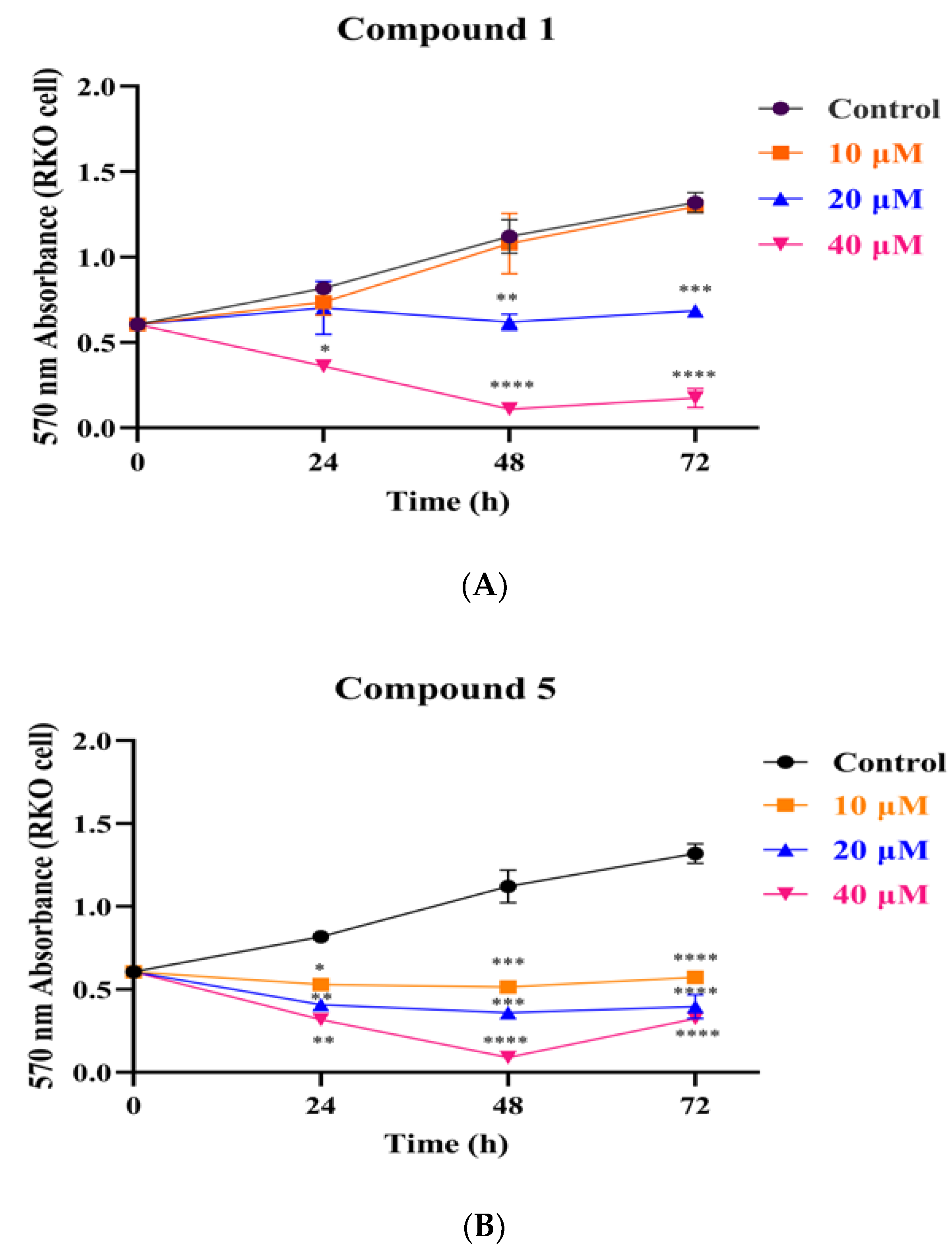
3.3. Compounds 1 and 5 Inhibited the Proliferation of RKO Cells
3.4. Compounds 1 and 5 Promoted Apoptosis in RKO Cells
3.5. Compound 5 Inhibited RKO Cell Growth via the Wnt/β-Catenin Signaling Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dai, C.; Wang, S.; De Souza, C.; Li, Y.-Y.; Zhou, C.; Qiu, R.; Xu, X.-Z.; Zhou, H.-L.; Wu, Y. Chemical constituents and chemotaxonomic study of Glycyrrhiza pallidiflora maxim. Biochem. Syst. Ecol. 2021, 94, 104204. [Google Scholar] [CrossRef]
- Li, K.; Ji, S.; Song, W.; Kuang, Y.; Lin, Y.; Tang, S.; Cui, Z.; Qiao, X.; Yu, S.; Ye, M. Glycybridins A–K, Bioactive Phenolic Compounds from Glycyrrhiza glabra. J. Nat. Prod. 2017, 80, 334–346. [Google Scholar] [CrossRef] [PubMed]
- Nassiri-Asl, M.; Hosseinzadeh, H. Review of the pharmacological effects of Vitis vinifera (Grape) and its bioactive compounds. Phytother. Res. 2009, 23, 1197–1204. [Google Scholar] [CrossRef]
- Chen, X.; Mukwaya, E.; Wong, M.S.; Zhang, Y. A systematic review on biological activities of prenylated flavonoids. Pharm. Biol. 2014, 52, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Qu, Q.; Zheng, Y.; Zhong, H.; Shan, C.; Wang, F.; Li, C.; Peng, G. Structural characterization and identification of flavonoid aglycones in three Glycyrrhizas pecies by liquid chromatography with photodiode array detection and quadrupole time-of-flight mass spectrometry. J. Sep. Sci. 2016, 39, 2068–2078. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wang, H.; Yang, M.; Peng, G.; Dong, T.T.X.; Xu, M.L.; Tsim, K.W.K. Prenylated Flavonoids from Roots of Glycyrrhiza uralensis Induce Differentiation of B16-F10 Melanoma Cells. Int. J. Mol. Sci. 2018, 19, 2422. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, L.; Hou, J.; Tian, S.; Liu, Y. Molecular mechanisms underlying the anticancer activities of licorice flavonoids. J. Ethnopharmacol. 2020, 267, 113635. [Google Scholar] [CrossRef]
