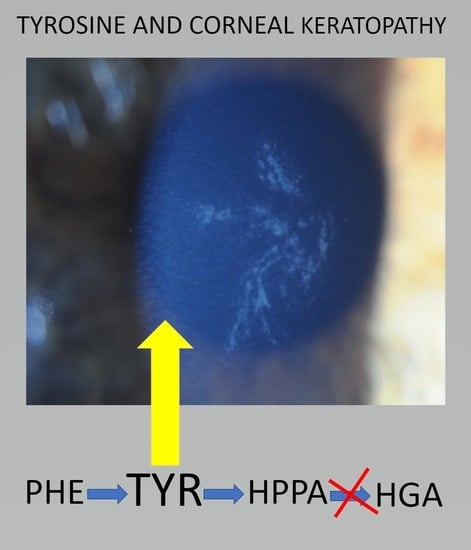
Comparing the Phenylalanine/Tyrosine Pathway and Related Factors between Keratopathy and No-Keratopathy Groups as Well as between Genders in Alkaptonuria during Nitisinone Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Procedures
2.3. Chemical Analyses
2.4. Statistical Analysis
3. Results
3.1. Demographics
3.2. Keratopathy (KP) and No-Keratopathy (NKP) Demographics
3.3. Female and Male Demographics
3.4. Comparison of Metabolite and Other Analytes in the KP and NKP Groups
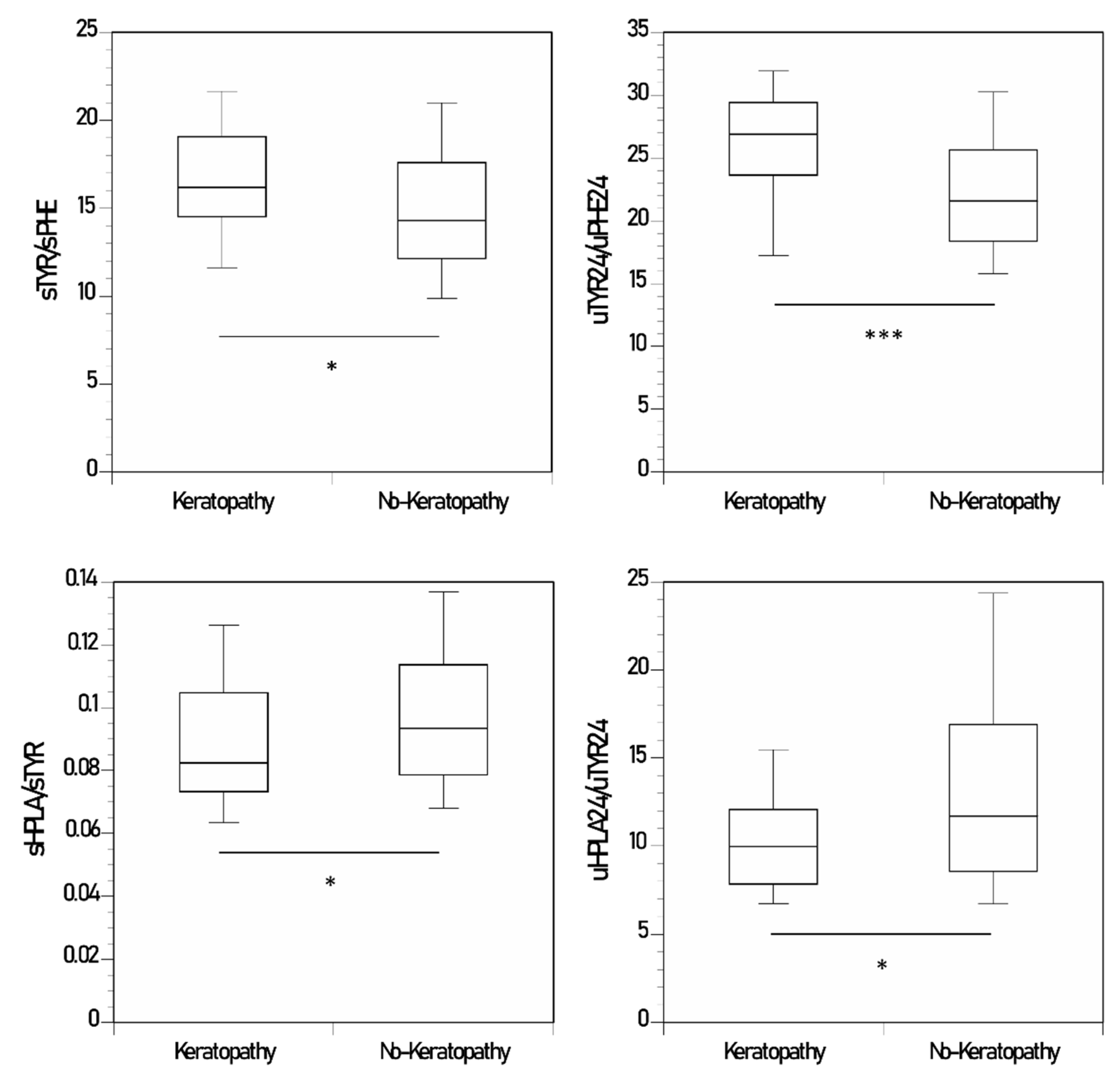
3.5. Keratopathy and No-Keratopathy Comparison of Ratio of Metabolites
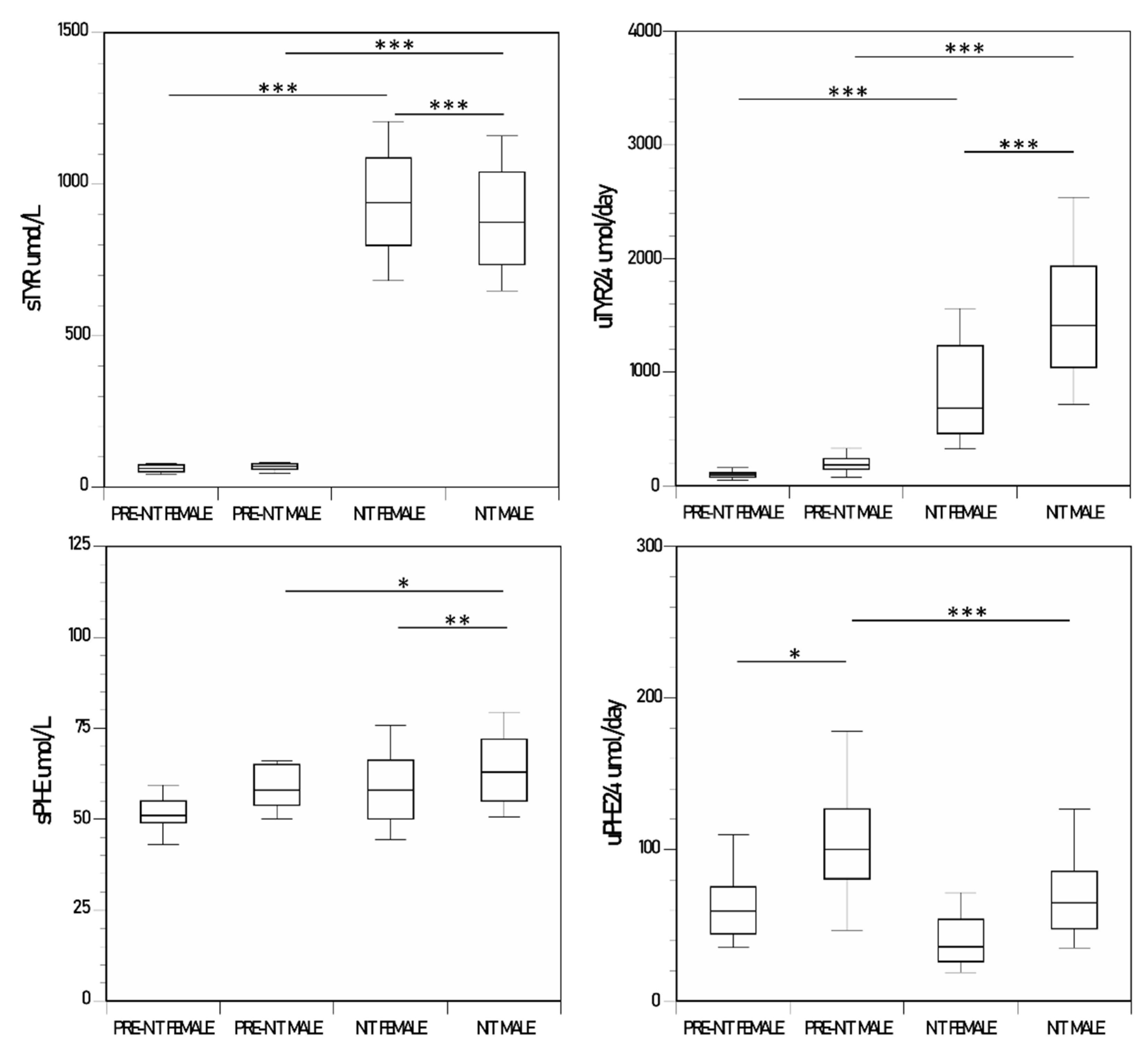
3.6. Comparison of Metabolite and Other Analytes in Males and Females
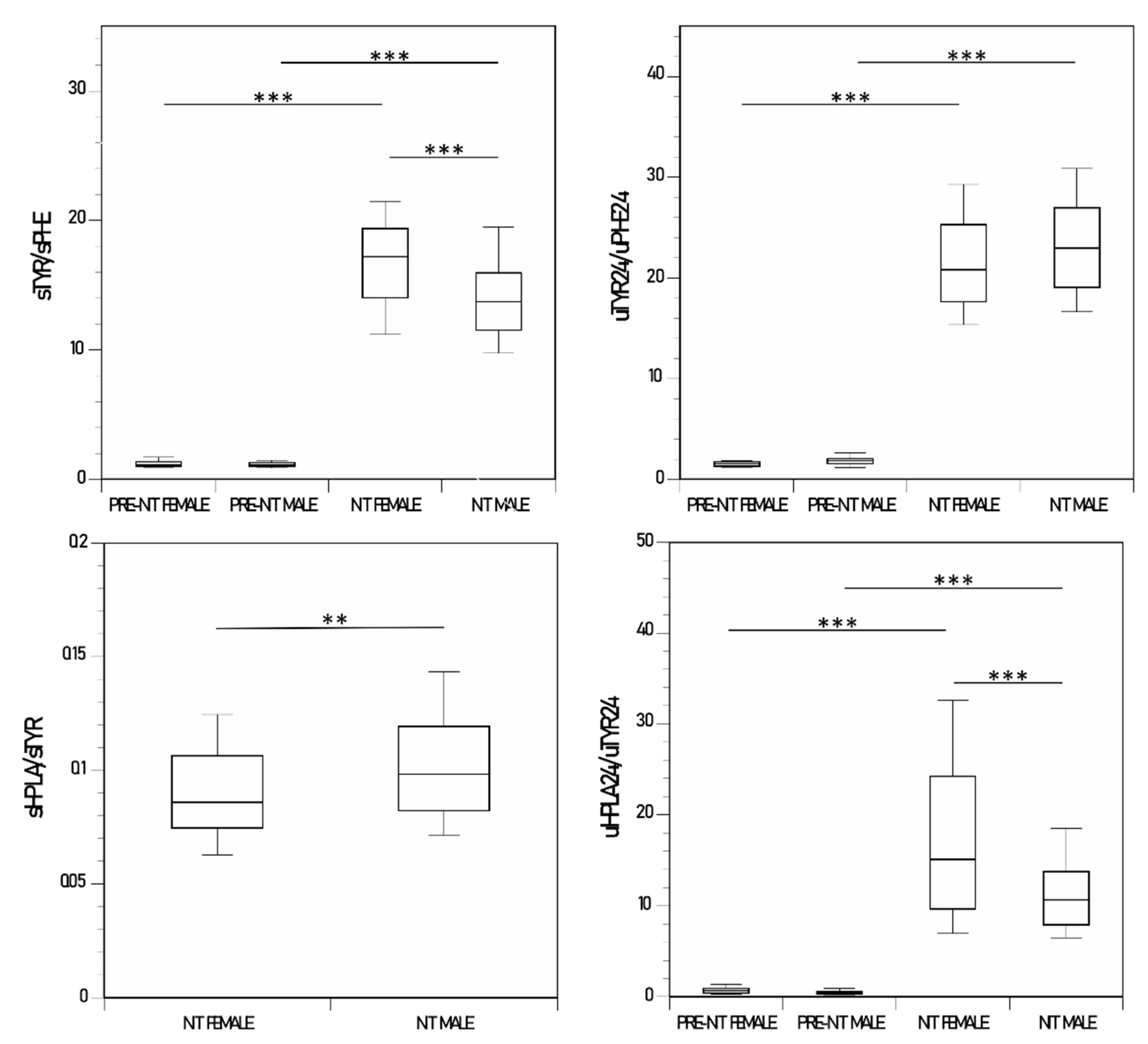
3.7. Female and Male Comparison of Ratio of Metabolites
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Jesús, V.R.; Adam, B.W.; Mandel, D.; Cuthbert, C.D.; Matern, D. Succinylacetone as primary marker to detect tyrosinemia type I in newborns and its measurement by newborn screening programs. Mol. Genet. Metab. 2014, 113, 67–75. [Google Scholar] [PubMed] [Green Version]
