The Discovery of the Mode of Action of Nitisinone
Abstract
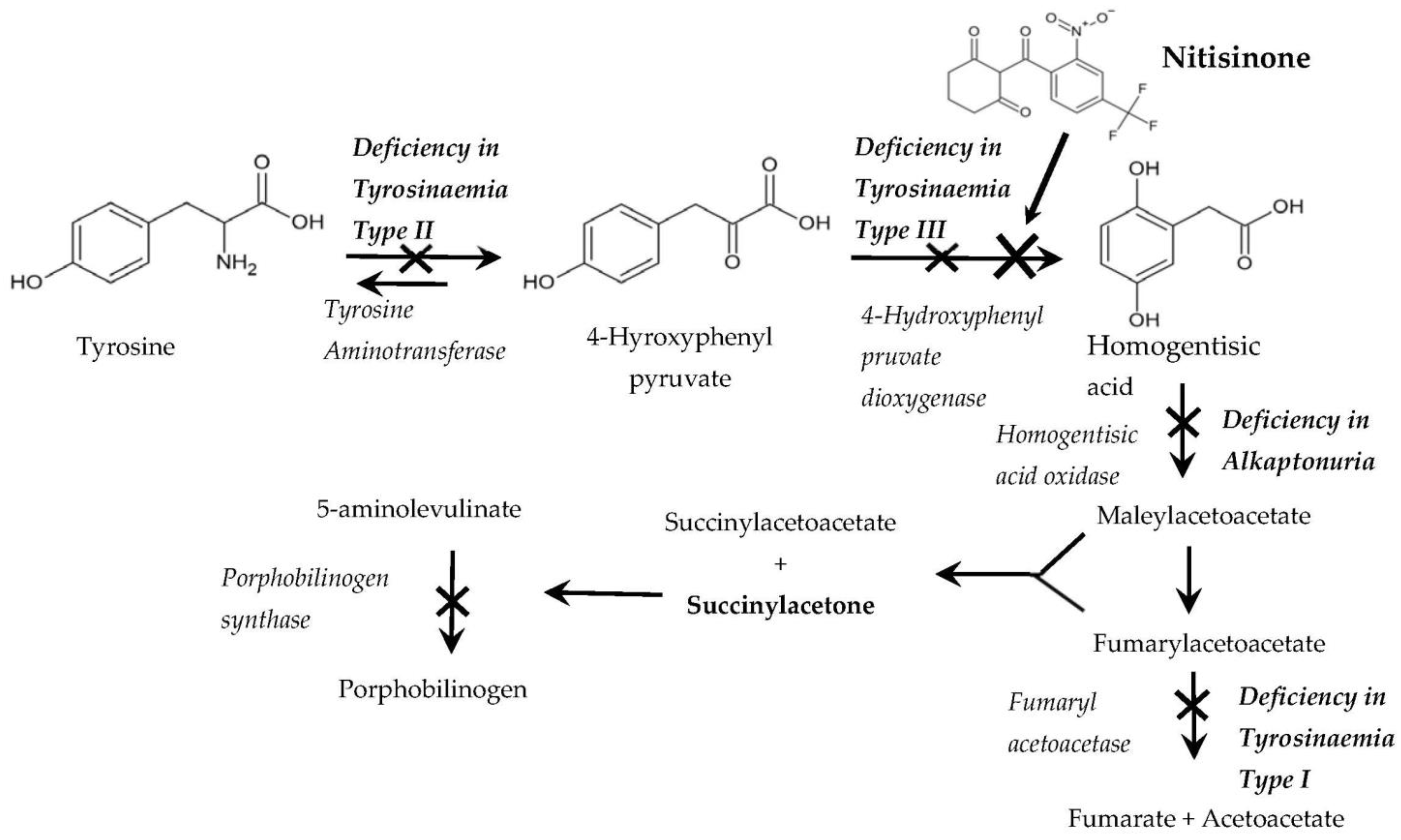
:1. Introduction
The Discovery of the Mode of Action of Nitisinone
2. Results and Conclusion
Use of Nitisinone as a Drug to Treat Rare Hereditary Disorders of Tyrosine Catabolism
Funding
Acknowledgments
Conflicts of Interest
References
- Prisbylla, M.P.; Onisko, B.C.; Shribbs, J.M. The novel mechanism of action of the herbicidal triketones. Proc. Brighton Crop Protection Conf. Weeds 1993, 2, 731–738. [Google Scholar]
- Beaudegnies, R.; Edmunds, A.J.; Fraser, T.E.; Hall, R.G.; Hawkes, T.R.; Mitchell, G.; Schaetzer, J.; Wendeborn, S.; Wibley, J. Herbicidal 4-hydroxyphenylpyruvate dioxygenase inhibitors-A review of the triketone chemistry story from a Syngenta perspective. Bioorganic Med. Chem. 2009, 17, 4134–4152. [Google Scholar] [CrossRef] [PubMed]
- Lock, E.A.; Gaskin, P.; Ellis, M.K.; Provan, W.M.; Robinson, M.; Smith, L.L.; Mutter, L.C. Tissue distribution of 2-(2-nitro-4-trifluoromethylbenzoyl)-cyclohexane-1,3-dione (NTBC): Effect on enzymes involved in tyrosine catabolism and relevance to ocular toxicity in the rat. Toxicol. Appl. Pharmacol. 1996, 141, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Lock, E.A.; Gaskin, P.; Ellis, M.; Provan, W.M.; Smith, L.L. Tyrosinemia produced by 2-(2-nitro-4-trifluoromethylbenzoyl)-cyclohexane-1,3-dione (NTBC) in experimental animals and its relationship to corneal injury. Toxicol. Appl. Pharmacol. 2006, 215, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Ellis, M.K.; Whitfield, A.C.; GOwANS, L.A.; Auton, T.R.; Provan, W.M.; Lock, E.A.; Smith, L.L. Inhibition of 4-hydroxyphenylpruvate dioxygenase by 2-(2-nitro-4-trifluoromethylbenzoyl)-cyclohexane-1,3-dione and 2-(2-chloro-4-methanesulphonylbenzoyl)-cyclohexane-1,3-dione. Toxicol. Appl. Pharmacol. 1995, 133, 12–19. [Google Scholar] [CrossRef]
- Rich, L.F.; Beard, M.E.; Burns, R.P. Excess dietary tyrosine and corneal lesions. Exp. Eye. Res. 1973, 17, 87–97. [Google Scholar] [CrossRef]
- Goldsmith, L.A. Tyrosinaemia II: Lessons in molecular pathology. J. Pediatr. 1983, 1, 25–34. [Google Scholar]
- Barroso, F.; Correia, J.; Bandeira, A.; Carmona, C.; Vilarinho, L.; Almeida, M.; Martins, E. Tyrosinaemia type III: A case report of sibling and a literature review. Rev. Paul. Pediatr. 2020, 38, e2018158. [Google Scholar] [CrossRef]