- Wang, K.-L.; Yu, Y.-C.; Chen, H.-Y.; Chiang, Y.-F.; Ali, M.; Shieh, T.-M.; Hsia, S.-M. Recent Advances in Glycyrrhiza glabra (Licorice)-Containing Herbs Alleviating Radiotherapy- and Chemotherapy-Induced Adverse Reactions in Cancer Treatment. Metabolites 2022, 12, 535. [Google Scholar] [CrossRef]
- Sharma, V.; Katiyar, A.; Agrawal, R.C. Glycyrrhiza glabra: Chemistry and Pharmacological Activity. Sweeteners 2017, 31, 87–100. [Google Scholar] [CrossRef]
- Popovich, D.G.; Yeo, S.Y.; Zhang, W. Ginseng (Panax quinquefolius) and Licorice (Glycyrrhiza uralensis) Root Extract Combinations Increase Hepatocarcinoma Cell (Hep-G2) Viability. Evid. Based Complement. Altern. Med. 2011, 2011, 408273. [Google Scholar] [CrossRef]
- Jo, E.-H.; Hong, H.-D.; Ahn, N.-C.; Jung, J.-W.; Yang, S.-R.; Park, J.-S.; Kim, S.-H.; Lee, Y.-S.; Kang, K.-S. Modulations of the Bcl-2/Bax Family Were Involved in the Chemopreventive Effects of Licorice Root (Glycyrrhiza uralensis Fisch) in MCF-7 Human Breast Cancer Cell. J. Agric. Food Chem. 2004, 52, 1715–1719. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Wang, L.-Q.; Liu, Y. Antitumor Activities of Widely-used Chinese Herb—Licorice. Chin. Herb. Med. 2014, 6, 274–281. [Google Scholar] [CrossRef]
- Rafi, M.M.; Vastano, B.C.; Zhu, N.; Ho, C.-T.; Ghai, G.; Rosen, R.T.; Gallo, M.A.; DiPaola, R.S. Novel Polyphenol Molecule Isolated from Licorice Root (Glycrrhiza glabra) Induces Apoptosis, G2/M Cell Cycle Arrest, and Bcl-2 Phosphorylation in Tumor Cell Lines. J. Agric. Food Chem. 2002, 50, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Çevik, D.; Yılmazgöz, B.; Kan, Y.; Güzelcan, E.A.; Durmaz, I.; Çetin-Atalay, R.; Kırmızıbekmez, H. Bioactivity-guided isolation of cytotoxic secondary metabolites from the roots of Glycyrrhiza glabra and elucidation of their mechanisms of action. Ind. Crop. Prod. 2018, 124, 389–396. [Google Scholar] [CrossRef]
- Esclapez, M.D.; García-Pérez, J.V.; Mulet, A.; Cárcel, J.A. Ultrasound-assisted extraction of natural products. Food Eng. Rev. 2011, 3, 108–120. [Google Scholar] [CrossRef]