- Peña-Quintana, L.; Scherer, G.; Curbelo-Estévez, M.L.; Jiménez-Acosta, F.; Hartmann, B.; La Roche, F.; Meavilla-Olivas, S.; Pérez-Cerdá, C.; García-Segarra, N.; Giguère, Y.; et al. Tyrosinemia type II: Mutation update, 11 novel mutations and description of 5 independent subjects with a novel founder mutation. Clin. Genet. 2017, 92, 306–317. [Google Scholar] [PubMed]
- Szymanska, E.; Sredzinsk, M.; Ciara, E.; Piekutowska-Abramczuk, D.; Ploski, R.; Rokicki, D.; Tylki-Szymanska, A. Tyrosinemia type III in an asymptomatic girl. Mol. Genet. Metab. Rep. 2015, 5, 48–50. [Google Scholar] [PubMed]
- Lindstedt, S.; Holme, E.; Lock, E.A.; Hjalmarson, O.; Strandvik, B. Treatment of Hereditary Tyrosinaemia Type I by Inhibition of 4-Hydroxyphenylpyruvate Dioxygenase. Lancet 1992, 340, 813–817. [Google Scholar] [PubMed]
- Ranganath, L.R.; Hughes, A.T.; Davison, A.S.; Khedr, M.; Olsson, B.; Rudebeck, M.; Imrich, R.; Norman, B.P.; Bou-Gharios, G.; Gallagher, J.A.; et al. Temporal adaptations in the phenylalanine/tyrosine pathway and related factors during nitisinone-induced tyrosinaemia in alkaptonuria. Mol. Genet. Metab. 2022; in press. [Google Scholar] [CrossRef]
- Pencharz, P.B.; Hsu, J.W.-C.; Ball, R.O. Aromatic amino acid requirements in healthy human subjects. J. Nutr. discussion 1597S–1598S. 2007, 137, 1576S–1578S. [Google Scholar]