- Mostofizadeh, N.; Najafi, R.; Hashemipour, M. A Case of Tyrosinemia Type III with Status Epilepticus and Mental Retardation. Adv. Biomed. Res. 2018, 7, 7. [Google Scholar] [CrossRef]
- Lindstedt, S.; Holme, E.; Lock, E.A.; Hjalmarson, O.; Strandvik, B. Treatment of hereditary tyrosinaemia type 1 by inhibition of 4-hydroxyphenylpyruvate dioxygenase. Lancet 1992, 340, 813–817. [Google Scholar] [CrossRef]
- Lock, E.A.; Ellis, M.K.; Gaskin, P.; Robinson, M.; Auton, T.R.; Provan, W.M.; Lee, D.L. From toxicological problem to therapeutic use: The discovery of the mode of action of 2-(2-nitro-4-trifluoromethylbenzoyl)-cyclohexane-1,3-dione (NTBC), its toxicology and development as a drug. J. Inherit. Metab. Dis. 1998, 21, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Thimm, E.; Richter-Werkle, R.; Kamp, G.; Molke, B.; Herebian, D.; Klee, D.; Mayatepek, E.; Spiekerkoetter, U. Neurocognitive outcome in patients with hypertyrosinemia type I after long-term treatment with NTBC. J. Inherit. Metab. Dis. 2012, 35, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Bendadi, F.; De Koning, T.J.; Visser, G.; Prinsen, H.C.; De Sain, M.G.; Verhoeven-Duif, N.; Van Hasselt, P.M. Impaired cognitive functioning in patients with tyrosinaemia type 1 receiving nitisinone. J. Pediatr. 2014, 164, 398–401. [Google Scholar] [CrossRef] [PubMed]
- Couce, M.L.; Sánchez-Pintos, P.; Aldámiz-Echevarría, L.; Vitoria, I.; Navas, V.; Martín-Hernández, E.; Díaz-Fernández, C. Evolution of tyrosinaemia type 1 disease in patients treated with Nitisinone in Spain. Medicine (Baltimore) 2019, 98, e17303. [Google Scholar] [CrossRef] [PubMed]
- Van Vliet, K.; van Ginkel, W.G.; Jahja, R.; Daly, A.; MacDonald, A.; De Laet, C.; van Spronsen, F.J. Emotional and behavioural problems, quality of life and metabolic control in NTBC-treated tyrosinaemia type 1 patients. Orphanet J. Rare Dis. 2019, 14, 285. [Google Scholar] [CrossRef]
- Spiekerkoetter, U.; Couce, M.L.; Das, A.M.; de Laet, C.; Dionisi-Vici, C.; Lund, A.M.; Schiff, M.; Spada, M.; Sparve, E.; Szamosi, J.; et al. Long-term safety and outcomes in hereditary tyrosinaemia type 1 with nitisinone treatment: A 15-year non-interventional, multicentre study. Lancet Diabetes-Endocrinol. 2021, 9, 427–435. [Google Scholar] [CrossRef]