- Luo, Z.; Wang, X.; Gao, Q.; Guo, Z.; Li, W.; Zhao, Y. Application of ultrasound-assisted processing in improving the purification effect and bioactivity of licorice flavonoids from licorice residues. Ind. Crop. Prod. 2021, 167, 113544. [Google Scholar] [CrossRef]
- Lin, Y.; Kuang, Y.; Li, K.; Wang, S.; Ji, S.; Chen, K.; Song, W.; Qiao, X.; Ye, M. Nrf2 activators from Glycyrrhiza inflata and their hepatoprotective activities against CCl4-induced liver injury in mice. Bioorg. Med. Chem. 2017, 25, 5522–5530. [Google Scholar] [CrossRef]
- Yang, E.-J.; Min, J.S.; Ku, H.-Y.; Choi, H.-S.; Park, M.-K.; Kim, M.K.; Song, K.-S.; Lee, D.-S. Isoliquiritigenin isolated from Glycyrrhiza uralensis protects neuronal cells against glutamate-induced mitochondrial dysfunction. Biochem. Biophys. Res. Commun. 2012, 421, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, T.; Shibata, S. Chemical Studies on the Oriental Plant Drugs. XXII. Some New Constituents of Licorice Root. (2) Glycyrol, 5-O-Methylglycyrol and Isoglycyrol. Chem. Pharm. Bull. 1969, 17, 729–734. [Google Scholar] [CrossRef]
- Singhal, A.K.; Sharma, R.P.; Thyagarajan, G.; Herz, W.; Govindan, S.V. New prenylated isoflavones and a prenylated dihydroflavonol from Millettia pachycarpa. Phytochemistry 1980, 19, 929–934. [Google Scholar] [CrossRef]
- Ji, S.; Li, Z.; Song, W.; Wang, Y.; Liang, W.; Li, K.; Tang, S.; Wang, Q.; Qiao, X.; Zhou, D.; et al. Bioactive Constituents of Glycyrrhiza uralensis (Licorice): Discovery of the Effective Components of a Traditional Herbal Medicine. J. Nat. Prod. 2016, 79, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, M.; Mimaki, Y.; Honda, S.; Tanaka, H.; Yokota, S.; Mae, T. Phenolics from Glycyrrhiza glabra roots and their PPAR-γ ligand-binding activity. Bioorg. Med. Chem. 2010, 18, 962–970. [Google Scholar] [CrossRef]
- Tsukayama, M.; Wada, H.; Kawamura, Y.; Yamashita, K.; Nishiuchi, M. Regioselective Synthesis of 6-Alkyl- and 6-Prenylpolyhydroxyisoflavones and 6-Alkylcoumaronochromone Derivatives. ChemInform 2005, 36, 1285–1289. [Google Scholar] [CrossRef]