- Milan, A.M.; Hughes, A.T.; Davison, A.S.; Khedr, M.; Rovensky, J.; Psarelli, E.E.; Cox, T.F.; Rhodes, N.P.; Gallagher, J.A.; Ranganath, L.R. Unmasking nature: Quantification of tyrosine flux in the ochronotic pathway during nitisinone treatment of Alkaptonuria. Sci. Rep. 2019, 9, 10024. [Google Scholar] [PubMed]
- Khedr, M.; Cooper, M.; Hughes, A.T.; Milan, A.M.; Davison, A.S.; Norman, B.P.; Sutherland, H.; Jarvis, J.C.; Fitzgerald, R.; Markinson, L.; et al. Nitisinone causes acquired tyrosinosis in alkaptonuria. J. Inherit. Metab. Dis. 2020, 43, 1014–1023. [Google Scholar]
- Stewart, R.M.; Briggs, M.C.; Jarvis, J.C.; Gallagher, J.A.; Ranganath, L. Reversible Keratopathy Due to Hypertyrosinaemia Following Intermittent Low-Dose Nitisinone in Alkaptonuria: A Case Report. JIMD Rep. 2014, 17, 1–6. [Google Scholar]
- Ranganath, L.R.; Khedr, M.; Evans, L.A.; Bygott, H.; Luangrath, E.; West, E. Vitiligo, alkaptonuria, and nitisinone—A report of four cases and review of the literature. JIMD Rep. 2022, 63, 351–360. [Google Scholar] [CrossRef]
- Ahmad, M.S.Z.; Ahmed, M.; Khedr, M.; Borgia, A.; Madden, A.; Ranganath, L.R.; Kaye, S. Association of alkaptonuria and low dose nitisinone therapy with cataract formation in a large cohort of patients with alkaptonuria. JIMD Rep. 2022, 1–10. [Google Scholar] [CrossRef]