- Introne, W.J. Nitisinone: Two decades treating hereditary tyrosinaemia type 1. Lancet Diabetes-Endocrinol. 2021, 9, 409–411. [Google Scholar] [CrossRef]
- Adams, D.R.; Menezes, S.; Jauregui, R.; Valivullah, Z.M.; Power, B.; Abraham, M.; Jeffrey, B.G.; Garced, A.; Alur, R.P.; Cunningham, D.; et al. One-year pilot study on the effects of nitisinone on melanin in patients with OCA-1B. JCI Insight 2019, 4, 124387. [Google Scholar] [CrossRef]
- Introne, W.J.; Perry, M.B.; Troendle, J.; Tsilou, E.; Kayser, M.A.; Suwannarat, P.; Gahl, W.A. A 3-year randomised therapeutic trial of nitisinone in alkaptonuria. Mol. Genet. Metab. 2011, 103, 307–314. [Google Scholar] [CrossRef]
- Ranganath, L.R.; Psarelli, E.E.; Arnoux, J.B.; Braconi, D.; Briggs, M.; Broijersen, A. Efficacy and safety of once-daily nitisinone for patients with alkaptonuria (SONIA 2): An international, multicentre, open-label, randomised controlled trial. Lancet Diabetes-Endocrinol. 2020, 8, 762–772. [Google Scholar] [CrossRef]
- Ranganath, L.; Khedr, M.; Milan, A.; Davison, A.; Hughes, A.; Usher, J.; Taylor, S.; Loftus, N.; Daroszewska, A.; West, E.; et al. Nitisinone arrests ochronosis and decreases rate of progression of Alkaptonuria: Evaluation of the effect of nitisinone in the United Kingdom National Alkaptonuria Centre. Mol. Genet. Metab. 2018, 125, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.S.Z.; Ahmed, M.; Khedr, M.; Borgia, A.; Madden, A.; Ranganath, L.R.; Kaye, S. Association of alkaptonuria and low dose Nitisinone therapy with cataract formation in a large cohort of patients. JIMD Rep. 2022, 63, 351–360. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lock, E.A. The Discovery of the Mode of Action of Nitisinone. Metabolites 2022, 12, 902. https://doi.org/10.3390/metabo12100902
Lock EA. The Discovery of the Mode of Action of Nitisinone. Metabolites. 2022; 12(10):902. https://doi.org/10.3390/metabo12100902
Chicago/Turabian StyleLock, Edward A. 2022. "The Discovery of the Mode of Action of Nitisinone" Metabolites 12, no. 10: 902. https://doi.org/10.3390/metabo12100902
APA StyleLock, E. A. (2022). The Discovery of the Mode of Action of Nitisinone. Metabolites, 12(10), 902. https://doi.org/10.3390/metabo12100902