- Hashidoko, Y.; Tahara, S.; Mizutani, J. Isoflavonoids of yellow lupin. Part I. New complex isoflavones in the roots of yellow lupin (Lupinus luteus L., cv. Barpine). Agric. Biol. Chem. 1986, 50, 1797–1807. [Google Scholar] [CrossRef]
- Yenesew, A.; Irungu, B.; Derese, S.; Midiwo, J.O.; Heydenreich, M.; Peter, M.G. Two prenylated flavonoids from the stem bark of Erythrina burttii. Phytochemistry 2003, 63, 445–448. [Google Scholar] [CrossRef]
- Máximoa, P.; Lourenço, A.; Feio, S.S.; Roseiro, J.C. A New Prenylisoflavone from Ulex jussiaei. Z. Naturforsch C J. Biosci. 2002, 57, 609–613. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fu, M.-Q.; Deng, D.; Feng, S.-X.; Huang, R.-M.; Tian, S.; Qiu, S.-X. Chemical Constituents from Roots of Flemingia Philippinensis. Chin. Herb. Med. 2012, 4, 8–11. [Google Scholar] [CrossRef]
- Saitoh, T.; Noguchi, H.; Shibata, S. A new isoflavone and the corresponding isoflavanone of licorice root. Chem. Pharm. Bull. 1978, 26, 144–147. [Google Scholar] [CrossRef]
- Ateba, S.B.; Njamen, D.; Ukowitz, K.; Zehl, M.; Kählig, H.; Hobiger, S.; Jungbauer, A.; Krenn, L. New flavonoids from the underground parts of Eriosema laurentii. Phytochem. Lett. 2016, 18, 144–149. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, X.-T.; Liang, J.-Y.; Min, Z.-D. Chemical Constituents from the Stems of Caesalpinia decapetala. Chin. J. Nat. Med. 2008, 6, 168–172. [Google Scholar] [CrossRef]
- Cho, S.; Park, J.-H.; Pae, A.N.; Han, D.; Kim, D.; Cho, N.-C.; No, K.T.; Yang, H.; Yoon, M.; Lee, C.; et al. Hypnotic effects and GABAergic mechanism of licorice (Glycyrrhiza glabra) ethanol extract and its major flavonoid constituent glabrol. Bioorg. Med. Chem. 2012, 20, 3493–3501. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-Y.; Kim, M.; Han, J. Stereospecific microbial production of isoflavanones from isoflavones and isoflavone glucosides. Appl. Microbiol. Biotechnol. 2011, 91, 1173–1181. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Li, X.; Song, P.; Zhang, Q.; Wu, Z.; Wang, J.; Li, X.; Xu, R.; Zhao, W.; Liu, Y.; et al. A vanillin derivative suppresses the growth of HT29 cells through the Wnt/β-catenin signaling pathway. Eur. J. Pharmacol. 2019, 849, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Sebio, A.; Kahn, M.; Lenz, H.-J. The potential of targeting Wnt/β-catenin in colon cancer. Expert Opin. Ther. Targets 2014, 18, 611–615. [Google Scholar] [CrossRef]