- van Ginkel, W.G.; Jahja, R.; Huijbregts, S.C.; Daly, A.; MacDonald, A.; De Laet, C.; Cassiman, D.; Eyskens, F.; Körver-Keularts, I.M.L.W.; Goyens, P.J.; et al. Neurocognitive outcome in tyrosinemia type 1 patients compared to healthy controls. Orphanet J. Rare Dis. 2016, 11, 87. [Google Scholar]
- Khedr, M.; Judd, S.; Briggs, M.C.; Hughes, A.T.; Milan, A.M.; Stewart, R.M.K.; Lock, E.A.; Gallagher, J.A.; Ranganath, L.R. Asymptomatic Corneal Keratopathy Secondary to Hypertyrosinaemia Following Low Dose Nitisinone and a Literature Review of Tyrosine Keratopathy in Alkaptonuria. JIMD Rep. 2018, 40, 31–37. [Google Scholar] [PubMed]
- Lock, E.A.; Ellis, M.K.; Gaskin, P.; Robinson, M.; Auton, T.R.; Provan, W.M.; Smith, L.L.; Prisbylla, M.P.; Mutter, L.C.; Lee, D.L. From toxicological problem to therapeutic use: The discovery of the mode of action of 2-(2-nitro-4-trifluoromethylbenzoyl)-1,3-cyclohexanedione (NTBC), its toxicology and development as a drug. J. Inherit. Metab. Dis. 1998, 21, 498–506. [Google Scholar] [PubMed]
- Lock, E.A.; Gaskin, P.; Ellis, M.K.; Provan, W.M.; Robinson, M.; Smith, L.L.; Prisbylla, M.P.; Mutter, L.C. Tissue distribution of 2-(2- nitro-4 trifluromethylbenzoyl)-1,3-cyclohexanedione (NTBC): Effect on enzymes involved in tyrosine catabolism and relevance to ocular toxicity in the rat. Toxicol. Appl. Pharmacol. 1996, 141, 439–447. [Google Scholar] [PubMed]
- O’Brien, W.M.; La Du, B.N.; Bunim, J.J. Biochemical, pathologic and clinical aspects of alcaptonuria, ochronosis and ochronotic arthropathy: Review of world literature (1584–1962). Am. J. Med. 1963, 34, 813–838. [Google Scholar]
- Phomphutkul, C.; Introne, W.J.; Perry, M.B.; Bernardini, I.; Murphey, M.D.; Fitzpatrick, D.L.; Anderson, P.D.; Huizing, M.; Anikster, Y.; Gerber, L.H.; et al. Natural History of Alkaptonuria. N. Eng. J. Med. 2002, 347, 2111–2121. [Google Scholar]
- Ranganath, L.R.; Psarelli, E.E.; Arnoux, J.-B.; Braconi, D.; Briggs, M.; Bröijersén, A.; Loftus, N.; Bygott, H.; Cox, T.F.; Davison, A.S.; et al. Efficacy and Safety of Once-Daily Nitisinone for Patients with Alkaptonuria (SONIA 2): An International, Multicentre, Open-Label, Randomised Controlled Trial. Lancet Diabetes Endocrinol. 2020, 8, 762–772. [Google Scholar]
- First Treatment for Rare Metabolic Disorder Alkaptonuria. Available online: https://www.ema.europa.eu/en/news/first-treatment-raremetabolic-disorder-alkaptonuria (accessed on 15 February 2022).