- Biyik, I.; Kalkan, U.; Simsek, S. The deep infiltrating endometriosis tissue has lower T-cadherin, E-cadherin, progesterone receptor and oestrogen receptor than endometrioma tissue. Taiwan. J. Obstet. Gynecol. 2021, 60, 1059–1065. [Google Scholar] [CrossRef]
- Kal, S.; Chakraborty, S.; Karmakar, S.; Ghosh, M.K. Wnt/β-catenin signaling and p68 conjointly regulate CHIP in colorectal carcinoma. BBA Mol. Cell Res. 2021, 1869, 119185. [Google Scholar] [CrossRef]
- Wang, M.; Yang, W.; Liu, X.; Liu, Q.; Zheng, H.; Wang, X.; Shen, T.; Wang, S.; Ren, D. Two new compounds with Nrf2 inducing activity from Glycyrrhiza uralensis. Nat. Prod. Res. 2021, 35, 4357–4364. [Google Scholar] [CrossRef]
- Ji, S.; Tang, S.; Li, K.; Li, Z.; Liang, W.; Qiao, X.; Wang, Q.; Yu, S.; Ye, M. Licoricidin inhibits the growth of SW480 human colorectal adenocarcinoma cells in vitro and in vivo by inducing cycle arrest, apoptosis and autophagy. Toxicol. Appl. Pharmacol. 2017, 326, 25–33. [Google Scholar] [CrossRef]
- Lin, Y.; Kuang, Y.; Li, K.; Wang, S.; Song, W.; Qiao, X.; Sabir, G.; Ye, M. Screening for bioactive natural products from a 67-compound library of Glycyrrhiza inflata. Bioorg. Med. Chem. 2017, 25, 3706–3713. [Google Scholar] [CrossRef]
- Miranda, C.L.; Stevens, J.F.; Helmrich, A.; Henderson, M.C.; Rodriguez, R.J.; Yang, Y.-H.; Deinzer, M.L.; Barnes, D.W.; Buhler, D.R. Antiproliferative and cytotoxic effects of prenylated flavonoids from hops (Humulus lupulus) in human cancer cell lines. Food Chem. Toxicol. 1999, 37, 271–285. [Google Scholar] [CrossRef]