- Introne, W.J.; Perry, M.B.; Troendle, J.; Tsilou, E.; Kayser, M.A.; Suwannarat, P.; O’Brien, K.E.; Bryant, J.; Sachdev, V.; Reynolds, J.C.; et al. A 3-year randomized therapeutic trial of nitisinone in Alkaptonuria. Mol. Genet. Metab. 2011, 103, 307–314. [Google Scholar]
- McKiernan, P.J. Nitisinone in the treatment of hereditary tyrosinaemia type I. Drugs 2006, 66, 743–750. [Google Scholar]
- Ranganath, L.R.; Khedr, M.; Milan, A.M.; Davison, A.S.; Hughes, A.T.; Usher, J.L.; Taylor, S.; Loftus, N.; Daroszewska, A.; West, E.; et al. Nitisinone arrests ochronosis and decreases rate of progression of Alkaptonuria: Evaluation of the effect of nitisinone in the United Kingdom National Alkaptonuria Centre. Mol. Genet. Metab. 2018, 125, 127–134. [Google Scholar] [PubMed]
- Hughes, A.T.; Milan, A.M.; Shweihdi, E.; Gallagher, J.A.; Ranganath, L.R. Method development and validation for analysis of phenylalanine, 4-hydroxyphenyllactic acid and 4-hydroxyphenylpyruvic acid in serum and urine. JIMD Rep. 2022, 63, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Kida, Y.; Ueda, H.; Tanaka, H.; Ichinose, M. Estimation of protein intake using urinary urea nitrogen in patients with early-stage liver cirrhosis. Hepatol. Int. 2007, 1, 382–386. [Google Scholar] [PubMed] [Green Version]
- Maroni, B.J.; Steinman, T.I.; Mitch, W.E. A method for estimating nitrogen intake of patients with chronic renal failure. Kidney Int. 1985, 27, 58–65. [Google Scholar]
- Ranganath, L.R.; Milan, A.M.; Hughes, A.T.; Davison, A.S.; Khedr, M.; Norman, B.P.; Bou-Gharios, G.; Gallagher, J.A.; Gornall, M.; Jackson, R.; et al. Characterization of changes in the tyrosine pathway by 24-hour profiling during nitisinone treatment in alkaptonuria. Mol. Genet. Metab. 2022, 30, 100846. [Google Scholar]
- Aktuglu-Zeybek, A.C.; Zubarioglu, T. Nitisinone: A review. Orphan Drugs Res. Rev. 2017, 7, 25–35. [Google Scholar]
- Rafii, M.; Chapman, K.; Owens, J.; Elango, R.; Campbell, W.W.; Ball, R.O.; Pencharz, P.B.; Courtney-Martin, G. Dietary Protein Requirement of Female Adults > 65 Years Determined by the Indicator Amino Acid Oxidation Technique Is Higher Than Current Recommendations. J. Nutr. 2015, 145, 18–24. [Google Scholar]
- Lonnie, M.; Hooker, E.; Brunstrom, J.M.; Corfe, B.M.; Green, M.A.; Watson, A.W.; Williams, E.A.; Stevenson, E.J.; Penson, S.; Johnstone, A.M. Protein for Life: Review of Optimal Protein Intake, Sustainable Dietary Sources and the Effect on Appetite in Ageing Adults. Nutrients 2018, 10, 360. [Google Scholar]
- Cox, T.F.; Ranganath, L. A quantitative assessment of alkaptonuria: Testing the reliability of two disease severity scoring systems. J. Inherit. Metab. Dis. 2011, 34, 1153–1162. [Google Scholar]
- Ranganath, L.R.; Cox, T.F. Natural history of alkaptonuria revisited: Analyses based on scoring systems. J. Inherit. Metab. Dis. 2011, 34, 1141–1151. [Google Scholar]
- Layton, A.T.; Sullivan, J.C. Recent advances in sex differences in kidney function. Am. J. Physiol. Renal Physiol. 2019, 316, F328–F331. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, M.A.; Didelija, I.C.; Marini, J.C. Arginase II Plays a Central Role in the Sexual Dimorphism of Arginine Metabolism in C57BL/6 Mice. J. Nutr. 2020, 150, 3133–3140. [Google Scholar] [CrossRef] [PubMed]
- Krumsiek, J.; Mittelstrass, K.; Do, K.T.; Stuckler, F.; Ried, J.; Adamski, J.; Peters, A.; Illig, T.; Kronenberg, F.; Friedrich, N.; et al. Gender-specific pathway differences in the human serum metabolome. Metabolomics 2015, 11, 1815–1833. [Google Scholar]
- Schuck, P.F.; Malgarin, F.; Cararo, J.H.; Cardoso, F.; Streck, E.L.; Ferreira, G.C. Phenylketonuria pathophysiology: On the role of metabolic alterations. Aging Dis. 2016, 6, 390–399. [Google Scholar]

| Age, Weight, uUREA24, uUREA/kg, uCREAT24 and Measured Metabolic Data in NIT-Treated AKU Patients Based on Keratopathy Status and Sex | ||||||
|---|---|---|---|---|---|---|
| Keratopathy Status | Sex | |||||
| Keratopathy (n = 35) | No-Keratopathy (n = 272) | Female | Male | |||
| Pre-NIT (n = 24) | NIT (n = 100) | Pre-NIT (n = 45) | NIT (n = 207) | |||
| Age years | 44.6 (10.4) *** | 51.4 (10.9) | 51.9 (9.6) | 54.0 (9.5) | 47.4 (11.9) | 49.5 (11.8) |
| Weight Kg | 75.9 (7.1) | 78.1 (16.3) | 66.3 (15.1) | 70.1 (16.3) | 79.2 (12.6) | 81.5 (13.5) |
| uUREA24 mmol/day | 326 (97) | 290 (148) | 277 (95) | 233 (102) | 333 (88) | 324 (148) |
| uUREA mmol/Kg | 4.3 (1.3) | 3.8 (1.9) | 4.3 (1.5) | 3.4 (1.6) | 4.2 (1.0) | 4.0 (1.8) |
| uCREAT24 mmol/day | 13.1 (7.8) * | 10.5 (6.6) | 8.2 (2.3) | 7.5 (3.1) | 11.4 (2.7) | 12.4 (7.3) |
| sHGA µmol/L | 1.9 (2.1) **** | 0.7 (1.1) | 27.9 (10.4) | 0.77 (1.5) | 31.7 (11.2) | 1.1 (2.6) |
| sTYR µmol/L | 982 (167) | 913 (231) | 62 (17) | 968 (256) | 67 (14) | 893 (214) |
| sPHE µmol/L | 61.4 (13.3) | 63.4 (14) | 52.1 (7.3) | 59.4 (15) | 59.3 (9.6) | 64.9 (13) |
| sHPPA µmol/L L | 36.6 (6.9) | 39.6 (18.7) | - | 39.4 (27) | - | 39.3 (12.3) |
| sHPLA µmol/L | 88.3 (29.9) | 90.9 (32.6) | - | 86.1 (38.3) | - | 89.6 (33.2) |
| sNIT µmol/L | 3.7 (2.7) ** | 5.1 (2.7) | - | 5.5 (3.3) | - | 4.4 (2.4) |
| uHGA24 µmol /day | 645 (882) **** | 202 (481) | 31,024 (13447) | 158 (521) | 37,098 (12575) | 298 (571) |
| uTYR24 µmol /day | 1672 (858) ** | 1290 (765) | 100 (46) | 867 (491) | 196 (88) | 1555 (801) |
| uPHE24 µmol /day | 68.6 (41.7) | 61.5 (38.7) | 65 (28) | 41 (21) | 152 (332) | 73 (41) |
| uHPPA24 µmol /day | 17,566 (6494) | 16,375 (8406) | 75 (39) | 13,497 (5719) | 189 (152) | 17,939 (8813) |