- Ko, H.-H.; Lu, Y.-H.; Yang, S.-Z.; Won, S.-J.; Lin, C.-N. Cytotoxic Prenylflavonoids from Artocarpus elasticus. J. Nat. Prod. 2005, 68, 1692–1695. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the 30 Years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Kim, E.J.; Choi, H.J.; Seon, M.R.; Lim, S.S.; Kang, Y.-H.; Choi, M.-S.; Lee, K.W.; Park, J.H.Y. Anti-carcinogenic effects of non-polar components containing licochalcone A in roasted licorice root. Nutr. Res. Pract. 2014, 8, 257–266. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, G.C.; Oliveira, L.F.S.; Predes, D.; Fokoue, H.H.; Kuster, R.M.; Oliveira, F.L.; Mendes, F.A.; Abreu, J.G. Piperine suppresses the Wnt/β-catenin pathway and has anti-cancer effects on colorectal cancer cells. Sci. Rep. 2020, 10, 11681. [Google Scholar] [CrossRef]
- Ferrarelli, L.K. Treating WNT-driven colorectal cancer. Science 2017, 356, 1346–1348. [Google Scholar] [CrossRef]
- Xu, M.-Y.; Kim, Y.S. Antitumor activity of glycyrol via induction of cell cycle arrest, apoptosis and defective autophagy. Food Chem. Toxicol. 2014, 74, 311–319. [Google Scholar] [CrossRef]
- Lu, S.; Yin, S.; Zhao, C.; Fan, L.; Hu, H. Synergistic anti-colon cancer effect of glycyrol and butyrate is associated with the enhanced activation of caspase-3 and structural features of glycyrol. Food Chem. Toxicol. 2020, 136, 110952. [Google Scholar] [CrossRef]
- Catalani, E.; Serafini, F.P.; Zecchini, S.; Picchietti, S.; Fausto, A.M.; Marcantoni, E.; Buonanno, F.; Ortenzi, C.; Perrotta, C.; Cervia, D. Natural products from aquatic eukaryotic microorganisms for cancer therapy: Perspectives on anti-tumour properties of ciliate bioactive molecules. Pharmacol. Res. 2016, 113, 409–420. [Google Scholar] [CrossRef]
- Bai, Z.-T.; Wu, Z.-R.; Xi, L.-L.; Li, X.; Chen, P.; Wang, F.-Q.; Meng, W.-B.; Zhou, W.-C.; Wu, X.-A.; Yao, X.-J.; et al. Inhibition of invasion by N -trans -feruloyloctopamine via AKT, p38MAPK and EMT related signals in hepatocellular carcinoma cells. Bioorg. Med. Chem. Lett. 2017, 27, 989–993. [Google Scholar] [CrossRef]
- Predes, D.; Oliveira, L.F.S.; Ferreira, L.S.S.; Maia, L.A.; Delou, J.M.A.; Faletti, A.; Oliveira, I.; Amado, N.G.; Reis, A.H.; Fraga, C.A.M.; et al. The Chalcone Lonchocarpin Inhibits Wnt/β-Catenin Signaling and Suppresses Colorectal Cancer Proliferation. Cancers 2019, 11, 1968. [Google Scholar] [CrossRef]
- Tong, X.; Zhuang, Z.; Wang, X.; Yang, X.; Bai, L.; Su, L.; Wei, P.; Su, X. ACBP suppresses the proliferation, migration, and invasion of colorectal cancer via targeting Wnt/beta-catenin signaling pathway. Biomed. Pharmacother. 2021, 137, 111209. [Google Scholar] [CrossRef] [PubMed]

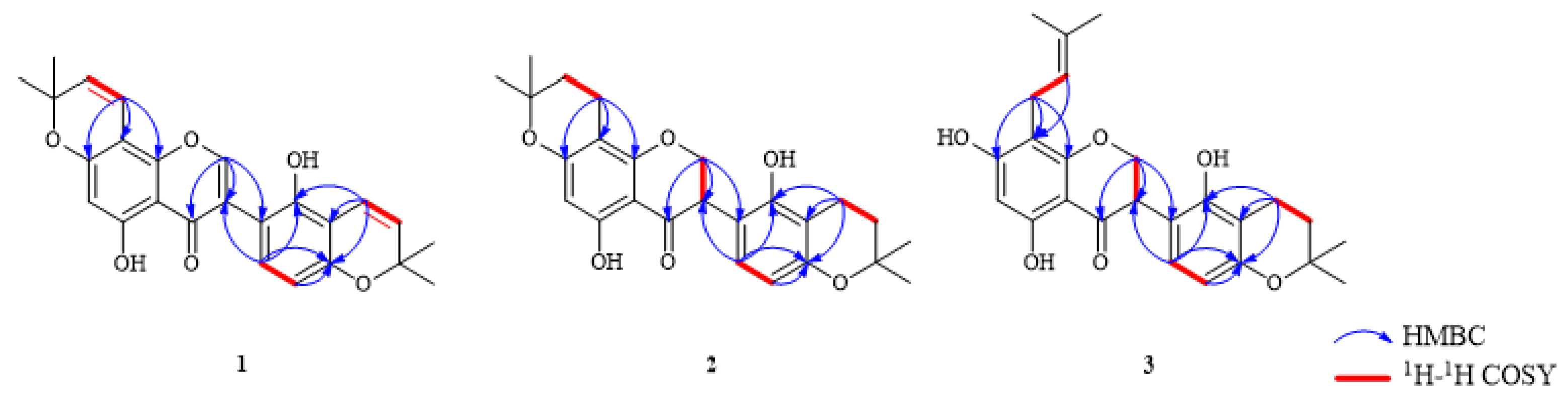
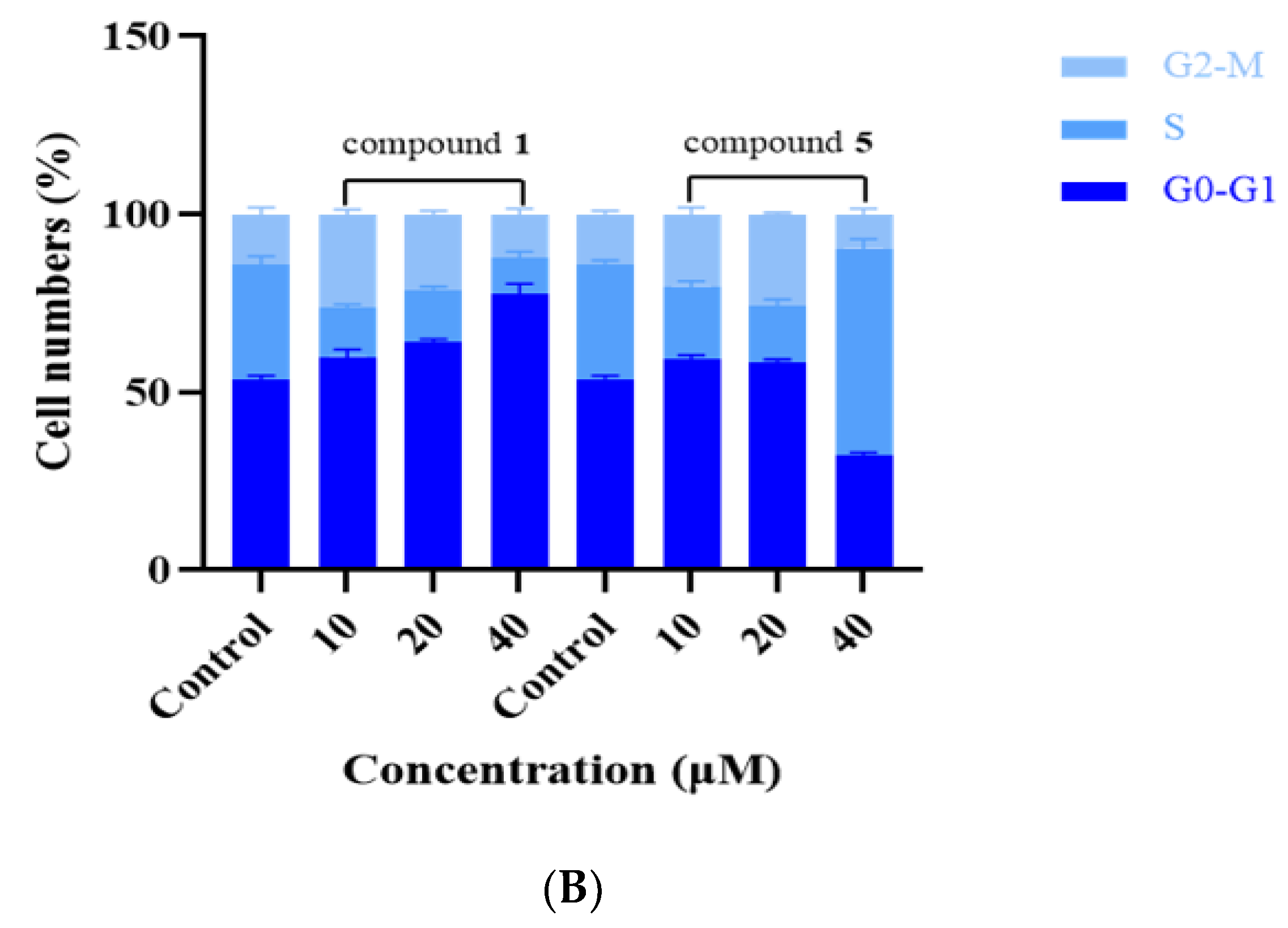
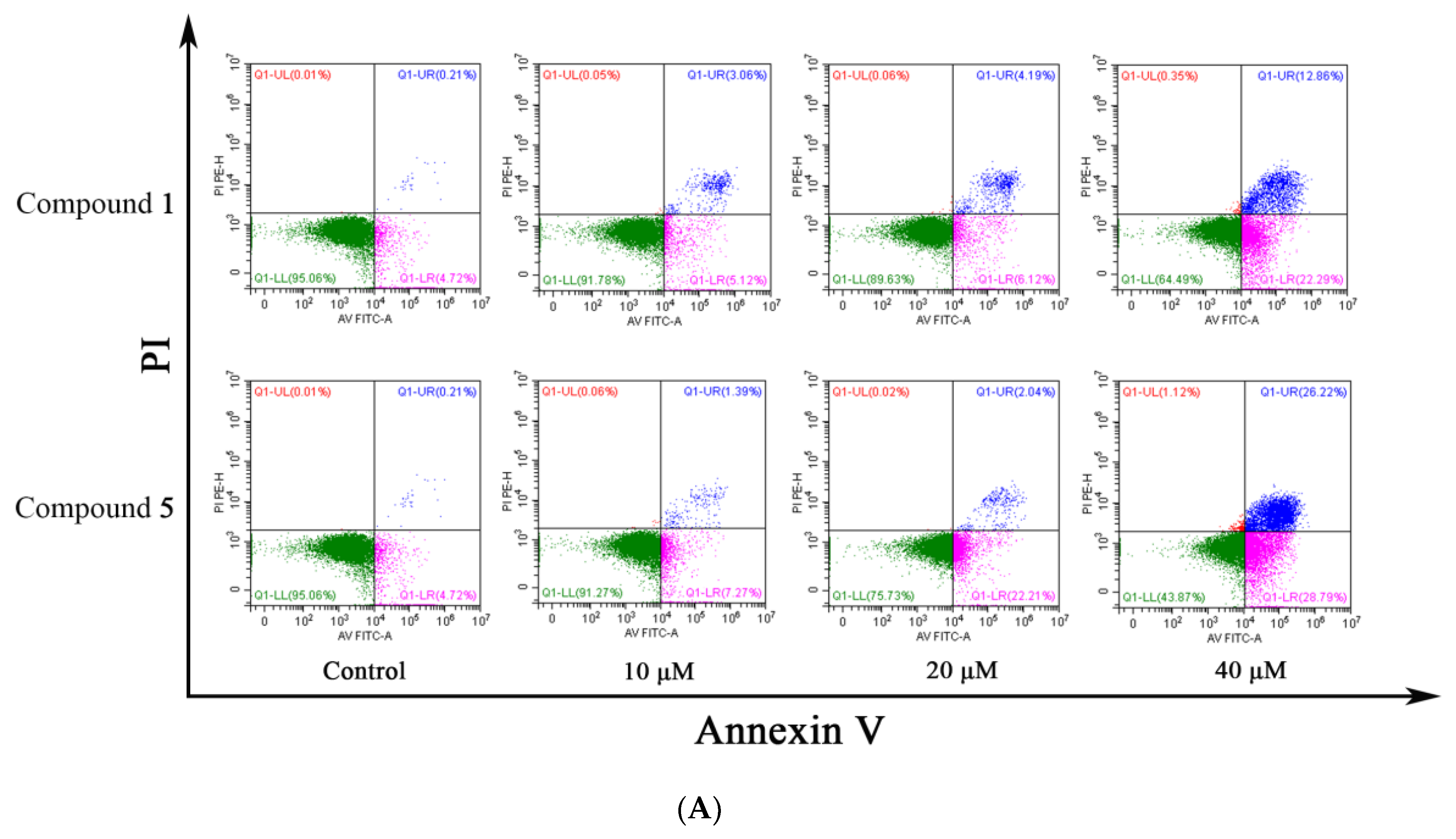
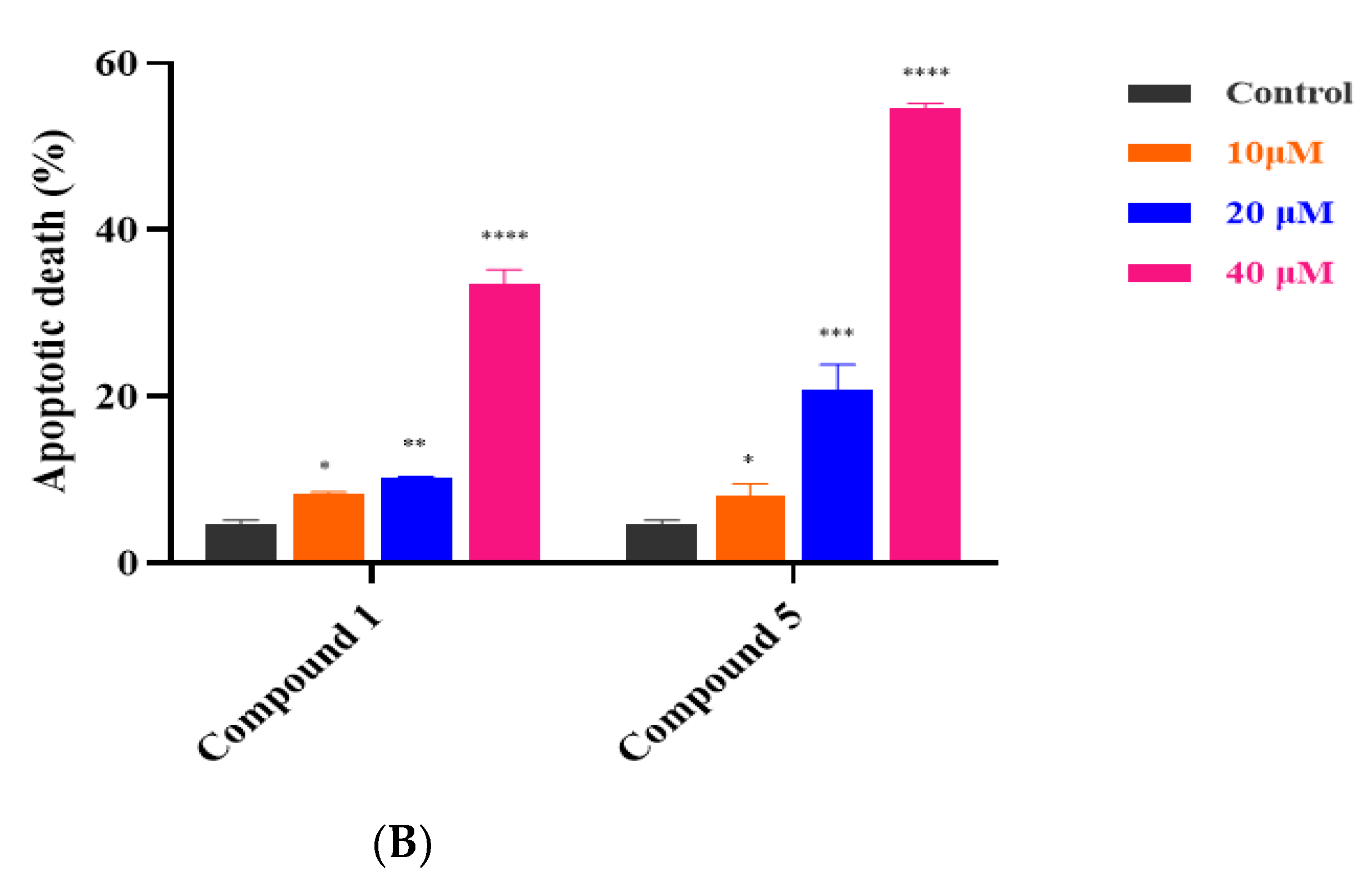
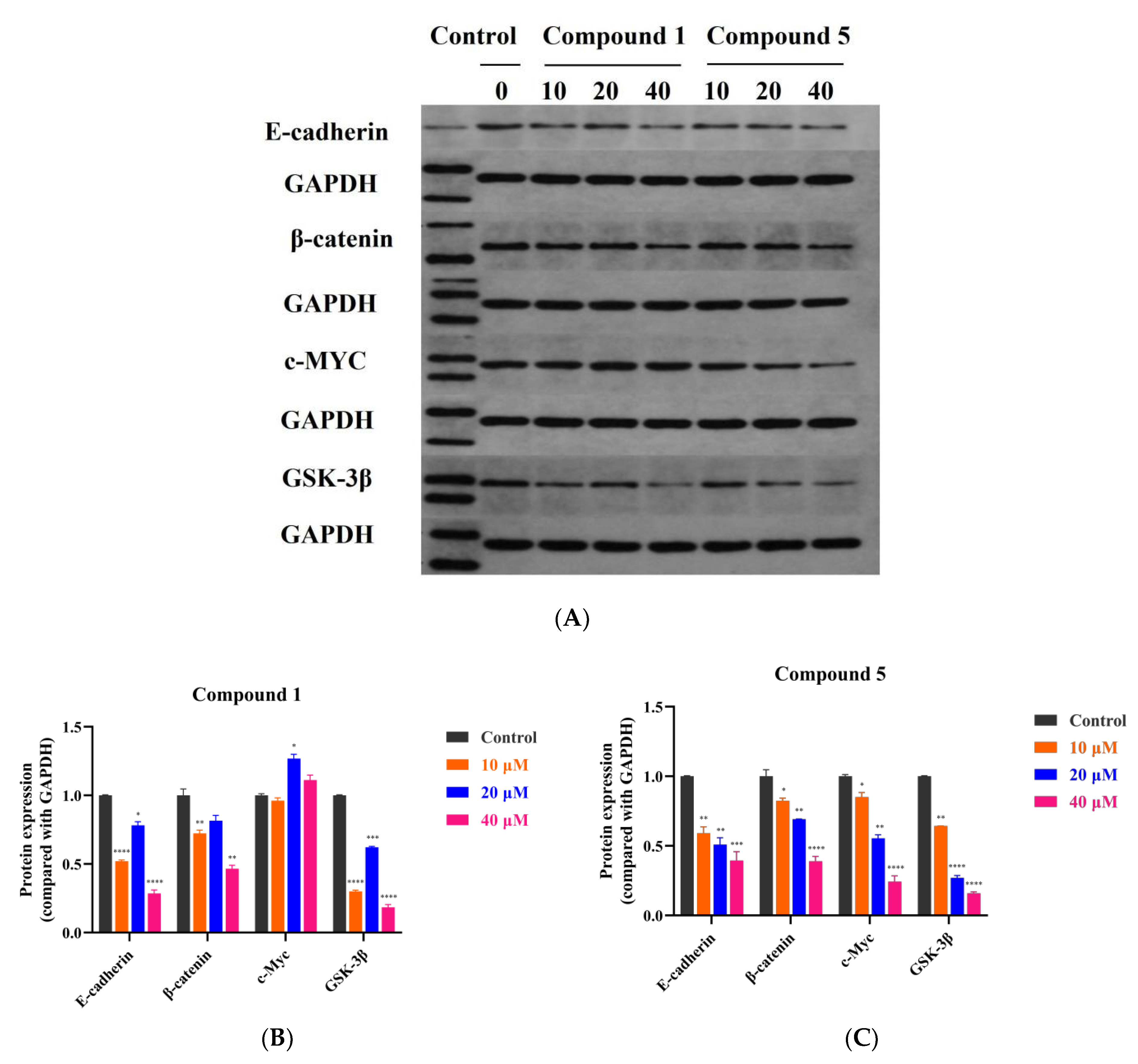
| Position | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|
| δH | δC | δH | δC | δH | δC | |
| 2α | 8.23, s | 155.7 | 4.50, dd (10.8,10.8) | 70.6 | 4.41, dd (10.4, 10.4) | 70.5 |
| 2β | 4.45, dd (10.8, 5.6) | 4.38, dd (10.4, 6.4) | ||||
| 3 | 120.7 | 4.15, dd (10.8, 5.6) | 47.2 | 4.09, dd (10.4, 6.4) | 47.2 | |
| 4 | 180.9 | 198.5 | 198.3 | |||
| 5 | 154.0 | 160.4 | 162.0 | |||
| 6 | 6.27, s | 99.9 | 5.85, s | 96.8 | 5.98, s | 95.9 |
| 7 | 161.8 | 162.1 | 164.9 | |||
| 8 | 101.3 | 100.7 | 107.3 | |||
| 9 | 159.2 | 161.4 | 160.2 | |||
| 10 | 105.7 | 103.0 | 102.6 | |||
| 1′ | 109.7 | 113.6 | 114.0 | |||
| 2′ | 151.9 | 152.4 | 152.3 | |||
| 3′ | 109.5 | 108.9 | 108.9 | |||
| 4′ | 152.1 | 155.7 | 155.6 | |||
| 5′ | 6.44, d (8.0) | 107.8 | 6.31, d (8.0) | 106.1 | 6.29, d (8.0) | 106.0 |
| 6′ | 6.93, d (8.0) | 131.7 | 6.76, d (8.0) | 128.7 | 6.75, d (8.0) | 128.6 |
| 1″ | 6.69, d (10.0) | 114.4 | 2.55, t (6.8) | 16.2 | 3.10, d (7.2) | 21.6 |
| 2″ | 5.79, d (10.0) | 128.7 | 1.77, t (6.8) | 31.6 | 5.10, t (7.2) | 123.5 |
| 3″ | 78.7. | 76.5 | 130.6 | |||
| 4″ | 1.45, s | 28.2 | 1.31, s | 27.4 | 1.62, s | 25.9 |
| 5″ | 1.45, s | 28.2 | 1.28, s | 26.5 | 1.67, s | 18.1 |
| 1‴ | 6.61, d (10.0) | 117.2 | 2.48, t (6.8) | 17.4 | 2.51, t (6.8) | 17.4 |
| 2‴ | 5.65, d (10.0) | 129.1 | 1.68, t (6.8) | 31.9 | 1.70, t (6.8) | 31.9 |
| 3‴ | 76.3 | 74.3 | 74.2 | |||
| 4‴ | 1.31, s | 27.9 | 1.16, s | 27.3 | 1.15, s | 27.4 |