| uHPLA24 µmol /day | 15,987 (6300) | 14211 (6081) | 63 (28) | 11,942 (4501) | 99 (76) | 15,585 (6441) |
| sHGA/sTYR | 0.002 (0.003) **** | 0.001 (0.001) | 0.46 (0.16) | 0.0008 (0.001) | 0.49 (0.18) | 0.003 (0.03) |
| sTYR/sPHE | 16.6 (4.0) * | 14.8 (4.1) | 1.2 (0.3) | 16.8 (4.2) | 1.1 (0.24) | 14.1 (3.9) |
| sHPPA/sTYR | 0.04 (0.009) | 0.05 (0.02) | - | 0.04 (0.03) | - | 0.045 (0.01) |
| sHPPA/sHPLA | 0.46 (0.17) | 0.47 (0.26) | - | 0.49 (0.37) | - | 0.46 (0.16) |
| sHPLA/sTYR | 0.09 (0.02) * | 0.10 (0.03) | - | 0.09 (0.03) | - | 0.1 (0.03) |
| uHGA24/uTYR24 | 0.43 (0.61) * | 0.18 (0.63) | 354 (157) | 0.22 (0.92) | 219 (107) | 0.21 (0.44) |
| uTYR24/uPHE24 | 25.9 (5.8) *** | 22.2 (5.7) | 1.53 (0.3) | 21.6 (5.9) | 1.9 (0.8) | 23.2 (5.8) |
| uHPPA24/uTYR24 | 11.3 (3.2) ** | 15.8 (9.5) | 0.85 (0.12) | 19.8 (11.7) | 0.85 (0.56) | 13.1 (6.6) |
| uHPPA24/uHPLA24 | 1.1 (0.32) | 1.2 (0.93) | 1.52 (1.0) | 1.3 (1.4) | 1.66 (1.3) | 1.18 (0.4) |
| uHPLA24/uTYR24 | 10.8 (4.8) * | 14.6 (10.9) | 0.74 (0.4) | 18.5 (13.6) | 0.49 (0.27) | 12.1 (7.7) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ranganath, L.R.; Milan, A.M.; Hughes, A.T.; Davison, A.S.; Khedr, M.; Imrich, R.; Rudebeck, M.; Olsson, B.; Norman, B.P.; Bou-Gharios, G.; et al. Comparing the Phenylalanine/Tyrosine Pathway and Related Factors between Keratopathy and No-Keratopathy Groups as Well as between Genders in Alkaptonuria during Nitisinone Treatment. Metabolites 2022, 12, 772. https://doi.org/10.3390/metabo12080772
Ranganath LR, Milan AM, Hughes AT, Davison AS, Khedr M, Imrich R, Rudebeck M, Olsson B, Norman BP, Bou-Gharios G, et al. Comparing the Phenylalanine/Tyrosine Pathway and Related Factors between Keratopathy and No-Keratopathy Groups as Well as between Genders in Alkaptonuria during Nitisinone Treatment. Metabolites. 2022; 12(8):772. https://doi.org/10.3390/metabo12080772
Chicago/Turabian StyleRanganath, Lakshminarayan R., Anna M. Milan, Andrew T. Hughes, Andrew S. Davison, Milad Khedr, Richard Imrich, Mattias Rudebeck, Birgitta Olsson, Brendan P. Norman, George Bou-Gharios, and et al. 2022. "Comparing the Phenylalanine/Tyrosine Pathway and Related Factors between Keratopathy and No-Keratopathy Groups as Well as between Genders in Alkaptonuria during Nitisinone Treatment" Metabolites 12, no. 8: 772. https://doi.org/10.3390/metabo12080772
APA StyleRanganath, L. R., Milan, A. M., Hughes, A. T., Davison, A. S., Khedr, M., Imrich, R., Rudebeck, M., Olsson, B., Norman, B. P., Bou-Gharios, G., & Gallagher, J. A. (2022). Comparing the Phenylalanine/Tyrosine Pathway and Related Factors between Keratopathy and No-Keratopathy Groups as Well as between Genders in Alkaptonuria during Nitisinone Treatment. Metabolites, 12(8), 772. https://doi.org/10.3390/metabo12080772