| 5‴ | 1.31, s | 27.9 | 1.13, s | 25.9 | 1.12, s | 25.9 |
| Compound | RKO | HT-29 | HCT-116 | A549 | Huh7 | MGC-803 |
|---|---|---|---|---|---|---|
| 5-FU | 28.2 ± 0.7 | 25.7 ± 2.9 | 29.8 ± 5.8 | 46.4 ± 10.5 | 33.6 ± 5.2 | 3.3 ± 0.3 |
| 1 | 31.4 ± 0.5 | 28.0 ± 1.5 | 28.3 ± 0.9 | 25.3 ± 0.4 | 26.5 ± 1.5 | 24.0 ± 0.2 |
| 2 | 32.1 ± 0.2 | 28.0 ± 1.4 | 31.2 ± 1.5 | >40 | 29.4 ± 0.4 | 26.1 ± 1.6 |
| 5 | 29.1 ± 3.1 | 11.9 ± 1.8 | 20.6 ± 7.3 | >40 | 24.3 ± 1.5 | 13.5 ± 4.3 |
| 6 | >40 | >40 | 25.8 ± 1.7 | 32.3 ± 3.8 | >40 | 25.4 ± 1.3 |
| 12 | >40 | 27.5 ± 0.2 | 31.1 ± 6.6 | 26.5 ± 3.1 | 34.4 ± 5.2 | 23.5 ± 0.6 |
| 15 | >40 | >40 | >40 | 31.13 ± 5.2 | >40 | >40 |
| 21 | >40 | >40 | >40 | 11.5 ± 6.9 | >40 | >40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Alafifi, J.H.; Chen, Q.; Shen, X.; Bi, C.; Wu, Y.; Jiang, Y.; Liu, Y.; Li, Y.; He, D.; et al. Three New Compounds, Licopyranol A–C, Together with Eighteen Known Compounds Isolated from Glycyrrhiza glabra L. and Their Antitumor Activities. Metabolites 2022, 12, 896. https://doi.org/10.3390/metabo12100896
Wang S, Alafifi JH, Chen Q, Shen X, Bi C, Wu Y, Jiang Y, Liu Y, Li Y, He D, et al. Three New Compounds, Licopyranol A–C, Together with Eighteen Known Compounds Isolated from Glycyrrhiza glabra L. and Their Antitumor Activities. Metabolites. 2022; 12(10):896. https://doi.org/10.3390/metabo12100896
Chicago/Turabian StyleWang, Shanshan, Jameel Hizam Alafifi, Qin Chen, Xue Shen, Chunmei Bi, Yangyang Wu, Yihan Jiang, Yanan Liu, Yimeng Li, Dian He, and et al. 2022. "Three New Compounds, Licopyranol A–C, Together with Eighteen Known Compounds Isolated from Glycyrrhiza glabra L. and Their Antitumor Activities" Metabolites 12, no. 10: 896. https://doi.org/10.3390/metabo12100896
APA StyleWang, S., Alafifi, J. H., Chen, Q., Shen, X., Bi, C., Wu, Y., Jiang, Y., Liu, Y., Li, Y., He, D., & Yang, Z. (2022). Three New Compounds, Licopyranol A–C, Together with Eighteen Known Compounds Isolated from Glycyrrhiza glabra L. and Their Antitumor Activities. Metabolites, 12(10), 896. https://doi.org/10.3390/metabo12100896